Effects of Larval Starvation Stress on the Life History and Adult Fitness of Fall Webworm, Hyphantria Cunea
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Insect Source and Rearing
2.2. Starvation Resistance
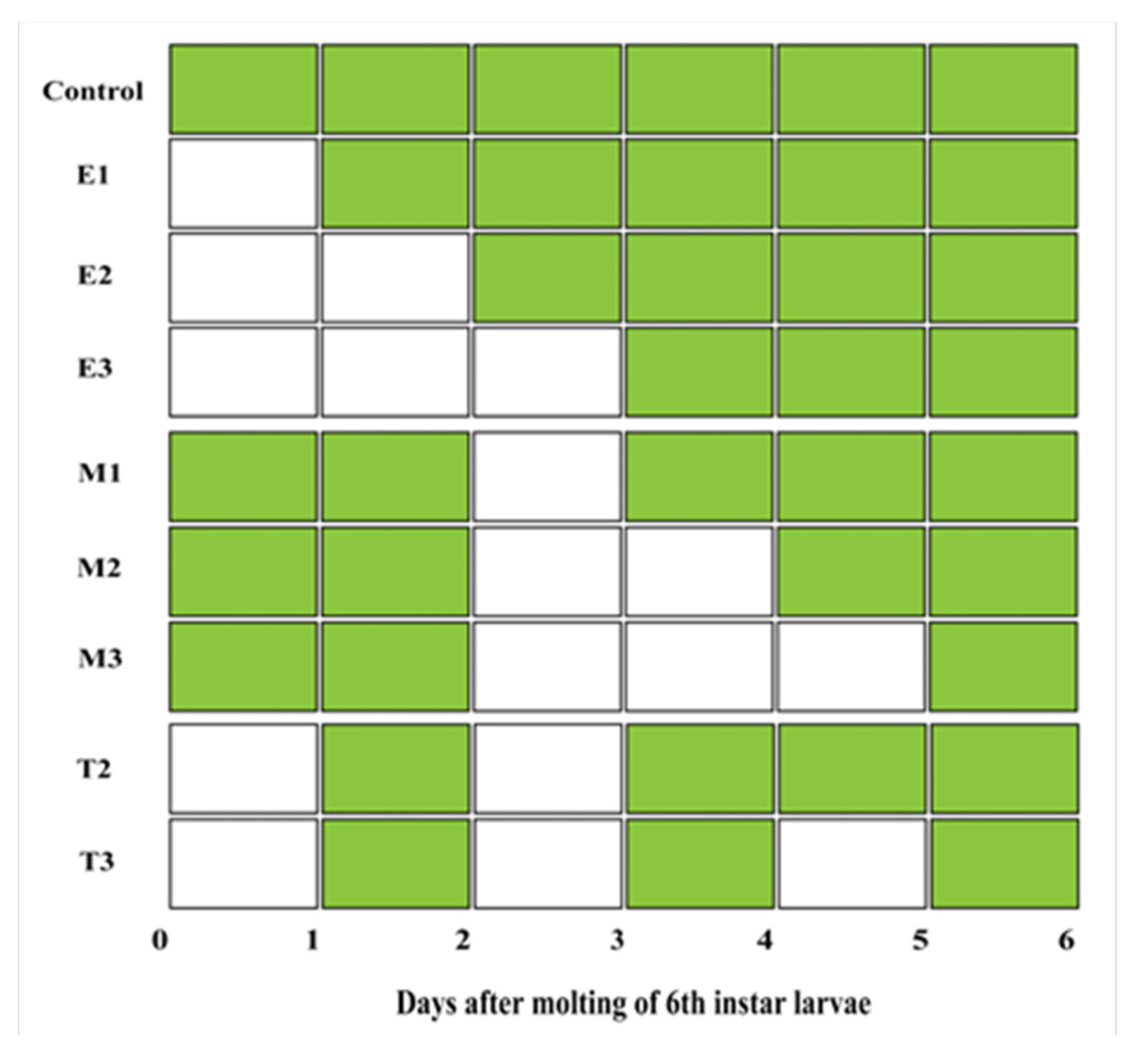
2.3. Complete Food Deprivation
2.4. Refeeding After Starvation
2.5. Measurement Parameters
2.6. Principal Component Analysis (PCA) of Life History Parameters
2.7. Data Analysis
3. Results
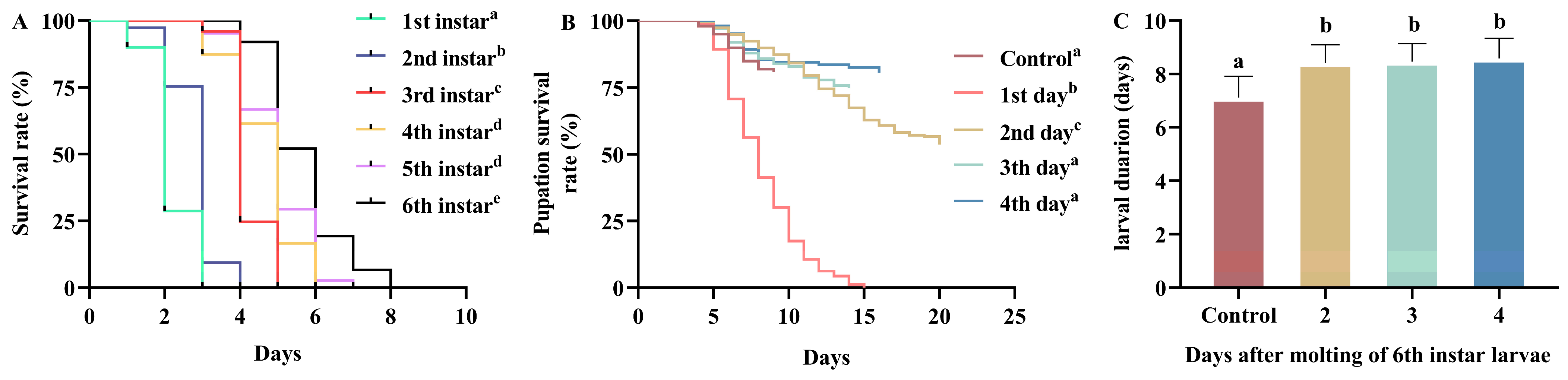
3.1. Survival Time
3.2. Complete Food Deprivation
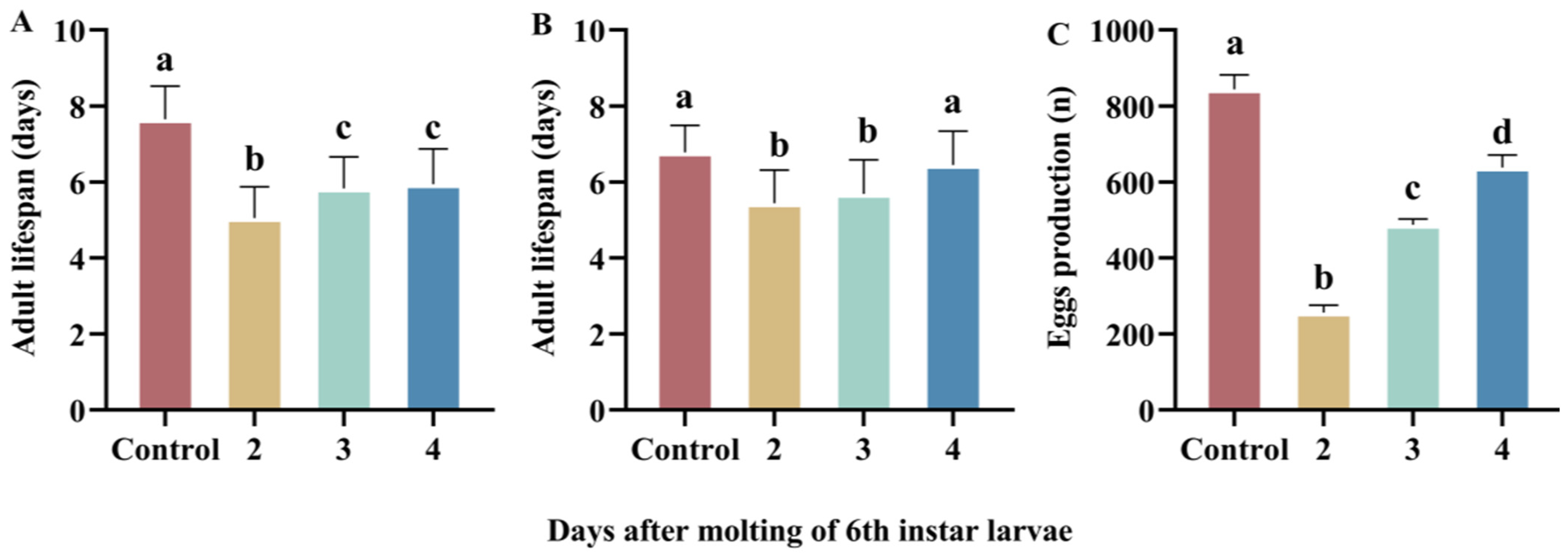
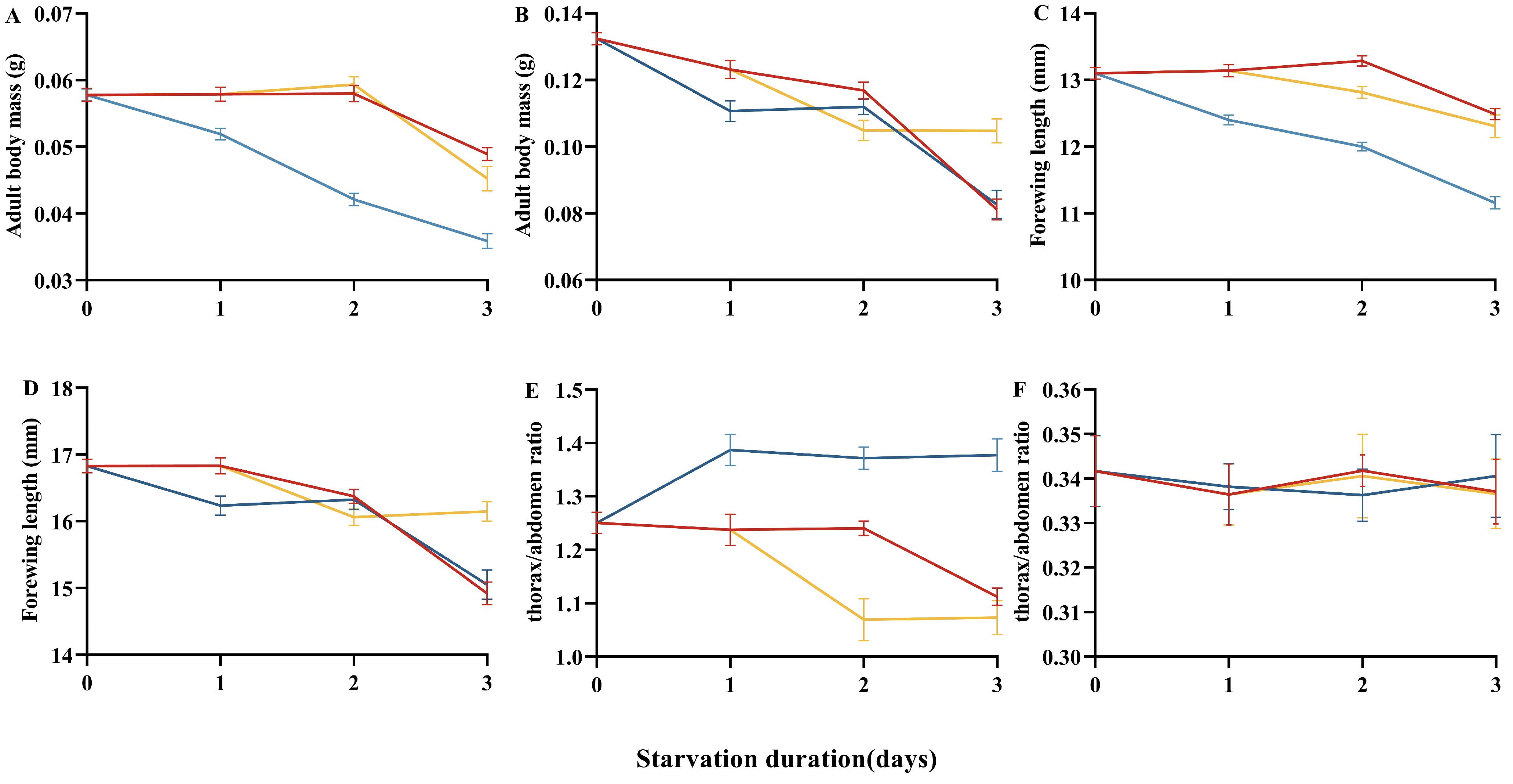
3.3. Resilience to Refeeding After Starvation
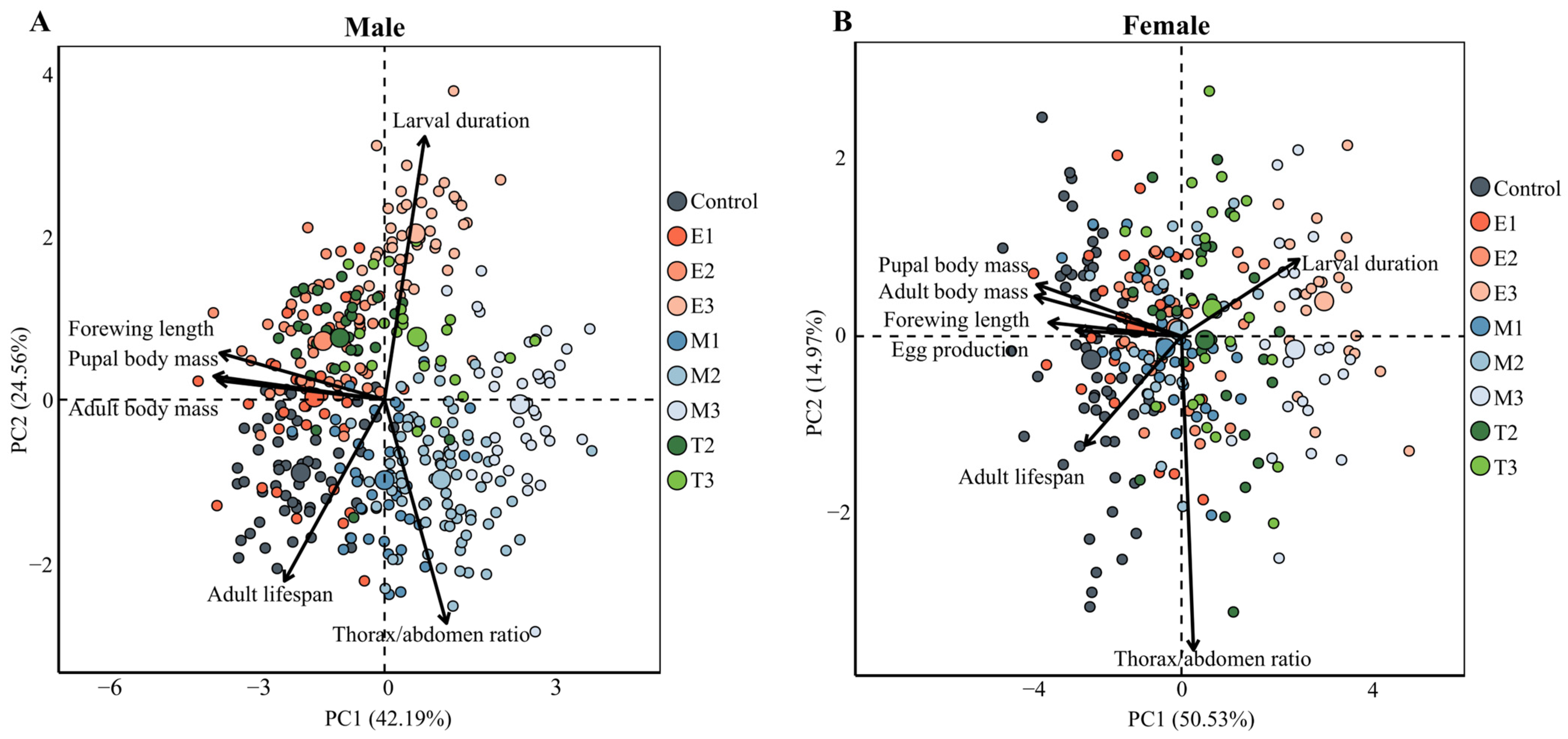
3.4. The Total Biological Response of H. cunea to Starvation Stress
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Parmesan, C. Ecological and evolutionary responses to recent climate change. Annu. Rev. Ecol. Evol. Syst. 2006, 37, 637–669. [Google Scholar] [CrossRef]
- Mccue, M.D. Starvation physiology: Reviewing the different strategies animals use to survive a common challenge. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2010, 156, 1–18. [Google Scholar] [CrossRef]
- Holtof, M.; Lenaerts, C.; Cullen, D.; Broeck, J.V. Extracellular nutrient digestion and absorption in the insect gut. Cell Tissue Res. 2019, 377, 397–414. [Google Scholar] [CrossRef]
- Devries, Z.C.; Kells, S.A.; Appel, A.G. Effects of starvation and molting on the metabolic rate of the bed bug (Cimex lectularius L.). Physiol. Biochem. Zool. 2015, 88, 53–65. [Google Scholar] [CrossRef]
- Zhang, D.W.; Xiao, Z.J.; Zeng, B.P.; Li, K.; Tang, Y.L. Insect behavior and physiological adaptation mechanisms under starvation stress. Front. Physiol. 2019, 10, 163. [Google Scholar] [CrossRef] [PubMed]
- Rion, S.; Kawecki, T.J. Evolutionary biology of starvation resistance: What we have learned from Drosophila. J. Evol. Biol. 2007, 20, 1655–1664. [Google Scholar] [CrossRef]
