Simple Summary
Ambrosia beetles are insects that infest stressed or dying trees and cultivate fungus as a food source. Some species, such as Xyleborus glabratus and Euwallacea fornicatus, may seriously damage fruit and forest trees, including avocado orchards, in addition to spreading harmful fungi that cause tree diseases. This review explores the use of naturally occurring fungi that infect insects, called entomopathogenic fungi (EPF), as an environmental-friendly approach to control these beetles. Although many of these fungi are effective in laboratory bioassays, their effect under real-life conditions, such as in infected orchards or forest trees, is still unknown. Studies must also consider that these beetles depend on their symbiotic fungi for survival. This review will focus on bioassays to assess the effectiveness of these fungi under field conditions. Adopting fungi as natural control strategies will help protect our forest and orchard ecosystems and reduce dependence on chemical pesticides.
Abstract
Ambrosia beetles, known for their symbiotic relationship with fungi cultivated within the tissues of host trees, have become significant pests, particularly when they serve as vectors for pathogenic fungi such as Raffaelea lauricola. Given the regulatory and environmental constraints for chemical application as a tool for their control, entomopathogenic fungi (EPF) represent a promising pest management alternative. This review presents an overview of bioassays assessing the pathogenicity and virulence of EPF against ambrosia beetles. Most studies have been performed in vivo (artificial diet) under laboratory conditions, focusing on exotic species and testing EPF genera such as Beauveria, Metarhizium, Isaria, and Purpureocillium. However, variations in inoculation methods, environmental conditions, and fungal formulations, have led to diverse results. In addition, the complex biology of these insects, particularly their dependence on symbiotic fungi, represents significant methodological challenges. Field trials (in situ bioassays) are still scarce, and there is a need to move toward standardized protocols and more objective experimental models that consider not only insects’ behavior but also ecological factors. Bridging this gap is essential for successfully implementing EPF-based strategies to assess ambrosia beetles’ biocontrol.
1. Introduction
Ambrosia beetles are a group of wood-boring beetles belonging to the subfamily Scolytinae []. They are characterized by their symbiotic relationship with microorganisms, particularly fungi, which are cultivated by female beetle on the walls of the galleries that they construct inside the host trees, serving as a food source for the beetles and their developing larvae [,]. Globally, approximately 6000 species within this subfamily are known, most of which play a key role in the wood decomposition within forest ecosystems, acting as primary colonizers of dead or weakened trees []. Among this group, around 50 species—mostly exotic—have the potential to attack and infect living trees. Beetles and symbiotic fungi cause significant damage to tree trunks, leading to their death, thus becoming an important phytosanitary threat. In tropical and subtropical regions, ambrosia beetles have been associated with damage to urban green areas and fruit trees such as avocado [,,].
In forest and orchard ecosystems, the use of chemical products for insect pests and vectors control is strictly regulated. Preventive treatments like bifenthrin and permethrin have shown effectiveness in reducing beetle population and preventing new infestations []. Although often effective, their application may have negative consequences on human health, non-target organisms, and the commercial value of wood and fruit products, thereby limiting their international trade [,,,]. Consequently, the management of ambrosia beetles has primarily focused on preventive strategies, involving continuous monitoring, along with cultural practices and semiochemical methods [,]. In recent years, there has also been growing interest in the potential of biological control agents, particularly parasitoids and entomopathogenic fungi (EPF) [,,,].
The use of EPF may be a viable initial alternative. Numerous studies have demonstrated their effectiveness under laboratory conditions [,]. However, it is essential to interpret these results with caution, as many were conducted under conditions that may not reflect real wood/gallery environments, often overlooking the beetles’ cryptic biology and nutritional needs [,].
This review provides an overview of bioassays used to evaluate entomopathogenic fungi (EPF) as biological control agents against ambrosia beetles, with a focus on assessing fungal pathogenicity and virulence. It primarily describes ambrosia beetles, excluding Platypodinae and Scolytinae species, considered true bark beetles (sensu stricto), such as Dendroctonus and Ips. References to these groups are included only when relevant for comparison or broader ecological or methodological context [].
2. Entomopathogenic Fungi
These microorganisms have emerged as important biological control agents against various insect pest species, particularly agriculture ones, such as aphids, thrips, whiteflies, and caterpillars, offering an ecological alternative to chemical pesticides []. In some cases, they exert minimal impact on non-target organisms and are compatible with other integrated pest management strategies [,]. These microorganisms primarily infect insects through their cuticle, penetrating into the hemolymph, where they produce toxic metabolites such as destruxins (produced by Metarhizium spp.) or beauvericin (produced by Beauveria spp.), that ultimately lead to host death [,]. In general, the infection cycle concludes with insect mycosis and the formation of conidia on the host surface, thus facilitating their dissemination and subsequent infection of other susceptible hosts (Figure 1).
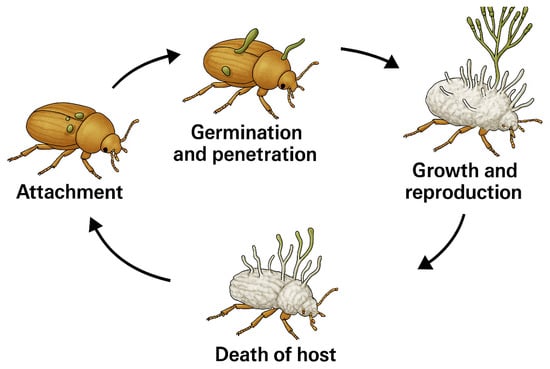
Figure 1.
Infectious cycle of entomopathogenic fungi.
The success of insect infection by EPF depends on a variety of biotic and abiotic factors. Relative humidity is critical for the development of infection and disease progression, as well as in the aerial sporulation on insect cadavers. Temperature significantly influences both fungal infection and persistence [,] and UV radiation plays a significant role in the depletion and inactivation of fungal propagules in epigeal habitats []. Biotic factors such as fungal species, spore density, host plant chemistry, and insect host species also affect virulence and pathogenicity [].
More than 750 species of EPF have been identified, including Beauveria bassiana, Metarhizium anisopliae, and Isaria fumosorosea, which are commonly used in commercial mycoinsecticides [,]. Despite their effectiveness, challenges remain in expanding commercial applications, including enhanced persistence and performance under adverse environmental conditions, such as high temperatures, low humidity, and ultraviolet radiation []. Ongoing research focuses on increasing the efficacy and formulation of EPF, as well as understanding their interactions with insect pests, to fully harness their potential in sustainable pest management programs [,].
3. Ambrosia Beetles
Approximately 3500 species of ambrosia beetles have been described worldwide to date []. Their defining feature is an obligate symbiotic association with fungi, a relationship that has independently evolved across multiple lineages, which confers a crucial ecological role as recyclers of organic matter (organic matter producers) [,]. The beetles excavate galleries in weakened, dying, or dead trees, where they cultivate symbiotic fungi, which are carried in internal or external specialized structures, known as mycangia. These fungi are the main nutritional resource for adults and larvae [,]. In some cases, the fungi obstruct the vascular system of healthy trees, leading to severe damage in forest and orchard ecosystems [,]. Several invasive species, such as members of the Euwallacea fornicatus complex and the genus Xylosandrus, have become established in several regions, including Europe, Argentina, and the United States, where they have caused severe damage to multiple tree species [,,].
