Simple Summary
This study investigated how short-term exposure to high temperatures affects Phytoseiulus persimilis, a predatory mite widely used to control spider mite pests in agriculture. As climate change leads to more frequent and intense heat waves, understanding how such heat stress impacts both pests and their natural enemies is crucial. The research exposed mite eggs and adults to high temperatures for four hours and monitored their development, survival, and reproduction. The results showed that, while warmer conditions sped up development, extremely high temperatures (42 °C) prevented eggs from hatching. Adult mites also lived shorter lives and produced fewer offspring at higher temperatures, with the best population growth observed at 36 °C. These findings suggest that rising temperatures could reduce the effectiveness of this beneficial predator, potentially threatening pest control in crops. By identifying the temperature limits and optimal conditions for P. persimilis, this research provides valuable insights for future pest management strategies. It helps farmers and agricultural planners prepare for climate challenges and maintain sustainable, chemical-free crop protection.
Abstract
Temperature is a critical factor affecting the development and population dynamics of many organisms. An organism’s ability to withstand extreme temperature events, such as heat waves, will become increasingly important as the severity, duration, and frequency of these events continue to rise worldwide due to global warming. Knowledge on the effects of heat stress on both pests and their natural enemies will thus be crucial for keeping biological control and pest control programs effective in future. This research aimed to study the effect of short-term heat stress on the predatory mite Phytoseiulus persimilis, which is one of the important natural enemies utilized as a biocontrol agent against spider mites such as Tetranychus urticae. The experiments assessed the immature developmental time of P. persimilis after a four-hour incubation of eggs at high temperatures, namely 36, 38, 40, and 42 °C, as well as 85 ± 5% RH and a 16:8 h photoperiod (L:D). After adult females emerged, they were exposed to the same conditions again and the population parameters were monitored. The results demonstrated that the immature development time decreased as temperature increased, with the shortest development duration of 5.30 days seen in eggs exposed to 40 °C, while the eggs exposed to 42 °C did not hatch. Female and male adult longevity decreased significantly as the temperature increased. Fecundity, the adult pre-ovipositional period, and the total pre-ovipositional period were lowest following the 40 °C treatment. The population parameters of P. persimilis, including r and λ, reached their highest values in mites treated at 36 °C, and were significantly higher than in the control group. Addressing these challenges through targeted research and adaptive management is essential to sustaining the efficiency of P. persimilis in biocontrol programs, particularly in the context of global climate change.
1. Introduction
Abiotic variables such as wind, humidity, and temperature have the most significant effects on biological control effectiveness. Numerous natural predators are commercially produced indoors, and hence must contend with environmental changes once they are released into the outdoors, often exceeding their typical range, which can cause injury or even death [1,2]. In particular, exposure to high temperatures can lead to altered metabolic rates, reduced fertility, and shortened lifespans, with a severe influence on population dynamics [3]. Consequently, a decrease in predation and survival, as well as the inability to increase the population, may potentially have an impact on biological control efficiency.
Organisms’ ability to withstand extreme temperature events, such as heat waves, will become increasingly important, as the severity, duration, and frequency of these events will continue to rise worldwide due to global warming [4,5,6,7]. Heat waves, defined as brief periods of stressfully high temperatures [8], usually happen in the summer when environmental temperatures generally fluctuate [9]. Brief heat stress can cause ecological and physiological harm to pests and their predators [10,11] and, in turn, have an impact on crop–pest dynamics [12] as well as on food pest–natural predator interactions [13]. Consequently, heat waves have large direct and indirect impacts on crop production [14]. Because climate models suggest the intensification of heat waves in the future, more frequent and severe occurrences of heat waves and mega heat waves are anticipated under the future warmer climate, and their distribution will vary significantly [5].
Temperature is a fundamental abiotic factor influencing the physiological processes, development, growth, and population dynamics of mites [9,15,16,17]. Optimal temperatures enhance mite survival; however, exceeding a certain threshold can lead to inhibited growth and mortality [18]. In natural environments, mites typically have sufficient time to acclimate to seasonal temperature variations. However, extreme temperatures can occur rapidly, giving mites limited time and resources to adapt to them and recover from the resultant damage [19]. Similarly to other arthropods, the consequences of heat stress on mites depend on the frequency, amplitude, and duration of the heat stress, as well as the feeding status, developmental stage, sex, and reproductive condition of the mites [20,21,22].