- Mccue, M.D.; Terblanche, J.S.; Benoit, J.B. Learning to starve: Impacts of food limitation beyond the stress period. J. Exp. Biol. 2017, 220, 4330–4338. [Google Scholar] [CrossRef] [PubMed]
- Łukowski, A.; Adamczyk, D.; Karolewski, P. Survival and recovery of the pine-tree lappet Dendrolimus pini when subjected to simulated starvation. Insects 2020, 11, 67. [Google Scholar] [CrossRef]
- Deblock, M.; Stoks, R. Short-term larval food stress and associated compensatory growth reduce adult immune function in a damselfly. Ecol. Entomol. 2008, 33, 796–801. [Google Scholar] [CrossRef]
- Adamo, S.A.; Davies, G.; Easy, R.; Kovalko, I.; Turnbull, K.F. Reconfiguration of the immune system network during food limitation in the caterpillar Manduca sexta. J. Exp. Biol. 2016, 219, 706–718. [Google Scholar]
- Etilé, E.; Despland, E. Developmental variation in the forest tent caterpillar: Life history consequences of a threshold size for pupation. Oikos 2008, 117, 135–143. [Google Scholar] [CrossRef]
- Helm, B.R.; Rinehart, J.P.; Yocum, G.D.; Greenlee, K.J.; Bowsher, J.H. Metamorphosis is induced by food absence rather than a critical weight in the solitary bee, Osmia lignaria. Proc. Natl. Acad. Sci. USA 2017, 114, 10924–10929. [Google Scholar] [CrossRef] [PubMed]
- Bauerfeind, S.S.; Fischer, K. Effects of larval starvation and adult diet-derived amino acids on reproduction in a fruit-feeding butterfly. Entomol. Exp. Et Appl. 2009, 130, 229–237. [Google Scholar] [CrossRef]
- Rosa, E.; Saastamoinen, M. Sex-dependent effects of larval food stress on adult performance under semi-natural conditions: Only a matter of size. Oecologia 2017, 184, 633–642. [Google Scholar] [CrossRef]