Many Scolytine beetles are highly mobile and invasive, with at least 163 species identified as having a cosmopolitan distribution []. Their global spread is facilitated by international trade in wood products and live plants, as well as by global warming [,,]. One of the most recent and notable cases is the South Asia native redbay ambrosia beetle (Xyleborus glabratus), which has become a major invasive pest in the United States since its detection in 2002 near Savannah, GA, USA, and rapidly spread throughout the southeastern United States, reaching Florida and becoming established in six other states by 2013 []. In 2014, its presence was documented in Louisiana, marking its first appearance west of the Mississippi river []. This beetle is a vector of the pathogenic fungus Raffaelea lauricola, which causes laurel wilt disease in the Lauraceae family trees, including avocados [,]. It has spread across 12 southeastern U.S. states, killing millions of trees []. Its presence on the continent poses a serious threat to countries like Mexico, the world’s leading avocado exporter, which also harbors a significant diversity of Lauraceae species []. Moreover, native beetles such as Xyleborus affinis, although not currently considered a phytosanitary issue, have been reported in some studies colonizing avocado trees in Mexican orchards. They also possess the potential to act as vector of R. lauricola [,,,].
4. Entomopathogenic Fungi Against Ambrosia Beetles
In recent years, research on EPF as biological control agents against ambrosia beetles has gained attention, primarily driven by the economic impact posed by various invasive and native species, as mentioned above. Table 1 provides a current representative summary of the most relevant studies regarding the application of EPF to this group of insects.

Table 1.
Studies developed with entomopathogenic fungi for the control of ambrosia beetles.
The most frequently used fungi belong to the genera Beauveria and Metarhizium, with Isaria and Purpureocillium (=Paecilomyces) used to a lesser extent. Some of the strains evaluated originated from reference and local collections [,,,,,,], and others were obtained from commercial products [,,]. Furthermore, the majority of strains were isolated from non-scolytine insects [,,,,], with only two studies involving strains isolated from scolytine beetles [,].
A detailed analysis of the available data reveals that all trials have been exclusively conducted under laboratory conditions. This methodological limitation has been widely recognized as a key barrier to applying biological control in practice. It highlights the urgent need for field-based evaluations, to develop more realistic and effective application strategies []. Moreover, current studies have primarily focused on exotic pest species, such as Xylosandrus crassiusculus, X. glabratus, and E. fornicatus. Three species—X. affinis, Xyleborus bispinatus, and Xyleborus volvulus—included in some trials, are not considered a high-risk species, which highlights a research gap regarding native or economically inconspicuous species that have the potential to vector phytopathogenic fungi [,].
Another noteworthy aspect is the considerable variability in EPF effectiveness, reported across studies. This variability can be explained by multiple factors. First, the susceptibility of the target insect widely varies among species and even between populations, which may be related to genetic, physiological, or ecological differences [,,]. Second, the pathogenicity and virulence of the fungi differ among genera, species, and strains, influencing the host’s response to the microorganism [,]. Moreover, fungal formulations—whether as dry spores, emulsions, or commercial preparations—may significantly affect efficacy, particularly due to differences in fungal adhesion to the insect cuticle [,]. Furthermore, the methodological diversity of laboratory trials makes it difficult to compare results across studies. Variables such as inoculation method and post-inoculation conditions significantly vary between experiments.
5. Bioassay Systems
A bioassay is an essential method in the evaluation of EPF for insect control, serving as one of the most critical stages in the development and eventual commercialization of biopesticides []. Bioassays allow for the verification of the fungal agents’ effectiveness against the target pests, which is fundamental for validating their practical application [,].
In vivo bioassays, typically conducted under laboratory conditions, aim to assess the pathogenicity and virulence of fungal strains against target insects in a controlled environment, which ensures replicability and variable control []. In contrast, in situ bioassays are performed in the host insect’s natural environment, allowing researchers to observe fungal efficacy under real-world conditions, with reduced experimental controls and increased environmental variability [,].
Studies have shown that the efficacy of EPF considerably differs across experimental settings. Under laboratory conditions, although efficacy varies depending on the insect species, mortality rates commonly ranges between 80% and 100%. However, in field conditions, effectiveness tends to significantly decline, with reported mortality rates ranging from 28% to 82% [,,]. This reduction is primarily associated with environmental factors such as ultraviolet radiation, temperature, and humidity, which may substantially influence the activity and persistence of EPF in natural settings [,].
5.1. In Vivo Bioassays on Ambrosia Beetles
When assessing the efficacy of an EPF against ambrosia beetles, it is essential to consider the following key elements: (1) the origin of the beetles used in the assays, (2) the method of inoculation, and (3) the bioassay conditions under which the insects are maintained, following inoculation.
5.1.1. Origin of Beetles
For laboratory assays aimed at evaluating the efficacy of EPF in ambrosia beetles, two main approaches have been used to obtain the insects: (1) direct collection from infested host plants in the field [,,,,] and (2) the establishment of laboratory colonies using artificial diets [,,,,,,,,]. The first approach, although frequently used due to its initial practicality, has important limitations, including the lack of control over critical variables such as quantity, physiological state, age, and health of the collected individuals []. Moreover, beetles obtained from natural environments are likely to carry microorganisms or natural enemies—such as bacteria, fungi, or nematodes—that may act as confounding factors in mortality assays and compromise the validity of the results [,]. In this regard, although some studies with ambrosia beetles have addressed this issue through molecular analyses to rule out latent infections or microbial contamination [], this is not yet a widespread practice. Nevertheless, it should be considered a standard measure when using field-collected individuals, especially in studies focused on assessing the pathogenicity or virulence of fungal strains.
On the other hand, the use of artificial diets for rearing ambrosia beetles in the laboratory represents a more robust alternative, and is therefore widely employed [,,,,,,]. This strategy allows for the maintenance of controlled populations, ensures homogeneous age among the individuals used, and significantly reduces the risk of contamination by external agents. It also facilitates the replication of experiments and the comparison between treatments under standardized conditions, which is crucial for the scientific validity of the results []. However, not all ambrosia beetle species easily adapt to rearing on artificial diets [], which presents an additional challenge for its widespread implementation.
5.1.2. Inoculation Methods
In laboratory studies focused on evaluating the pathogenicity and virulence of EPF against ambrosia beetles, various inoculation methodologies have been employed []. One of the most used methods, due to its simplicity, reproducibility, and effectiveness, is the immersion of insects in suspensions with known concentrations of conidia [,,,,]. This technique enables uniform contact with the inoculum and typically results in a high fungal load on the insect’s body surface.
Another documented strategy involves the direct application of the inoculum using manual sprayers or pneumatic devices, such as the Potter spray tower [,,,,,]. In addition, indirect inoculation methods have been implemented, which consist of depositing the inoculum on surfaces such as filter paper or plant material, over which the insects are allowed to walk for a specific period [,,,,,].
Each of these strategies involves differences in the number of conidia that adhere to the insect’s body. Immersion typically results in a significantly higher fungal load, with the possibility that conidia may penetrate cavities such as spiracles, the mouth, or the anus, potentially accelerating systemic infection []. In contrast, indirect methods generally result in a lower initial load, typically limited to regions such as the legs or the ventral surface [,]. However, factors such as the duration of exposure to the inoculated surface can significantly influence the amount of inoculum that adheres []. Interestingly, comparative studies between direct and indirect methods have not shown significant differences in terms of beetle mortality or survival time [,,], suggesting that fungal effectiveness may depend more on its intrinsic virulence than on the initial quantity of conidia.
