Effects of the Ecdysone Receptor on the Regulation of Reproduction in Coccinella septempunctata
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Insects
2.2. Ladybug Diets
2.3. Sample Handling and Collection
2.4. RNA Extraction and cDNA Synthesis
2.5. RNA Interference
2.5.1. Synthesis of EcR dsRNA
2.5.2. Injection of dsRNA
2.5.3. Effects of RNAi on Ladybug Adults
2.6. Quantitative Real-Time PCR (qPCR)
2.7. Data Analysis
3. Results
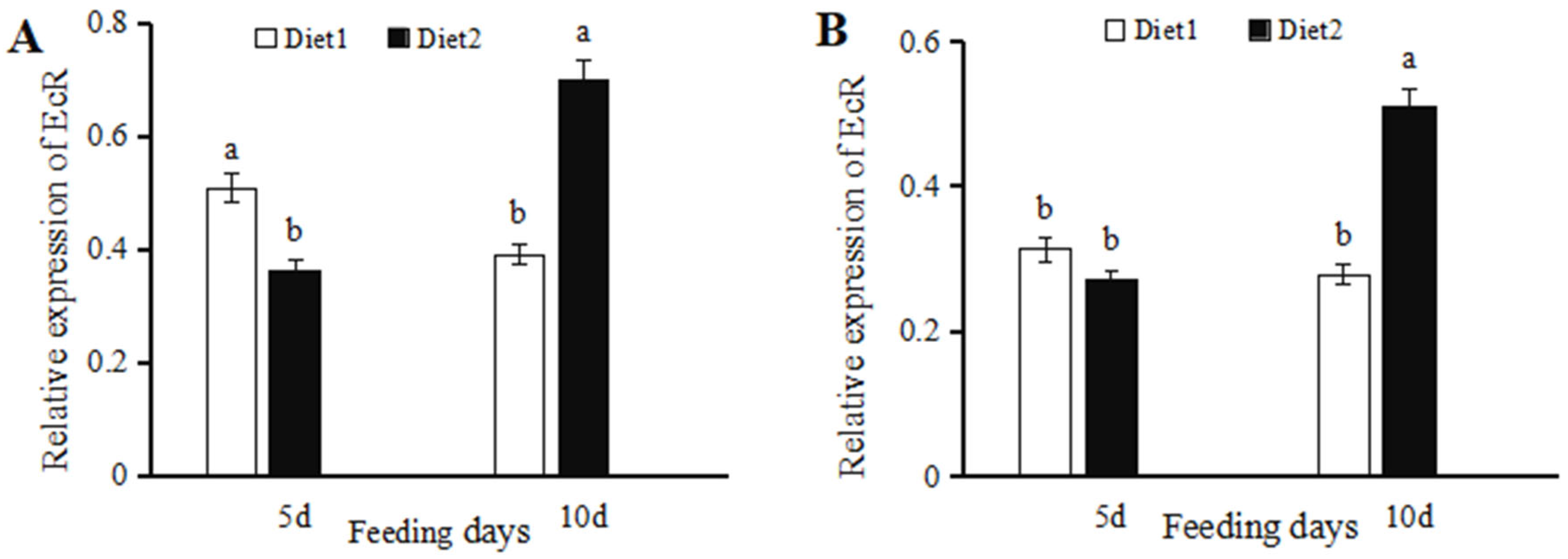
3.1. 20-Hydroxyecdysone-Mediated Changes in EcR Transcription
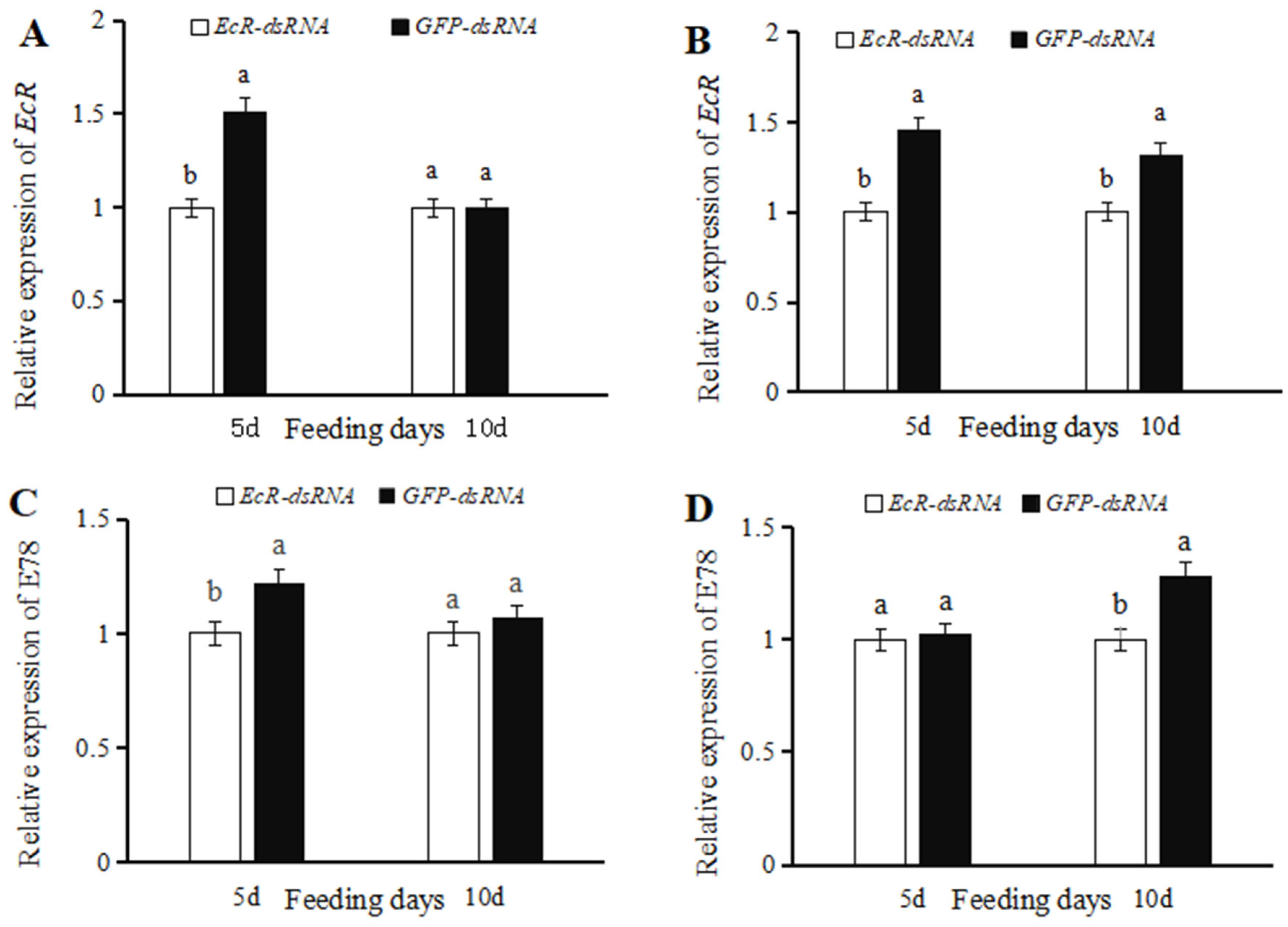
3.2. Effects of dsRNA on EcR Expression
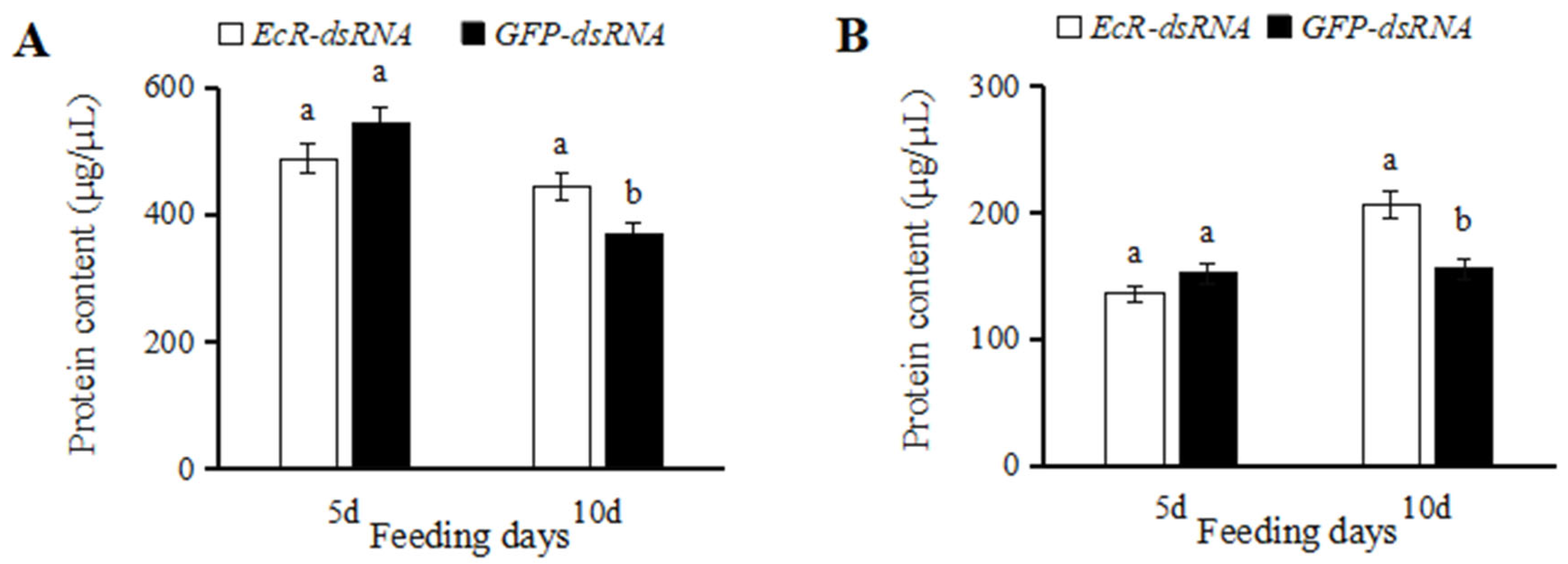
3.3. Effects of dsRNA Injection on Ovaries and Testes
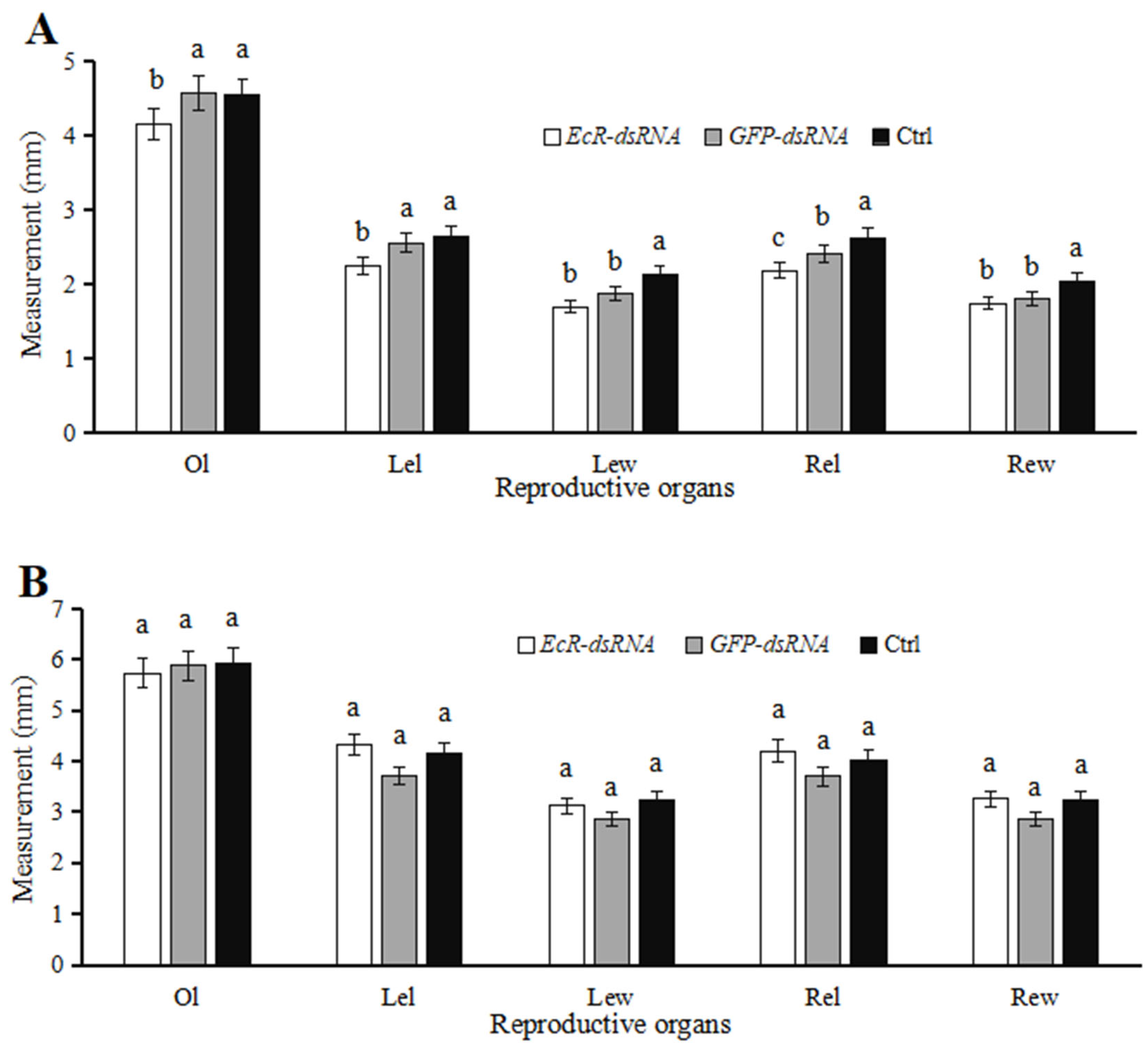
3.4. Effects of dsRNA Injection on Development of Reproductive Systems
3.5. Effect of dsRNA Injection on Egg Production and Hatching
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Cartwright, B.O.; Eikenbary, R.D.; Angalet, G.W.; Ampbell, R.K. Release and establishment of Coccinella septempunctata in Oklahoma. Environ. Entomol. 1979, 8, 819–823. [Google Scholar] [CrossRef]
- Hance, T.; Dixon, A.F.G. Insect predator-prey dynamics: Ladybird beetles and biological control. J. Insect Conserv. 2006, 10, 375–376.s. [Google Scholar] [CrossRef]
- Hodek, I.; Michaud, J.P. Why is Coccinella septempunctata so successful? (A point-of-view). Eur. J. Entomol. 2008, 105, 1–12. [Google Scholar] [CrossRef]
- Duan, Y.J.; He, H.G.; Pu, D.Q.; Yang, H.; Chen, Q.; Lu, Q.C. Basic research status of Coccinella septempunctata. J. Biosaf. 2021, 30, 20–28. [Google Scholar]
- Smirnoff, W.A. An artificial diet for rearing Coccinellid beetles. Can. Entomol. 1958, 90, 563–565. [Google Scholar] [CrossRef]
