Synergistic Insecticidal Activity of Plant Volatile Compounds: Impact on Neurotransmission and Detoxification Enzymes in Sitophilus zeamais
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Insecticide Activity
2.2.1. Insects
2.2.2. Fumigant Toxicity Assay
2.2.3. Topical Contact Toxicity Assay
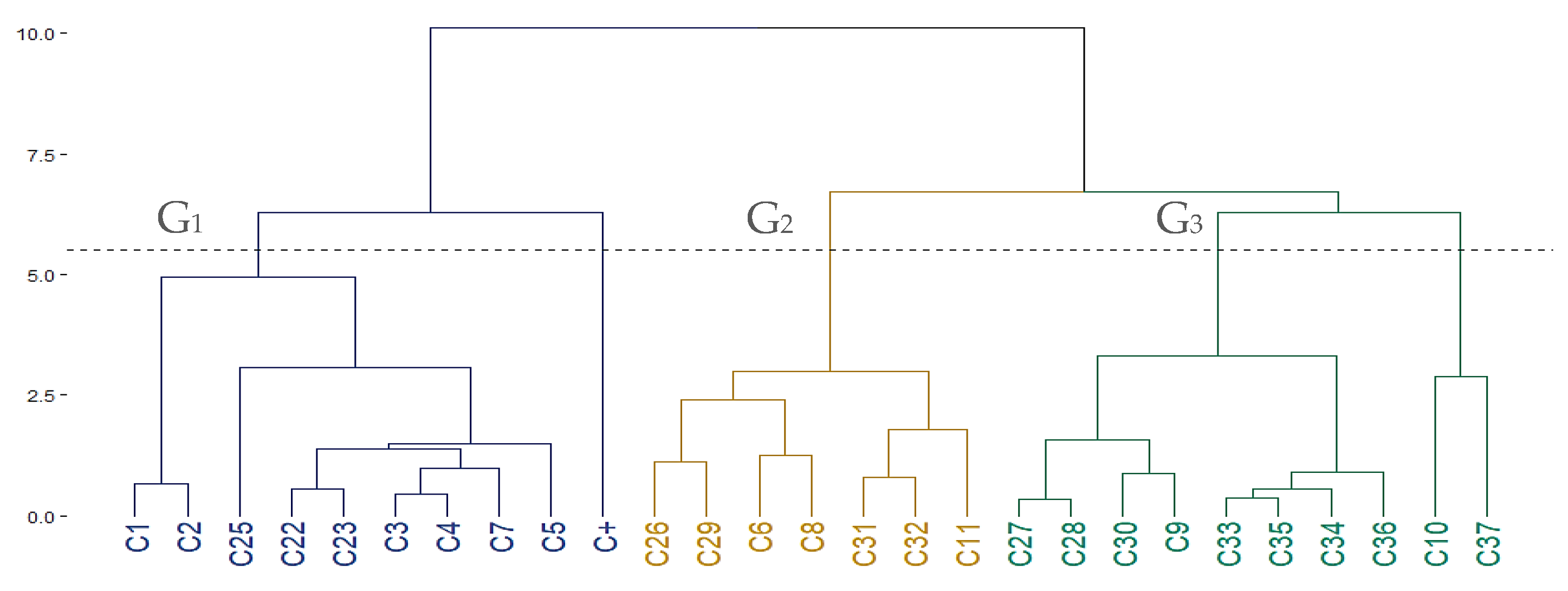
2.2.4. Cluster Statistical Analysis
2.3. Design of Mixtures of Bioactive Volatile Metabolites
2.3.1. Pre-Design of the Mixtures
2.3.2. Response Surface Model (RSM)
2.3.3. Effect of Insecticidal Interaction
2.4. Enzymatic Effects of the Most Active Mixtures and Their Components
2.4.1. Enzyme Extraction from S. zeamais
2.4.2. Protein Quantification
2.4.3. GST Activity Assay
2.4.4. CAT Activity Assay
2.4.5. AChE Activity Assay
3. Results and Discussion
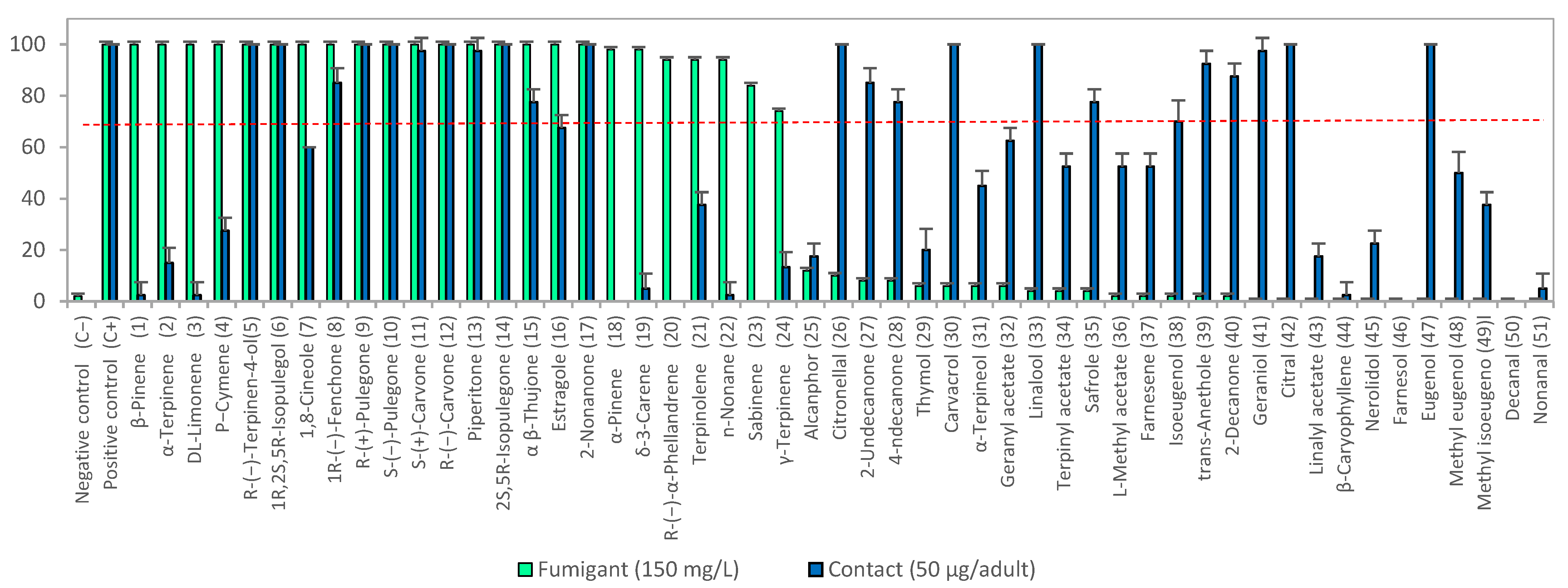
3.1. Insecticide Activity
3.1.1. Fumigant Toxicity
3.1.2. Contact Toxicity
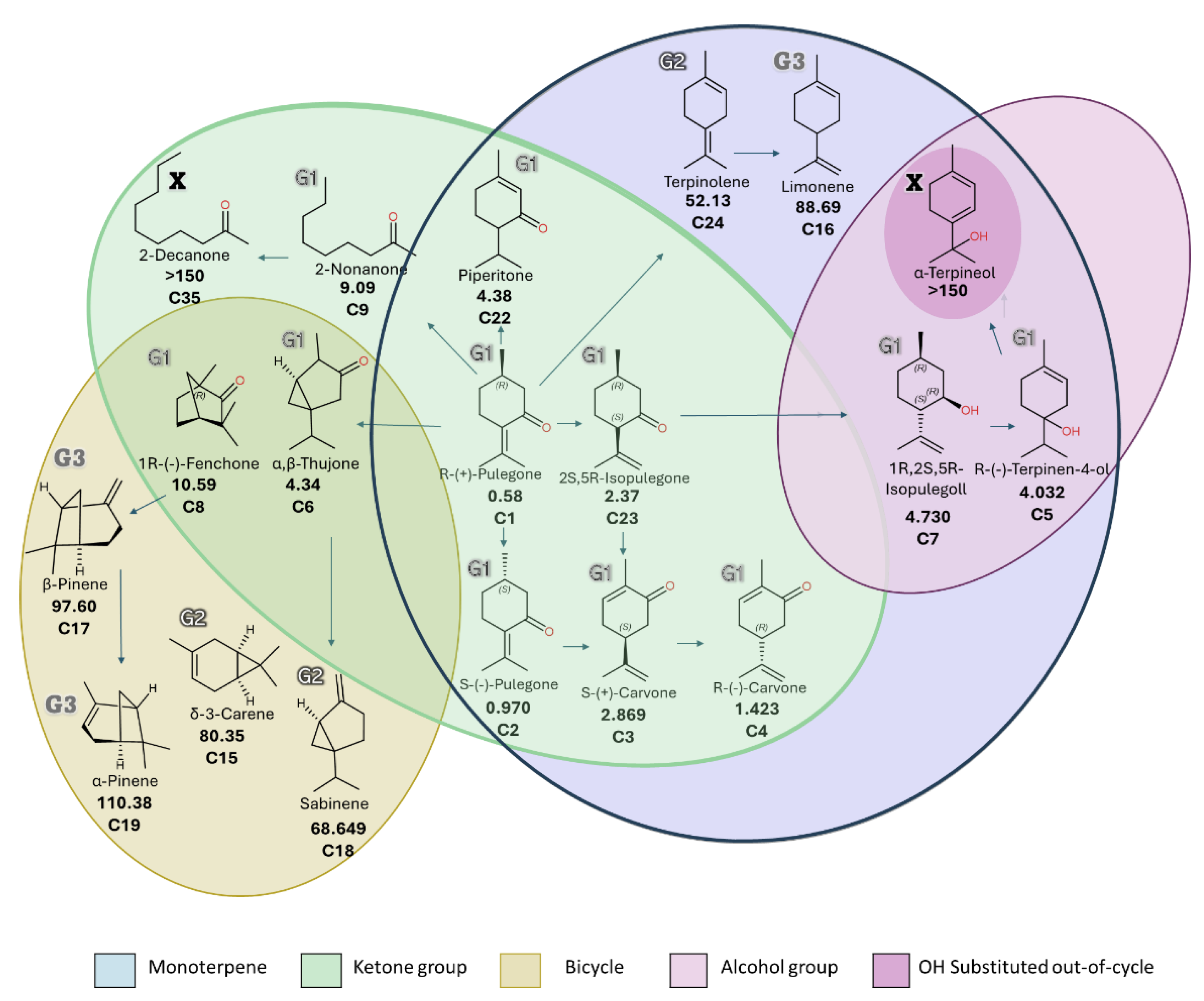
3.2. Design of Mixtures of Bioactive Volatile Metabolites and Their Insecticide Effect
3.2.1. Mixture Pre-Design and RSM Modeling
3.2.2. Toxicity Effect of Predicted Mixtures by RMS
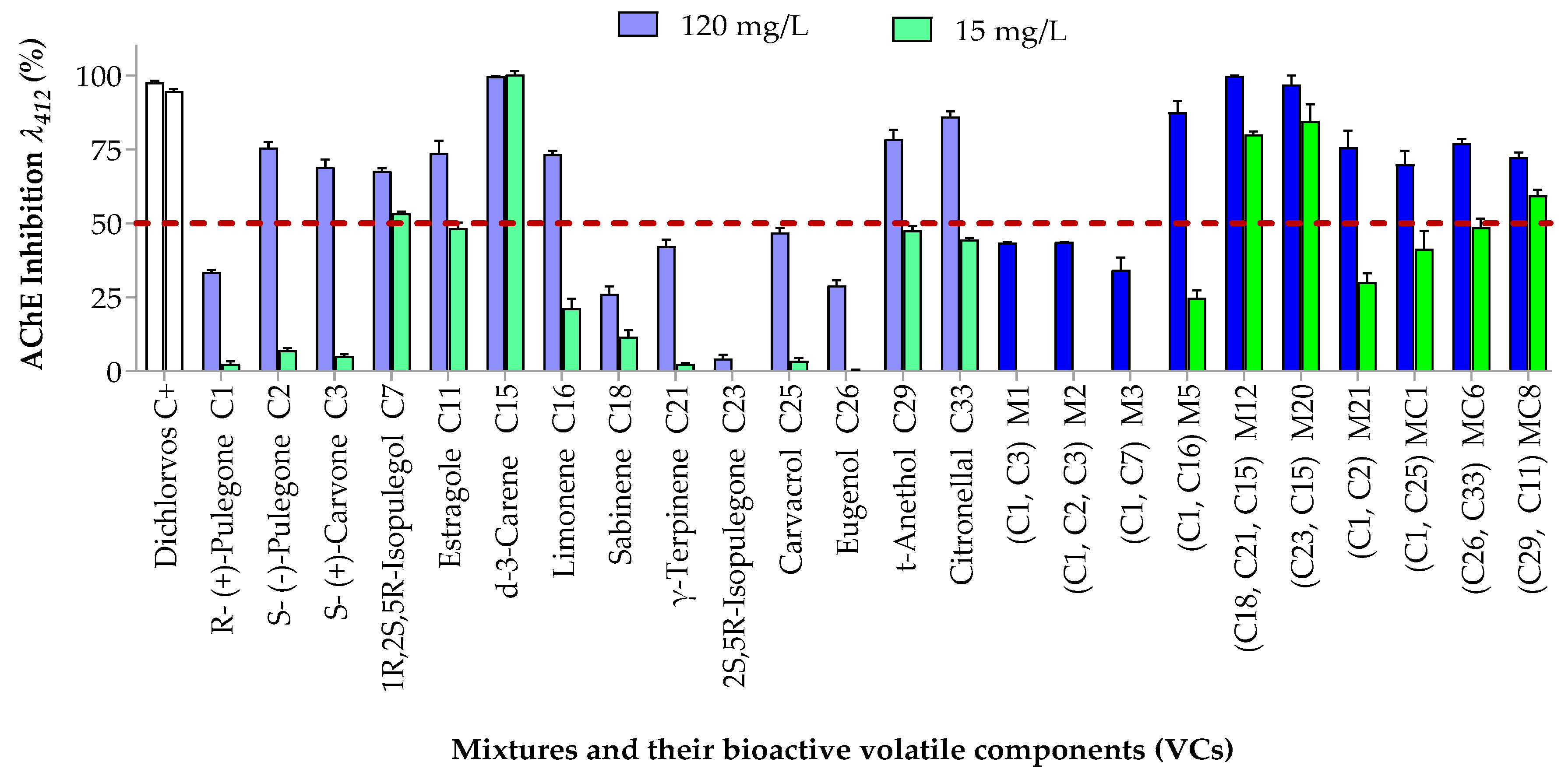
3.2.3. Interaction Analysis: Synergism and Antagonism
3.3. Effects of the Most Active Mixtures and Their Components on the Nervous System and Detoxifying Enzymes in S. zeamais
3.3.1. Inhibitory Effects on Acetylcholinesterase (AChE)
3.3.2. Inhibitory Effects on Catalase (CAT) Activity
3.3.3. Inhibitory Effects on Glutathione S-Transferase (GST) Activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Garutti, M.; Nevola, G.; Mazzeo, R.; Cucciniello, L.; Totaro, F.; Bertuzzi, C.A.; Caccialanza, R.; Pedrazzoli, P.; Puglisi, F. The Impact of Cereal Grain Composition on the Health and Disease Outcomes. Front. Nutr. 2022, 9, 888974. [Google Scholar] [CrossRef] [PubMed]
- Laskowski, W.; Górska-Warsewicz, H.; Rejman, K.; Czeczotko, M.; Zwolińska, J. How Important Are Cereals and Cereal Products in the Average Polish Diet? Nutrients 2019, 11, 679. [Google Scholar] [CrossRef] [PubMed]
- Alagbe, T.O.; Loko, Y.L.E.; Djègbè, I.; Gandjala, J.; Gavoedo, D.; Tamò, M. Post-Harvest Conservation Practices, Related Insect Pests of Stored Pearl Millet (Pennisetum glaucum (L) R. Br.), and Their Management in Northern Benin. J. Basic. Appl. Zool. 2025, 86, 7. [Google Scholar] [CrossRef]
- Stopar, K.; Trdan, S.; Bartol, T.; Arthur, F.H.; Athanassiou, C.G. Research on Stored Products: A Bibliometric Analysis of the Leading Journal of the Field for the Years 1965–2020. J. Stored Prod. Res. 2022, 98, 101980. [Google Scholar] [CrossRef]
- Almeida, D.M.; Conceição, V.d.S.; da Silva Dias, F.; Dias de Almeida Duarte, B.M.; Figueredo, L.R.; Jatoba Irarrazabal, V.M.; Araujo de Jesus, M.; Macedo, G.F.; Magalhaes, C.M.; Mendes, B.B.; et al. Evaluating the Population Dynamics of a Maize Weevil under Varying Initial Population Sizes. J. Stored Prod. Res. 2025, 111, 102562. [Google Scholar] [CrossRef]
- Cortese, D.; de Oliveira, G.S.; Fernandes, M.G. Influence of Temperature and Maize Genotypes on the Population Dynamics of Sitophilus zeamais Motschulsky 1885 (COLEOPTERA: CURCULIONIDAE) and Grain Quality during Storage. J. Stored Prod. Res. 2025, 111, 102564. [Google Scholar] [CrossRef]
- Pathak, V.M.; Verma, V.K.; Rawat, B.S.; Kaur, B.; Babu, N.; Sharma, A.; Dewali, S.; Yadav, M.; Kumari, R.; Singh, S.; et al. Current Status of Pesticide Effects on Environment, Human Health and It’s Eco-Friendly Management as Bioremediation: A Comprehensive Review. Front. Microbiol. 2022, 13, 962619. [Google Scholar] [CrossRef]
- Akhter, S.; Naik, V.K.; Naladi, B.J.; Rathore, A.; Yadav, P.; Lal, D. The Ecological Impact of Pesticides on Non-Target Organisms in Agricultural Ecosystems. Adv. Biores. 2024, 15, 322–334. [Google Scholar]
- Garrido-Miranda, K.A.; Giraldo, J.D.; Schoebitz, M. Essential Oils and Their Formulations for the Control of Curculionidae Pests. Front. Agron. 2022, 4, 876687. [Google Scholar] [CrossRef]
- Mssillou, I.; Saghrouchni, H.; Saber, M.; Zannou, A.J.; Balahbib, A.; Bouyahya, A.; Allali, A.; Lyoussi, B.; Derwich, E. Efficacy and Role of Essential Oils as Bio-Insecticide against the Pulse Beetle Callosobruchus maculatus (F.) in Post-Harvest Crops. Ind. Crops Prod. 2022, 189, 115786. [Google Scholar] [CrossRef]