Despite the practicality of direct inoculation strategies, their results should be interpreted with caution, especially when aiming to extrapolate them to field conditions. The application of EPF in forest systems or agroecosystems with woody plants presents considerable challenges in terms of coverage and efficacy []. Unlike low-growing herbaceous crops, where target insects—often sessile—are almost fully covered by applications, achieving effective contact between the pathogen and the host. In contrast, the use of EPF is difficult in trees [,]. In these settings, applications are typically limited to trunks or canopies through spraying, where the likelihood of reaching the beetles—generally found inside galleries—is low []. In this context, bioassays using indirect inoculations may offer a more realistic model of EPF performance under natural conditions, as they more accurately simulate the type of contact that occurs in the field. Therefore, despite their methodological complexity, such approaches should be promoted and generalized in future research.
Among the indirect inoculation methods, filter paper has been occasionally used in studies with ambrosia beetles [,,,]. Although it is easy to manage and widely accessible, its high absorbent potential may reduce the availability of conidia on the surface, thereby decreasing the efficacy of insect contact and sometimes yielding inconsistent results [,]. Alternatively, the use of plant material such as branches, bark, or logs has proven to be a more realistic option that mimics the insect’s natural environment and has been successfully used in numerous studies [,,,,]. In these assays, reported mortality rates have ranged from 60 to 100%, highlighting the potential of EPF under exposure conditions that more closely resemble those in the field.
5.2. Post-Inoculation Conditions
As mentioned above, ambrosia beetles are insects with an obligate symbiotic relationship. They are considered “farmer” beetles because they cultivate the fungi that serve as their food source during both adult and immature stages []. Bioassays conducted with this type of insect must undoubtedly take this mutualistic relationship into account. In our personal experience, in the case of X. affinis, these insects do not survive for more than five days in humid chambers when isolated (unpublished data). Therefore, at least for this species, it is not possible to maintain beetles without their food source. The same may be expected for other ambrosia beetle species. A considerable portion of the reported studies consistently disregard the insect’s feeding, keeping them under starvation conditions in humid chambers after being directly inoculated [,,,,,,], or maintaining them throughout the trial period in contact with the filter paper on which the inoculum was placed [,]. These studies report low mortality in control groups, among different beetles, suggesting that starvation was not a significant factor and raising the possibility of a species-specific tolerance to food deprivation.
The relationship between insect starvation and their susceptibility to EPF has been marginally studied. However, available evidence suggests that lack of food significantly influences such susceptibility. Nutritional stress, including food deprivation and poor diet, has been shown to increase the vulnerability of several insect species to B. bassiana [,].
One way to counteract the effects of food deprivation in ambrosia beetles during bioassays is through the use of plant material, such as branches or logs, or by providing artificial diets that support the cultivation of their symbiotic fungi []. In indirect inoculation assays using branches or logs, it is implicitly assumed that the substrate serves for gallery formation, which nourishes the insect.
Based on the experience of our research group, we have concluded that the mere contact between the insect and a plant substrate does not necessarily guarantee colonization. It is common for branches or logs to be placed inside containers, which increases temperature, humidity, and the concentration of volatile compounds. These conditions often induce erratic behavior in the beetles, which tend to focus more on escaping the container than on actively boring into the substrate. In such cases, beetles often make only superficial perforations in the bark without developing internal galleries. As a result, they are deprived of food and use a considerable amount of energy, chaotically wandering over the surface. This phenomenon must be considered to avoid misinterpreting the lack of boring or gallery formation as a potential effect of EPF infection. One method that might help minimize this behavior is to soak the plant material with ethanol, a simple chemical compound to which ambrosia beetles respond [,]. Recently, methodological strategies have been implemented in which beetles are placed in small tubes attached directly to the surface of the log, allowing for direct and controlled contact with the substrate []. The issues described above are less common when artificial diets are used; although, as previously noted, this depends on the species under study and the specific formulation of the diet [,]. Taking this into consideration, parameters such as boring activity, gallery formation, and progeny are highly informative. In studies that have evaluated boring activity, rates have ranged from 28% to 83% in natural branches and from 65% to 95% in artificial diets [,,]. Regarding progeny, reductions of up to 97% have been documented [,].
A crucial factor to consider when using natural or artificial diets in bioassays is the removal of conidia during gallery formation by the insect. This variable has been appropriately considered by some authors, who in their bioassays chose to keep insects in humid chambers after inoculation and before placing them onto the substrate, thereby avoiding conidial removal []. This strategy may be advantageous for the fungal infection process and represents a critical point to consider when interpreting and designing bioassays involving ambrosia beetles. Among the few studies addressing conidial removal, it is estimated that boring activity may remove more than 70% of the conidia in about 12 h in artificial diet settings [], which is a significant amount, especially considering that the number of infective units adhered to the insect depends on the inoculation method, as mentioned above. Therefore, to achieve higher mortality rates, it is necessary to apply higher doses in such a way that the insect acquires enough infective units, thereby counteracting the removal effect during gallery formation. This has been observed in beetles such as X. affinis, where immersion inoculation with B. bassiana required a dose of 1 × 109 conidia/mL to achieve mortality rates slightly above 50% [], when beetles were released onto artificial diet after inoculation.
5.3. In Situ Bioassays on Ambrosia Beetles
To date, there is no evidence of field evaluations using EPF for the control of ambrosia beetles. However, the accumulated experience from studies conducted on bark beetles of the genera Dendroctonus, Ips, and Pityophthorus provides a useful framework for proposing methodological guidelines that facilitate the design and implementation of similar trials for ambrosia beetles.
Field trials have yielded mixed results. Some studies report a significant reduction in tree mortality and bark beetle infestations [,,,]; others have observed limited or no effects under similar conditions [,,]. The variability in efficacy is likely influenced by environmental factors such as ambient temperature, ultraviolet radiation, and phytochemicals produced by host trees, all of which may affect the viability and infectivity of entomopathogenic fungi []. Moreover, for fungal applications to be effective, it is critical to consider the complex ecological interactions between bark beetles and their environment, including their symbiotic associations and specific habitat characteristics []. The development of specialized formulations or delivery systems has shown promising results in improving infection success in natural environments, particularly in species such as Scolytus amygdali and Dendroctonus ponderosae [,].
Among the most effective experimental approaches are the spraying of log sections—commonly referred to as trap logs—under field conditions [,,,], and the use of autodissemination traps baited with pheromones to enhance beetle attraction and contact with the pathogen [,,,]. In addition, trials conducted in controlled environments have tested the release of beetles previously inoculated with entomopathogenic fungi, offering insights into the pathogen’s transmission potential [,]. Nonetheless, some researchers have raised concerns about the extrapolation of these laboratory or semi-natural results to real-world conditions, citing limited environmental representativeness and potential overestimation of field efficacy.
6. Future Directions
Despite the notable progress in evaluating EPF against ambrosia beetles under laboratory conditions, their practical implementation still faces significant challenges. One of the key future priorities should be the transition toward field-based bioassays that allow for validation of laboratory-observed efficacy under real environmental variables such as humidity, temperature, and UV radiation, all of which directly influence the persistence and activity of EPF [,,,].
