Mating Behavior of Cyclocephala barrerai Martínez (Coleoptera: Melolonthidae)
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Insects
2.2. Study of the Age at the Beginning of the First Period of Sexual Activity and Its Duration
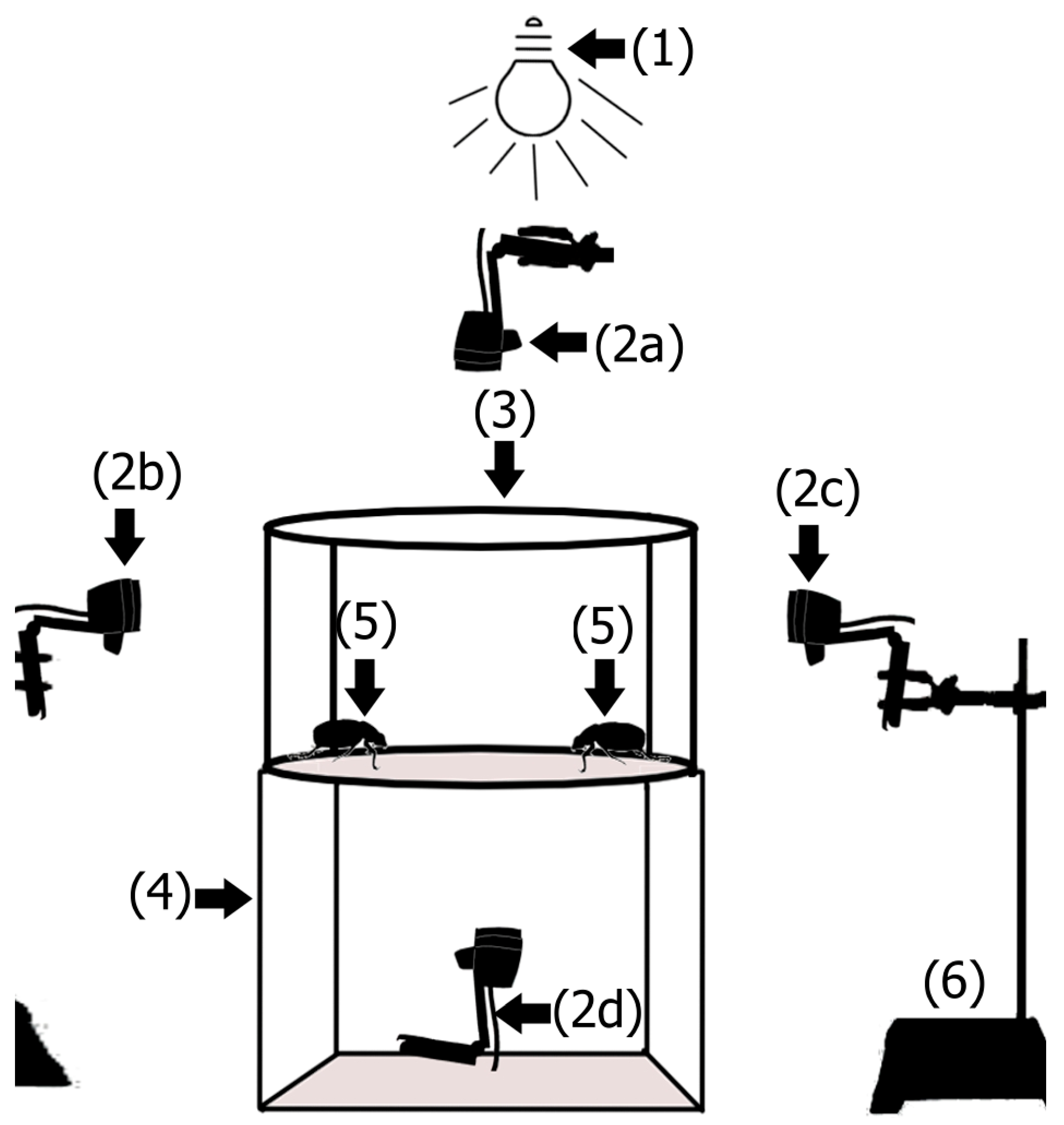
2.3. Recording of Mating Behavior
- (1)
- Virgin adults (n = 26 couples): adults who were in their first period of mating activity, 25 to 27 days post-emergence, and had no earlier mating experience.
- (2)
- Once-mated adults (n = 15 couples): these adults came from the previous bioassay and were in their second period of activity. The age of these insects ranged from 34 to 36 days post-emergence.
- (1)
- Premating—This phase begins when the insects emerge from the soil and are allocated to the bioassay arena, where they search for and court a potential mating partner [61]. Blue color was used in the ethogram to represent this phase.
- (2)
- Mating—This phase involves the union of the reproductive apparatus, insertion of the male’s aedeagus into the female, and ejaculation by the male [62]. Red color was used in the ethogram to represent this phase.
- (3)
- Postmating—This phase begins after the male transfers an ejaculate and withdraws his aedeagus [30]. Gray color was used in the ethogram to represent this phase.
