Death-Leading Envenomization of Rabbits with Snake Versus Scorpion Venoms: A Comparative Forensic Investigation of Postmortem Decomposition and Beetle Succession
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
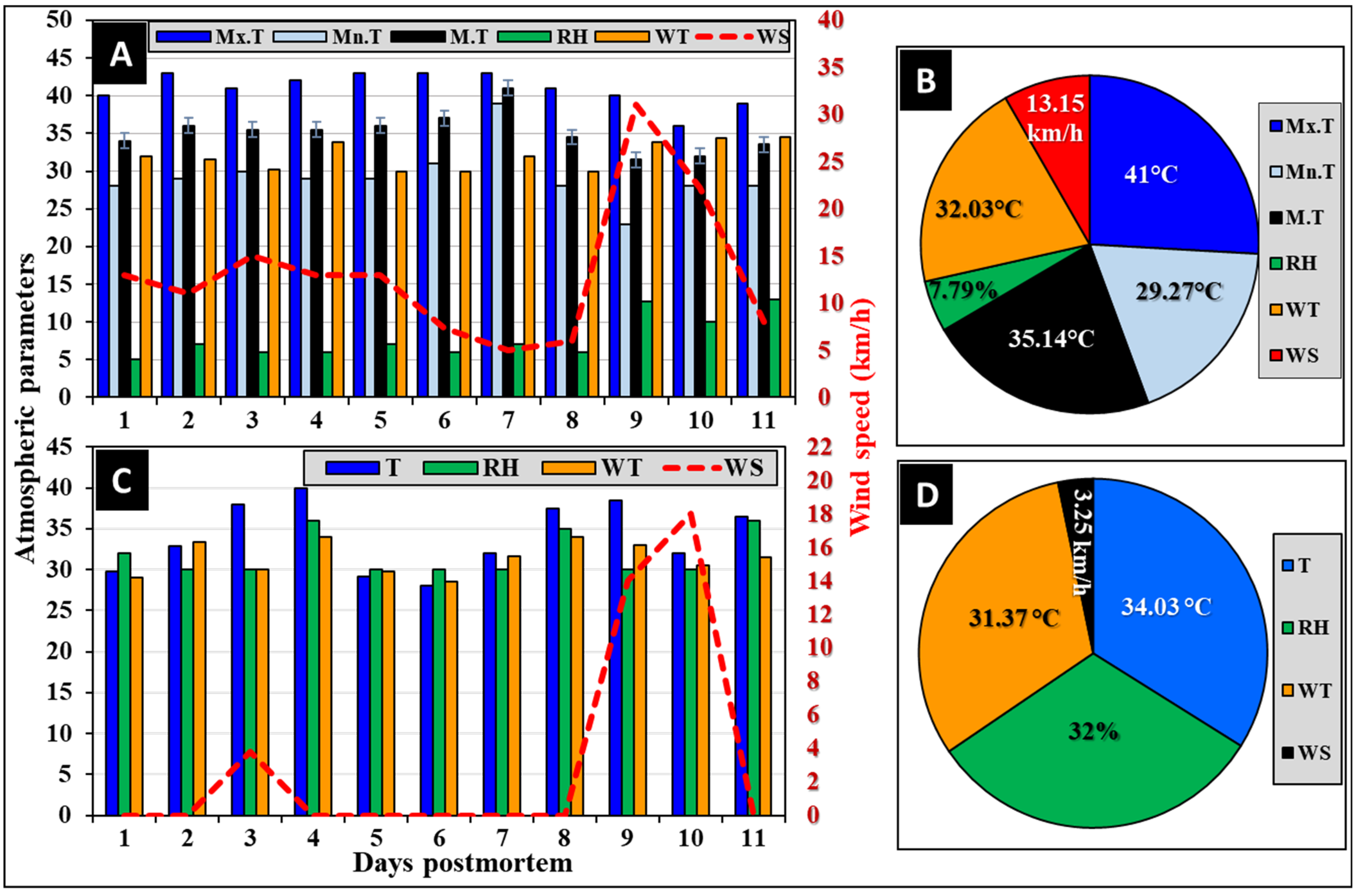
2.1. Meteorological Parameters
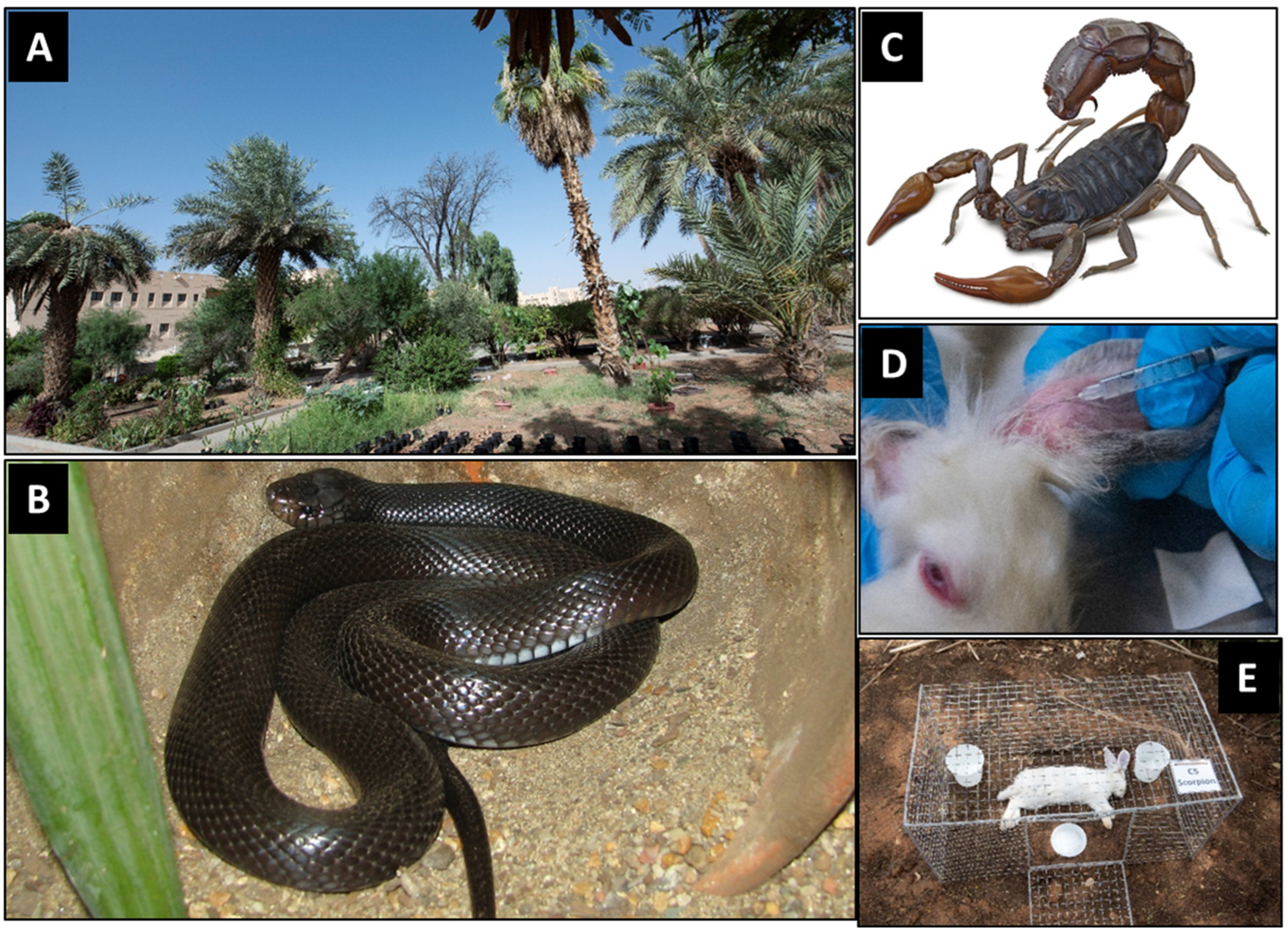
2.2. Experimental Site
2.3. Experimental Animals
2.3.1. Rabbits and Mice
2.3.2. Snakes and Scorpions
2.4. Collection of Venoms
2.5. Lethality Assay
2.6. Envenomization of Rabbits
2.7. Experimental Design
2.8. Decomposition Process
2.9. Beetles Collection and Identification
2.10. Statistical Analysis
3. Results
3.1. Venoms Lethality
3.2. Meteorological Measurements
3.2.1. Atmospheric Parameters
3.2.2. On-Site Recorded Weather Parameters
3.3. Decomposition Stages
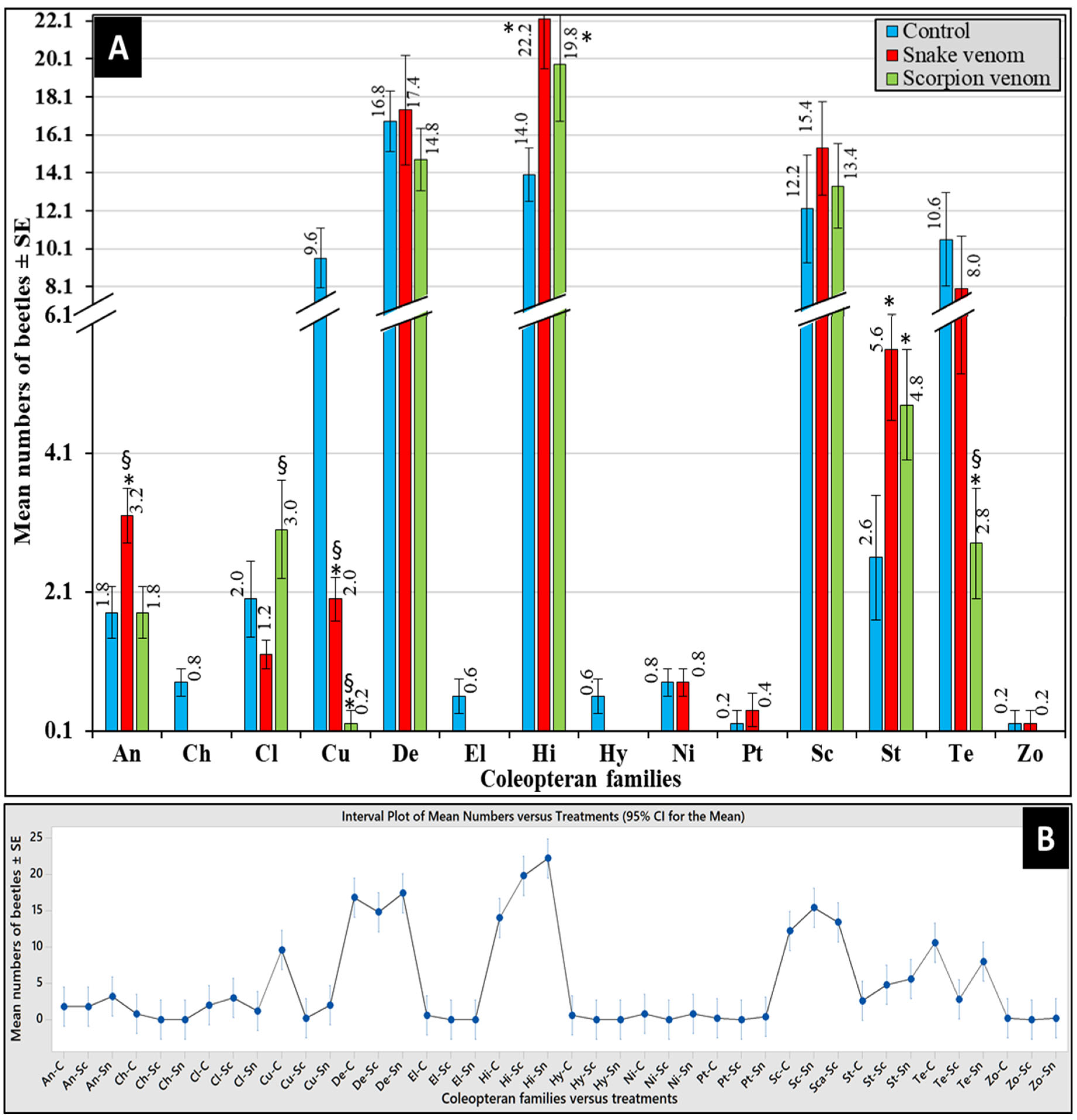
3.4. Abundance of Corpse Associated Beetles
3.5. Differential Abundance of Beetles
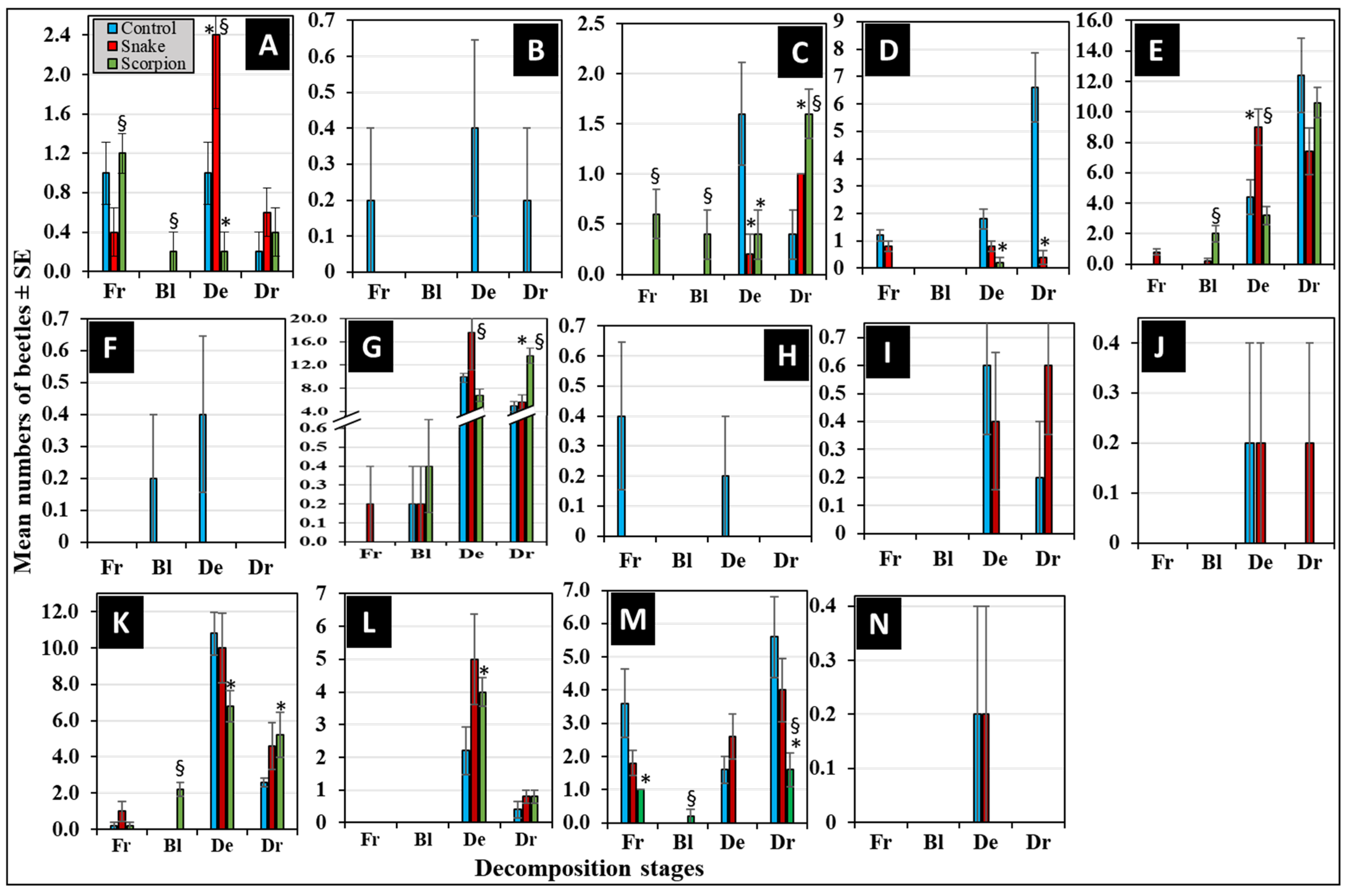
3.6. Differential Succession of Beetles
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Byrd, J.H.; Brundage, A. Forensic entomology. In Veterinary Forensic Medicine and Forensic Sciences, 1st ed.; Byrd, J.H., Norris, P., Bradley-Siemens, N., Eds.; CRC Press, Taylor and Francis Group: Boca Raton, FL, USA, 2020; pp. 67–111. [Google Scholar]
- Amendt, J.; Richards, C.S.; Campobasso, C.P.; Zehner, R.; Hall, M.J. Forensic entomology: Applications and limitations. Forensic Sci. Med. Pathol. 2011, 7, 379–392. [Google Scholar] [CrossRef] [PubMed]
- Charabidze, D.; Gosselin, M.; Hedouin, V. Use of necrophagous insects as evidence of cadaver relocation: Myth or reality? PeerJ 2017, 5, e3506. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Kumawat, R.; Singh, G.; Jangir, S.S.; Kushwaha, P.; Rana, M. Forensic entomology: A novel approach in crime investigation. GSC Biol. Pharm. Sci. 2022, 19, 165–174. [Google Scholar] [CrossRef]
- Correa, R.C.; Caneparo, M.F.C.; Vairo, K.P.; de Lara, A.G.; Moura, M.O. What have we learned from the dead? A compilation of three years of cooperation between entomologists and crime scene investigators in Southern Brazil. Rev. Bras. Entomol. 2019, 63, 224–231. [Google Scholar] [CrossRef]
- Hu, G.L.; Wang, M.; Wang, Y.; Liao, M.Q.; Hu, J.Y.; Zhang, Y.N.; Yu, Y.M.; Wang, J.F. Estimation of post-mortem interval based on insect species present on a corpse found in a suitcase. Forensic Sci. Int. 2020, 306, 110046. [Google Scholar] [CrossRef]
- Coe, J. Postmortem chemistry: Practical considerations and a review of the literature. J. Forensic Sci. 1974, 19, 13–32. [Google Scholar] [CrossRef]