- Oviedo-Sarmiento, J.S.; Bustos Cortes, J.J.; Delgado Ávila, W.A.; Cuca Suárez, L.E.; Herrera Daza, E.; Patiño-Ladino, O.J.; Prieto-Rodríguez, J.A. Fumigant Toxicity and Biochemical Effects of Selected Essential Oils toward the Red Flour Beetle, Tribolium castaneum (Coleoptera: Tenebrionidae). Pestic. Biochem. Physiol. 2021, 179, 104941. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Qiao, S.; Zhang, S. Essential Oils in Grain Storage: A Comprehensive Review of Insecticidal and Antimicrobial Constituents, Mechanisms, and Applications for Grain Security. J. Stored Prod. Res. 2025, 111, 102537. [Google Scholar] [CrossRef]
- Jankowska, M.; Rogalska, J.; Wyszkowska, J.; Stankiewicz, M. Molecular Targets for Components of Essential Oils in the Insect Nervous System—A Review. Molecules 2018, 23, 34. [Google Scholar] [CrossRef]
- Siddiqui, J.A.; Fan, R.; Naz, H.; Bamisile, B.S.; Hafeez, M.; Ghani, M.I.; Wei, Y.; Xu, Y.; Chen, X. Insights into Insecticide-Resistance Mechanisms in Invasive Species: Challenges and Control Strategies. Front. Physiol. 2023, 13, 1112278. [Google Scholar] [CrossRef] [PubMed]
- Gupta, I.; Singh, R.; Muthusamy, S.; Sharma, M.; Grewal, K.; Singh, H.P.; Batish, D.R. Plant Essential Oils as Biopesticides: Applications, Mechanisms, Innovations, and Constraints. Plants 2023, 12, 2916. [Google Scholar] [CrossRef]
- Santana, A.d.S.; Baldin, E.L.L.; Santos, T.L.B.d.; Baptista, Y.A.; Santos, M.C.d.; Lima, A.P.S.; Tanajura, L.S.; Vieira, T.M.; Crotti, A.E.M. Synergism between Essential Oils: A Promising Alternative to Control Sitophilus zeamais (Coleoptera: Curculionidae). Crop Prot. 2022, 153, 105882. [Google Scholar] [CrossRef]
- Ebadollahi, A.; Jalali Sendi, J. A Review on Recent Research Results on Bio-Effects of Plant Essential Oils against Major Coleopteran Insect Pests. Toxin Rev. 2015, 34, 76–91. [Google Scholar] [CrossRef]
- Patiño-Bayona, W.R.; Plazas, E.; Bustos-Cortes, J.J.; Prieto-Rodríguez, J.A.; Patiño-Ladino, O.J. Essential Oils of Three Hypericum Species from Colombia: Chemical Composition, Insecticidal and Repellent Activity against Sitophilus zeamais Motsch. (Coleoptera: Curculionidae). Rec. Nat. Prod. 2021, 15, 111–121. [Google Scholar] [CrossRef]
- Liu, Z.; Li, Q.X.; Song, B. Pesticidal Activity and Mode of Action of Monoterpenes. J. Agric. Food Chem. 2022, 70, 4556–4571. [Google Scholar] [CrossRef]
- Opiyo, S.A.; Njoroge, P.W.; Ndirangu, E.G. A Review Pesticidal Activity of Essential Oils against Sitophilus oryzae, Sitophilus granaries and Sitophilus zeamais. IOSR J. Appl. Chem. 2022, 15, 39–51. Available online: https://www.researchgate.net/publication/366191547 (accessed on 3 March 2025).
- Patiño-Bayona, W.R.; Nagles Galeano, L.J.; Bustos Cortes, J.J.; Delgado Ávila, W.A.; Herrera Daza, E.; Suárez, L.E.C.; Prieto-Rodríguez, J.A.; Patiño-Ladino, O.J. Effects of Essential Oils from 24 Plant Species on Sitophilus zeamais Motsch (Coleoptera, Curculionidae). Insects 2021, 12, 532. [Google Scholar] [CrossRef] [PubMed]
- Qasim, M.; Islam, W.; Rizwan, M.; Hussain, D.; Noman, A.; Khan, K.A.; Ghramh, H.A.; Han, X. Impact of Plant Monoterpenes on Insect Pest Management and Insect-Associated Microbes. Heliyon 2024, 10, e39120. [Google Scholar] [CrossRef] [PubMed]
- Drosdoski, S.D.; Sinópolis Gigliolli, A.A.; Cabral, L.C.; Julio, A.H.F.; Bespalhok, D.D.N.; Santini, B.L.; Lapenta, A.S. Characterization of Esterases in the Involvement of Insecticide Resistance in Sitophilus oryzae and Sitophilus zeamais (Coleoptera: Curculionidae). Int. J. Trop. Insect Sci. 2024, 44, 1103–1115. [Google Scholar] [CrossRef]
- Yu, J. Chemical Composition of Essential Oils and Their Potential Applications in Postharvest Storage of Cereal Grains. Molecules 2025, 30, 683. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, A.; Beato, M.; Usseglio, V.L.; Camina, J.; Zygadlo, J.A.; Dambolena, J.S.; Zunino, M.P. Phenolic Compounds as Controllers of Sitophilus zeamais: A Look at the Structure-Activity Relationship. J. Stored Prod. Res. 2022, 99, 102038. [Google Scholar] [CrossRef]
- Yildirim, E.; Emsen, B.; Kordali, S. Insecticidal Effects of Monoterpenes on Sitophilus zeamais Motschulsky (Coleoptera: Curculionidae). J. Appl. Bot. Food Qual. 2013, 86, 198–204. [Google Scholar] [CrossRef]