2.4. Statistical Analysis
3. Results
3.1. Age at the Beginning of the First Period of Sexual Activity and Its Duration
3.2. Mating Behavior of Virgin and Once-Mated Adult Cyclocephala barrerai
3.2.1. Male Mating Behavior
3.2.2. Female Mating Behavior
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kokko, H.; Klug, H.; Jennions, M.D.; Shuker, D.M.; Simmons, L.W. Mating systems. In The Evolution of Insect Mating Systems; Shuker, D.M., Simmons, L.W., Eds.; Oxford University Press: Oxford, UK; New York, NY, USA, 2014; pp. 42–58. [Google Scholar]
- Parker, G.A. Sperm competition and its evolutionary consequences in the insects. Biol. Rev. 1970, 45, 525–567. [Google Scholar] [CrossRef]
- Markow, T.A.; Hanson, S.J. Multivariate analysis of Drosophila courtship. Proc. Natl. Acad. Sci. USA 1981, 78, 430–434. [Google Scholar] [CrossRef] [PubMed]
- Bonduriansky, R. The evolution of male mate choice in insects: A synthesis of ideas and evidence. Biol. Rev. 2001, 76, 305–339. [Google Scholar] [CrossRef] [PubMed]
- Eberhard, W.G. Copulatory courtship and cryptic female choice in insects. Biol. Rev. 1991, 66, 1–31. [Google Scholar] [CrossRef]
- Jagadeeshan, S.; Singh, R.S. A time-sequence functional analysis of mating behaviour and genital coupling in Drosophila: Role of cryptic female choice and male sex-drive in the evolution of male genitalia. J. Evol. Biol. 2006, 19, 1058–1070. [Google Scholar] [CrossRef]
- Alcock, J. Postinsemination associations between males and females in insects: The mate-guarding hypothesis. Annu. Rev. Entomol. 1994, 39, 1–21. [Google Scholar] [CrossRef]
- Alexander, R.D.; Marshall, D.C.; Cooley, J.R. Evolutionary perspectives on insect mating. In The Evolution of Mating Systems in Insects and Arachnids; Choe, J.C., Crespi, B.J.E., Eds.; Cambridge University Press: Cambridge, UK, 1997; pp. 4–31. [Google Scholar]
- Lenteren, J.C. van Fundamental knowledge about insect reproduction: Essential to develop sustainable pest management. Invertebr. Reprod. & Dev. 1999, 36, 1–15. [Google Scholar] [CrossRef]
- De Resende, B.O.; Ferreira, V.R.S.; Brasil, L.S.; Calvão, L.B.; Mendes, T.P.; de Carvalho, F.G.; Mendoza-Penagos, C.C.; Bastos, R.C.; Brito, J.S.; Oliveira-Junior, J.M.B.; et al. Impact of environmental changes on the behavioral diversity of the Odonata (Insecta) in the Amazon. Sci. Rep. 2021, 11, 9742. [Google Scholar] [CrossRef]
- Boake, C.R.B.; Shelly, T.E.; Kaneshiro, K.Y. Sexual selection in relation to pest-management strategies. Annu. Rev. Entomol. 1996, 41, 211–229. [Google Scholar] [CrossRef]
- Rockstein, M.; Miquel, J. Chapter 6—Aging in Insects. In The Physiology of Insecta, 2nd ed.; Rockstein, M., Ed.; Academic Press: Cambridge, MA, USA, 1973; pp. 371–478. ISBN 978-0-12-591601-1. [Google Scholar]
- Beck, S.D. Insect Photoperiodism; Academic Press: San Diego, CA, USA, 1980; ISBN 9780120843800. [Google Scholar]
- Cordero, C. Ejaculate substances that affect female insect reproductive physiology and behavior: Honest or arbitrary traits? J. Theor. Biol. 1995, 174, 453–461. [Google Scholar] [CrossRef]
- Carmel, I.; Tram, U.; Heifetz, Y. Mating induces developmental changes in the insect female reproductive tract. Curr. Opin. Insect Sci. 2016, 13, 106–113. [Google Scholar] [CrossRef] [PubMed]
- Dukas, R. Evolutionary biology of insect learning. Annu. Rev. Entomol. 2008, 53, 145–160. [Google Scholar] [CrossRef] [PubMed]
- Klein, A.L.; de Araújo, A.M. Courtship behavior of Heliconius erato phyllis (Lepidoptera, Nymphalidae) towards virgin and mated females: Conflict between attraction and repulsion signals? J. Ethol. 2010, 28, 409–420. [Google Scholar] [CrossRef]
- Engqvist, L.; Cordes, N.; Schwenniger, J.; Bakhtina, S.; Schmoll, T. Female remating behavior in a lekking Moth. Ethology 2014, 120, 662–671. [Google Scholar] [CrossRef]
- Schlaepfer, M.A.; McNeil, J.N. Are virgin male lepidopterans more successful in mate acquisition than previously mated individuals? A study of the European corn borer, Ostrinia nubilalis (Lepidoptera: Pyralidae). Can. J. Zool. 2000, 78, 2045–2050. [Google Scholar] [CrossRef]
- Cherman, M.A.; Morón, M.Á. Validación de la familia Melolonthidae Leach, 1819 (Coleoptera: Scarabaeoidea). Acta Zoológica Mex. 2014, 30, 201–220. [Google Scholar]
- Ramírez-Salinas, C.; Castro-Ramírez, A.E. El complejo” gallina ciega”(Coleoptera: Melolonthidae) en el cultivo de maíz, en El Madronal, municipio de Amatenango Del Valle, Chiapas, México. Acta Zoológica Mex. 2000, 79, 17–41. [Google Scholar] [CrossRef]
- Oliveira, L.J.; Garcia, M.A. Flight, feeding and reproductive behavior of Phyllophaga cuyabana (Moser) (Coleoptera: Melolonthidae) adults. Pesqui. Agropecuária Bras. 2003, 38, 179–186. [Google Scholar] [CrossRef]
- Rodrigues, S.R.; Morón, M.A.; Gomes, E.S.; Bento, J.M.S. Morphology of immature stages and mating behavior in Liogenys fusca (Blanchard) (Coleoptera, Melolonthidae, Melolonthinae). Rev. Bras. Entomol. 2016, 60, 284–289. [Google Scholar] [CrossRef]
- Nieves-Silva, E.; Aragón-García, A.; Robledo-Quintos, N.R.; Romero-López, A.A.; Pérez-Torres, B.C. Respuesta fisiológica y comportamental de Macrodactylus nigripes a compuestos volátiles del durazno. Southwest. Entomol. 2021, 46, 773–780. [Google Scholar] [CrossRef]
- Romero-López, A.A.; Arzuffi, R.; Valdez, J.; Morón, M.Á. Morfología y protrusión-retracción de la cámara genital femenina de Phyllophaga obsoleta (Coleoptera: Melolonthidae). Acta Zoológica Mex. 2009, 25, 315–321. [Google Scholar] [CrossRef][Green Version]