- Byrd, J.H.; Tomberlin, J.K. Forensic Entomology: The Utility of Arthropods in Legal Investigations; CRC Press, Taylor & Francis Group: Boca Raton, FL, USA, 2019; ISBN 978-0-8153-5020-0. [Google Scholar]
- Kulshrestha, P.; Satpathy, D.K. Use of beetles in forensic entomology. Forensic Sci. Int. 2001, 120, 15–17. [Google Scholar] [CrossRef]
- McKenna, D.D.; Farrell, B.D. Beetles (Coleoptera). In The Timetree of Life; Hedges, S.B., Kumar, S., Eds.; Oxford University Press: New York, NY, USA, 2009; pp. 278–289. [Google Scholar]
- Alajmi, R.; Abdel-Gaber, R.; Haddadi, R. Molecular identification of forensically important beetles in Saudi Arabia based on mitochondrial 1 s rRNA gene. Entomol. Res. 2020, 50, 343–350. [Google Scholar] [CrossRef]
- Charabidze, D.; Colard, T.; Vincent, B.; Pasquerault, T.; Hedouin, V. Involvement of larder beetles (Coleoptera: Dermestidae) on human cadavers: A review of 81 forensic cases. Int. J. Leg. Med. 2014, 128, 1021–1030. [Google Scholar] [CrossRef]
- Lyu, Z.; Wan, L.H.; Yang, Y.Q.; Tang, R.; Xu, L.Z. A checklist of beetles (Insecta, Coleoptera) on pig carcasses in the suburban area of southwestern China: A preliminary study and its forensic relevance. J. Forensic Leg. Med. 2016, 41, 42–48. [Google Scholar] [CrossRef]
- Pushkin, S.V.; Tsymbal, B.M.; Nagdalian, A.A.; Nuzhnaya, K.V.; Sutaeva, A.N.; Ramazanova, S.Z.; Maschenko-Grigorova, A.N.; Mishvelov, A.E. The use of model groups of necrobiont beetles (Coleoptera) for the diagnosis of time and place of death. Entomol. Appl. Sci. Lett. 2019, 6, 46–56. [Google Scholar]
- White, J. Venomous animals: Clinical Toxinology. In Molecular, Clinical and Environmental Toxicology; Luch, A., Ed.; Birkhäuser: Basel, Switzerland, 2010; Volume 2, pp. 233–291. [Google Scholar] [CrossRef]
- Chippaux, J.-P. Snakebite in Africa: Current situation and urgent needs. In Handbook of Venoms and Toxins of Reptiles, 2nd ed.; CRC Press, Taylor & Francis Group: London, UK, 2021; pp. 593–612. [Google Scholar] [CrossRef]
- Fathinia, B.; Rastegar, P.N.; Darvishnia, H.; Rajabizadeh, M. The snake fauna of Ilam Province, southwestern Iran. Iran. J. Anim. Biosyst. 2010, 6, 9–23. [Google Scholar]
- Al-Sadoon, M.K.; Paray, B.A.; Al-Otaibi, H. Survey of the reptilian fauna of the Kingdom of Saudi Arabia. VI. The snake fauna of Turaif region. Saudi J. Biol. Sci. 2017, 24, 925–928. [Google Scholar] [CrossRef]
- McCartney, J.A.; Seiffert, E.R. A late Eocene snake fauna from the Fayum Depression, Egypt. J. Vertebr. Paleontol. 2016, 36, e1029580. [Google Scholar] [CrossRef]
- Ernst, C.H.; Ernst, E.M. Snakes of the United States and Canada; Smithsonian Books: Washington, DC, USA, 2003; Volume 790. [Google Scholar]