It is also necessary to standardize inoculation methods and post-inoculation conditions, as current strategies exhibit high variability in terms of mortality, gallery formation, and conidia removal [,,,]. It is recommended to increase the use of plant material as a substrate in bioassays to more realistically replicate natural conditions [,,,], as well as to explore new formulations that enhance conidial adherence and persistence on the insect cuticle [,].
Taken together, several EPF species, particularly Beauveria bassiana and Metarhizium brunneum, have shown significant potential to control ambrosia beetles. However, their effectiveness may vary depending on environmental conditions, target species, and the specific fungal strain used. Therefore, further testing under field conditions is essential to accurately selecting strains for this pest management tool assessment.
Furthermore, the inclusion of native beetle species in future studies is essential, given their potential to become significant pests and act as vectors of pathogens [,,,]. Addressing these aspects will be key to advancing toward sustainable and effective biological control strategies for these insects.
Author Contributions
Conceptualization; J.E.C.-A.; literature review: J.E.C.-A. and P.T.-G.; writing: J.E.C.-A. and P.T.-G.; editing: J.E.C.-A. and P.T.-G.; language review: P.T.-G. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Acknowledgments
To Ricardo Gómez Flores for reviewing and editing the document. This review represents the culmination of a cycle of work on ambrosia beetles and entomopathogenic fungi, an experience that has shaped the professional life of the first author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Vega, F.E.; Hofstetter, R.W. (Eds.) Bark Beetles: Biology and Ecology of Native and Invasive Species; Academic Press: London, UK, 2014; ISBN 978-0-124-17156-5. [Google Scholar]
- Six, D.L. Ecological and evolutionary determinants of bark beetle—Fungus symbioses. Insects 2012, 3, 339–366. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.M.; Hulcr, J. Scolytus and other economically important bark and ambrosia beetles. In Bark Beetles: Biology and Ecology of Native and Invasive Species; Vega, F.E., Hofstetter, R.W., Eds.; Academic Press: New York, NY, USA, 2015; pp. 495–531. [Google Scholar]
- Haack, R.A.; Rabaglia, R.J. Exotic bark and ambrosia beetles in the USA: Potential and current invaders. In Potential Invasive Pest of Agricultural Crops; Peña, J., Ed.; CAB International: London, UK, 2013; pp. 48–74. [Google Scholar]
- Baniszewski, J.A.; Barnett, J.; Reding, M.E.; Ranger, C.M. Seasonal dominance of exotic ambrosia beetles compared to native species within deciduous and coniferous woodlots. Biol. Invasions 2024, 26, 1651–1668. [Google Scholar] [CrossRef]
- Fraedrich, S.W.; Harrington, T.C.; Rabaglia, R.J.; Ulyshen, M.D.; Mayfiel, A.E.; Hanula, J.L.; Eickwort, J.M.; Miller, D.R. A fungal symbiont of the redbay ambrosia beetle causes a lethal wilt in redbay and other Lauraceae in the southeastern United States. Plant Dis. 2008, 92, 215–224. [Google Scholar] [CrossRef] [PubMed]
- Peña, J.E.; Crane, J.H.; Capinera, J.L.; Duncan, R.E.; Kendra, P.E.; Ploetz, R.C.; McLean, S.; Brar, G.; Thomas, M.C.; Cave, R.D. Chemical Control of the Redbay Ambrosia Beetle, Xyleborus glabratus, and Other Scolytinae (Coleoptera: Curculionidae). Fla. Entomol. 2011, 94, 882–896. [Google Scholar] [CrossRef]
- Cueva, F.D. El control biológico como estrategia para apoyar las exportaciones agrícolas no tradicionales en Perú: Un análisis empírico. Contab. Neg. 2012, 7, 81–100. (In Spanish) [Google Scholar]
- Mendell, B.C.; Lang, A.H.; Caldwell, W.; Garrett, D.L. Chemical use and forest certification: Productivity and economic implications. J. For. 2015, 113, 367–371. [Google Scholar] [CrossRef]
- Holmes, S.B.; MacQuarrie, C.J. Chemical control in forest pest management. Can. Entomol. 2016, 148, S270–S295. [Google Scholar] [CrossRef]
- Hejazi, M.; Grant, J.H.; Peterson, E. Trade impact of maximum residue limits in fresh fruits and vegetables. Food Policy 2022, 106, 102203. [Google Scholar] [CrossRef]
- Gugliuzzo, A.; Biedermann, P.H.; Carrillo, D.; Castrillo, L.A.; Egonyu, J.P.; Gallego, D.; Khalid, H.; Hulcr, J.; Jactel, H.; Kajimura, H.; et al. Recent advances toward the sustainable management of invasive Xylosandrus ambrosia beetles. J. Pest Sci. 2021, 94, 615–637. [Google Scholar] [CrossRef]
- Martini, X.; Hughes, M.A.; Conover, D.; Smith, J. Use of semiochemicals for the management of the redbay ambrosia beetle. Insects 2020, 11, 796. [Google Scholar] [CrossRef]
- Peña, J.E.; Weihman, S.W.; McLean, S.; Cave, R.D.; Carrillo, D.; Duncan, R.E.; Evans, G.; Krauth, S.; Thomas, M.C.; Lu, S.S.; et al. Predators and parasitoids associated with Scolytinae in Persea species (Laurales: Lauraceae) and other Lauraceae in Florida and Taiwan. Fla. Entomol. 2015, 98, 903–910. [Google Scholar] [CrossRef]
- Fuentes-Guardiola, L.T.; Sánchez-González, J.A.; Birrueta-Valencia, M.M.; Arredondo-Bernal, H.C. Hymenoptera parasítica asociada a especies de Xyleborus Eichhoff en aguacate en Colima, México. Southwest. Entomol. 2019, 44, 271–279. (In Spanish) [Google Scholar] [CrossRef]
- Reverchon, F.; Contreras-Ramos, S.M.; Eskalen, A.; Guerrero-Analco, J.A.; Quiñones-Aguilar, E.E.; Rios-Velasco, C.; Velázquez- Fernández, J.B. Microbial biocontrol strategies for ambrosia beetles and their associated phytopathogenic fungi. Food Syst. 2021, 5, 737977. [Google Scholar] [CrossRef]
- Jacobo-Macías, E.R.; Robles-Bermúdez, A.; Cambero-Campos, O.J.; Coronado-Blanco, J.M.; Isiordia-Aquino, N.; Ruíz-Cancino, E.; Robles-Navarrete, A.P. Parasitoides himenópteros presentes en especies ambrosiaes y descortezadores recolectados de Persea americana Mill. (Laurales: Lauraceae), en Nayarit, México. Acta Zool. Mex 2022, 38, 1–12. (In Spanish) [Google Scholar]