- Rodrigues, S.R.; Gomes, E.S.; Bento, J.M.S. Sexual dimorphism and mating behavior in Anomala testaceipennis. J. Insect Sci. 2014, 14, 210. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Nóbrega, R.L.; Maia, A.C.D.; De Lima, C.H.M.; Felix, K.E.S.; De Souza, T.B.; Pontes, W.J.T. Behavioral traits and sexual recognition: Multiple signaling in the reproductive behavior of Cyclocephala distincta (Melolonthidae, Cyclocephalini). An. Acad. Bras. Cienc. 2022, 94, e20200694. [Google Scholar] [CrossRef]
- Santos, G.K.N.; Navarro, D.M.d.A.F.; Maia, A.C.D. Cuticular lipid profiles of selected species of cyclocephaline beetles (Melolonthidae, Cyclocephalini). Bull. Entomol. Res. 2024, 114, 124–133. [Google Scholar] [CrossRef]
- Garcia, M.A.; Oliveira, L.J.; Oliveira, M.C.N. de Aggregation behavior of Phyllophaga cuyabana (Moser) (Coleoptera: Melolonthidae): Relationships between sites chosen for mating and offspring distribution. Neotrop. Entomol. 2003, 32, 537–542. [Google Scholar] [CrossRef]
- Arakaki, N.; Sadoyama, Y.; Kishita, M.; Nagayama, A.; Oyafuso, A.; Ishimine, M.; Ota, M.; Akino, T.; Fukaya, M.; Hirai, Y.; et al. Mating behavior of the scarab beetle Dasylepida ishigakiensis (Coleoptera: Scarabaeidae). Appl. Entomol. Zool. 2004, 39, 669–674. [Google Scholar] [CrossRef]
- Ferreira, K.R.; Rodrigues, S.R. The mating behavior of Leucothyreus marginaticollis Blanchard, 1843 (Coleoptera: Scarabaeidae: Rutelinae). Biota Neotrop. 2017, 17, e20170330. [Google Scholar] [CrossRef]
- Ferreira, K.R.; Gomes, E.S.; Rodrigues, S.R. Description of the third instar and mating behavior of Liogenys suturalis (Blanchard) (Coleoptera: Scarabaeidae). Coleopt. Bull. 2018, 72, 457–464. [Google Scholar] [CrossRef]
- Moore, M.R.; Cave, R.D.; Branham, M.D. Synopsis of the cyclocephaline scarab beetles (Coleoptera, Scarabaeidae, Dynastinae). Zookeys 2018, 745, 1–99. [Google Scholar] [CrossRef]
- Moore, M.R.; Cave, R.D.; Branham, M.A. Annotated catalog and bibliography of the cyclocephaline scarab beetles (Coleoptera, Scarabaeidae, Dynastinae, Cyclocephalini). Zookeys 2018, 2018, 101–378. [Google Scholar] [CrossRef]
- Rodrigues, S.R.; Barbosa, C.A.F.; Fuhrmann, J.; Amaro, R.A. Mating behavior and description of immature stages of Cyclocephala melanocephala (Fabricius, 1775) (Coleoptera: Scarabaeidae: Dynastinae), identification key and remarks on known immatures of Cyclocephalini species. Rev. Bras. Entomol. 2018, 62, 205–219. [Google Scholar] [CrossRef]
- Saldanha, F.G.; Rodrigues, S.R.; Amaro, R.A.; Fuhrmann, J. Description of mating behavior, life cycle, and antennal sensilla of Cyclocephala putrida Burmeister, 1847 (Coleoptera, Scarabaeidae, Dynastinae). Biota Neotrop. 2020, 20, 1–8. [Google Scholar] [CrossRef]
- Souza, T.B.; Maia, A.C.D.; Albuquerque, C.M.R.; Iannuzzi, L. Biology and management of the masked chafer Cyclocephala distincta Burmeister (Melolonthidae, Dynastinae, Cyclocephalini). Rev. Bras. Entomol. 2015, 59, 37–42. [Google Scholar] [CrossRef]
- Stechauner-Rohringer, R.; Pardo-Locarno, L.C. Redescripcion de inmaduros, ciclo de vida, distribucion e importancia agricola de Cyclocephala lunulata Burmeister (Coleoptera: Melolonthidae: Dynastinae) en Colombia. Bol. Cient. Mus. Hist. Nat. 2010, 14, 203–220. [Google Scholar]
- Benítez-Herrera, L.-N.; Cibrián-Tovar, J.; Romero-López, A.-A. Behavioral and histological evidence of participation by pheromones in precopulatory behavior of Cyclocephala lunulata. Southwest. Entomol. 2024, 49, 173–184. [Google Scholar] [CrossRef]
- Johnson, J.P. Cyclocephala (ochrosidia) borealis in connecticut 1. J. Agric. Res. 1941, 62, 79–86. [Google Scholar]
- Potter, D.A. Flight activity and sex attraction of Northern and Southern masked chafers1 in Kentucky Turfgrass2. Ann. Entomol. Soc. Am. 1980, 73, 414–417. [Google Scholar] [CrossRef]
- Aragón, A.; Morón, M.-A.; Tapia-Rojas, A.M.; Rojas-García, R. Fauna de Coleoptera Melolonthidae en el rancho “La joya”, Atlixco, Puebla. México. Acta Zool. Mex. 2001, 83, 143–164. [Google Scholar] [CrossRef]
- Minor, P.; Morón, M.Á. Coleópteros lamelicornios (Coleoptera: Scarabaeoidea) de la barranca de Huehuetitla, Tlaxcala, México. Acta Zoológica Mex. 2016, 32, 310–322. [Google Scholar] [CrossRef][Green Version]
- Márquez, J.; Reyes-Hernández, J.L.; Cerón-Gómez, R.; Escoto-Moreno, J.A.; Ramírez-Ponce, A. Coleópteros Scarabaeoidea (Insecta: Coleoptera) de Aguascalientes, México. Acta Zoológica Mex. 2022, 38, 1–51. [Google Scholar] [CrossRef]
- Márquez-Manzano, J.P.; Aragón-García, A.; Cuate-Mozo, V.A.; Pérez-Torres, B.C. Diversidad de gallinas ciegas (Coleoptera: Melolonthidae) asociadas a pastos ornamentales de la ciudad de Puebla, México. Ent. Mex. 2024, 11, 1–5. [Google Scholar]
- Romero-López, A.A.; Morón, M.A.; Aragón, A.; Villalobos, F.J. La “Gallina Ciega” (Coleoptera: Scarabaeoidea: Melolonthidae) vista como un “ingeniero del suelo”. Southwest. Entomol. 2010, 35, 331–343. [Google Scholar] [CrossRef]
- Morón, M.A.; Lugo-García, G.A.; Aragón-García, A. Description of the third instar larvae of five species of Cyclocephala (Coleoptera, Melolonthidae, Dynastinae) from Mexico. Rev. Bras. Entomol. 2014, 58, 219–228. [Google Scholar] [CrossRef]
- Sanchez-Cruz, A.; Tapia-Maruri, D.; Villa-Ayala, P.; Robledo, N.; Romero-López, A.A.; Rojas, J.C.; Jiménez-Pérez, A. Antennal sensilla of Cyclocephala barrerai (Coleoptera: Melolonthidae): Morphology, sexual dimorphism, allometric relationships, and function. Environ. Entomol. 2024, 53, nvae087. [Google Scholar] [CrossRef]