- Al-Asmari, A.K.; Al-Saif, A.A.; Abdo, N.; Al-Moutaery, K.; Al-Harbi, N. A review of the scorpion fauna of Saudi Arabia. Egypt. J. Nat. Hist. 2013, 6, 1–21. [Google Scholar] [CrossRef]
- Lourenço, W.R. A historical approach to scorpion studies with special reference to the 20th and 21st centuries. J. Venom. Anim. Toxins Incl. Trop. Dis. 2014, 20, 8. [Google Scholar] [CrossRef]
- Kachel, H.S.; Al-Khazali, A.M.; Hussen, F.S.; Yağmur, E.A. Checklist and review of the scorpion fauna of Iraq (Arachnida: Scorpiones). Arachnol. Mitteilungen 2021, 61, 1–10. [Google Scholar] [CrossRef]
- Chippaux, J.-P. Snake Venoms and Envenomations; Krieger Publishing Company: Malabar, FL, USA, 2006. [Google Scholar]
- White, J. Bites and stings from venomous animals: A global overview. Ther. Drug Monit. 2000, 22, 65–68. [Google Scholar] [CrossRef]
- Kularatne, S.; Senanayake, N. Venomous snake bites, scorpions, and spiders. Handb. Clin. Neurol. 2014, 120, 987–1001. [Google Scholar] [CrossRef]
- Forrester, J.A.; Holstege, C.P.; Forrester, J.D. Fatalities from venomous and nonvenomous animals in the United States (1999–2007). Wilderness Environ. Med. 2012, 23, 146–152. [Google Scholar] [CrossRef]
- Forrester, J.A.; Weiser, T.G.; Forrester, J.D. An update on fatalities due to venomous and nonvenomous animals in the United States (2008–2015). Wilderness Environ. Med. 2018, 29, 36–44. [Google Scholar] [CrossRef]
- Forrester, J.D.; Forrester, J.A.; Tennakoon, L.; Staudenmayer, K. Mortality, hospital admission, and healthcare cost due to injury from venomous and non-venomous animal encounters in the USA: 5-year analysis of the National Emergency Department Sample. Trauma Surg. Acute Care Open 2018, 3, e000250. [Google Scholar] [CrossRef] [PubMed]
- Chippaux, J.-P.; Goyffon, M. Epidemiology of scorpionism: A global appraisal. Acta Trop. 2008, 107, 71–79. [Google Scholar] [CrossRef]
- Dehghani, R.; Fathi, B. Scorpion sting in Iran: A review. Toxicon 2012, 60, 919–933. [Google Scholar] [CrossRef] [PubMed]
- Santos, M.S.; Silva, C.G.; Neto, B.S.; Grangeiro Júnior, C.R.; Lopes, V.H.; Teixeira Júnior, A.G.; Bezerra, D.A.; Luna, J.V.; Cordeiro, J.B.; Júnior, J.G. Clinical and epidemiological aspects of scorpionism in the world: A systematic review. Wilderness Environ. Med. 2016, 27, 504–518. [Google Scholar] [CrossRef] [PubMed]
- Amr, Z.S.; Baker, M.A.A.; Warrell, D.A. Terrestrial venomous snakes and snakebites in the Arab countries of the Middle East. Toxicon 2020, 177, 1–15. [Google Scholar] [CrossRef]