- Łukowski, A.; Ciesielska, K.; Adamczyk, D.; Karolewski, P. Starvation of pine-tree lappet Dendrolimus pini fourth instar larvae and recovery on different pine species—Can they recover from transport and start a new way of life? For. Ecol. Manag. 2021, 500, 119662. [Google Scholar] [CrossRef]
- Dmitriew, C.; Rowe, L. The effects of larval nutrition on reproductive performance in a food-limited adult environment. PLoS ONE 2011, 6, e17399. [Google Scholar] [CrossRef]
- Nestel, D.; Papadopoulos, N.T.; Pascacio-Villafán, C.; Righini, N.; Altuzar-Molina, A.R.; Aluja, M. Resource allocation and compensation during development in holometabolous insects. J. Insect Physiol. 2016, 95, 78–88. [Google Scholar] [CrossRef]
- Zhao, X.; Geng, Y.; Hu, T.; Xie, C.; Xu, W.; Zuo, Z.; Xue, M.; Hao, D. Ecological strategies of Hyphantria cunea (Lepidoptera: Arctiidae) response to different larval densities. Front. Ecol. Evol. 2023, 11, 1177029. [Google Scholar] [CrossRef]
- Pigeon, G.; Loe, L.E.; Bischof, R.; Bonenfant, C.; Forchhammer, M.; Irvine, R.J.; Ropstad, E.; Stien, A.; Veiberg, V.; Albon, S. Silver spoon effects are constrained under extreme adult environmental conditions. Ecology 2019, 100, e02886. [Google Scholar] [CrossRef]
- Monaghan, P. Early growth conditions, phenotypic development and environmental change. Philos. Trans. R. Soc. B Biol. Sci. 2008, 363, 1635–1645. [Google Scholar] [CrossRef]
- Boggs, C.L.; Freeman, K.D. Larval food limitation in butterflies: Effects on adult resource allocation and fitness. Oecologia 2005, 144, 353–361. [Google Scholar] [CrossRef] [PubMed]
- Boggs, C.L. Understanding insect life histories and senescence through a resource allocation lens. Funct. Ecol. 2009, 23, 27–37. [Google Scholar] [CrossRef]
- Tobin, P.C.; Robinet, C. Advances in understanding and predicting the spread of invading insect populations. Curr. Opin. Insect Sci. 2022, 54, 100985. [Google Scholar] [CrossRef]