- Sanchez-Cruz, A.; Tapia-Maruri, D.; Jiménez-Pérez, A. Reproductive apparatus, gonadic maturation, and allometry of Cyclocephala barrerai Martínez (Coleoptera: Melolonthidae: Dynastinae). Insects 2022, 13, 638. [Google Scholar] [CrossRef]
- Sanchez-Cruz, A.; Jiménez-Pérez, A.; Benítez-Herrera, L.N.; Villa-Ayala, P.; Rosete-Enríquez, M.; Romero-López, A.A. Notas sobre apareamiento, supervivencia y preferencia alimentaria para Cyclocephala barrerai Martínez (Coleoptera: Melolonthidae). In Coleópteros Edafícolas y Microorganismos Asociados: Diversidad y Ecología; García, A.A., Miguel, B., Rincón, N., Eds.; Benemérita Universidad Autónoma de Puebla: Puebla, Mexico, 2025; pp. 169–186. ISBN 978-607-2637-39-9. [Google Scholar]
- Sanchez-Cruz, A.; Robledo, N.; Rosete-Enríquez, M.; Romero-López, A.A. Attraction of adults of Cyclocephala lunulata and Cyclocephala barrerai (Coleoptera: Scarabaeoidea: Melolonthidae) towards bacteria volatiles isolated from their genital chambers. Molecules 2020, 25, 4430. [Google Scholar] [CrossRef]
- Souza, T.B.; Maia, A.C.D.; Schlindwein, C.; de Albuquerque, L.S.C.; Iannuzzi, L. The life of Cyclocephala celata Dechambre, 1980 (Coleoptera: Scarabaeidae: Dynastinae) in captivity with descriptions of the immature stages. J. Nat. Hist. 2014, 48, 275–283. [Google Scholar] [CrossRef]
- Singh, S.R.; Singh, B.N. Female remating in Drosophila: Comparison of duration of copulation between first and second matings in six species. Curr. Sci. 2004, 86, 465–470. [Google Scholar]
- Ridley, M. Mating frequency and fecundity in insects. Biol. Rev. 1988, 63, 509–549. [Google Scholar] [CrossRef]
- Calisi, R.M.; Bentley, G.E. Lab and field experiments: Are they the same animal? Horm. Behav. 2009, 56, 1–10. [Google Scholar] [CrossRef]
- Markow, T.A. Reproductive behavior of Drosophila melanogaster and D. nigrospiracula in the field and in the laboratory. J. Comp. Psychol. 1988, 102, 169–173. [Google Scholar] [CrossRef]
- Reisen, W.K.; Knop, N.F.; Peloquin, J.J. Swarming and mating behavior of laboratory and field strains of Culex tarsalis (Diptera: Culicidae). Ann. Entomol. Soc. Am. 1985, 78, 667–673. [Google Scholar] [CrossRef]
- Pérez-Staples, D.; Shelly, T.E.; Yuval, B. Female mating failure and the failure of ‘mating’ in sterile insect programs. Entomol. Exp. Appl. 2013, 146, 66–78. [Google Scholar] [CrossRef]
- Bakthavatsalam, N. Chapter 19—Semiochemicals. In Ecofriendly Pest Management for Food Security; Omkar, Ed.; Academic Press: San Diego, CA, USA, 2016; pp. 563–611. ISBN 978-0-12-803265-7. [Google Scholar]
- Friard, O.; Gamba, M. BORIS: A free, versatile open-source event-logging software for video/audio coding and live observations. Methods Ecol. Evol. 2016, 7, 1325–1330. [Google Scholar] [CrossRef]
- Okada, Y.; Hasegawa, E. Size-dependent precopulatory behavior as mate-securing tactic in the Japanese stag beetle, Prosopocoilus inclinatus (Coleoptera; Lucanidae). J. Ethol. 2005, 23, 99–102. [Google Scholar] [CrossRef]
- Brennan, P.L.R.; Orbach, D.N. Chapter Three—Copulatory Behavior and Its Relationship to Genital Morphology. In Advances in the Study of Behavior; Naguib, M., Barrett, L., Healy, S.D., Podos, J., Simmons, L.W., Zuk, M., Eds.; Academic Press: Cambridge, MA, USA, 2020; Volume 52, pp. 65–122. [Google Scholar]
- Teal, P.E.A.; McLaughlin, J.R.; Tumlinson, J.H. Analysis of the reproductive behavior of Heliothis virescens (F.)1 under laboratory conditions2. Ann. Entomol. Soc. Am. 1981, 74, 324–330. [Google Scholar] [CrossRef]
- Gansner, E.R.; Koutsofios, E.; North, S.C.; Vo, K.-P. A technique for drawing directed graphs. IEEE Trans. Softw. Eng. 1993, 19, 214–230. [Google Scholar] [CrossRef]
- Ellson, J.; Gansner, E.R.; Koutsofios, E.; North, S.C.; Woodhull, G. Graphviz and Dynagraph—Static and Dynamic Graph Drawing Tools BT—Graph Drawing Software; Jünger, M., Mutzel, P., Eds.; Springer: Berlin/Heidelberg, Germany, 2004; pp. 127–148. ISBN 978-3-642-18638-7. [Google Scholar]
- Boggs, C.L. Understanding insect life histories and senescence through a resource allocation lens. Funct. Ecol. 2009, 23, 27–37. [Google Scholar] [CrossRef]
- Aragón-Gacía, A.; Morón, M.Á.; López Olguín, J.F.; Cervantes Peredo, L.M. Ciclo de vida y conducta de adultos de cinco especies de Phyllophaga Harris, 1827 (Coleoptera: Melolonthidae; Melolonthinae). Acta Zoológica Mex. (Nueva Ser.) 2005, 21, 87–99. [Google Scholar] [CrossRef]
- Metcalf, R.L. Ultramicrochemistry of insect semiochemicals. Microchim. Acta 1998, 129, 167–180. [Google Scholar] [CrossRef]
- Facundo, H.T.; Linn, C.E.; Villani, M.G.; Roelofs, W.L. Emergence, mating, and postmating behaviors of the oriental beetle (Coleoptera: Scarabaeidae). J. Insect Behav. 1999, 12, 175–192. [Google Scholar] [CrossRef]
- Costa, A.; Reeve, J.D. Olfactory experience modifies semiochemical responses in a bark beetle predator. J. Chem. Ecol. 2011, 37, 1166–1176. [Google Scholar] [CrossRef]
- Favaris, A.P. Semiochemicals Associated with the Reproductive Behavior of Cyclocephala paraguayensis Arrow. Ph.D. Thesis, University of São Paulo, São Paulo, Brazil, 2021. [Google Scholar]
- Ghose, S.L. The Effects of Xanthosoma spp. (Araceae) Temperature, Scent, and Flowers on the Mating Frequency of Cyclocephala sexpunctata (Scarabaeidae); Monteverde Institute: Tropical Ecology and Conservation: Monteverde, Costa Rica, 2009; p. 285. Available online: https://digitalcommons.usf.edu/tropical_ecology/285 (accessed on 15 January 2025).