- WHO. Snakebite Envenoming: A Strategy for Prevention and Control; WHO: Geneva, Switzerland, 2019; pp. 1–70. Available online: https://www.who.int/publications/i/item/9789241515641 (accessed on 12 April 2025).
- Schneemann, M.; Cathomas, R.; Laidlaw, S.; El Nahas, A.; Theakston, R.D.G.; Warrell, D.A. Life-threatening envenoming by the Saharan horned viper (Cerastes cerastes) causing micro-angiopathic haemolysis, coagulopathy and acute renal failure: Clinical cases and review. QJM 2004, 97, 717–727. [Google Scholar] [CrossRef]
- Rahav, G.; Weiss, A.T. Scorpion sting-induced pulmonary edema: Scintigraphic evidence of cardiac dysfunction. Chest 1990, 97, 1478–1480. [Google Scholar] [CrossRef]
- du Toit-Prinsloo, L.; Morris, N.K.; Meyer, P.; Saayman, G. Deaths from bee stings: A report of three cases from Pretoria, South Africa. Forensic Sci. Med. Pathol. 2016, 12, 81–85. [Google Scholar] [CrossRef][Green Version]
- Hughes, R.L. A fatal case of acute renal failure from envenoming syndrome after massive bee attack: A case report and literature review. Am. J. Forensic Med. Pathol. 2019, 40, 52–57. [Google Scholar] [CrossRef]
- Haddad Junior, V.; Amorim, P.C.H.d.; Haddad Junior, W.T.; Cardoso, J.L.C. Venomous and poisonous arthropods: Identification, clinical manifestations of envenomation, and treatments used in human injuries. Rev. Soc. Bras. Med. Trop. 2015, 48, 650–657. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.; Al-Kathiri, W.H.; Balkhi, B.; Samrkandi, O.; Al-Khalifa, M.S.; Asiri, Y. The burden of bites and stings management: Experience of an academic hospital in the Kingdom of Saudi Arabia. Saudi Pharm. J. 2020, 28, 1049–1054. [Google Scholar] [CrossRef] [PubMed]
- Al-Sadoon, M.K.; Albeshr, M.F.; Paray, B.A.; Al-Mfarij, A.R. Envenomation and the bite rate by venomous snakes in the kingdom of Saudi Arabia over the period (2015–2018). Saudi J. Biol. Sci. 2021, 28, 582–586. [Google Scholar] [CrossRef]
- Alhamoud, M.A.; Al Fehaid, M.S.; Alhamoud, M.A.; Alzoayed, M.H.; Alkhalifah, A.A.; Menezes, R.G. Scorpion stings in Saudi Arabia: An overview. Acta Bio Medica Atenei Parm. 2021, 92, e2021273. [Google Scholar] [CrossRef]
- Al-Sadoon, M.; Jarrar, B. Epidemiological study of scorpion stings in Saudi Arabia between 1993 and 1997. J. Venom. Anim. Toxins Incl. Trop. Dis. 2003, 9, 54–64. [Google Scholar] [CrossRef]
- Jarrar, B.M.; Al-Rowaily, M.A. Epidemiological aspects of scorpion stings in Al-Jouf province, Saudi Arabia. Ann. Saudi Med. 2008, 28, 183–187. [Google Scholar] [CrossRef]
- National Center for Meteorology. Meteorological services. Available online: https://ncm.gov.sa/Ar/EService/met/Pages/default.aspx (accessed on 5 June 2023).