- Castrillo, L.A.; Griggs, M.H.; Vandenberg, J.D. Granulate ambrosia beetle, Xylosandrus crassiusculus (Coleoptera: Curculionidae), survival and brood production following exposure to entomopathogenic and mycoparasitic fungi. Biol. Control 2013, 67, 220–226. [Google Scholar] [CrossRef]
- Nel, W.J.; Slippers, B.; Wingfield, M.J.; Yilmaz, N.; Hurley, B.P. Efficacy of commercially available entomopathogenic agents against the polyphagous shot hole borer in South Africa. Insects 2023, 14, 361. [Google Scholar] [CrossRef]
- Mann, A.J.; Davis, T.S. Entomopathogenic fungi to control bark beetles: A review of ecological recommendations. Pest Manag. Sci. 2021, 77, 3841–3846. [Google Scholar] [CrossRef]
- Singh, D.; Raina, T.K.; Singh, J. Entomopathogenic fungi: An effective biocontrol agent for management of insect populations naturally. J. Pharm. Sci. Res. 2017, 9, 833. [Google Scholar]
- Zimowska, B.; Krol, E.D. Entomopathogenic fungi and their biocenotic importance. Adv. Microbiol. 2019, 58, 471–483. [Google Scholar] [CrossRef]
- Butt, T.M.; Coates, C.J.; Dubovskiy, I.M.; Ratcliffe, N.A. Entomopathogenic fungi: New insights into host–pathogen interactions. Adv. Genet. 2016, 94, 307–364. [Google Scholar]
- Shahid, A.A.; Rao, Q.A.; Bakhsh, A.; Husnain, T. Entomopathogenic fungi as biological controllers: New insights into their virulence and pathogenicity. Arch. Biol. Sci. Belgrade 2012, 64, 21–42. [Google Scholar] [CrossRef]
- Berestetskiy, A.; Hu, Q. The chemical ecology approach to reveal fungal metabolites for arthropod pest management. Microorganisms 2021, 9, 1379. [Google Scholar] [CrossRef] [PubMed]
- Bugti, G.A.; Bin, W.; Memon, S.A.; Khaliq, G.; Jaffar, M.A. Entomopathogenic fungi: Factors involved in successful microbial control of insect pests. J. Entomol. 2020, 17, 74–83. [Google Scholar] [CrossRef]
- Quesada-Moraga, E.; González-Mas, N.; Yousef-Yousef, M.; Garrido-Jurado, I.; Fernández-Bravo, M. Key role of environmental competence in successful use of entomopathogenic fungi in microbial pest control. J. Pest Sci. 2024, 97, 1–15. [Google Scholar] [CrossRef]
- Jaronski, S.T. Ecological factors in the inundative use of fungal entomopathogens. BioControl 2010, 55, 159–185. [Google Scholar] [CrossRef]
- Rajula, J.; Rahman, A.; Krutmuang, P. Entomopathogenic fungi in Southeast Asia and Africa and their possible adoption in biological control. Biol. Control 2020, 151, 104399. [Google Scholar] [CrossRef]
- Maina, U.M.; Galadima, I.B.; Gambo, F.M.; Zakaria, D.J.J.O.E. A review on the use of entomopathogenic fungi in the management of insect pests of field crops. J. Entomol. Zool. Stud. 2018, 6, 27–32. [Google Scholar]
- Kidanu, S.; Hagos, L. Research and application of entomopathogenic fungi as pest management option: A review. J. Env. Earth Sci. 2020, 10, 31–39. [Google Scholar]
- Dar, S.A.; Rather, B.A.; Kandoo, A.A. Insect pest management by entomopathogenic fungi. J. Entomol. Zool. Stud. 2017, 5, 1185–1190. [Google Scholar]
- Mayers, C.G.; Harrington, T.C.; Biedermann, P.H. Mycangia define the diverse ambrosia beetle–fungus symbioses. In The Convergent Evolution of Agriculture in Humans and Insects; MIT Press: Cambridge, MA, USA, 2022; pp. 105–142. [Google Scholar]
- Klepzig, K.D.; Six, D.L. Bark beetle-fungal symbiosis: Context dependency in complex associations. Symbiosis 2004, 37, 189–205. [Google Scholar]
- Farrell, B.D.; Sequeira, A.S.; O'Meara, B.C.; Normark, B.B.; Chung, J.H.; Jordal, B.H. The evolution of agriculture in beetles (Curculionidae: Scolytinae and Platypodinae). Evolution 2001, 55, 2011–2027. [Google Scholar] [CrossRef] [PubMed]
- Hulcr, J.; Stelinski, L.L. The ambrosia symbiosis: From evolutionary ecology to practical management. Annu. Rev. Entomol. 2017, 62, 285–303. [Google Scholar] [CrossRef] [PubMed]
- Joseph, R.; Keyhani, N.O. Fungal mutualisms and pathosystems: Life and death in the ambrosia beetle mycangia. Appl. Microbiol. Biotechnol. 2021, 105, 3393–3410. [Google Scholar] [CrossRef] [PubMed]
- Kirkendall, L.R.; Biedermann, P.H.W.; Jordal, B.H. Evolution and diversity of bark and ambrosia beetles. In Bark Beetles: Biology and Ecology of Native and Invasive Species; Elsevier Inc.: Amsterdam, The Netherlands, 2015; pp. 85–156. ISBN 9780124171732. [Google Scholar]
- Harrington, T.; Fraedrich, S.; Aghayeva, D. Raffaelea lauricola a new ambrosia beetle symbiont and pathogen on the Lauraceae. Mycotaxon 2008, 104, 399–404. [Google Scholar]
- Schuler, H.; Witkowski, R.; Van de Vossenberg, B.; Hoppe, B.; Mittelbach, M.; Bukovinszki, T.; Schwembacher, S.; van de Meulengraaf, B.; Lange, U.; Rode, S.; et al. Recent invasion and eradication of two members of the Euwallacea fornicatus species complex (Coleoptera: Curculionidae: Scolytinae) from tropical greenhouses in Europe. Biol. Invasions 2023, 25, 299–307. [Google Scholar] [CrossRef]
- Landi, L.; Braccini, C.L.; Knížek, M.; Pereyra, V.A.; Marvaldi, A.E. A newly detected exotic ambrosia beetle in Argentina: Euwallacea interjectus (Coleoptera: Curculionidae: Scolytinae). Flor. Entomol. 2019, 102, 240–242. [Google Scholar] [CrossRef]
- Stouthamer, R.; Rugman-Jones, P.; Thu, P.Q.; Eskalen, A.; Thibault, T.; Hulcr, J.; Liangjomg, W.; Jordal, B.H.; ChiYu, C.; Cooperband, M.; et al. Tracing the origin of a cryptic invader: Phylogeography of the Euwallacea fornicatus (Coleoptera: Curculionidae: Scolytinae) species complex. Agric. For. Entomol. 2017, 19, 366–375. [Google Scholar] [CrossRef]
- Grégoire, J.C.; Jactel, H.; Hulcr, J.; Battisti, A.; Inward, D.; Petter, F.; Grousset, F. Cosmopolitan Scolytinae: Strong common drivers but too many singularities for accurate prediction. NeoBiota 2023, 84, 81–105. [Google Scholar] [CrossRef]
- Bentz, B.J.; Jönsson, A.M. Modeling Bark Beetles responses to climate change. In Bark Beetles: Biology and Ecology of Native and Invasive Species; Elsevier Inc.: Amsterdam, The Netherlands, 2015; pp. 533–553. ISBN 9780124171732. [Google Scholar]