- Gippet, J.M.W.; Liebhold, A.M.; Fenn-Moltu, G.; Bertelsmeier, C. Human-mediated dispersal in insects. Curr. Opin. Insect Sci. 2019, 35, 96–102. [Google Scholar] [CrossRef]
- Guo, J.-W.; Li, P.; Zhang, J.; Liu, X.-D.; Zhai, B.-P.; Hu, G. Cnaphalocrocis medinalis moths decide to migrate when suffering nutrient shortage on the first day after emergence. Insects 2019, 10, 364. [Google Scholar] [CrossRef]
- Zhao, L.; Wang, X.; Liu, Z.; Torson, A.S. Energy consumption and cold hardiness of diapausing fall webworm pupae. Insects 2022, 13, 853. [Google Scholar] [CrossRef]
- Zhang, X.; Fan, Z.; Zhang, R.; Kong, X.; Liu, F.; Fang, J.; Zhang, S.; Zhang, Z. Bacteria-mediated RNAi for managing fall webworm, Hyphantria cunea: Screening target genes and analyzing lethal effect. Pest Manag. Sci. 2023, 79, 1566–1577. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.Y. Occurrence regularity of Huphantria cunea in jiangsu distict of shanghai. For. Pest Dis. 2022, 14, 27–31. [Google Scholar]
- Slansky, F. Phagism relationships among butterflies. J. N. Y. Entomol. Soc. 1976, 84, 91–105. [Google Scholar]
- Loewy, K.J.; Flansburg, A.L.; Grenis, K.; Kjeldgaard, M.K.; Mccarty, J.; Montesano, L.; Vernick, J.; Murphy, S.M. Life history traits and rearing techniques for fall webworms (Hyphantria cunea Drury) in Colorado. J. Lepid. Soc. 2013, 67, 196–205. [Google Scholar]
- Girish, T.N.; Pradeep, B.E.; Parkash, R. Heat-and humidity-induced plastic changes in body lipids and starvation resistance in the tropical fly Zaprionus indianus during wet and dry seasons. J. Exp. Biol. 2018, 221, jeb174482. [Google Scholar]
- Papach, A.; Williams, G.R.; Neumann, P. Evolution of starvation resistance in an invasive insect species, Aethina tumida (Coleoptera: Nitidulidae). Ecol. Evol. 2020, 10, 9003–9010. [Google Scholar] [CrossRef] [PubMed]
- Gergs, A.; Jager, T. Body size-mediated starvation resistance in an insect predator. J. Anim. Ecol. 2014, 83, 758–768. [Google Scholar] [CrossRef]