- Al-Qurashi, A.S.; Mashaly, A.M.; Alagmi, R.; Al-Khalifa, M.S.; Mansour, L.; Al-Omar, S.Y.; Sharaf, M.R.; Aldawood, A.S.; Al-Dhafer, H.M.; Hunter, T. A preliminary investigation of rabbit carcass decomposition and attracted ants (Hymenoptera: Formicidae) on the seaward coastal beach of Al-Jubail City, Saudi Arabia. J. Med. Entomol. 2023, 61, 318–330. [Google Scholar] [CrossRef]
- Almutawa, M.e.Y.; Al-Khalifa, M.S.; Al-Dhafer, H.M.; Abdel-Dayem, M.S.; Ebaid, H.; Ahmed, A.M. Forensic investigation of carcass decomposition and dipteran fly composition over the summer and winter: A comparative analysis of indoor versus outdoor at a multi-story building. J. Med. Entomol. 2024, 61, 877–890. [Google Scholar] [CrossRef]
- Al Farhan, A.H.; Aldjain, I.M.; Thomas, J.; Miller, A.G.; Knees, S.G.; Llewellyn, O.; Akram, A. Botanic gardens in the Arabian Peninsula. Sibbaldia Int. J. Bot. Gard. Hortic. 2008, 6, 189–203. [Google Scholar] [CrossRef]
- Khalil, A.; Zidan, M.M.; Alajmi, R.; Ahmed, A.M. Impact of envenomation with snake venoms on rabbit carcass decomposition and differential adult dipteran succession patterns. J. Med. Entomol. 2023, 60, 40–50. [Google Scholar] [CrossRef]
- Mapara, M.; Thomas, B.S.; Bhat, K. Rabbit as an animal model for experimental research. Dent. Res. J. 2012, 9, 111. [Google Scholar] [CrossRef]
- Dautartas, A.; Kenyhercz, M.W.; Vidoli, G.M.; Jantz, L.M.; Mundorff, A.; Steadman, D.W. Differential decomposition among pig, rabbit, and human remains. J. Forensic Sci. 2018, 63, 1673–1683. [Google Scholar] [CrossRef] [PubMed]
- Khalil, A.; Salem, A.M.; Shaurub, E.-S.H.; Ahmed, A.M.; Al-Khalaf, A.A.; Zidan, M.M. Envenomation with snake venoms as a cause of death: A forensic investigation of the decomposition stages and the impact on differential succession pattern of carcass-attracted coleopteran beetles. Insects 2024, 15, 902. [Google Scholar] [CrossRef] [PubMed]
- Park, J.K.; Kim, C.J.; Do, Y. Post mortem insect colonization and body weight loss in rabbit carcasses. Entomol. Res. 2020, 50, 594–600. [Google Scholar] [CrossRef]
- Zeariya, M.; Hammad, K.M.; Fouda, M.A.; Al-Dali, A.G.; Kabadaia, M.M. Forensic-insect succession and decomposition patterns of dog and rabbit carcasses in different habitats. J. Entomol. Zool. Stud. 2015, 3, 473–482. [Google Scholar]
- Alqahtan, A.R. Ecological notes on snake diversity in Tathleeth District, Aseer Region, Southwest of Saudi Arabia. Egypt. Acad. J. Biol. Sci. B Zool. 2017, 9, 61–70. [Google Scholar] [CrossRef]
- Masood, M.F. Ecological distribution of snakes’ fauna of Jazan region of Saudi Arabia. Egypt. Acad. J. Biol. Sci. B Zool. 2012, 4, 183–197. [Google Scholar] [CrossRef]
- Alshammari, A.M. Four new records of snake species in Ar’ar region, Northern border of Saudi Arabi. Jordan J. Nat. Hist. 2022, 9, 41–48. [Google Scholar]
- Wikipedia. Sinai-Desert-Cobra. By Ltshears. Available online: https://en.wikipedia.org/wiki/File:Sinai-Desert-Cobra.jpg (accessed on 15 December 2024).
- Alqahtani, A.R.; Badry, A.; Abd Al Galil, F.M.; Amr, Z.S. Morphometric and meristic diversity of the species Androctonus crassicauda (Olivier, 1807) (Scorpiones: Buthidae) in Saudi Arabia. PeerJ 2022, 10, e14198. [Google Scholar] [CrossRef]
- Al-Asmari, A.; Al-Saief, A.; Abdo, N.; Al-Moutaery, K. New additions to the scorpion fauna of Riyadh region, Saudi Arabia. J. Venom. Anim. Toxins Incl. Trop. Dis. 2009, 15, 612–632. [Google Scholar] [CrossRef]
- Alqahtani, A.R.; Elgammal, B.; Ghaleb, K.I.; Badry, A. The scorpion fauna of the southwestern part of Saudi Arabia. Egypt. Acad. J. Biol. Sci. B Zool. 2019, 11, 19–29. [Google Scholar] [CrossRef][Green Version]
- Timokhanov, V. Scorpion Androctonus crassicauda. 2009. Available online: https://freelance.ru/science_art/work-298408.html (accessed on 15 December 2024).
- Al-Sadoon, M.K.; Orabi, G.M.; Badr, G. Toxic effects of crude venom of a desert cobra, Walterinnesia aegyptia, on liver, abdominal muscles and brain of male albino rats. Pak. J. Zool. 2013, 45, 1359–1366. [Google Scholar]
- Boghozian, A.; Nazem, H.; Fazilati, M.; Hejazi, S.H.; Sheikh Sajjadieh, M. Toxicity and protein composition of venoms of Hottentotta saulcyi, Hottentotta schach and Androctonus crassicauda, three scorpion species collected in Iran. Vet. Med. Sci. 2021, 7, 2418–2426. [Google Scholar] [CrossRef] [PubMed]
- Calvete, J.J.; Pla, D.; Els, J.; Carranza, S.; Damm, M.; Hempel, B.-F.; John, E.B.; Petras, D.; Heiss, P.; Nalbantsoy, A. Combined molecular and elemental mass spectrometry approaches for absolute quantification of proteomes: Application to the venomics characterization of the two species of desert black cobras, Walterinnesia aegyptia and Walterinnesia morgani. J. Proteome Res. 2021, 20, 5064–5078. [Google Scholar] [CrossRef]
- Al-Asmari, A.K.; Kunnathodi, F.; Al Saadon, K.; Idris, M.M. Elemental analysis of scorpion venoms. J. Venom Res. 2016, 7, 16–20. [Google Scholar]
- Broad, A.; Sutherland, S.; Coulter, A.R. The lethality in mice of dangerous Australian and other snake venom. Toxicon 1979, 17, 661–664. [Google Scholar] [CrossRef]
- WHO. World Health Organization Expert Committee on Biological Standardization, Sixty-Seventh Report; Technical Report Series; World Health Organization: Geneva, Switzerland, 2017; Volume 1004, pp. 1–591. Available online: https://iris.who.int/bitstream/handle/10665/255657/9789241210133-eng.pdf (accessed on 15 December 2024).