- Yang, D.L.; Hao, J.; Wang, Y.N.; Xi, G.S. Research progress of ecdysone in adult insects. Chin. Bull. Life Sci. 2014, 26, 874–881. [Google Scholar]
- Niwa, R.; Niwa, Y.S. Enzymes for ecdysteroid biosynthesis: Their biological functions in insects and beyond. Biosci. Biotech. Bioch. 2014, 78, 1283–1292. [Google Scholar] [CrossRef]
- Zhu, J.S. Non-genomic action of juvenile hormone modulates the synthesis of 20- hydroxyecdysone in Drosophila. Sci. Bull. 2022, 67, 117–118. [Google Scholar] [CrossRef]
- Feyereisen, R. Insect CYP genes and P450 enzymes. Insect Mol. Miol. 2012, 8, 236–316. [Google Scholar]
- Wang, J.J.; Hu, Q.B. A review of ecdysone receptor. J. Environ. Entomol. 2017, 39, 721–729. [Google Scholar]
- Ru, Y.T.; Wang, Y.; Zhou, J.G.; Wang, D.Y.; Ma, Y.Y.; Jiang, Y.R.; Gao, Q.; Qin, L. The expression patterns of ecdysone receptor and ultraspiracle genes in Antheraea pernyi during development and hormone-induced process. Sci. Seric. 2017, 43, 0594–0602. [Google Scholar]
- Fallon, A.M.; Hagedorn, H.H.; Wyatt, G.R.; Laufer, H. Activation of vitellogenin synthesis in the mosquito Aedes aegypti by ecdysone. J. Insect Physiol. 1974, 20, 1815–1823. [Google Scholar] [CrossRef]
- Zhou, J.; Li, J.; Wang, Q.; Luo, Y.Q. The regulation of ecdysteroid on insect growth and productive processes. Chinese J. Applied Entomol. 2013, 50, 1413–1418. [Google Scholar]
- König, A.; Yatsenko, A.S.; Weiss, M.; Shcherbata, H. Ecdysteroids affect Drosophila ovarian stem cell niche formation and early germline differentiation. EMBO J. 2011, 30, 1549–1562. [Google Scholar]