- Leite, N.A.; Teatini, B.C.; Mendes, S.M.; da Silva, A.F. Effect of starvation and feeding on desiccated cover crops (Urochloa spp.), in different time periods, on the survival and biomass of Spodoptera frugiperda. Crop Prot. 2022, 153, 105888. [Google Scholar] [CrossRef]
- Gols, R.; Croijmans, L.; Dicke, M.; van Loon, J.J.A.; Harvey, J.A. Plant quantity affects development and reproduction of a gregarious butterfly more than plant quality. Entomol. Exp. Et Appl. 2022, 170, 646–655. [Google Scholar] [CrossRef]
- Yang, N.-W.; Zang, L.-S.; Wang, S.; Guo, J.-Y.; Xu, H.-X.; Zhang, F.; Wan, F.-H. Biological pest management by predators and parasitoids in the greenhouse vegetables in China. Biol. Control 2014, 68, 92–102. [Google Scholar] [CrossRef]
- Chen, K.W.; Chen, Y. Slow-growth high-mortality: A meta-analysis for insects. Insect Sci. 2018, 25, 337–351. [Google Scholar] [CrossRef]
- Yang, F.; Hu, G.; Shi, J.J.; Zhai, B.P. Effects of larval density and food stress on life-history traits of Cnaphalocrocis medinalis (Lepidoptera: Pyralidae). J. Appl. Entomol. 2015, 139, 370–380. [Google Scholar] [CrossRef]
- Fei, M.; Gols, R.; Zhu, F.; Harvey, J.A. Plant quantity affects development and survival of a gregarious insect herbivore and its endoparasitoid wasp. PLoS ONE 2016, 11, e0149539. [Google Scholar] [CrossRef]
- Reim, E.; Eichhorn, D.; Roy, J.D.; Steinhoff, P.O.M.; Fischer, K. Nutritional stress reduces flight performance and exploratory behavior in a butterfly. Insect Sci. 2019, 26, 897–910. [Google Scholar] [CrossRef]
- Pincheira-Donoso, D.; Hunt, J. Fecundity selection theory: Concepts and evidence. Biol. Rev. 2017, 92, 341–356. [Google Scholar] [CrossRef]
- Jahant-Miller, C.; Miller, R.; Parry, D. Size-dependent flight capacity and propensity in a range-expanding invasive insect. Insect Sci. 2022, 29, 879–888. [Google Scholar] [CrossRef]