- Finney, D.J. Probit Analysis, 3rd ed.; Cambridge University: London, UK, 1971; Volume 333. [Google Scholar] [CrossRef]
- Ozkan, O.; Kar, S.; Güven, E.; Ergun, G. Comparison of proteins, lethality and immunogenic compounds of Androctonus crassicauda (Olivier, 1807) (Scorpiones: Buthidae) venom obtained by different methods. J. Venom. Anim. Toxins Incl. Trop. Dis. 2007, 13, 844–856. [Google Scholar] [CrossRef]
- Jacob, S.; Nair, A.B.; Morsy, M.A. Dose conversion between animals and humans: A practical solution. Indian J. Pharm. Educ. Res. 2022, 56, 600–607. [Google Scholar] [CrossRef]
- Al-Qahtni, A.; Mashaly, A.; Haddadi, R.; Al-Khalifa, M. Seasonal impact of heroin on rabbit carcass decomposition and insect succession. J. Med. Entomol. 2021, 58, 567–575. [Google Scholar] [CrossRef]
- Conlee, K.; Stephens, M.; Rowan, A.N.; King, L.A. Carbon dioxide for euthanasia: Concerns regarding pain and distress, with special reference to mice and rats. Lab. Anim. 2005, 39, 137–161. [Google Scholar] [CrossRef]
- Velásquez, Y. A checklist of arthropods associated with rat carrion in a montane locality of northern Venezuela. Forensic Sci. Int. 2008, 174, 68–70. [Google Scholar] [CrossRef] [PubMed]
- Lewis, A.; Benbow, M. When entomological evidence crawls away: Phormia regina en masse larval dispersal. J. Med. Entomol. 2011, 48, 1112–1119. [Google Scholar] [CrossRef] [PubMed]
- Majer, J.D. The use of pitfall traps for sampling ants—A critique. Mem. Mus. Vic. 1997, 56, 323–329. [Google Scholar] [CrossRef]
- Mashaly, A.; Mahmoud, A.; Ebaid, H. Relative insect frequency and species richness on sun-exposed and shaded rabbit carrions. J. Med. Entomol. 2020, 57, 1006–1011. [Google Scholar] [CrossRef]
- Parmenter, R.R.; MacMahon, J.A. Carrion decomposition and nutrient cycling in a semiarid shrub–steppe ecosystem. Ecol. Monogr. 2009, 79, 637–661. [Google Scholar] [CrossRef]
- Goff, M.L. Early postmortem changes and stages of decomposition. In Current Concepts in Forensic Entomology; Amendt, J., Goff, M.L., Campobasso, C.P., Grassberger, M., Eds.; Springer: Dordrecht, The Netherlands; Heidelberg, Germany; London, UK; New York, NY, USA, 2010; pp. 1–24. [Google Scholar] [CrossRef]
- Mashaly, A.M.A. Carrion beetles succession in three different habitats in Riyadh, Saudi Arabia. Saudi J. Biol. Sci. 2017, 24, 430–435. [Google Scholar] [CrossRef]
- Litchfield, J.J.; Wilcoxon, F. A simplified method of evaluating dose-effect experiments. J. Pharmacol. Exp. Ther. 1949, 96, 99–113. [Google Scholar] [CrossRef]
- Matsuda, K. Changes in the insecticide susceptibility of the American serpentine leafminer, Liriomyza trifolii (Diptera: Agromyzidae), in indoor successively reared and crop field populations over 25 years. Appl. Entomol. Zool. 2021, 57, 71–80. [Google Scholar] [CrossRef]
- Morrison, D.A. How to improve statistical analysis in parasitology research publications. Int. J. Parasitol. 2002, 32, 1065–1070. [Google Scholar] [CrossRef]
- Novotný, V.; Basset, Y. Rare species in communities of tropical insect herbivores: Pondering the mystery of singletons. Oikos 2000, 89, 564–572. [Google Scholar] [CrossRef]
- Sakuma, M. Probit analysis of preference data. Appl. Entomol. Zool. 1998, 33, 339–347. [Google Scholar] [CrossRef]
- Indra, L.; Lösch, S.; Errickson, D.; Finaughty, D. Forensic experiments on animal scavenging: A systematic literature review on what we have and what we need. Forensic Sci. Int. 2023, 353, 111862. [Google Scholar] [CrossRef] [PubMed]
- Chippaux, J.-P. Snakebite envenomation turns again into a neglected tropical disease! J. Venom. Anim. Toxins Incl. Trop. Dis. 2017, 23, 38. [Google Scholar] [CrossRef]
- Weathers, K.C.; Strayer, D.L.; Likens, G.E. Fundamentals of Ecosystem Science; Elsevier, Academic Press: San Diego, CA, USA, 2012; ISBN 978-0-12-088774-3. [Google Scholar]
- Haglund, W.D.; Sorg, M.H. Advances in Forensic Taphonomy: Method, Theory, and Archaeological Perspectives, 1st ed.; CRC Press: Boca Raton, FL, USA; London, UK; New York, NY, USA; Washington, DC, USA, 2002. [Google Scholar]
- Vanin, S.; Zanotti, E.; Gibelli, D.; Taborelli, A.; Andreola, S.; Cattaneo, C. Decomposition and entomological colonization of charred bodies—A pilot study. Croat. Med. J. 2013, 54, 387–393. [Google Scholar] [CrossRef] [PubMed]
- Hwang, B.C.; Giardina, C.P.; Litton, C.M.; Francisco, K.S.; Pacheco, C.; Thomas, N.; Uehara, T.; Metcalfe, D.B. Impacts of insect frass and cadavers on soil surface litter decomposition along a tropical forest temperature gradient. Ecol. Evol. 2022, 12, e9322. [Google Scholar] [CrossRef]
- Iancu, L.; Dean, D.E.; Purcarea, C. Temperature influence on prevailing necrophagous Diptera and bacterial taxa with forensic implications for postmortem interval estimation: A review. J. Med. Entomol. 2018, 55, 1369–1379. [Google Scholar]
- Mashaly, A.M.A.; Al-Mekhlafi, F.A. Differential Diptera succession patterns on decomposed rabbit carcasses in three different habitats. J. Med. Entomol. 2016, 53, 1192–1197. [Google Scholar] [CrossRef]
- Dalal, J.; Sharma, S.; Bhardwaj, T.; Dhattarwal, S.K.; Verma, K. Seasonal study of the decomposition pattern and insects on a submerged pig cadaver. J. Forensic Leg. Med. 2020, 74, 102023. [Google Scholar] [CrossRef]
- Fancher, J.; Aitkenhead-Peterson, J.; Farris, T.; Mix, K.; Schwab, A.; Wescott, D.; Hamilton, M. An evaluation of soil chemistry in human cadaver decomposition islands: Potential for estimating postmortem interval (PMI). Forensic Sci. Int. 2017, 279, 130–139. [Google Scholar] [CrossRef]
- Dangerfield, C.R.; Frehner, E.H.; Buechley, E.R.; Sekercioglu, C.H.; Brazelton, W.J. Succession of bacterial communities on carrion is independent of vertebrate scavengers. PeerJ 2020, 8, e9307. [Google Scholar] [CrossRef]