- Herrera, J.M.; Zunino, M.P.; Massuh, Y.; Pizzollito, R.P.; Dambolena, J.S.; Gañan, N.A.; Zygadlo, J.A. Fumigant Toxicity of Five Essential Oils Rich in Ketones against Sitophilus zeamais (Motschulsky). AgriScientia 2014, 31, 35–41. [Google Scholar] [CrossRef]
- Sierra-Quitian, A.G.; Prieto-Rodríguez, J.A.; Patiño-Ladino, O.J. Insecticidal Activity of Monoterpenoids Against Sitophilus zeamais Motschulsky and Tribolium castaneum Herbst: Preliminary Structure—Activity Relationship Study. Int. J. Mol. Sci. 2025, 26, 3407. [Google Scholar] [CrossRef]
- Abbott, W.S. The Value of the Dry Substitutes for Liquid Lime. Econ. Entomol. 1925, 18, 265–267. [Google Scholar] [CrossRef]
- Sakuma, M. Probit Analysis of Preference Data. Appl. Entomol. Zool. 1998, 33, 339–347. [Google Scholar] [CrossRef]
- Holliday, J.D.; Rodgers, S.L.; Willett, P.; Chen, M.Y.; Mahfouf, M.; Lawson, K.; Mullier, G. Clustering Files of Chemical Structures Using the Fuzzy K-Means Clustering Method. J. Chem. Inf. Comput. Sci. 2004, 44, 894–902. [Google Scholar] [CrossRef] [PubMed]
- Charrad, M.; Ghazzali, N.; Boiteau, V.; Niknafs, A. Nbclust: An R Package for Determining the Relevant Number of Clusters in a Data Set. J. Stat. Softw. 2014, 61, 1–36. [Google Scholar] [CrossRef]
- Murtagh, F. Ward’s Hierarchical Agllomerative Clustering Method: Which Algorithms Implement Ward’s Criterio? J. Classif. 2014, 31, 274–295. [Google Scholar] [CrossRef]
- Cornell, J. Experiments with Mixtures: Designs, Models, and the Analysis of Mixture Data; John Wiley & Sons: New York, NY, USA, 2002; ISBN 0471393673. [Google Scholar]
- Lazcano Díaz, E.; Padilla Camberos, E.; Castillo Herrera, G.A.; Estarrón Espinosa, M.; Espinosa Andrews, H.; Paniagua Buelnas, N.A.; Gutiérrez Ortega, A.; Martínez Velázquez, M. Development of Essential Oil-Based Phyto-Formulations to Control the Cattle Tick Rhipicephalus microplus Using a Mixture Design Approach. Exp. Parasitol. 2019, 201, 26–33. [Google Scholar] [CrossRef]
- Cedergreen, N.; Svendsen, C.; Backhaus, T. Chemical Mixtures: Concepts for Predicting Toxicity. In Encyclopedia of Environmental Management; CRC Press: Boca Raton, FL, USA, 2013; pp. 2572–2581. [Google Scholar] [CrossRef]
- Bezerra, M.A.; Lemos, V.A.; Novaes, C.G.; de Jesus, R.M.; Filho, H.R.S.; Araújo, S.A.; Alves, J.P.S. Application of Mixture Design in Analytical Chemistry. Microchem. J. 2020, 152, 104336. [Google Scholar] [CrossRef]
- Patt, J.M.; Tarshis Moreno, A.M.; Niedz, R.P. Response Surface Methodology Reveals Proportionality Effects of Plant Species in Conservation Plantings on Occurrence of Generalist Predatory Arthropods. PLoS ONE 2020, 15, e0231471. [Google Scholar] [CrossRef]
- Lederer, S.; Dijkstra, T.M.H.; Heskes, T. Additive Dose Response Models: Defining Synergy. Front. Pharmacol. 2019, 10, 1384. [Google Scholar] [CrossRef]
- Chou, T. Drug Combination Studies and Their Synergy Quantification Using the Chou-Talalay Method. Cancer Res. 2010, 70, 440–447. [Google Scholar] [CrossRef]
- Chou, T.C. Theoretical Basis, Experimental Design, and Computerized Simulation of Synergism and Antagonism in Drug Combination Studies. Pharmacol. Rev. 2006, 58, 621–681. [Google Scholar] [CrossRef]
- Zamora Espitia, H. Selected Methods of Experimental Biochemistry; Universidad Nacional de Colombia, Faculty of Sciences: Bogotá, Colombia, 2008; Volume 176, pp. 40–150. [Google Scholar]
- Holdgate, G.A.; Meek, T.D.; Grimley, R.L. Mechanistic Enzymology in Drug Discovery: A Fresh Perspective. Nat. Rev. Drug Discov. 2018, 17, 115–132. [Google Scholar] [CrossRef]
- Valipour Nouroozi, R.; Valipour Nouroozi, M.; Ahmadizadeh, M. Determination of Protein Concentration Using Bradford Microplate Protein Quantification Assay. Int. Electron. J. Med. 2015, 4, 11–17. [Google Scholar] [CrossRef]
- Bradford, M.M. Determinación de Proteínas: Método de Bradford. Anal. Biochem. 1976, 254, 1976. [Google Scholar]
- Habig, W.H.; Pabst, M.J.; Jakoby, W.B. Glutathione S-Transferases. The First Enzymatic Step in Mercapturic Acid Formation. J. Biol. Chem. 1974, 249, 7130–7139. [Google Scholar] [CrossRef]