- Morris, L.X.; Spradling, A.C. Steroid signaling within Drosophila ovarian epithelial cells sex-specifically modulates early germ cell development and meiotic entry. PLoS ONE 2012, 7, e46109. [Google Scholar] [CrossRef]
- Parthasarathy, R.; Sheng, Z.T.; Sun, Z.Y.; Palli, S.R. Ecdysteroid regulation of ovarian growth and oocyte maturation in the red flour beetle, Tribolium castaneum. Insect Biochem. Molec. 2010, 40, 429–439. [Google Scholar] [CrossRef]
- Swevers, L.; Iatrou, K. Ecdysteroids and ecdysteroid signaling pathways during insect oogenesis. In Ecdysone: Structures and Functions; Springer: Dordrecht, The Netherlands, 2009; pp. 127–164. [Google Scholar]
- Happ, G.M. Maturation of the male reproductive system and its endocrine regulation. Annu. Rev. Entomol. 1992, 37, 303–320. [Google Scholar] [CrossRef]
- Shen, G.W.; Lin, Y.; Lv, Y.H.; Wang, J.Y.; Xing, R.M.; Xia, Q.Y. Regulation of ecdysone on vitellogenin gene expression in silkworm Bombyx mori. Chin. J. Biochem. Mol. Biol. 2014, 30, 1106–1112. [Google Scholar]
- Cheng, Y.; Zhou, Y.H.; Ran, H.Y.; Li, F.L. Effects of different hormones as dietary supplements on biological characteristics of Coccinella septempunctata L. J. Appl. Entomol. 2023, 147, 1–7. [Google Scholar] [CrossRef]
- Cheng, Y.; Zhou, Y.; Li, C.; Jin, J.X. Cloning and functional analysis of the juvenile hormone receptor gene CsMet in Coccinella septempunctata. J. Insect Sci. 2024, 24, ieae065. [Google Scholar] [CrossRef]
- Cheng, Y.; Zhou, Y.; Li, C. Functional analysis of genes encoding juvenile hormone receptor Met and transcription factor Kr-h1 in the reproductive capacity of Coccinella septempunctata males. Insects 2025, 16, 49. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.H.; Cheng, Y.; Jin, J.X.; Li, W.H.; Li, F.L. Large scale production and release application of Coccinella septempunctata. Southwest China J. Agric. Sci. 2017, 30, 602–605. [Google Scholar]