- Xu, X.; He, S.; Wu, J. The effect of starvation and subsequent feeding on the reproductive potential of the grain aphid, Sitobion avenae. Entomol. Exp. Et Appl. 2012, 144, 294–300. [Google Scholar] [CrossRef]
- Lorenz, M.W. Synthesis of lipids in the fat body of Gryllus bimaculatus: Age-dependency and regulation by adipokinetic hormone. Arch. Insect Biochem. Physiol. 2001, 47, 198–214. [Google Scholar] [CrossRef] [PubMed]
- Gruntenko, N.; Wen, D.; Karpova, E.; Adonyeva, N.; Liu, Y.; He, Q.; Faddeeva, N.; Fomin, A.; Li, S.; Rauschenbach, I. Altered juvenile hormone metabolism, reproduction and stress response in Drosophila adults with genetic ablation of the corpus allatum cells. Insect Biochem. Mol. Biol. 2010, 40, 891–897. [Google Scholar] [CrossRef] [PubMed]
- Rehman, N.; Varghese, J. Larval nutrition influences adult fat stores and starvation resistance in Drosophila. PLoS ONE 2021, 16, e0247175. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Kaftanoglu, O.; Brent, C.S.; Page, R.E.; Amdam, G.V. Starvation stress during larval development facilitates an adaptive response in adult worker honey bees (Apis mellifera L.). J. Exp. Biol. 2016, 219, 949–959. [Google Scholar] [CrossRef]
- Esperk, T.; Toomas, T. Size compensation in moth larvae: Attention to larval instars. Physiol. Entomol. 2010, 35, 222–230. [Google Scholar] [CrossRef]
- Zhang, W.; Liang, G.; Ma, L.; Jiang, T.; Xiao, H. Dissecting the role of juvenile hormone binding protein in response to hormone and starvation in the cotton bollworm, Helicoverpa armigera (Hübner) (Lepidoptera: Noctuidae). J. Econ. Entomol. 2019, 112, 1411–1417. [Google Scholar] [CrossRef]
- Xu, L.C.; Nunes, C.; Wang, V.R.; Saito, A.; Chen, T.; Basak, P.; Chang, J.J.; Koyama, T.; Suzuki, Y. Distinct nutritional and endocrine regulation of prothoracic gland activities underlies divergent life history strategies in Manduca sexta and Drosophila melanogaster. Insect Biochem. Mol. Biol. 2020, 119, 103335. [Google Scholar] [CrossRef]