- Haynes, K.F.; Potter, D.A. Chemically mediated sexual attraction of male Cyclocephala lurida (Coleoptera: Scarabaeidae) and other Scarabaeid beetles to immature stages. Environ. Entomol. 1995, 24, 1302–1306. [Google Scholar] [CrossRef]
- Romero-López, A.A.; Aragón, A.; Arzuffi, R. Estudio comparativo del comportamiento sexual de cuatro especies de Phyllophaga (Coleoptera: Melolonthidae). Entomol. Mex. 2007, 6, 275–281. [Google Scholar]
- Rodrigues, S.R.; Fuhrmann, J.; Amaro, R.A. Aspects of mating behavior and antennal sensilla in Anomala inconstans Burmeister, 1844 (Coleoptera: Scarabaeidae: Rutelinae). Biota Neotrop. 2019, 19, e20180664. [Google Scholar] [CrossRef]
- Dukas, R.; Clark, C.W.; Abbott, K. Courtship strategies of male insects: When is learning advantageous? Anim. Behav. 2006, 72, 1395–1404. [Google Scholar] [CrossRef]
- Eberhard, W.G.; Kariko, S.J. Copulation behavior inside and outside the beetle Macrohaltica jamaicensis (Coleoptera, Chrysomelidae). J. Ethol. 1996, 14, 59–72. [Google Scholar] [CrossRef]
- Eberhard, W.G. Copulatory courtship and genital mechanics of three species of Macrodactylus (Coleoptera Scarabaeidae Melolonthinae). Ethol. Ecol. Evol. 1993, 5, 19–63. [Google Scholar] [CrossRef]
- Tallamy, D.W.; Darlington, M.B.; Pesek, J.D.; Powell, B.E. Copulatory courtship signals male genetic quality in cucumber beetles. Proc. R. Soc. Lond. Ser. B Biol. Sci. 2003, 270, 77–82. [Google Scholar] [CrossRef]
- Eberhard, W.G. Rapid divergent evolution of sexual morphology: Comparative tests of antagonistic coevolution and traditional female choise. Evolution 2004, 58, 1947–1970. [Google Scholar] [CrossRef]
- Cayetano, L.; Maklakov, A.A.; Brooks, R.C.; Bonduriansky, R. Evolution of male and female genitalia following release from sexual selection. Evolution 2011, 65, 2171–2183. [Google Scholar] [CrossRef] [PubMed]
- Eberhard, W.G. Female Control: Sexual Selection by Cryptic Female Choice; Princeton University Press: Princeton, NJ, USA, 1996; Volume 69, ISBN 9780691010847. [Google Scholar]
- Wagner, W.E. Measuring female mating preferences. Anim. Behav. 1998, 55, 1029–1042. [Google Scholar] [CrossRef] [PubMed]
- Somjee, U. Positive allometry of sexually selected traits: Do metabolic maintenance costs play an important role? BioEssays 2021, 43, 2000183. [Google Scholar] [CrossRef] [PubMed]

| Abbreviation of the Elements of the Act | Name of the Elements of the Act | Description |
|---|---|---|
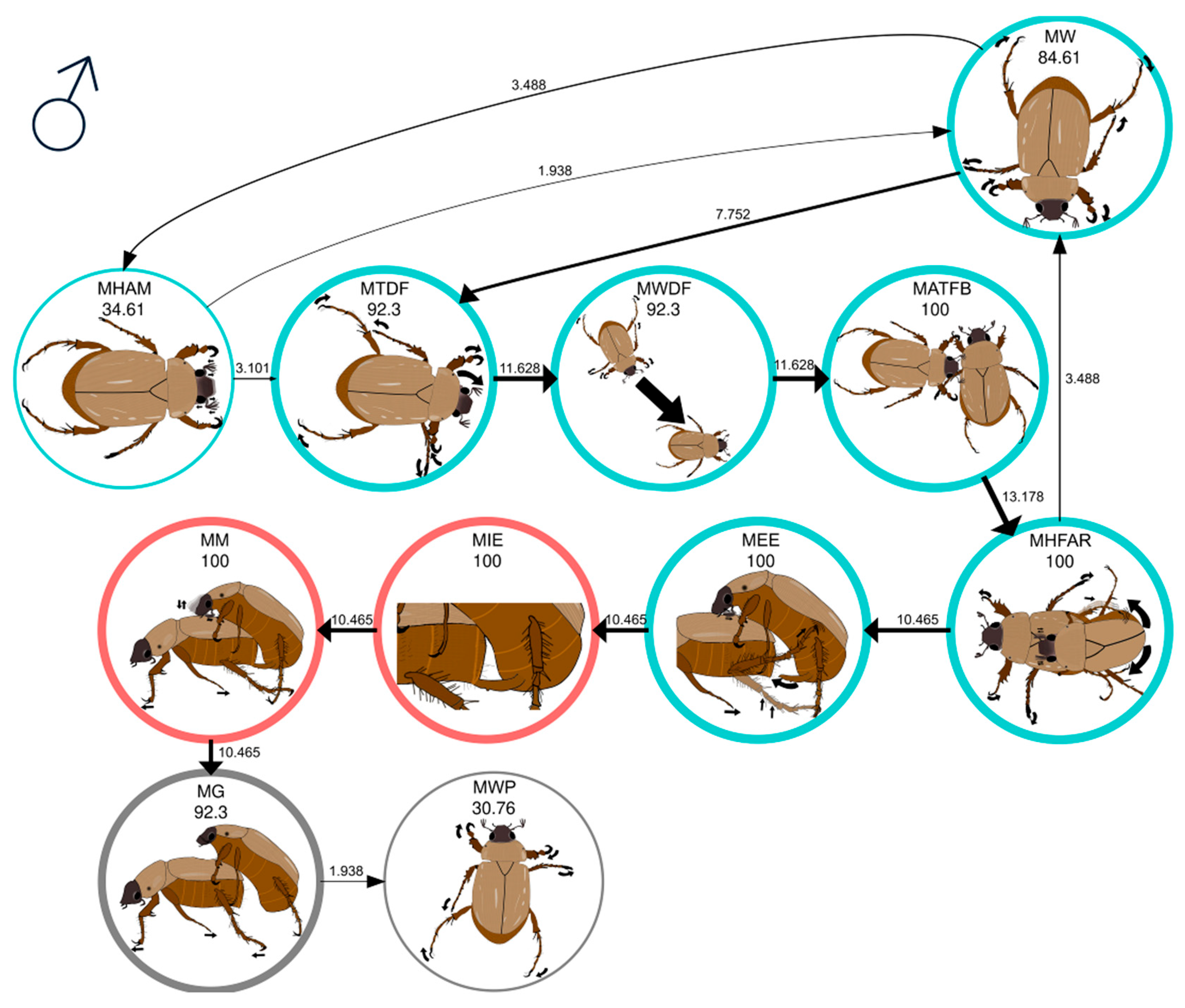
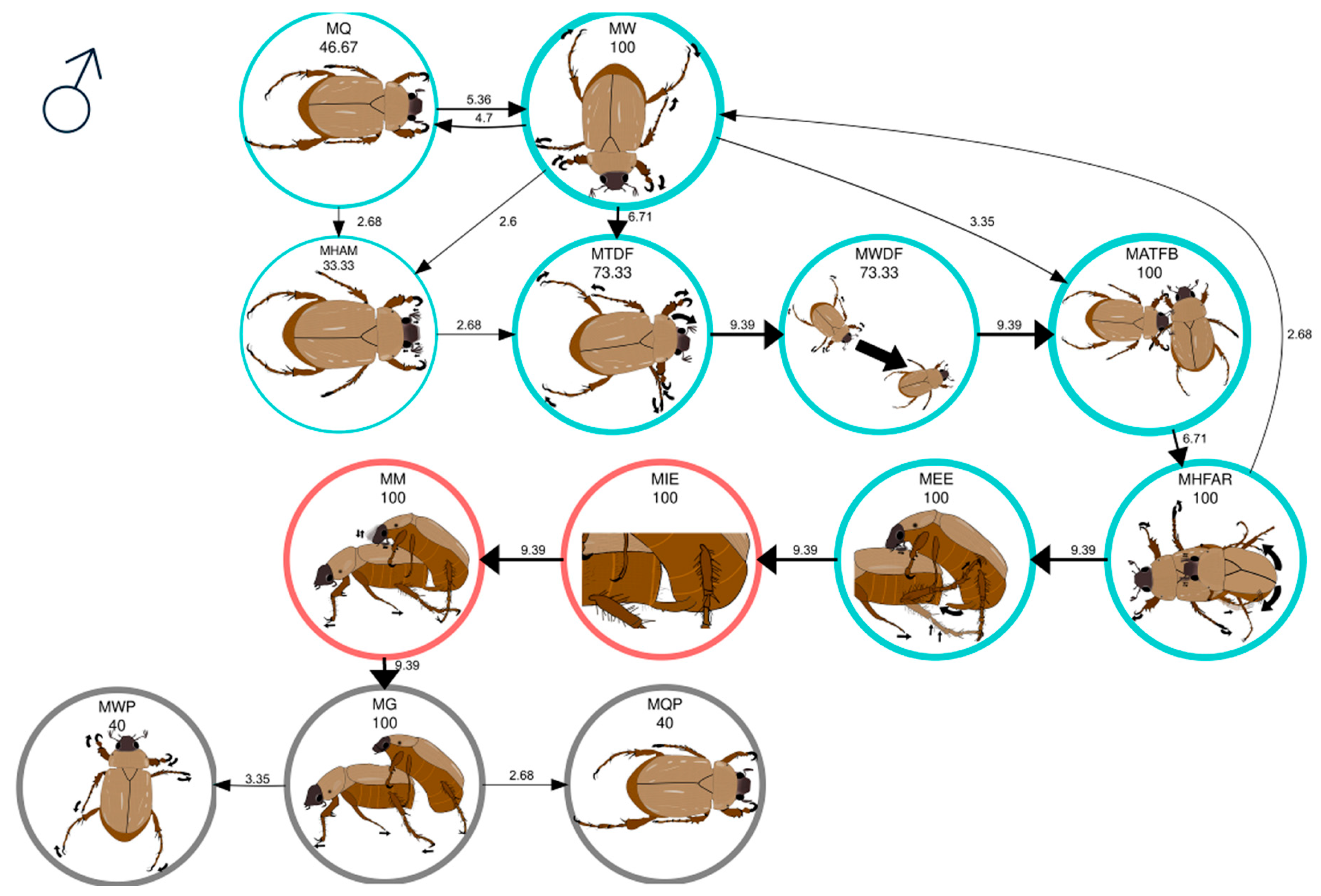
| MW | Male walks | The male walks along the periphery of the arena without any apparent direction or orientation. |
| MQ | Male is quiet | The male is still with his head down. |
| MHAM | Male head movement and antennation | The male moves his antennae up and down, accompanied by head movements and alternating contraction and extension of the antennae. |
| MTDF | Male turns in direction of female | The male turns his head and thorax in the direction the female is walking. |
| MWDF | Male walks in direction of female | The male, wandering, changes course and walks directly toward the female. |