- Cheng, Y.; Zhi, J.R.; Li, F.L.; Wang, H.; Zhou, Y.H.; Jin, J.X. Transcriptome sequencing of Coccinella septempunctata adult (Coleoptera: Coccinellidae) feeding on artificial diet and Aphis craccivora. PLoS ONE 2020, 15, e0236249. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.Y.; Wang, J.; Wang, M.Z.; Gao, F.; Zhang, H.Z.; Li, Y.Y.; Zang, L.S.; Zhang, L.S. Cloning and expression analysis of juvenile hormone epoxide hydrolase in Coccinella septempunctata. Plant Prot. 2019, 45, 156–162. [Google Scholar]
- Pfaffl, M.W. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic. Acids Res. 2001, 29, e45. [Google Scholar] [CrossRef]
- Tang, Q.Y.; Zhang, C.X. Data Processing System (DPS) software with experimental design, statistical analysis and data mining developed for use in entomological research. Insect Sci. 2013, 20, 254–260. [Google Scholar] [CrossRef]
- Wang, Z.S.; Zhong, X.C.; Qiu, X.J.; Hu, S.Y.; Guo, F. Observations on the reproduction of Coccinella septempunctata L. Acta Entomol. Sin. 1977, 20, 397–404. [Google Scholar]
- Mello, T.R.P.; Aleixo, A.C.; Pinheiro, D.G.; Nunes, F.M.F.; Bitondi, M.M.G.; Hartfelder, K.; Barchuk, A.R.; Simoes, Z.L.P. Developmental regulation of ecdysone receptor (EcR) and EcR-controlled gene expression during pharate-adult development of honeybees (Apis mellifera). Front. Genet. 2014, 5, 445. [Google Scholar] [CrossRef]
- Yu, J.; Song, H.Y.; Wang, Y.; Liu, Z.G.; Wang, H.F.; Xu, B.H. 20-hydroxyecdysone upregulates ecdysone receptor (EcR) gene to promote pupation in the honeybee, Apis Mellifera Ligustica. Integr. Comp. Biol. 2023, 63, icad077. [Google Scholar] [CrossRef]