- Kaur, G.; Quilici, D.R.; Woolsey, R.J.; Petereit, J.; Nuss, A.B. Starvation-Induced Changes to the Midgut Proteome and Neuropeptides in Manduca sexta. Insects 2024, 15, 325. [Google Scholar] [CrossRef] [PubMed]
- Booth, K.; Cambron, L.; Fisher, N.; Greenlee, K.J. Immune defense varies within an instar in the tobacco hornworm, Manduca sexta. Physiol. Biochem. Zool. 2015, 88, 226–236. [Google Scholar] [CrossRef] [PubMed]
- Saastamoinen, M.; van der Sterren, D.; Vastenhout, N.; Zwaan, B.J.; Brakefield, P.M. Predictive adaptive responses: Condition-dependent impact of adult nutrition and flight in the tropical butterfly Bicyclus anynana. Am. Nat. 2010, 176, 686–698. [Google Scholar] [CrossRef] [PubMed]
- Trumbo, S.T.; Xhihani, E. Mass–size relationships, starvation and recovery in an engorging feeder. Physiol. Entomol. 2015, 40, 257–263. [Google Scholar] [CrossRef]
- Boggs, C.L.; Niitepõld, K. Effects of larval dietary restriction on adult morphology, with implications for flight and life history. Entomol. Exp. Et Appl. 2016, 159, 189–196. [Google Scholar] [CrossRef]
- Tarusikirwa, V.L.; Cuthbert, R.N.; Mutamiswa, R.; Nyamukondiwa, C. Context-dependent integrated stress resistance promotes a global invasive pest. Insect Sci. 2022, 29, 1790–1804. [Google Scholar] [CrossRef]
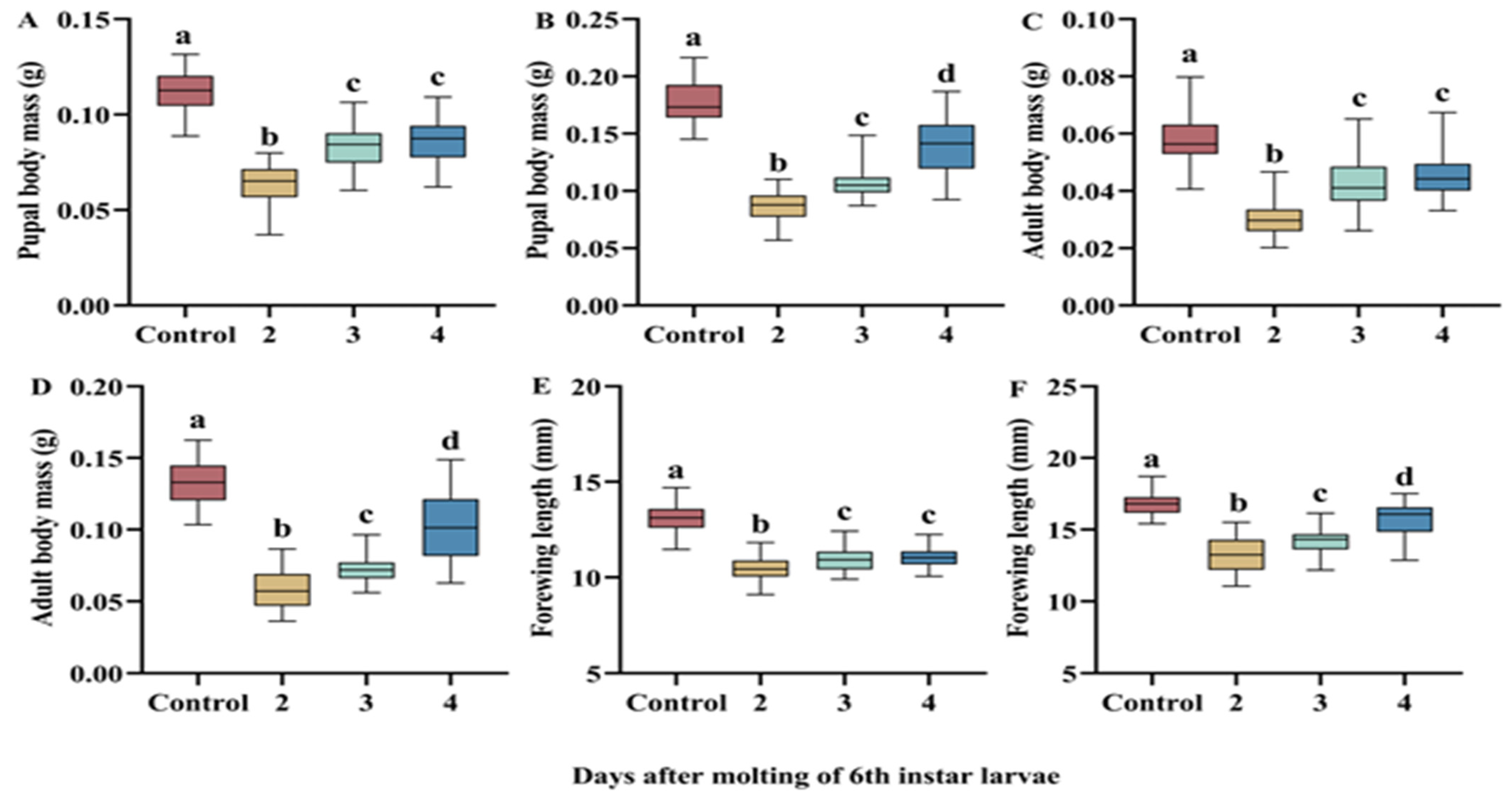
| Treatment | Pupation Survival Rate (%) | Larval Duration (Days ± SE) | Pupal Body Mass (mg ± SE) | |
|---|---|---|---|---|
| Male | Female | |||
| Control | 82.7 a | 7.1 ± 0.1 a | 111.77 ± 1.08 a | 177.63 ± 2.57 a |
| E1 | 82.0 a | 8.6 ± 0.1 b | 107.55 ± 1.13 a | 164.19 ± 2.07 b |
| E2 | 78.0 ab | 10.7 ± 0.1 cf | 107.19 ± 1.20 a | 157.30 ± 2.98 b |
| E3 | 61.3 bc | 12.7 ± 0.1 d | 94.52 ± 1.07 c | 118.05 ± 4.17 c |