- Fettig, C.J.; Asaro, C.; Nowak, J.T.; Dodds, K.J.; Gandhi, K.J.; Moan, J.E.; Robert, J. Trends in bark beetle impacts in North America during a period (2000–2020) of rapid environmental change. J. For. 2022, 120, 693–713. [Google Scholar] [CrossRef]
- Kendra, P.E.; Montgomery, W.S.; Niogret, J.; Epsky, N.D. An uncertain future for American Lauraceae: A lethal threat from redbay ambrosia beetle and laurel wilt disease (a review). Am. J. Plant Sci. 2013, 4, 727–738. [Google Scholar] [CrossRef]
- Fraedrich, S.W.; Johnson, C.W.; Menard, R.D.; Harrington, T.C.; Olatinwo, R.; Best, G.S. First report of Xyleborus glabratus (Coleoptera: Curculionidae: Scolytinae) and laurel wilt in Louisiana, USA: The disease continues westward on sassafras. Fla. Entomol. 2015, 98, 1266–1268. Available online: https://www.jstor.org/stable/24587650 (accessed on 17 April 2025). [CrossRef]
- Hughes, M.A.; Smith, J.A.; Ploetz, R.C.; Kendra, P.E.; Mayfield, A.E., III; Hanula, J.L.; Hulcr, J.; Stelinski, L.L.; Cameron, S.; Riggins, J.J. Recovery plan for laurel wilt on redbay and other forest species caused by Raffaelea lauricola and disseminated by Xyleborus glabratus. Plant Health Progr. 2015, 16, 173–210. [Google Scholar] [CrossRef]
- Cloonan, K.R.; Montgomery, W.S.; Narvaez, T.I.; Carrillo, D.; Kendra, P.E. Community of bark and ambrosia beetles (Coleoptera: Curculionidae: Scolytinae and Platypodinae) in agricultural and forest ecosystems with laurel wilt. Insects 2022, 13, 971. [Google Scholar] [CrossRef] [PubMed]
- Lira-Noriega, A.; Soberón, J.; Equihua, J. Potential invasion of exotic ambrosia beetles Xyleborus glabratus and Euwallacea sp. in Mexico: A major threat for native and cultivated forest ecosystems. Sci. Rep. 2018, 8, 10179. [Google Scholar] [CrossRef]
- Carrillo, D.; Dunca, R.; Ploetz, R.; Peña, J.E. Lateral transfer of a phytopathogenic symbiont among native and exotic ambrosia beetles. Plant Pathol. 2014, 63, 54–62. [Google Scholar] [CrossRef]
- Castrejón-Antonio, J.E.; Montesinos-Matías, R.; Acevedo-Reyes, N.; Tamez-Guerra, P.; Ayala-Zermeño, M.Á.; Berlanga-Padilla, A.M.; Arredondo-Bernal, H.C. Especies de Xyleborus (Coleoptera: Curculionidae: Scolytinae) asociados a huertos de aguacate en Colima, México. Acta Zool. Mex. 2017, 33, 146–150. (In Spanish) [Google Scholar] [CrossRef]
- Angeles-Restrepo, M.; Ochoa-Ascencio, S.; Fernández-Pavía, S.; Vázquez-Marrufo, G.; Equihua-Martínez, A.; Barrieto-Priego, A.F.; Correa-Suarez, M.; Saucedo-Carabez, J.R. Identificación de escarabajos ambrosiaes (Coleópteros: Curculionidae) asociados a árboles de aguacate en Michoacán, México. Folia Entomol. Mex. 2019, 5, 80–88. (In Spanish) [Google Scholar]
- Kendra, P.E.; Guillén, L.; Tabanca, N.; Montgomery, W.S.; Schnell, E.Q.; Deyrup, M.A.; Cloonan, K.R. Risk assessment of Hass avocado and Mexican Lauraceae for attack by redbay ambrosia beetle (Coleoptera: Curculionidae: Scolytinae). Agric. For. Entomol. 2023, 25, 285–302. [Google Scholar] [CrossRef]
- Castrillo, L.A.; Griggs, M.H.; Ranger, C.M.; Reding, M.E.; Vandenberg, J.D. Virulence of commercial strain of Beauveria bassiana and Metarhizium brunneum (Ascomycota: Hypocreales) against adult Xylosandrus germanus (Coleoptera: Curculionidae) and impact on brood. Biol. Control 2011, 58, 121–126. [Google Scholar] [CrossRef]
- Carrillo, D.; Dunlap, C.; Avery, P.; Navarrete, J.; Dunca, R.; Jackson, M.; Peña, J.E. Entomopathogenic fungi as biological control agents for the vector of the laurel wilt disease, the redbay ambrosia beetle, Xyleborus glabratus (Coleoptera: Curculionidae). Biol. Control 2015, 81, 44–50. [Google Scholar] [CrossRef]
- Avery, P.B.; Bojorque, V.; Gámez, C.; Duncan, R.E.; Carrillo, D.; Cave, R.D. Spore acquisition and survival of ambrosia beetles associated with the laurel wilt pathogen in avocados after exposure to entomopathogenic fungi. Insects 2018, 9, 49. [Google Scholar] [CrossRef] [PubMed]
- Toksöz, S.; Oksal, E.; Saruhan, I.; Kepenekci, I. Pathogenicity of the entomopathogenic fungus, Purpureocillium lilacinum TR1 against ambrosia beetles, Xylosandrus germanus (Blandford) and Xyleborus dispar (Fabricius) (Coleoptera: Curculionidae: Scolytinae). Mun. Ent. Zool. 2018, 13, 471–481. [Google Scholar]
- Kushiyev, R.; Tuncer, C.; Erper, I.; Ozdemir, I.O.; Saruhan, I. Efficacy of native entomopathogenic fungus, Isaria fumosorosea, against bark and ambrosia beetles, Anisandrus dispar Fabricius and Xylosandrus germanus Blandford (Coleoptera: Curculionidae: Scolytinae). Egypt. J. Biol. Pest Control 2018, 28, 55. [Google Scholar] [CrossRef]
- Tuncer, C.; Kushiyev, R.; Erper, I.; Ozdemir, I.O.; Saruhan, I. Efficacy of native isolates of Metarhizium anisopliae and Beauveria bassiana against the invasive ambrosia beetle, Xylosandrus germanus Blandford (Coleoptera: Curculionidae: Scolytinae). Egypt. J. Biol. Pest Control 2019, 29, 28. [Google Scholar] [CrossRef]
- Castrejón-Antonio, J.E.; Tamez-Guerra, P.; Montesinos-Matías, R.; Ek-Ramos, M.J.; Garza-López, P.M.; Arredondo-Bernal, H.C. Selection of Beauveria bassiana (Hypocreales: Cordycipitaceae) strains to control Xyleborus affinis (Curculionidae: Scolytinae) females. PeerJ 2020, 8, e9472. [Google Scholar] [CrossRef]
- Reynoso-López, E.A.; Méndez-Hernández, J.E.; Ek-Ramos, J.; Montesinos-Matías, R.; Loera, O. Metarhizium robertsii in combination with Trichoderma asperellum reduce the malathion doses used to control ambrosia beetles: The case of Xyleborus affinis. Biocontrol Sci. Technol. 2021, 31, 1080–1097. [Google Scholar] [CrossRef]
- Castrejón-Antonio, J.E.; Tamez-Guerra, P.; García-Ortiz, N.; Muñiz-Paredes, F.; Sánchez-Rangel, J.C.; Montesinos-Matías, R. Biocontrol of Xyleborus affinis (Curculionidae: Scolitinae) females and progeny by Beauveria bassiana (Hypocreales: Cordycipitaceae) in a sawdust artificial diet model. Insects 2023, 14, 477. [Google Scholar] [CrossRef]