- Ables, E.T.; Drummond-Barbosa, D. The steroid hormone ecdysone functions with intrinsic chromatin remodeling factors to control female germ line stem cells in Drosophila. Cell Stem Cell 2010, 7, 581–592. [Google Scholar] [CrossRef]
- Carney, G.E.; Bender, M. The Drosophila ecdysone receptor (EcR) gene is required matemally for normal oogenesis. Genetics 2000, 154, 1203–1211. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.X.; Zheng, W.Y.; Xue, H.; Jiao, P.Y.; Chen, L.Z. Effects of silencing ecdysone receptor gene on the fecundity of Adelphocris suturalis. Plant Prot. 2023, 49, 164–177. [Google Scholar]
- Polanska, M.A.; Maksimiuk-Ramirez, E.; Ciuk, M.A.; Kotwica, J.; Bebas, P. Clock-controlled rhythm of ecdysteroid levels in the haemolymph and testes and its relation to sperm release in the egyptian cotton leafworm, Spodoptera littoralia. J. Insect Physiol. 2009, 55, 426–434. [Google Scholar] [CrossRef]
- Xu, J.; Raman, C.; Zhu, F.; Tan, A.; Palli, S.R. Identification of nuclear receptors involved in regulation of male reproduction in the red flour beetle, Tribolium castaneum. J. Insect Physiol. 2012, 58, 710–717. [Google Scholar] [CrossRef] [PubMed]
- Attardo, G.M.; Hanseni, A.; Raikhel, A.S. Nutritional regulation of vitellogenesis in mosquitoes: Implications for anautogeny. Insect Biochem. Molec. 2005, 35, 661–675. [Google Scholar] [CrossRef]
- Wang, X.L.; Hou, Y.; Saha, T.T.; Pei, G.F.; Raikhel, A.S.; Zou, Z. Hormone and receptor interplay in the regulation of mosquito lipid metabolism. Proc. Nati. Acad. Sci. USA 2017, 114, E2709–E2718. [Google Scholar] [CrossRef]