| M1 | 84.7 a | 7.8 ± 0.1 e | 94.27 ± 1.07 c | 153.45 ± 3.27 b |
| M2 | 78.7 ab | 8.2 ± 0.2 be | 82.46 ± 1.26 e | 150.28 ± 2.78 b |
| M3 | 74.7 ab | 9.7 ± 0.2 f | 70.90 ± 1.29 f | 119.27 ± 4.64 c |
| T2 | 62.0 bc | 9.5 ± 0.1 f | 100.85 ± 1.58 b | 150.86 ± 3.37 b |
| T3 | 54.0 c | 10.2 ± 0.1 f | 87.71 ± 2.22 cd | 153.02 ± 4.29 b |
| Treatment | Adult Lifespan (Days ± SE) | Egg Production (n ± SE) | |
|---|---|---|---|
| Male | Female | ||
| Control | 7.7 ± 0.2 a | 6.8 ± 0.1 a | 840.7 ± 36.2 a |
| E1 | 7.5 ± 0.2 a | 6.5 ± 0.2 a | 805.9 ± 40.1 ab |
| E2 | 7.5 ± 0.1 a | 6.4 ± 0.2 ab | 800.8 ± 39.1 ab |
| E3 | 6.3 ± 0.1 b | 5.5 ± 0.2 b | 560.3 ± 30.0 c |
| M1 | 7.4 ± 0.2 a | 6.2 ± 0.2 ab | 796.0 ± 43.2 ab |
| M2 | 7.4 ± 0.1 a | 6.3 ± 0.2 ab | 777.6 ± 42.4 ab |
| M3 | 6.3 ± 0.2 b | 5.5 ± 0.2 b | 541.0 ± 37.0 c |
| T2 | 7.5 ± 0.2 a | 6.1 ± 0.2 ab | 656.1 ± 27.7 bc |
| T3 | 6.7 ± 0.5 b | 5.9 ± 0.2 ab | 633.1 ± 31.2 bc |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Zhang, L.; Zheng, J.; Li, T.; Zhao, L. Effects of Larval Starvation Stress on the Life History and Adult Fitness of Fall Webworm, Hyphantria Cunea. Insects 2025, 16, 410. https://doi.org/10.3390/insects16040410
Zhang Y, Zhang L, Zheng J, Li T, Zhao L. Effects of Larval Starvation Stress on the Life History and Adult Fitness of Fall Webworm, Hyphantria Cunea. Insects. 2025; 16(4):410. https://doi.org/10.3390/insects16040410
Chicago/Turabian StyleZhang, Yuan, Lin Zhang, Junchao Zheng, Tongpu Li, and Lvquan Zhao. 2025. "Effects of Larval Starvation Stress on the Life History and Adult Fitness of Fall Webworm, Hyphantria Cunea" Insects 16, no. 4: 410. https://doi.org/10.3390/insects16040410
APA StyleZhang, Y., Zhang, L., Zheng, J., Li, T., & Zhao, L. (2025). Effects of Larval Starvation Stress on the Life History and Adult Fitness of Fall Webworm, Hyphantria Cunea. Insects, 16(4), 410. https://doi.org/10.3390/insects16040410