- Cobaugh, K.L.; Schaeffer, S.M.; DeBruyn, J.M. Functional and structural succession of soil microbial communities below decomposing human cadavers. PLoS ONE 2015, 10, e0130201. [Google Scholar] [CrossRef] [PubMed]
- Soon, L.P.; See, K.L.; Ahmad, N.W.; Abdullah, K.; Hasmi, A.H. A scoping review on factors affecting cadaveric decomposition rates. J. Forensic Sci. Crim. Investig. 2017, 2, 555584. [Google Scholar] [CrossRef]
- Card, A.; Cross, P.; Moffatt, C.; Simmons, T. The effect of clothing on the rate of decomposition and Diptera colonization on Sus scrofa carcasses. J. Forensic Sci. 2015, 60, 979–982. [Google Scholar] [CrossRef]
- Mashaly, A.M.; Mahmoud, A.; Ebaid, H. Influence of clothing on decomposition and presence of insects on rabbit carcasses. J. Med. Entomol. 2019, 56, 921–926. [Google Scholar] [CrossRef]
- Al-Khalifa, M.; Mashaly, A.; Al-Qahtni, A. Impacts of antemortem ingestion of alcoholic beverages on insect successional patterns. Saudi J. Biol. Sci. 2021, 28, 685–692. [Google Scholar] [CrossRef] [PubMed]
- Abd El-Aziz, F.E.; Eldeeb, S.M.; Abdellah, N.Z.; Shaltout, E.S.; Ebrahem, N.E. Influence of scorpion venom on decomposition and arthropod succession. Egypt. Acad. J. Biol. Sci. B Zool. 2022, 14, 209–219. [Google Scholar] [CrossRef]
- Edstrom, A. Venomous and Poisonous Animals; Krieger Publishing Company: Malabar, FL, USA, 1992. [Google Scholar]
- Biery, T.L. Venomous Arthropod Handbook: Envenomization Symptoms/Treatment, Identification, Biology and Control; Disease Surveillance Branch, Epidemiology Division, USAF School of Aerospace. U.S. Government Printing Office: Washington, DC, USA, 1977. [Google Scholar]
- Cichutek, K.; Epstein, J.; Griffiths, E.; Hindawi, S.; Jivapaisarnpong, T.; Klein, H.; Minor, P.; Moftah, F.; Reddy, V.; Slamet, L. WHO Expert Committee on Biological Standardization Sixty-seventh report. Tech. Rep. Ser. WHO 2017, 1004, 1–591. [Google Scholar]
- Coelho, P.; Sousa, P.; Harris, D.; van der Meijden, A. Deep intraspecific divergences in the medically relevant fat-tailed scorpions (Androctonus, Scorpiones). Acta Trop. 2014, 134, 43–51. [Google Scholar] [CrossRef]
- El-Aziz, A.; Kasem, S.M.; Ebrahem, N.E. Evaluation of the toxicity of scorpion venom and digoxin on human cardiovascular system and in decomposition arthropods succession using rat carrions. Egypt. Acad. J. Biol. Sci. B Zool. 2022, 14, 1–16. [Google Scholar] [CrossRef]
- Abdou, R.H.; Ibrahim, A.E. Effects of Egyptian cobra (Naja haje) venom on postmortem changes and some biochemical parameters in rats. Forensic Sci. 2015, 4, 186–190. [Google Scholar]
- Abd El-Gawad, A.; Badawy, R.M.; Abd El-Bar, M.M.; Kenawy, M.A. Successive waves of dipteran flies attracted to warfarin-intoxicated rabbit carcasses in Cairo, Egypt. J. Basic Appl. Zool. 2019, 80, 56. [Google Scholar] [CrossRef]
- Al-Sadoon, M.K.; Fahim, A.; Salama, S.F.; Badr, G. The effects of LD50 of Walterinnesia aegyptia crude venom on blood parameters of male rats. Afr. J. Microbiol. Res. 2012, 6, 653–659. [Google Scholar]
- Oukkache, N.; Jaoudi, R.E.; Ghalim, N.; Chgoury, F.; Bouhaouala, B.; Mdaghri, N.E.; Sabatier, J.-M. Evaluation of the lethal potency of scorpion and snake venoms and comparison between intraperitoneal and intravenous injection routes. Toxins 2014, 6, 1873–1881. [Google Scholar] [CrossRef]
- Biologyinsights. LD50 Values: Mechanisms, Influences, and Pharmacological Applications. Pathology and Diseases. 2024. Available online: https://biologyinsights.com/ld50-values-mechanisms-influences-and-pharmacological-applications/?form=MG50AV53 (accessed on 20 December 2024).
- WHO. Progress in the Characterization of Venoms and Standardization of Antivenoms; WHO: Geneva, Switzerland, 1981; Available online: https://iris.who.int/bitstream/handle/10665/37282/WHO_OFFSET_58.pdf?sequence=1 (accessed on 15 December 2024).
- Al-Saleh, S.S.; Khan, S. Purification and characterization of phosphodiesterase i from Walterinnesia aegyptia venom. Prep. Biochem. Biotechnol. 2011, 41, 262–277. [Google Scholar] [CrossRef]
- Abid, I.; Jemel, I.; Alonazi, M.; Ben Bacha, A. A new group II phospholipase A2 from Walterinnesia aegyptia venom with antimicrobial, antifungal, and cytotoxic potential. Processes 2020, 8, 1560. [Google Scholar] [CrossRef]
- Aziz, T.M.A.E.; Bourgoin-Voillard, S.; Combemale, S.; Beroud, R.; Fadl, M.; Seve, M.; De Waard, M. Fractionation and proteomic analysis of the Walterinnesia aegyptia snake venom using OFFGEL and MALDI-TOF-MS techniques. Electrophoresis 2015, 36, 2594–2605. [Google Scholar] [CrossRef]
- Al-Jammaz, I. Physiological effect of LD50 of Walterinnesia aegyptia crude venom on rat metabolism over various periods of time. Pak. J. Biol. Sci. 2001, 4, 1429–1431. [Google Scholar]
- Alhazza, I. Effect of Walterinnesia aegyptia, Cerastes cerastes and Bitis arietans venoms on liver functions of male rats. Pak. J. Zool. 2001, 33, 157–165. [Google Scholar]
- Khalil, F.; Zaki, K.; Naguib, M. Effect of black snake (Walterinnesia aegyptia) venom on the respiratory activity of some tissues of the rabbit. Z. Vgl. Physiol. 1964, 48, 270–276. [Google Scholar] [CrossRef]
- Postma, T.L. Neurotoxic animal poisons and venoms. Clin. Neurotoxicol. 2009, 55, 463–489. [Google Scholar]
- Safdarian, M.; Vazirianzadeh, B.; Ghorbani, A.; Pashmforoosh, N.; Baradaran, M. Intraspecific differences in Androctunus crassicauda venom and envenomation symptoms. EXCLI J. 2022, 21, 1222. [Google Scholar] [CrossRef] [PubMed]
- Dekeirsschieter, J.; Verheggen, F.; Gohy, M.; Hubrecht, F.; Bourguignon, L.; Lognay, G.; Haubruge, E. Cadaveric volatile organic compounds released by decaying pig carcasses (Sus domesticus L.) in different biotopes. Forensic Sci. Int. 2009, 189, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Al-Dakhil, A.A.; Alharbi, S.A. A preliminary investigation of the entomofauna composition of forensically important necrophagous insects in Al-Madinah Al-Munawwarah region, Kingdom of Saudi Arabia. J. Taibah Univ. Sci. 2020, 14, 1127–1133. [Google Scholar] [CrossRef]