| MATFB | Male antennation on female’s body | The male touches the lateral area of the thorax or abdomen of the female with his antennae. |
| MHFAR | Male holds female and antennal rubbing | The male holds the female’s elytra with his tarsi and rubs his antennae on her elytra. |
| MEE | Male extends aedeagus | The male folds his abdomen and exposes the aedeagus. |
| MIE | Male introduces aedeagus | The male inserts the aedeagus into the female’s pygidium. |
| MM | Male mounting the female | The male mounts the female, holding her with the tarsal claws of his forelegs. The male inserts the aedeagus into the female’s pygidium, rubs its antennae on her elytra, and occasionally moves its abdomen. |
| MG | Male guarding the female | The male is positioned on top of the female, holding her with the tarsal claws of his forelegs. The aedeagus is neither inserted nor exposed, and no antennal rubs are observed. |
| MWP | Male walking (postcopulatory) | The male releases the female and walks away. |
| MQP | Male quiet (postcopulatory) | The male is still with his head down. Only in remating behavior. |
| MW | MHAM | MTDF | MWDF | MATFB | MHFAR | MEE | MIE | MM | MG | MWP | Σ | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MW | Obs | 9 * | 20 * | 29 | |||||||||
| Exp | 1 | 3.14 | |||||||||||
| χ2 | 64 | 90.52 | |||||||||||
| T | 3.48 | 7.75 | |||||||||||
| MHAM | Obs | 5 * | 8 * | 13 | |||||||||
| Exp | 0.7 | 1.41 | |||||||||||
| χ2 | 26.14 | 30.8 | |||||||||||
| T | 1.93 | 3.1 | |||||||||||
| MTDF | Obs | 30 * | 30 | ||||||||||
| Exp | 3.48 | ||||||||||||
| χ2 | 202.1 | ||||||||||||
| T | 11.62 | ||||||||||||
| MWDF | Obs | 30 * | 30 | ||||||||||
| Exp | 3.95 | ||||||||||||
| χ2 | 171.7 | ||||||||||||
| T | 11.62 | ||||||||||||
| MATFB | Obs | 34 * | 34 | ||||||||||
| Exp | 4.48 | ||||||||||||
| χ2 | 194.5 | ||||||||||||
| T | 13.17 | ||||||||||||
| MHFAR | Obs | 9 * | 27 * | 36 | |||||||||
| Exp | 1.95 | 3.76 | |||||||||||
| χ2 | 24.48 | 143.6 | |||||||||||
| T | 3.48 | 10.46 | |||||||||||
| MEE | Obs | 27 * | 27 | ||||||||||
| Exp | 3.76 | ||||||||||||
| χ2 | 143.6 | ||||||||||||
| T | 10.46 | ||||||||||||
| MIE | Obs | 27 * | 27 | ||||||||||
| Exp | 3.76 | ||||||||||||
| χ2 | 143.6 | ||||||||||||
| T | 10.46 | ||||||||||||
| MM | Obs | 27 * | 27 | ||||||||||
| Exp | 3.76 | ||||||||||||
| χ2 | 143.6 | ||||||||||||
| T | 10.46 | ||||||||||||
| MG | Obs | 5 * | 5 | ||||||||||
| Exp | 0.09 | ||||||||||||
| χ2 | 267.8 | ||||||||||||
| T | 1.93 | ||||||||||||
| Σ | 14 | 9 | 28 | 30 | 30 | 34 | 27 | 27 | 27 | 27 | 5 | 258 |
| MQ | MW | MHAM | MTDF | MWDF | MATFB | MHFAR | MEE | MIE | MM | MG | MWP | MQP | Σ | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MQ | Obs | 8 * | 4 * | 12 | |||||||||||
| Exp | 0.96 | 0.64 | |||||||||||||
| χ2 | 51.6 | 17.6 | |||||||||||||
| T | 5.3 | 2.7 | |||||||||||||
| MW | Obs | 7 * | 4 * | 10 * | 5 * | 26 | |||||||||
| Exp | 1.22 | 1.39 | 2.44 | 3.31 | |||||||||||
| χ2 | 27.3 | 4.9 | 23.4 | 0.86 | |||||||||||
| T | 4.7 | 2.6 | 6.7 | 3.35 | |||||||||||
| MHAM | Obs | 4 * | 4 | ||||||||||||