- Castrejón-Antonio, J.E.; Tamez-Guerra, P.; Montesinos-Matías, R.; Sánchez-Rangel, J.C.; Mendoza-Muñoz, N. Compatibility of Beauveria bassiana with Inverted Emulsions in Contaminated Surface Tests Against Xyleborus affinis. Southwest. Entomol. 2025, 50, 210–227. [Google Scholar] [CrossRef]
- Liu, J.; Jiang, N.; Gao, H.; Lai, S.; Zhou, Y.; Hao, D.; Dai, L. Laboratory Exploration of Several Potential Biocontrol Methods Against the Ambrosia Beetle, Euwallacea interjectus. Insects 2025, 16, 56. [Google Scholar] [CrossRef]
- Sibao, W.A.N.G.; Xuexia, M.I.A.O.; Weiguo, Z.H.A.O.; Huang, B.; Meizhen, F.A.N.; Zengzhi, L.I.; Huang, Y. Genetic diversity and population structure among strains of the entomopathogenic fungus, Beauveria bassiana, as revealed by inter-simple sequence repeats (ISSR). Mycol. Res. 2025, 109, 1364–1372. [Google Scholar] [CrossRef]
- Rohrlich, C.; Merle, I.; Mze Hassani, I.; Verger, M.; Zuin, M.; Besse, S.; Robène, I.; Nibouche, S.; Costet, L. Variation in physiological host range in three strains of two species of the entomopathogenic fungus Beauveria. PLoS ONE 2018, 13, e0199199. [Google Scholar] [CrossRef] [PubMed]
- Ullah, S.; Raza, A.B.M.; Alkafafy, M.; Sayed, S.; Hamid, M.I.; Majeed, M.Z.; Riaz, M.A.; Gaber, N.M.; Asim, M. Isolation, identification and virulence of indigenous entomopathogenic fungal strains against the peach-potato aphid, Myzus persicae Sulzer (Hemiptera: Aphididae), and the fall armyworm, Spodoptera frugiperda (JE Smith) (Lepidoptera: Noctuidae). Egypt. J. Biol. Pest Control 2022, 32, 2. [Google Scholar] [CrossRef]
- Hussain, A.; Tian, M.Y.; He, Y.R.; Ruan, L.; Ahmed, S. In vitro and in vivo culturing impacts on the virulence characteristics of serially passed entomopathogenic fungi. J. Food Agric. Environ. 2010, 8, 481–487. [Google Scholar]
- Robertson, J.L.; Jones, M.M.; Olguin, E.; Alberts, B. Bioassays with Arthropods, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2017; pp. 1–10. ISBN 978-1-4822-1708-7. [Google Scholar]
- Beris, E. Evaluation and environmental testing of entomopathogenic fungi for their effectiveness as bio-control agents of major vineyard pests. Plant Prot. 2021, 5, 13–23. Available online: https://esciencepress.net/journals/index.php/PP/article/view/3571 (accessed on 17 April 2025).
- Liu, Y.C.; Ni, N.T.; Chang, J.C.; Li, Y.H.; Lee, M.R.; Kim, J.S.; Nai, Y.S. Isolation and selection of entomopathogenic fungi from soil samples and evaluation of fungal virulence against insect pests. J. Vis. Exp. 2021, 175, 62882. [Google Scholar] [CrossRef]
- Haverty, M.I.; Robertson, J.L. Laboratory bioassays for selecting candidate insecticides and application rates for field tests on the western spruce budworm. J. Econ. Entomol. 1982, 75, 179. [Google Scholar] [CrossRef]
- Draganova, S.A.; Doychev, D.D.; Pilarska, D.K.; Takov, D.I. Bioassays of entomopathogenic fungi against xylophagous insects in Bulgaria: Laboratory and field experiments. Acta Zool. Bulg. 2017, 69, 411–419. [Google Scholar]
- Graf, T.; Scheibler, F.; Niklaus, P.A.; Grabenweger, G. From lab to field: Biological control of the Japanese beetle with entomopathogenic fungi. Front. Insect Sci. 2023, 3, 1138427. [Google Scholar] [CrossRef]
- Abalo, M.; Scorsetti, A.C.; Vianna, M.F.; Russo, M.L.; De Abajo, J.M.; Pelizza, S.A. Field evaluation of entomopathogenic fungi formulations against Rachiplusia nu (Lepidoptera: Noctuidae) in soybean crop. J. Plant Prot. Res. 2022, 62, 403–410. [Google Scholar] [CrossRef]
- Wakil, W.; Kavallieratos, N.G.; Nika, E.P.; Qayyum, M.A.; Yaseen, T.; Ghazanfar, M.U.; Yasin, M. Combinations of Beauveria bassiana and spinetoram for the management of four important stored-product pests: Laboratory and field trials. Environ. Sci. Pollut. Res. 2023, 30, 27698–27715. [Google Scholar] [CrossRef]
- Foster, R.N.; Jaronski, S.; Reuter, K.C.; Black, L.R.; Schlothauer, R.; Harper, J.; Jech, L.E. Simulated aerial sprays for field cage evaluation of Beauveria bassiana and Metarhizium brunneum (Ascomycetes: Hypocreales) against Anabrus simplex (Orthoptera: Tettigoniidae) in Montana. Biocontrol Sci. Technol. 2011, 21, 1331–1350. [Google Scholar] [CrossRef]
- Mann, A.J.; Davis, T.S. Plant secondary metabolites and low temperature are the major limiting factors for Beauveria bassiana (Bals.-Criv.) Vuill. (Ascomycota: Hypocreales) growth and virulence in a bark beetle system. BioControl 2020, 141, 104130. [Google Scholar] [CrossRef]
- Draganova, S.A.; Takov, D.I.; Doychev, D.D. Naturally-occurring entomopathogenic fungi on three bark beetle species (Coleoptera: Curculionidae) in Bulgaria. Pestic. Fitomed. 2010, 25, 59–63. [Google Scholar] [CrossRef]
- Katumanyane, A.; Slippers, B.; Wondafrash, M.; Malan, A.P.; Hurley, B.P. Natural infection of white grubs (Coleoptera: Scarabaeidae) with entomopathogenic nematodes in the KwaZulu-Natal province of South Africa. J. Helminthol. 2023, 97, e54. [Google Scholar] [CrossRef]
- Cohen, A.C. Insect Diets: Science and Technology, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2012; pp. 1–19. [Google Scholar]
- Castrillo, L.A.; Griggs, M.H.; Vandenberg, J.D. Brood production by Xylosandrus germanus (Coleoptera: Curculionidae) and growth of its fungal symbiont on artificial diet based on sawdust of different tree species. Environ. Entomol. 2012, 41, 822–827. [Google Scholar] [CrossRef]
- Cruz, L.F.; Rocio, S.A.; Duran, L.G.; Menocal, O.; Garcia-Avila, C.D.J.; Carrillo, D. Developmental biology of Xyleborus bispinatus (Coleoptera: Curculionidae) reared on an artificial medium and fungal cultivation of symbiotic fungi in the beetle's galleries. Fungal Ecol. 2018, 35, 116–126. [Google Scholar] [CrossRef]
- Maner, M.L.; Hanula, J.L.; Braman, S.K. Rearing redbay ambrosia beetle, Xyleborus glabratus (Coleoptera: Curculionidae: Scolytinae), on semi-artificial media. Fla. Entomol. 2013, 96, 1042–1051. [Google Scholar] [CrossRef]