- Kamoshida, Y.; Fujiyama-Nakamur, S.; Kimura, S.; Suzuki, E.; Lim, J.; Shiozaki-Sato, Y.; Kato, S.; Takeyama, K. Ecdysone receptor (EcR) suppresses lipid accumulation in the Drosophila fat body via transcription control. Biochem. Biophys. Res. Commun. 2012, 421, 203–207. [Google Scholar] [CrossRef]





| Primer Name | Sequence | Annealing Temperature |
|---|---|---|
| EcR-F EcR-R | AAAGGACCAACACCAAGGCA TCGTCACACTCCGTCGAAAG | 55.5 |
| EcR-dsRNA-F EcR-dsRNA-R | taatacgactcactatagggCGATGACTTGCTGCTGGTTA taatacgactcactatagggGTCGTAGCTGCCTGATGACA | |
| GFP-dsRNA-F GFP-dsRNA-R E78-F E78-R | taatacgactcactatagggGCCAACACTTGTCACTACTT taatacgactcactatagggGGAGTATTTTGTTGATAATGGTCTG AGCTCCTGTTTCATGATGCGG ATTCATCCCCGGTCGAATGTG | 58.0 |
| Actin-F Actin-R | GATTCGCCATCCAGGACATCTC TCCTTGCTCAGCTTGTTGTAGTC | 60.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, Y.; Zhou, Y.; Li, C. Effects of the Ecdysone Receptor on the Regulation of Reproduction in Coccinella septempunctata. Insects 2025, 16, 643. https://doi.org/10.3390/insects16060643
Cheng Y, Zhou Y, Li C. Effects of the Ecdysone Receptor on the Regulation of Reproduction in Coccinella septempunctata. Insects. 2025; 16(6):643. https://doi.org/10.3390/insects16060643
Chicago/Turabian StyleCheng, Ying, Yuhang Zhou, and Cao Li. 2025. "Effects of the Ecdysone Receptor on the Regulation of Reproduction in Coccinella septempunctata" Insects 16, no. 6: 643. https://doi.org/10.3390/insects16060643
APA StyleCheng, Y., Zhou, Y., & Li, C. (2025). Effects of the Ecdysone Receptor on the Regulation of Reproduction in Coccinella septempunctata. Insects, 16(6), 643. https://doi.org/10.3390/insects16060643