| Exp | 0.37 | ||||||||||||||
| χ2 | 35.6 | ||||||||||||||
| T | 2.68 | ||||||||||||||
| MTDF | Obs | 14 * | 14 | ||||||||||||
| Exp | 1.31 | ||||||||||||||
| χ2 | 123 | ||||||||||||||
| T | 9.4 | ||||||||||||||
| MWDF | Obs | 14 * | 14 | ||||||||||||
| Exp | 1.78 | ||||||||||||||
| χ2 | 83.8 | ||||||||||||||
| T | 9.4 | ||||||||||||||
| MATFB | Obs | 10 * | 10 | ||||||||||||
| Exp | 0.67 | ||||||||||||||
| χ2 | 130 | ||||||||||||||
| T | 6.7 | ||||||||||||||
| MHFAR | Obs | 4 * | 14 * | 18 | |||||||||||
| Exp | 1.45 | 1.69 | |||||||||||||
| χ2 | 4.4 | 89.6 | |||||||||||||
| T | 2.68 | 9.4 | |||||||||||||
| MEE | Obs | 14 * | 14 | ||||||||||||
| Exp | 1.31 | ||||||||||||||
| χ2 | 123 | ||||||||||||||
| T | 9.4 | ||||||||||||||
| MIE | Obs | 14 * | 14 | ||||||||||||
| Exp | 1.31 | ||||||||||||||
| χ2 | 123 | ||||||||||||||
| T | 9.4 | ||||||||||||||
| MM | Obs | 14 * | 14 | ||||||||||||
| Exp | 1.31 | ||||||||||||||
| χ2 | 123 | ||||||||||||||
| T | 9.4 | ||||||||||||||
| MG | Obs | 5 * | 4 * | 9 | |||||||||||
| Exp | 0.3 | 0.24 | |||||||||||||
| χ2 | 73.6 | 58.9 | |||||||||||||
| T | 3.3 | 2.7 | |||||||||||||
| Σ | 7 | 12 | 8 | 14 | 14 | 19 | 10 | 14 | 14 | 14 | 14 | 5 | 4 | 149 |
| Abbreviation of the Elements of the Act | Name of the Elements of the Act | Description |
|---|---|---|
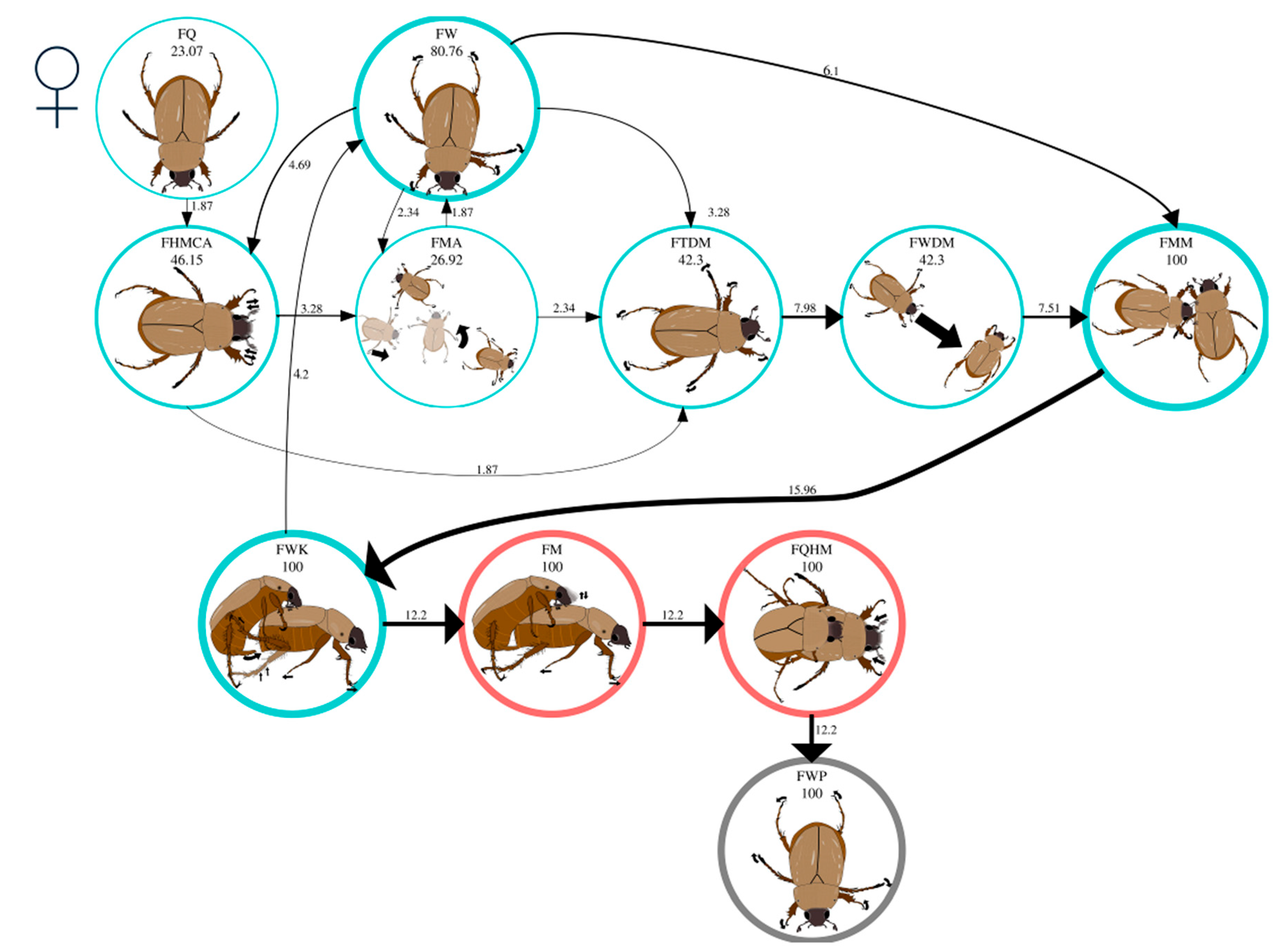
| FW | The female walks. | The female walks on the periphery of the arena without apparent direction or orientation. |
| FQ | The female is quiet. | The female is still with her head down. |
| FHMCA | The female moves her head and antennae. | The female moves her head up and down and contracts and extends her antennae. |
| FTDM | The female turns in the direction of the male. | The female turns her head and thorax towards the male. |
| FWDM | The female walks in the direction of the male. | The female walks straight toward the male, occasionally extending her antennae. |
| FMA | The female moves away from the male. | The female stops walking on the periphery of the arena or has no apparent orientation and moves away from the male. |
| FMM | The female meets the male. | The male and female meet and interact. |
| FWK | The female walks forward while kicking the male. | As the male attempts to grasp the lateral areas of the female’s elytra, the female moves forward while continuously thrusting back one of the second or third pair of legs, slamming it against the male and pushing him backward. |
| FM | The female is in mounting. | The female walks with the male on top. The male has inserted his aedeagus into the female’s pygidium and moves his head and antennae. |
| FQHM | The female is quiet and has head movements. | The female stops and moves her head up and down, stretching and contracting her antennae. |
| FWP | The female walks (postcopulatory). | The female walks with or without the male on top of her, and the male’s aedeagus is not inserted. |
| FW | FHC | FMA | FTDM | FWDM | FMM | FWK | FM | FQHM | FWP | Σ | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| FW | Obs | 10 * | 5 * | 7 * | 35 | |||||||
| Exp | 2.3 | 1.97 | 2.62 | |||||||||