- Mizuno, T.; Kajimura, H. Effects of ingredients and structure of semi-artificial diet on the reproduction of an ambrosia beetle, Xyleborus pfeili (Ratzeburg) (Coleoptera: Curculionidae: Scolytinae). Appl. Entomol. Zool. 2009, 44, 363–370. [Google Scholar] [CrossRef]
- Biedermann, P.H.; Klepzig, K.D.; Taborsky, M. Fungus cultivation by ambrosia beetles: Behavior and laboratory breeding success in three xyleborine species. Environ. Entomol. 2009, 38, 1096–1105. [Google Scholar] [CrossRef]
- Gokulakrishnaa, R.K.; Thirunavukkarasu, S. Bioassay techniques in entomological research. Int. J. Plant Soil Sci. 2023, 35, 363–373. [Google Scholar] [CrossRef]
- Mannino, M.C.; Huarte-Bonnet, C.; Davyt-Colo, B.; Pedrini, N. Is the insect cuticle the only entry gate for fungal infection? Insights into alternative modes of action of entomopathogenic fungi. J. Fungi 2019, 5, 33. [Google Scholar] [CrossRef] [PubMed]
- Skrzecz, I.; Sierpińska, A.; Tumialis, D. Entomopathogens in the integrated management of forest insects: From science to practice. Pest Manag. Sci. 2024, 80, 2503–2514. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; Huang, Y.; Thomson, S.J.; Elliott, R.B. Effects of conidial densities and spray volume of Metarhizium anisopliae and Beauveria bassiana fungal suspensions on conidial viability, droplet size and deposition coverage in bioassay using a novel bioassay spray system. Biocontrol Sci. Technol. 2013, 23, 362–366. [Google Scholar] [CrossRef]
- Behle, R.W. Importance of direct spray and spray residue contact for infection of Trichoplusia ni larvae by field applications of Beauveria bassiana. J. Econ. Entomol. 2006, 99, 1120–1128. [Google Scholar] [CrossRef]
- Dara, S.K.; Montalva, C.; Barta, M. Microbial control of invasive forest pests with entomopathogenic fungi: A review of the current situation. Insects 2019, 10, 341. [Google Scholar] [CrossRef]
- Hulcr, J.; Rountree, N.R.; Diamond, S.E.; Stelinski, L.L.; Fierer, N.; Dunn, R.R. Mycangia of ambrosia beetles host communities of bacteria. Microb. Ecol. 2012, 64, 784–793. [Google Scholar] [CrossRef]
- Donegan, K.; Lighthart, B. Effect of several stress factors on the susceptibility of the predatory insect, Chrysoperla carnea (Neuroptera: Chrysopidae), to the fungal pathogen Beauveria bassiana. J. Invertebr. Pathol. 1989, 54, 79–84. [Google Scholar] [CrossRef]
- Lord, J.C. Dietary stress increases the susceptibility of Tribolium castaneum to Beauveria bassiana. J. Econ. Entomol. 2010, 103, 1542–1546. [Google Scholar] [CrossRef]
- Brar, G.S.; Capinera, J.L.; Kendra, P.E.; McLean, S.; Peña, J.E. Life cycle, development, and culture of Xyleborus glabratus (Coleoptera: Curculionidae: Scolytinae). Fla. Entomol. 2013, 96, 1158–1167. [Google Scholar] [CrossRef]
- Ranger, C.M.; Reding, M.E.; Persad, A.B.; Herms, D.A. Ability of stress-related volatiles to attract and induce attacks by Xylosandrus germanus and other ambrosia beetles. Agric. For. Entomol. 2010, 12, 177–185. [Google Scholar] [CrossRef]
- Castrillo, L.A.; Mayfield, A.E., III; Griggs, M.H.; Camp, R.; Mudder, B.; Taylor, A.; Vandenberg, J.D. Mortality and reduced brood production in walnut twig beetles, Pityophthorus juglandis (Coleoptera: Curculionidae), following exposure to commercial strains of entomopathogenic fungi Beauveria bassiana and Metarhizium brunneum. Biol. Control 2017, 114, 79–86. [Google Scholar] [CrossRef]
- Olatinwo, R.; Walters, S.; Strom, B. Impact of Beauveria bassiana (Ascomycota: Hypocreales) on the Small Southern Pine Engraver (Coleoptera: Scolytidae) in a Loblolly Pine Bolt Assay. J. Entomol. Sci. 2018, 53, 180–191. [Google Scholar] [CrossRef]
- Kreutz, J.; Zimmermenn, G.; Mahron, H.; Vaupel, O.; Mosbacher, G. Preliminary investigations on the use of Beauveria bassiana (Bals.) Vuill. and other control methods against the bark beetle Ips typographus L. (Col., Scolytidae) in the Weld. IOBC/WPRS Bull. 2000, 23, 167–173. [Google Scholar]
- Kreutz, J.; Vaupel, O.; Zimmermann, G. Efficacy of Beauveria bassiana (Bals.) Vuill. against the spruce bark beetle, Ips typographus L., in the laboratory under various conditions. J. Appl. Entomol. 2004, 128, 384–389. [Google Scholar] [CrossRef]
- Davis, T.S.; Mann, A.J.; Malesky, D.; Jankowski, E.; Bradley, C. Laboratory and field evaluation of the entomopathogenic fungus Beauveria bassiana (Deuteromycotina: Hyphomycetes) for population management of spruce beetle, Dendroctonus rufipennis (Coleoptera: Scolytinae), in felled trees and factors limiting pathogen success. Environ. Entomol. 2018, 47, 594–602. [Google Scholar] [CrossRef]
- Grodzki, W.; Kosibowicz, M. An attempt to use the fungus Beauveria bassiana (Bals.) Vuill. in forest protection against the bark beetle Ips typographus (L.) in the field. For. Res. Pap. 2015, 76, 5–17. [Google Scholar] [CrossRef]
- Batta, Y.A. Biocontrol of almond bark beetle (Scolytus amygdali Geurin-Meneville, Coleoptera: Scolytidae) using Beauveria bassiana (Bals.) Vuill. (Deuteromycotina: Hyphomycetes). J. Appl. Microbiol. 2007, 103, 1406–1414. [Google Scholar] [CrossRef]
- Fernandez, K.X.; Pokorny, S.; Ishangulyeva, G.; Ullah, A.; Todorova, S.I.; Erbilgin, N.; Carroll, A.; Vederas, J.C. Beauveria bassiana exhibits strong virulence against Dendroctonus ponderosae in greenhouse and field experiments. Appl. Microbiol. Biotechnol. 2023, 107, 3341–3352. [Google Scholar] [CrossRef]
- Fora, C.G.; Boja, N.; Moatăr, M.; Tóth, F.; Balog, A. Effect of entomopathogenic fungi, Beauveria bassiana (Cordycipitaceae), on the bark beetle, Ips typographus (L.), under field conditions. Insects 2022, 13, 885. [Google Scholar] [CrossRef]
- Srei, N.; Lavallée, R.; Guertin, C. Susceptibility of Dendroctonus simplex to Hypocreales fungi: Towards the development of a biological control strategy. J. Appl. Entomol. 2017, 141, 487–495. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).