- Sonker, R.; Rawat, S.; Singh, K. Succession and life cycle of beetles on the exposed carcass. Int. J. Sci. Innov. Res. 2015, 1, 46–50. [Google Scholar]
- Evans, A.V.; Bellamy, C.L. An Inordinate Fondness for Beetles; University of California Press, Nevraumont Publishing Company: New York, NY, USA, 2000. [Google Scholar]
- Van Laerhoven, S.L.; Anderson, G.S. Insect succession on buried carrion in two biogeoclimatic zones of British Columbia. J Forensic Sci. 1999, 44, 32–43. [Google Scholar] [CrossRef]
- Matuszewski, S.; Frątczak, K.; Konwerski, S.; Bajerlein, D.; Szpila, K.; Jarmusz, M.; Szafałowicz, M.; Grzywacz, A.; Mądra, A. Effect of body mass and clothing on carrion entomofauna. Int. J. Leg. Med. 2016, 130, 221–232. [Google Scholar] [CrossRef]
- Matuszewski, S.; Konwerski, S.; Frątczak, K.; Szafałowicz, M. Effect of body mass and clothing on decomposition of pig carcasses. Int. J. Leg. Med. 2014, 128, 1039–1048. [Google Scholar] [CrossRef]
- Wang, Y.; Ma, M.-Y.; Jiang, X.-Y.; Wang, J.-F.; Li, L.-L.; Yin, X.-J.; Wang, M.; Lai, Y.; Tao, L.-Y. Insect succession on remains of human and animals in Shenzhen, China. Forensic Sci. Int. 2017, 271, 75–86. [Google Scholar] [CrossRef]


| Venom Types | LD50 (mg/kg) (Lower–Upper) | LD95 (mg/kg) (Lower–Upper) | Slope ± SE |
|---|---|---|---|
| W. aegyptia | 0.053 * (0.052–0.054) | 0.066 ** (0.063–0.069) | 18.29 ± 3.22 |
| A. crassicauda | 1.416 * (1.288–1.558) | 2.516 ** (2.251–2.813) | 6.59 ± 0.91 |
| Types of Venoms | Days Postmortem | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | ||||||||
| Control | ||||||||||||||||||
| W. eagiptia | ||||||||||||||||||
| A. crassicauda | ||||||||||||||||||
| Keys | Fresh stage | Bloated stage | Decay stage | Dry stage | ||||||||||||||
| Coleopteran Families (Total Number) | Beetle Species (Total Number) | Treatments | Control | W. aegyptia | A. crassicauda | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DS | Fr | Bl | De | Dr | Fr | Bl | De | Dr | Fr | Bl | De | Dr | ||
| HPM * | 0–31 | 31–45 | 45–93 | 93→ | 0–21 | 21–31 | 31–93 | 93→ | 0–21 | 21–45 | 45–117 | 117→ | ||
| Anthicidae (38) | O. formicarius (38) | + | − | + | + | + | − | + | + | + | + | + | + | |
| Chrysomelidae (4) | C. acaciae (4) | + | − | + | + | − | − | − | − | − | − | − | − | |
| Cleridae (31) | N. rufipes (23) | − | − | + | + | − | − | + | + | − | − | + | + | |
| Necrobia sp. (8) | − | − | + | − | − | − | − | + | + | + | − | − | ||
| Curculionidae (59) | Dinoderus sp. (57) | + | − | + | + | + | − | + | + | − | − | + | − | |
| C. rhizophorae (2) | + | − | − | − | − | − | − | − | − | − | − | − | ||
| Dermestidae (250) | D. maculatus (127) | − | − | + | + | − | + | + | + | − | + | + | + | |
| D. frischi (114) | − | − | + | + | − | − | + | + | − | + | + | + | ||
| A. posticalis (9) | − | − | + | + | + | − | + | − | − | − | + | − | ||
| Elateridae (3) | A. grisescens (3) | − | + | + | − | − | − | − | − | − | − | − | − | |
| Histeridae (298) | S. chalcites (294) | − | + | + | + | + | + | + | + | − | + | + | + | |
| S. caerulescens (4) | − | − | − | − | − | − | + | − | − | − | + | + | ||
| Hybosoridae (3) | H. illigeri (3) | + | − | + | − | − | − | − | − | − | − | − | − | |
| Nitidulidae (9) | C. hemipterus (8) | − | − | + | + | − | − | + | + | − | − | − | − | |
| U. humeralis (1) | − | − | − | − | − | − | + | − | − | − | − | − | ||
| Ptinidae (3) | S. paniceum (3) | − | − | + | − | − | − | − | + | + | − | − | − | |
| Scarabaeidae (218) | A. adustus (181) | + | − | + | + | + | − | + | + | − | + | + | + | |
| R. saoudi (10) | − | − | + | + | + | − | + | + | − | + | + | + | ||
| M. insanabilis (27) | − | − | + | + | + | − | + | + | + | + | + | + | ||
| Staphylinidae (66) | Philonthus sp. (62) | − | − | + | + | − | − | + | + | − | − | + | + | |
| Leptacinus sp. (4) | − | − | − | − | − | − | + | − | − | − | − | − | ||
| Tenebrionidae (110) | M. pincticollis (22) | + | − | + | + | + | − | − | + | + | − | − | − | |
| T. crinite (31) | + | − | + | + | + | − | − | + | + | − | − | + | ||
| A. diapernius (11) | − | − | − | + | − | − | − | − | − | − | − | − | ||
| O. punctulatus (45) | + | − | + | + | + | − | + | + | + | + | − | + | ||
| A. cancellate (1) | − | − | − | − | + | − | − | − | − | − | − | − | ||
| Zopheridae (2) | Synchita sp. (2) | − | − | + | − | − | − | + | − | − | − | − | − | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Qurashi, A.S.; Al-Khalifa, M.S.; Al Dhafer, H.M.; Abdel-Dayem, M.S.; Ebaid, H.; Ahmed, A.M. Death-Leading Envenomization of Rabbits with Snake Versus Scorpion Venoms: A Comparative Forensic Investigation of Postmortem Decomposition and Beetle Succession. Insects 2025, 16, 625. https://doi.org/10.3390/insects16060625
Al-Qurashi AS, Al-Khalifa MS, Al Dhafer HM, Abdel-Dayem MS, Ebaid H, Ahmed AM. Death-Leading Envenomization of Rabbits with Snake Versus Scorpion Venoms: A Comparative Forensic Investigation of Postmortem Decomposition and Beetle Succession. Insects. 2025; 16(6):625. https://doi.org/10.3390/insects16060625
Chicago/Turabian StyleAl-Qurashi, Afnan Saleh, Mohammed Saleh Al-Khalifa, Hathal Mohammed Al Dhafer, Mahmoud Saleh Abdel-Dayem, Hossam Ebaid, and Ashraf Mohamed Ahmed. 2025. "Death-Leading Envenomization of Rabbits with Snake Versus Scorpion Venoms: A Comparative Forensic Investigation of Postmortem Decomposition and Beetle Succession" Insects 16, no. 6: 625. https://doi.org/10.3390/insects16060625
APA StyleAl-Qurashi, A. S., Al-Khalifa, M. S., Al Dhafer, H. M., Abdel-Dayem, M. S., Ebaid, H., & Ahmed, A. M. (2025). Death-Leading Envenomization of Rabbits with Snake Versus Scorpion Venoms: A Comparative Forensic Investigation of Postmortem Decomposition and Beetle Succession. Insects, 16(6), 625. https://doi.org/10.3390/insects16060625