| χ2 | 25.77 | 4.66 | 7.32 | |||||||||
| T | 4.69 | 2.34 | 3.28 | |||||||||
| FQ | Obs | 4 * | 4 | |||||||||
| Exp | 0.26 | |||||||||||
| χ2 | 53.12 | |||||||||||
| T | 1.87 | |||||||||||
| FHMCA | Obs | 7 * | 4 * | 11 | ||||||||
| Exp | 0.61 | 0.82 | ||||||||||
| χ2 | 66.93 | 12.33 | ||||||||||
| T | 3.28 | 1.87 | ||||||||||
| FMA | Obs | 4 * | 5 * | 9 | ||||||||
| Exp | 0.54 | 0.67 | ||||||||||
| χ2 | 22.17 | 27.98 | ||||||||||
| T | 1.87 | 2.34 | ||||||||||
| FTDM | Obs | 17 * | 17 | |||||||||
| Exp | 1.35 | |||||||||||
| χ2 | 181.4 | |||||||||||
| T | 7.98 | |||||||||||
| FWDM | Obs | 16 * | 16 | |||||||||
| Exp | 2.17 | |||||||||||
| χ2 | 88.14 | |||||||||||
| T | 7.51 | |||||||||||
| FMM | Obs | 34 * | 34 | |||||||||
| Exp | 5.42 | |||||||||||
| χ2 | 150.7 | |||||||||||
| T | 15.96 | |||||||||||
| FWK | Obs | 9 * | 26 * | 35 | ||||||||
| Exp | 2.13 | 4.27 | ||||||||||
| χ2 | 22.15 | 110.5 | ||||||||||
| T | 4.22 | 12.2 | ||||||||||
| FM | Obs | 26 * | 26 | |||||||||
| Exp | 3.17 | |||||||||||
| χ2 | 164.41 | |||||||||||
| T | 12.2 | |||||||||||
| FQHM | Obs | 26 * | 26 | |||||||||
| Exp | 3.17 | |||||||||||
| χ2 | 164.4 | |||||||||||
| T | 12.2 | |||||||||||
| Σ | 13 | 14 | 12 | 16 | 17 | 29 | 34 | 26 | 26 | 26 | 213 |
| FW | FTDM | FWDM | FMM | FWK | FM | FQHM | FWP | Σ | ||
|---|---|---|---|---|---|---|---|---|---|---|
| FW | Obs | 10 * | 9 * | 19 | ||||||
| Exp | 1.6 | 3.16 | ||||||||
| χ2 | 41.6 | 10.7 | ||||||||
| T | 8.7 | 7.9 | ||||||||
| FTDM | Obs | 10 * | 10 | |||||||
| Exp | 0.87 | |||||||||
| χ2 | 94.8 | |||||||||
| T | 8.77 | |||||||||
| FWDM | Obs | 10 * | 10 | |||||||
| Exp | 1.66 | |||||||||
| χ2 | 41.6 | |||||||||
| T | 8.7 | |||||||||
| FMM | Obs | 21 * | 21 | |||||||
| Exp | 3.86 | |||||||||
| χ2 | 75.8 | |||||||||
| T | 18.4 | |||||||||
| FWK | Obs | 6 * | 15 * | 21 | ||||||
| Exp | 1.1 | 2.76 | ||||||||
| χ2 | 21.6 | 54.1 | ||||||||
| T | 5.26 | 13.15 | ||||||||
| FM | Obs | 18 * | 18 | |||||||
| Exp | 2.84 | |||||||||
| χ2 | 80.8 | |||||||||
| T | 15.7 | |||||||||
| FQHM | Obs | 15 * | 15 | |||||||
| Exp | 1.97 | |||||||||
| χ2 | 85.9 | |||||||||
| T | 13.15 | |||||||||
| Σ | 6 | 10 | 10 | 19 | 21 | 15 | 18 | 15 | 114 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sanchez-Cruz, A.; Villa-Ayala, P.; Jiménez-Pérez, A. Mating Behavior of Cyclocephala barrerai Martínez (Coleoptera: Melolonthidae). Insects 2025, 16, 613. https://doi.org/10.3390/insects16060613
Sanchez-Cruz A, Villa-Ayala P, Jiménez-Pérez A. Mating Behavior of Cyclocephala barrerai Martínez (Coleoptera: Melolonthidae). Insects. 2025; 16(6):613. https://doi.org/10.3390/insects16060613
Chicago/Turabian StyleSanchez-Cruz, Abraham, Patricia Villa-Ayala, and Alfredo Jiménez-Pérez. 2025. "Mating Behavior of Cyclocephala barrerai Martínez (Coleoptera: Melolonthidae)" Insects 16, no. 6: 613. https://doi.org/10.3390/insects16060613
APA StyleSanchez-Cruz, A., Villa-Ayala, P., & Jiménez-Pérez, A. (2025). Mating Behavior of Cyclocephala barrerai Martínez (Coleoptera: Melolonthidae). Insects, 16(6), 613. https://doi.org/10.3390/insects16060613