- Dowd, A.J.; Steven, A.; Morou, E.; Hemingway, J.; Vontas, J.; Paine, M.J.I. A Simple Glutathione Transferase-Based Colorimetric Endpoint Assay for Insecticide Detection. Enzyme Microb. Technol. 2009, 45, 164–168. [Google Scholar] [CrossRef]
- Sinha, A.K. Colorimetric Assay of Catalase. Anal. Biochem. 1972, 47, 389–394. [Google Scholar] [CrossRef] [PubMed]
- Hadwan, M.H. New Method for Assessment of Serum Catalase Activity. Indian J. Sci. Technol. 2016, 9, 1–5. [Google Scholar] [CrossRef]
- Ellman, G.; Courtney, D.; Andres, V.; Featherston, R. A New and Rapid Colorimetric of Acetylcholinesterase Determination. Biochem. Pharmecology 1961, 7, 88–95. [Google Scholar] [CrossRef]
- Albadrani, H.M.; Alsaweed, M.; Jamal, Q.M.S.; Alasiry, S.M.; Jahan, S.; Hamed, M.; Kamal, M.; Rehman, M.T.; Iqbal, D. In-Vitro Enzyme Inhibition, Kinetics, Molecular Docking and Dynamics Simulation Approaches to Decoding the Mechanism of Ficus virens in Cholinesterase Inhibition. J. Taibah Univ. Sci. 2024, 18, 2403813. [Google Scholar] [CrossRef]
- Rodriguez, A.M.; Zunino, M.P.; Dambolena, J.S. Optimización de Ensayos de Inhibición de Acetilcolinesterasa En Sitophilus zeamais (Mots.). Rev. Fac. Cienc. Exactas Físicas y Naturales 2018, 5, 51. [Google Scholar]
- Su, J.; Liu, H.; Gou, K.; Chen, L.; Yang, M.; Chen, Q. Research Advances and Detection Methodologies for Microbe-Derived Acetylcholinesterase Inhibitors: A Systemic Review. Molecules 2017, 22, 176. [Google Scholar] [CrossRef] [PubMed]
- Herrera, J.M.; Zunino, M.P.; Dambolena, J.S.; Pizzolitto, R.P.; Gañan, N.A.; Lucini, E.I.; Zygadlo, J.A. Terpene Ketones as Natural Insecticides against Sitophilus zeamais. Ind. Crops Prod. 2015, 70, 435–442. [Google Scholar] [CrossRef]
- Quan, M.; Liu, Q.Z.; Liu, Z.L. Identification of Insecticidal Constituents from the Essential Oil from the Aerial Parts Stachys riederi var. japonica. Molecules 2018, 23, 1200. [Google Scholar] [CrossRef] [PubMed]
- Rajendran, S.; Sriranjini, V. Plant Products as Fumigants for Stored-Product Insect Control. J. Stored Prod. Res. 2008, 44, 126–135. [Google Scholar] [CrossRef]
- Chu, S.S.; Du, S.S.; Liu, L.Z. Fumigant Compounds from the Essential Oils of Chinese Blumea bolsamifera Leaves against Maize Weevil (Sitophilus zeamais). J. Chem. 2013, 2013, 289874. [Google Scholar] [CrossRef]
- Fang, R.; Jiang, C.H.; Wang, X.Y.; Zhang, H.M.; Liu, Z.L.; Zhou, L.; Du, S.S.; Deng, Z.W. Insecticidal Activity of Essential Oil of Carum Carvi Fruits from China and Its Main Components against Two Grain Storage Insects. Molecules 2010, 15, 9391–9402. [Google Scholar] [CrossRef]
- Chu, S.S.; Feng Hu, J.; Liu, Z.L. Composition of Essential Oil of Chinese Chenopodium Ambrosioides and Insecticidal Activity against Maize Weevil, Sitophilus zeamais. Pest Manag. Sci. 2011, 67, 714–718. [Google Scholar] [CrossRef]
- Prieto, J.a.; Pabón, L.C.; Patiño, Ó.J.; Delgado, W.a.; Cuca, L.E. Constituyentes Químicos, Actividad Insecticida y Antifúngica de Los Aceites Esenciales de Hojas de Dos Especies Colombianas Del Género Ocotea (Lauraceae). Rev. Colomb. Química 2010, 39, 199–209. [Google Scholar]
- Suthisut, D.; Fields, P.G.; Chandrapatya, A. Fumigant Toxicity of Essential Oils from Three Thai Plants (Zingiberaceae) and Their Major Compounds against Sitophilus zeamais, Tribolium castaneum and Two Parasitoids. J. Stored Prod. Res. 2011, 47, 222–230. [Google Scholar] [CrossRef]
- Dambolena, J.S.; Zunino, M.P.; Herrera, J.M.; Pizzolitto, R.P.; Areco, V.A.; Zygadlo, J.A. Terpenes: Natural Products for Controlling Insects of Importance to Human Health—A Structure-Activity Relationship Study. Psyche 2016, 17, 4595823. [Google Scholar] [CrossRef]
- Mossa, A.T.H. Green Pesticides: Essential Oils as Biopesticides in Insect-Pest Management. J. Environ. Sci. Technol. 2016, 9, 354–378. [Google Scholar] [CrossRef]
- Jang, Y.S.; Yang, Y.C.; Choi, D.S.; Ahn, Y.J. Vapor Phase Toxicity of Marjoram Oil Compounds and Their Related Monoterpenoids to Blattella germanica (Orthoptera: Blattellidae). J. Agric. Food Chem. 2005, 53, 7892–7898. [Google Scholar] [CrossRef] [PubMed]
- Ripathi, A.K.; Upadhyay, S.; Bhuiyan, M.; Bhattacharya, P.R. A Review of Essential Oils as Biopesticide in Insect-Pest Management. J. Pharmacogn. Phytother. 2009, 1. Available online: https://www.researchgate.net/publication/255988644 (accessed on 30 January 2025).
- Lee, S.; Peterson, C.J.; Coats, J.R. Fumigation Toxicity of Monoterpenoids to Several Stored Product Insects. J. Stored Prod. Res. 2003, 39, 77–85. [Google Scholar] [CrossRef]
- Gharbi, K.; Tay, J.-W.; Gharbi, K.; Tay, J.-W. Fumigant Toxicity of Essential Oils against Frankliniella occidentalis and F. insularis (Thysanoptera: Thripidae) as Affected by Polymer Release and Adjuvants. Insects 2022, 13, 493. [Google Scholar] [CrossRef]
- Enan, E. Insecticidal Activity of Essential Oils: Octopaminergic Sites of Action. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2001, 130, 325–337. [Google Scholar] [CrossRef]
- Asawalam, E.F.; Emosairue, S.O.; Hassanali, A. Contribution of Different Constituents to the Toxicity of the Essential Oil Constituents of Vernonia amygdalina (Compositae) and Xylopia aetiopica (Annonaceae) on Maize Weevil, Sitophilus zeamais Motschulsky (Coleoptera: Curculionidae). Afr. J. Biotechnol. 2008, 7, 2957–2962. [Google Scholar]
- Huang, Y.; Ho, S.H.; Lee, H.C.; Yap, Y.L. Insecticidal Properties of Eugenol, Isoeugenol and Methyleugenol and Their Effects on Nutrition of Sitophilus zeamais Motsch. (Coleoptera: Curculionidae) and Tribolium castaneum (Herbst) (Coleoptera: Tenebrionidae). J. Stored Prod. Res. 2002, 38, 403–412. [Google Scholar] [CrossRef]
- Suthisut, D.; Fields, P.G.; Chandrapatya, A. Contact Toxicity, Feeding Reduction, and Repellency of Essential Oils from Three Plants from the Ginger Family (Zingiberaceae) and Their Major Components against Sitophilus zeamais and Tribolium castaneum. J. Econ. Entomol. 2011, 104, 1445–1454. [Google Scholar] [CrossRef]
- Chu, S.S.; Wang, C.F.; Du, S.S.; Liu, S.L.; Liu, Z.L. Toxicity of the Essential Oil of Illicium difengpi Stem Bark and Its Constituent Compounds towards Two Grain Storage Insects. J. Insect Sci. 2011, 11, 152. [Google Scholar] [CrossRef]
- Seo, S.M.I.; Junheon, K.; Eunae, K.; Park, H.M.I.; Kim, Y.J.; Park, I.L.K. Structure-Activity Relationship of Aliphatic Compounds for Nematicidal Activity against Pine Wood Nematode (Bursaphelenchus xylophilus). J. Agric. Food Chem. 2010, 58, 1823–1827. [Google Scholar] [CrossRef]
- Ben Hamouda, A.; Ben Bnina, E.; Chaieb, I.; Laarif, A.; Ben Jannet, H. Cyclic and Acyclic Alcohols: A Structure-Activity Relationship Study Correlation between Insecticidal Activity and Chemical Structure. Int. J. Trop. Insect Sci. 2021, 41, 961–968. [Google Scholar] [CrossRef]
- Crespo, Y.A.; Bravo Sánchez, L.R.; Quintana, Y.G.; Cabrera, A.S.T.; Bermúdez del Sol, A.; Mayancha, D.M.G. Evaluation of the Synergistic Effects of Antioxidant Activity on Mixtures of the Essential Oil from Apium graveolens L., Thymus vulgaris L. and Coriandrum sativum L. Using Simplex-Lattice Design. Heliyon 2019, 5, e01942. [Google Scholar] [CrossRef]
- Scalerandi, E.; Flores, G.A.; Palacio, M.; Defagó, M.T.; Carpinella, M.C.; Valladares, G.; Bertoni, A.; Palacios, S.M. Understanding Synergistic Toxicity of Terpenes as Insecticides: Contribution of Metabolic Detoxification in Musca domestica. Front. Plant Sci. 2018, 9, 1579. [Google Scholar] [CrossRef]
- Food and Drug Administration (FDA). Substances Added to Food (Formerly EAFUS). Available online: https://www.hfpappexternal.fda.gov/scripts/fdcc/index.cfm?id=PULEGONE&set=FoodSubstances&utm_source=chatgpt.com (accessed on 1 April 2025).
- Guldiken, B.; Catalkaya, G.; Ozkan, G.; Ceylan, F.D.; Capanoglu, E. Toxicological Effects of Commonly Used Herbs and Spices. In Toxicology: Oxidative Stress and Dietary Antioxidants; Academic Press: Cambridge, MA, USA, 2021; pp. 201–213. [Google Scholar] [CrossRef]
- Pavela, R. Acute Toxicity and Synergistic and Antagonistic Effects of the Aromatic Compounds of Some Essential Oils against Culex quinquefasciatus Say Larvae. Parasitol. Res. 2015, 114, 3835–3853. [Google Scholar] [CrossRef]
- Peschiutta, M.L.; Achimón, F.; Brito, V.D.; Pizzolitto, R.P.; Zygadlo, J.A.; Zunino, M.P. Fumigant Toxicity of Essential Oils against Sitophilus zeamais (Motschulsky) (Coleoptera: Curculionidae): A Systematic Review and Meta-Analysis. J. Pest Sci. 2022, 95, 1037–1056. [Google Scholar] [CrossRef]
- López, M.D.; Pascual-Villalobos, M.J. Mode of Inhibition of Acetylcholinesterase by Monoterpenoids and Implications for Pest Control. Ind. Crops Prod. 2010, 31, 284–288. [Google Scholar] [CrossRef]
- Hayek-Orduz, Y.; Acevedo-Castro, D.A.; Saldarriaga Escobar, J.S.; Eli Ortiz-Domínguez, B.; Villegas-Torres, M.F.; Caicedo, P.A.; Barrera-Ocampo, A. DyphAI Dynamic Pharmacophore Modeling with AI: A Tool for Efficient Screening of New Acetylcholinesterase Inhibitors. Front. Chem. 2025, 13, 1479763. [Google Scholar] [CrossRef]
- Rajkumar, V.; Gunasekaran, C.; Christy, I.K.; Dharmaraj, J.; Chinnaraj, P.; Paul, C.A. Toxicity, Antifeedant and Biochemical Efficacy of Mentha piperita L. Essential Oil and Their Major Constituents against Stored Grain Pest. Pestic. Biochem. Physiol. 2019, 156, 138–144. [Google Scholar] [CrossRef]
- Regnault-Roger, C.; Vincent, C.; Arnason, J.T. Essential Oils in Insect Control: Low-Risk Products in a High-Stakes World. Annu. Rev. Entomol. 2012, 57, 405–424. [Google Scholar] [CrossRef]
- Sun, W.; Kadima, T.; Pickard, M.; Dunford, H. Catalase Activity of Chloroperoxidase and Its Interaction with Peroxidase Activity. Biochem. Cell Biol. 2011, 72, 321–331. [Google Scholar] [CrossRef]
- Seomoon, D.; Lee, K.; Kim, H.; Lee, P.H. Inter- and intramolecular palladium-catalyzed allyl cross-coupling reactions using allylindium generated in situ from allyl acetates, indium, and indium trichloride. Chem. A Eur. J. 2007, 13, 5197–5206. [Google Scholar] [CrossRef] [PubMed]
- Malosh, C.F.; Ready, J.M. Catalytic cross-coupling of alkylzinc halides with α-chloroketones. J. Am. Chem. Soc. 2004, 126, 10240–10241. [Google Scholar] [CrossRef] [PubMed]
- Cheallaigh, A.N.; Mansell, D.J.; Toogood, H.S.; Tait, S.; Lygidakis, A.; Scrutton, N.S.; Gardiner, J.M. Chemoenzymatic Synthesis of the Intermediates in the Peppermint Monoterpenoid Biosynthetic Pathway. J. Nat. Prod. 2018, 81, 1546–1552. [Google Scholar] [CrossRef]



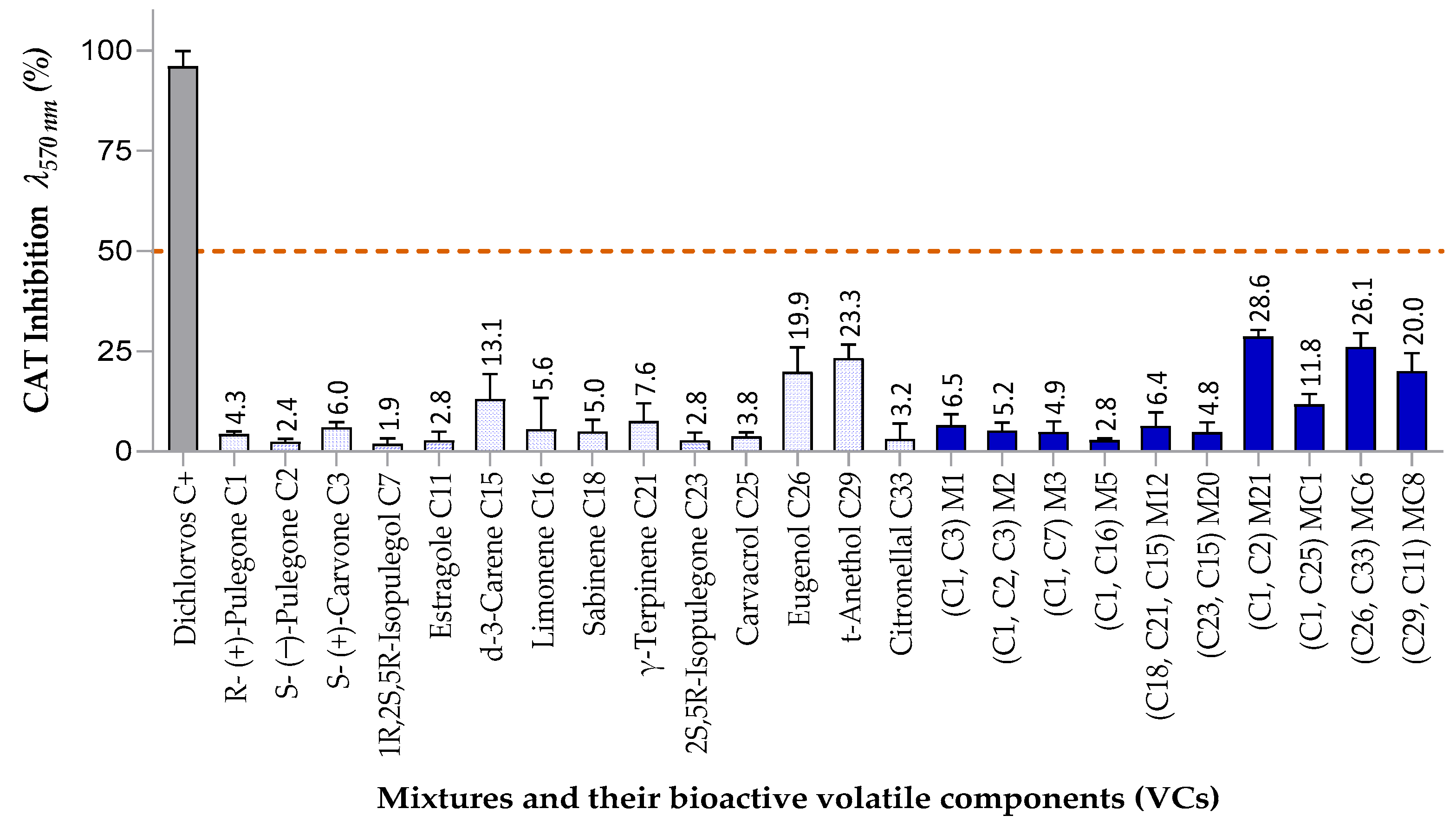
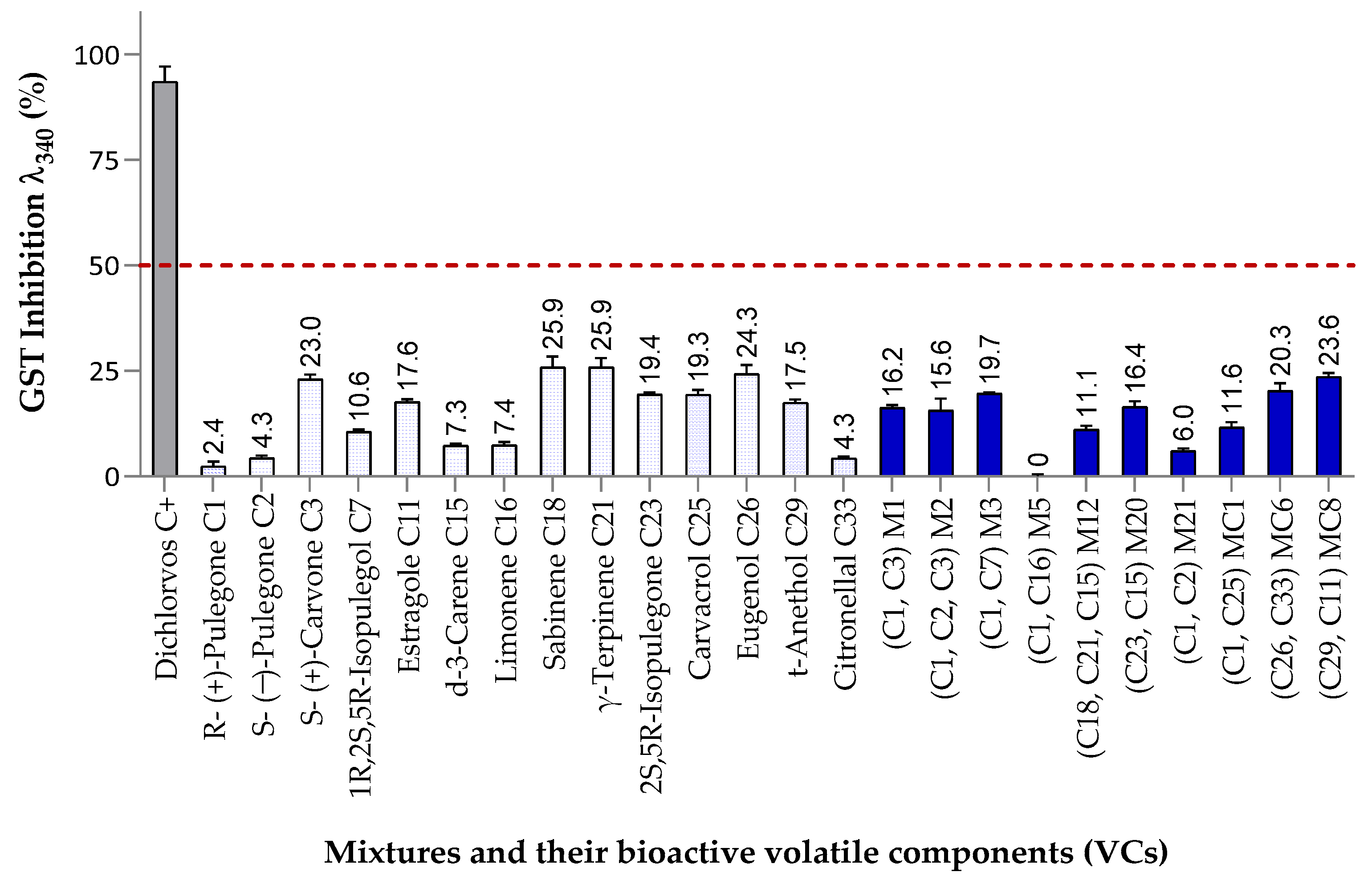
| C | Compound | Fumigant Toxicity | Contact Toxicity | ||||||
|---|---|---|---|---|---|---|---|---|---|
| LC50 a (95% FL) | LC90 b (95% FL) | c (±SD) | LD50 d (95% FL) | LD90 e (95% FL) | c (±SD) | ||||
| mg L−1 | µmol L−1 | mg L−1 | µmol L−1 | µg/Adult | µg/Adult | ||||
| C1 | R-(+)-Pulegone a | 0.58 (0.46–0.71) | 3.81 (3.03–4.69) | 0.92 (0.77–1.26) | 6.07 (5.09–8.25) | 3.73 (±0.84) | 4.85 (4.35–5.32) | 7.40 (6.79–8.28) | 0.51 (±0.060) |
| C2 | S-(−)-Pulegone a | 0.97 (0.69–1.21) | 6.37 (4.54–7.98) | 1.68 (1.39–2.37) | 11.06 (9.17–15.56) | 1.79 (±0.44) | 7.44 (7.00–7.89) | 9.64 (9.05–10.54) | 0.58 (±0.075) |
| C3 | S-(+)-Carvone a | 2.87 (1.99–3.75) | 19.10 (13.26–24.99) | 5.31 (4.26–8.25) | 35.36 (28.36–54.95) | 0.52 (±0.14) | 12.68 (10.91–14.60) | 23.236 (20.46–27.42) | 0.12 (±0.015) |
| C4 | R-(−)-Carvone a | 1.42 (1.14–1.72) | 9.48 (7.57–11.48) | 2.17 (1.84–2.88) | 14.42 (12.23–19.20) | 1.73 (±0.40) | 16.89 (14.56–19.35) | 28.45 (24.99–34.24) | 0.11 (±0.012) |
| C5 | R-(−)-Terpinen-4-ol a | 4.03 (3.27–4.91) | 26.14 (21.22–31.82) | 6.13 (5.18–8.46) | 39.76 (33.56–54.87) | 0.61 (±0.15) | 19.64 (18.42–21.45) | 24.82 (22.64–29.63) | 0.25 (±0.035) |
| C6 | α, β-Thujone (70:10) | 4.34 (3.49–5.30) | 28.54 (22.90–34.79) | 6.81 (5.75–8.94) | 44.70 (37.75–58.73) | 0.52 (±0.11) | 32.04 (29.44–34.42) | 44.67 (41.56–49.34) | 0.10 (±0.013) |
| C7 | 1R,2S,5R-Isopulegol | 4.73 (3.86–5.68) | 30.66 (25.05–36.84) | 7.19 (6.13–9.43) | 46.64 (39.75–61.17) | 0.52 (±0.11) | 18.99 (17.7–20.15) | 26.65 (25.07–28.90) | 0.17 (±0.019) |
| C8 | 1R-(−)-Fenchone a | 10.59 (8.56–13.16) | 69.59 (56.24–86.45) | 17.06 (14.18–23.81) | 112.05 (93.12–156.40) | 0.20 (±0.046) | 34.65 (32.19–37.08) | 48.33 (45.12–52.78) | 0.09 (±0.010) |
| C9 | 2-Nonanone | 9.09 (7.49–11.04) | 63.92 (52.63–77.64) | 13.73 (11.61–18.91) | 96.54 (81.59–132.94) | 0.28 (±0.067) | 31.86 (30.27–33.17) | 39.69 (38.01–42.15) | 0.16 (±0.021) |
| C10 | 1.8-Cineole a | 12.96 (10.38–16.10) | 84.04 (67.31–104.36) | 21.68 (18.02–29.56) | 140.57 (116.82–119.66) | 0.15 (±0.031) | 56.55 (53.14–60.22) | 79.45 (73.78–87.99) | 0.056 (±0.002) |
| C11 | Estragole | 30.46 (22.40–39.81) | 205.56 (151.13–268.62) | 57.45 (45.99–87.88) | 387.67 (310.36–593.03) | 0.05 (±0.012) | 45.59 (38.53–53.40) | 79.66 (68.62–99.73) | 0.038 (±0.005) |
| C12 | p-Cymene a | 28.68 (23.08–35.59) | 213.67 (171.95–256.12) | 46.96 (39.01–65.39) | 349.89 (290.68–487.22) | 0.07 (±0.016) | - | - | - |
| C13 | α-Terpinene a | 60.24 (47.80–72.75) | 442.21 (350.86–533.98) | 96.19 (81.75–125.28) | 706.07 (600.09–919.60) | 0.04 (±0.007) | - | - | - |
| C14 | R-(−)-α-Phellandrene a | 88.87 (69.70–108.27) | 652.36 (511.64–794.79) | 142.53 (119.87–197.61) | 1046.26 (879.93–1450.53) | 0.02 (±0.006) | - | - | - |
| C15 | δ-3-Carene a | 80.35 (64.09–97.18) | 589.79 (470.47–713.38) | 135.83 (114.92–178.25) | 997.04 (843.60–1308.47) | 0.02 (±0.005) | - | - | - |
| C16 | DL-Limonene (1:1) | 88.69 (74.72–103.70) | 651.06 (548.49–761.24) | 136.14 (117.96–171.69) | 999.33 (865.87–1260.27) | 0.03 (±0.005) | - | - | - |
| C17 | β-Pinene a | 97.60 (77.15–116.76) | 716.41 (566.36–857.12) | 151.34 (129.39–198.56) | 1.110.93 (949.79–1457.51) | 0.02 (±0.005) | - | - | - |
| C18 | Sabinene a | 68.65 (56.89–79.75) | 503.92 (417.59–585.44) | 102.10 (89.08–128.61) | 749.50 (653.90–944.04) | 0.04 (±0.008) | - | - | - |
| C19 | α-Pinene a | 110.38 (90.76–130.60) | 810.25 (666.22–958.70) | 178.81 (152.92–235.051) | 1312.53 (1122.51–1725.39) | 0.02 (±0.004) | - | - | - |
| C20 | n-Nonane | 109.12 (86.72–127.88) | 850.81 (676.19–997.02) | 171.55 (147.92–228.44) | 1337.49 (1153.25–1781.07) | 0.02 (±0.005) | - | - | - |
| C21 | γ-Terpinene a | 107.95 (82.31–146.97) | 792.42 (604.23–1078.84) | 187.96 (148.32–224.83) | 1379.72 (1088.75–2384.44) | 0.02 (±0.005) | - | - | - |
| C22 | Piperitone | 4.38 (3.72–5.35) | 28.77 (24.45–35.13) | 6.19 (5.26–8.89) | 40.67 (34.54–54.43) | 0.70 (±0.19) | 9.45 (8.61–10.41) | 14.70 (13.25–16.96) | 0.24 (±0.031) |
| C23 | 2S,5R-Isopulegone | 2.37 (1.86–2.89) | 15.57 (12.19–18.96) | 3.88 (3.29–5.18) | 25.46 (21.47–34.05) | 0.85 (±0.19) | 11.14 (9.64–12.49) | 18.48 (16.56–21.64) | 0.17 (±0.022) |
| C24 | Terpinolene a | 52.13 (34.83–72.33) | 337.92 (225.81–468.91) | 88.83 (69.69–154.50) | 575.86 (451.79–1001.61) | 0.035 (±0.012) | |||
| C25 | Carvacrol | - | - | - | - | - | 8.71 (7.92–9.56) | 13.45 (12.19–15.38) | 0.27 (±0.033) |
| C26 | Eugenol | - | - | - | - | - | 20.9 (19.22–22.65) | 30.82 (28.35–34.42) | 0.13 (±0.015) |
| C27 | Citral | - | - | - | - | - | 21.42 (19.34–23.61) | 32.68 (29.77–36.82) | 0.11 (±0.013) |
| C28 | Linalool | - | - | - | - | - | 21.87 (19.09–24.23) | 36.23 (33.20–40.64) | 0.09 (±0.011) |
| C29 | trans-Anethol | - | - | - | - | - | 26.25 (22.85–29.37) | 46.08 (41.56–52.95) | 0.06 (±0.008) |
| C30 | Geraniol | - | - | - | - | - | 28.13 (25.70–30.49) | 41.06 (37.98–45.41) | 0.01 (±0.011) |
| C31 | Isoeugenol | - | - | - | - | - | 29.1 (24.40–34.21) | 66.2 (57.28–80.01) | 0.03 (±0.004) |
| C32 | Safrole | - | - | - | - | - | 36.31 (30.15–42.37) | 83.04 (73.50–96.63) | 0.03 (±0.003) |
| C33 | Citronellal | - | - | - | - | - | 36.94 (34.60–39.14) | 50.64 (47.46–55.39) | 0.09 (±0.012) |
| C34 | 4-Undecanone | - | - | - | - | - | 37.05 (34.09–40.01) | 53.95 (49.63–60.68) | 0.08 (±0.010) |
| C35 | 2-Decanone | - | - | - | - | - | 38.04 (35.29–40.84) | 57.17 (52.97–63.12) | 0.07 (±0.007) |
| C36 | 2-Undecanone | - | - | - | - | - | 42.86 (40.60–45.30) | 57.13 (53.43–62.83) | 0.09 (±0.011) |
| C37 | Geranyl acetate | - | - | - | - | - | 73.00 (65.98–81.05) | 126.06 (112.84–146.08) | 0.02 (±0.003) |
| C+ | Dichlorvos | 2.17 (1.33–3.81) | 9.84 (6.01–17.22) | 4.57 (3.21–8.75) | 20.68 (14.53–39.59) | 0.51 (±0.15) | - | - | - |
| C+ | Cypermethrin | - | - | - | - | - | 10.492 (0.00–29.96) | 35.79 (21.98–84.37) | 0.08 (±0.019) |
| Fumigant Toxicity | Contact Toxicity | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Code | Pre-Designed Mixes | RSM Estimated Mixtures | Code | Pre-Designed Mixes | RSM Estimated Mixtures | ||||||||
| (A + B + C) | Components (Ratio) | ppm mg/L a | Mortality (%) ± SE | (A + B + C) | Components (Ratio) | µg/ Adult b | Mortality (%) ± SE | ||||||
| M1 | C1 | C3 | C4 | C1: C3 0.73: 0.27 | 0.6 | 67.5 ± 5.0 | MC1 | C1 | C25 | C26 | C1: C25 0.79: 0.21 | 5 | 73.3 ± 5.8 |
| M2 | C1 | C2 | C3 | C1: C2: C3 0.65: 0.14: 0.21 | 0.6 | 87.5 ± 5.0 | MC2 | C9 | C25 | C26 | C1 1 | ND | |
| M3 | C1 | C3 | C7 | C1: C7 0.67: 0.33 | 0.6 | 52.5 ± 5.0 | MC3 | C1 | C25 | C34 | C1: C25 0.79: 0.21 | ND | |
| M4 | C1 | C3 | C5 | C1: C3 0.73: 0.27 | ND | MC4 | C25 | C25 | C34 | C25 1 | ND | ||
| M5 | C1 | C3 | C16 | C1: C16 0.74: 0.26 | 0.6 | 60.0 ± 8.2 | MC5 | C1 | C5 | C10 | C1: 1.00 (combiner C10) * | ND | |
| M6 | C22 | C3 | C16 | C3: C22 0.80: 0.20 | 2.9 | 35.0 ± 5.7 | MC6 | C26 | C37 | C33 | C26: C33 0.70: 0.30 | 20 | 57.5 ± 9.6 |
| M7 | C8 | C3 | C10 | C8: C3 0.1: 0.90 | 2.9 | 7.5 ± 5.0 | MC7 | C27 | C37 | C28 | C27: C37 0.59: 0.41 | 22 | 37.5 ± 5.0 |
| M8 | C8 | C7 | C6 | C9: 1.00 (all combinations) * | ND | MC8 | C29 | C11 | C28 | C29: C11 0.60: 0.40 | 22 | 50.0 ± 8.2 | |
| M9 | C12 | C9 | C10 | C9: 1.00 | ND | MC9 | C22 | C10 | C28 | C10: C28: C22 0.65: 0.14: 0.21 | 9.5 | 40.0 ± 8.2 | |
| M10 | C20 | C19 | C17 | C17: 1.00 | ND | ||||||||
| M11 | C14 | C19 | C16 | C16: 1.00 | ND | ||||||||
| M12 | C18 | C21 | C15 | C18: C21: C15 0.46: 0.31: 0.23 | 66.4 | 97.0 ± 5.8 | |||||||
| M13 | C14 | C12 | C16 | C12: 1.00 | ND | ||||||||
| M14 | C10 | C16 | C19 | C10: C16 0.75: 0.25 | 13 | 7.5 ± 5.0 | |||||||
| M15 | C11 | C12 | C13 | C11: 1.00 (all combinations) * | ND | ||||||||
| M16 | C10 | C16 | C18 | C10: C16 0.75: 0.25 | ND | ||||||||
| M17 | C10 | C5 | C18 | C5:1.00 (combiner C5) * | ND | ||||||||
| M18 | C1 | C22 | C18 | C1: C22 0.75: 0.25 | 0.6 | 40.0 ± 5.0 | |||||||
| M19 | C3 | C15 | C18 | C3: 1.00 | ND | ||||||||
| M20 | C6 | C23 | C15 | C23:C15 0.74: 0.26 | 2.9 | 70.0 ± 8.2 | |||||||
| Mixes A (Ratio) B (Ratio) C (Ratio) | Fumigant Toxicity | Contact Toxicity | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| a (LC-95%) mg/L | b ± SD | DRI c | CI50 d | Interaction | e (LC-95%) µg/Adult | b ± SD | DRI a | CI50 d | Interaction | |
| M1 C1 (0.73) C3 (0.27) | 0.54 (0.38–0.73) 0.58 2.87 | 2.54 ± 0.78 | 1.41 18.1 | 0.77 | Moderate synergism | 9.17 (4.09–13.97) 4.85 12.68 | 0.16 ± 0.60 | 0.70 4.77 | 1.63 | Antagonism |
| M2 C1 (0.65) C2 (0.14) C3 (0.21) | 0.48 (0.35–0.55) 0.58 0.97 2.87 | 5.84 ± 1.55 | 1.89 14.3 27.7 | 0.64 | Synergism | 6.36 (3.85–9.31) 4.85 7.44 12.68 | 0.31 ± 0.11 | 1.02 7.43 7.04 | 1.10 | Additive |
| M3 C1 (0.67) C7 (0.33) | 0.63 (0.45–0.81) 0.58 4.73 | 3.36 ± 1.02 | 1.31 21.9 | 0.81 | Moderate synergism | 9.01 (6.00–12.74) 4.85 18.99 | 0.22 ± 0.71 | 0.78 6.28 | 1.44 | Moderate antagonism |
| M5 C1 (0.74) C16 (0.26) | 0.65 (0.52–0.70) 0.58 88.69 | 3.74 ± 0.91 | 1.17 511.1 | 0.86 | Slight synergism | 9.33 (6.39–13.58) 4.85 - | 0.23 ± 0.07 | 0.68 123.4 | 1.47 * | Antagonism |
| M12 C18 (0.46) C21 (0.31) C15 (0.23) | 39.22 (30.85–49.13) 68.65 107.95 80.35 | 0.06 ± 0.01 | 3.71 8.40 8.40 | 0.51 | Synergism | - | - | - | - | - |
| M20 C23 (0.74) C15 (0.26) | 2.06 (1.18–2.92) 2.37 80.35 | 0.64 ± 0.17 | 4.21 49.8 | 0.26 | Strong synergism | 15.61 (10.83–23.00) 11.14 - | 0.14 ± 0.05 | 0.92 27.3 | 1.09 * | Additive |
| M21 C1 (0.50) C2 (0.50) | 0.63 (0.45–0.83) 0.58 0.97 | 2.43 ± 0.65 | 1.77 2.90 | 0.91 | Additive | 8.17 (4.88–11.78) 4.85 7.44 | 0.23 ± 0.72 | 1.15 1.80 | 1.42 | Moderate antagonism |
| MC1 C1 (0.79) C25 (0.21) | 1.56 (0.86–2.40) 0.58 - | 0.66 ± 0.18 | 0.45 732.7 | 2.19 * | Antagonism | 7.25 (4.11–10.80) 4.85 8.71 | 0.26 ± 0.09 | 0.83 5.54 | 1.40 | Moderate antagonism |
| MC6 C26 (0.70) C33 (0.30) | - - | - | - | - | - | 18.33 (10.79–23.95) 20.90 36.94 | 0.14 ± 0.05 | 1.59 6.62 | 0.78 | Moderate synergism |
| MC8 C29 (0.60) C11 (0.40) | 48.87 (36.01–67.79) - 30.46 | 0.06 ± 0.01 | 8.00 1.44 | 0.82 * | Slight synergism | 42.43 (22.16–60.30) 26.25 45.59 | 0.045 ± 0.02 | 0.97 2.54 | 1.42 | Moderate antagonism |
| C+ Dichlorvos (fumigant) Cypermethrin (contact) | 2.17 (1.53–3.81) | 0.51 ± 0.15 | 10.49 (0.10–19.96) | 0.045 ± 0.02 | ||||||
| Volatile Compounds or Mixtures [A ((Ratio):B ((Ratio):C ((Ratio)] | AChE Effect | ||
|---|---|---|---|
(mg/L) | Inhibitor Type | ||
| C2 (0.50) | 36.60 ± 3.29 | 50.27 ± 0.74 | C |
| C3 (1.00) | 66.27 ± 4.35 | 56.76 ± 0.18 | C |
| C7 (1.00) | 18.15 ± 0.64 | 282.43 ± 4.14 | C |
| C11 (0.40) | 27.41 ± 1.64 | 159.17 ± 6.34 | C |
| C15 (0.23) | 0.19 ± 0.06 | 2.85 ± 0.05 | NC |
| C16 (0.26) | 26.69 ± 2.90 | 79.64 ± 0.55 | NC |
| C29 (0.60) | 4.24 ± 0.36 | 43.65 ± 3.46 | C |
| C33 (0.30) | 7.72 ± 0.78 | 198.55 ± 3.51 | C |
| M5 [C1 (0.74):C16 (0.26)] | 30.19 ± 5.78 | 207.85 ± 2.05 | C |
| M12 [C18 (0.46):C21 (0.31):C15 (0.23)] | 0.81 ± 0.06 | 3.27 ± 0.06 | NC |
| M20 [C23 (0.74):C15 (0.26)] | 0.61 ± 0.05 | 1.44 ± 2.98 × 10−3 | C |
| M21 [C1 (0.50):C2 (0.50)] | 30.24 ± 4.60 | 159.15 ± 7.35 | C |
| MC1 [C1 (0.79):C25 (0.21)] | 22.32 ± 6.78 | 94.46 ± 3.57 | C |
| MC6 [C26 (0.70):C33 (0.30)] | 4.45 ± 0.68 | 30.45 ± 3.51 | C |
| MC8 [C29 (0.60):C11 (0.40)] | 5.28 ± 0.72 | 44.61 ± 1.64 | C |
| C+ | 9.60 × 10−3 ± 2.08 × 10−3 | - | - |
| Mixes A (Ratio) B (Ratio) C (Ratio) | Interaction of Components in Mixtures | ||
|---|---|---|---|
| DRI a | CI b | Interaction | |
| M5 | 0.21 | Strong synergism | |
| C1 (0.74) | 109.80 | ||
| C16 (0.26) | 5.10 | ||
| M12 | 1.95 | Antagonism | |
| C18 (0.46) | 4 268.45 | ||
| C21 (0.31) | 786.24 | ||
| C15 (0.23) | 0.51 | ||
| M20 | 2.31 | Antagonism | |
| C23 (0.74) | 42 279.60 | ||
| C15 (0.26) | 0.43 | ||
| M21 | 0.29 | Strong synergism | |
| C1 (0.50) | 162.21 | ||
| C2 (0.50) | 3.53 | ||
| MC1 | 0.04 | Very strong | |
| C1 (0.79) | 139.10 | synergism | |
| C25 (0.21) | 28.14 | ||
| MC6 | 0.13 | Strong synergism | |
| C26 (0.70) | 81.17 | ||
| C33 (0.30) | 8.75 | ||
| MC8 | 0.52 | Synergism | |
| C29 (0.60) | 2.37 | ||
| C11 (0.40) | 9.70 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Galeano, L.J.N.; Prieto-Rodríguez, J.A.; Patiño-Ladino, O.J. Synergistic Insecticidal Activity of Plant Volatile Compounds: Impact on Neurotransmission and Detoxification Enzymes in Sitophilus zeamais. Insects 2025, 16, 609. https://doi.org/10.3390/insects16060609
Galeano LJN, Prieto-Rodríguez JA, Patiño-Ladino OJ. Synergistic Insecticidal Activity of Plant Volatile Compounds: Impact on Neurotransmission and Detoxification Enzymes in Sitophilus zeamais. Insects. 2025; 16(6):609. https://doi.org/10.3390/insects16060609
Chicago/Turabian StyleGaleano, Leidy J. Nagles, Juliet A. Prieto-Rodríguez, and Oscar J. Patiño-Ladino. 2025. "Synergistic Insecticidal Activity of Plant Volatile Compounds: Impact on Neurotransmission and Detoxification Enzymes in Sitophilus zeamais" Insects 16, no. 6: 609. https://doi.org/10.3390/insects16060609
APA StyleGaleano, L. J. N., Prieto-Rodríguez, J. A., & Patiño-Ladino, O. J. (2025). Synergistic Insecticidal Activity of Plant Volatile Compounds: Impact on Neurotransmission and Detoxification Enzymes in Sitophilus zeamais. Insects, 16(6), 609. https://doi.org/10.3390/insects16060609