The impact of heat stress on mites has been extensively documented. Most studies revealed that heat stress adversely affects mite behavior, growth, reproduction, survival, and progeny fitness [9,20,23,24]. Additionally, heat stress causes harmful carryover effects on physiological traits across generations [25].
In agricultural systems, predatory mites such as Phytoseiulus persimilis Athias-Henriot play an important role in managing pest populations in many countries. Their advantage, among others, is that, as a specialist predator of spider mites, they can deal with dense spiderwebs [26]; they also have a high predation rate [27], making them an important biological control agent extensively used to suppress spiders, particularly the two-spotted spider mite Tetranychus urticae Koch, under field and greenhouse conditions [28,29]. The optimal temperature range for P. persimilis is between 17 and 28 °C [18]. Constant temperatures at or above 35 °C result in immature P. persimilis individuals not reaching adulthood [30], while, at 40 °C, adult females cease movement. The mean temperature at which heat coma is encountered is 41.1 °C [31]. However, although entry into heat coma effectively identifies the upper lethal temperature, the breeding of thermo-resistant lines of P. persimilis increased their resistance to temperatures of 40–42 °C by 8–11 times [32]. When this mite was exposed to extreme heat waves during its juvenile development, it developed faster, reached a smaller size at maturity [11], and consumed more prey [33]. Reproduction in P. persimilis increased under extreme heat waves but was not affected by juvenile acclimation [34]. Predator females also laid smaller eggs during extreme heat waves [35]. The effect of the short-term exposure of P. persimilis eggs and adults to high temperatures on the life table parameters of this predatory mite, to our knowledge, is currently unknown.
The present study, therefore, aimed to examine P. persimilis’ responses, namely its development, reproduction, and life table parameters, to short-term exposure to elevated temperatures, to comprehend its tolerance to a high-temperature environment. Knowing the effects of heat stress on various life stages of natural predators could yield essential insights for studies in natural predator ecology [20,21,36]. Furthermore, pest control via natural predators through introduction, conservation, or augmentation would be more efficacious if the heat resistance of biocontrol agents across different life stages were understood [37,38]. Moreover, the results can offer better directions for commercial predatory mite breeding implementation when releasing P. persimilis at high temperatures outdoors and/or indoors.
2. Materials and Methods
2.1. Rearing of Prey and Predator
Adults of the species T. urticae and P. persimilis were collected from cucumber and bean greenhouses in Pakdasht County in Tehran province. These were kept fresh on detached bean leaves that were put upside down on a layer of moist sponge in a 15 × 10 × 5 cm plastic container. Dampened tissue was placed around the sponge borders to provide moisture and keep mites from escaping. A 2 cm diameter hole was drilled into the container’s lid and covered with fine cloth mesh to provide ventilation. In a growth chamber, the containers were maintained at a 16:8 (L:D) photoperiod, 25 ± 0.5 °C, and 75 ± 5% relative humidity. Phytoseiulus persimilis were moved to a fresh container every second day. The predatory mite colony was maintained on T. urticae in this way for three months before using the mites in experiments.
2.2. Test Arena
Phaseolus calcaratus Roxburgh “Goli” seedlings were cultivated in greenhouses in pots 15 cm in diameter until they reached the fourth or fifth leaf stage. Tetranychus urticae were kept on detached bean leaves (3 cm in diameter) with thin veins. In a plastic dish measuring 1 cm deep and 6 cm in diameter, each leaf disk was placed upside down on a layer of moist sponge. To keep mites from escaping and to provide moisture, dampened tissue was placed around the border of the sponge. A 2 cm diameter hole in the middle of the container lid covered by a fine cloth mesh provided ventilation and prevented the mites from escaping.
2.3. Experimental Design
Thirty T. urticae eggs and a young gravid female P. persimilis were placed in the test arena. The female was removed 24 h later, leaving just one predator egg in the experimental arena. The eggs in the test arenas were then exposed to high temperatures of 36 °C, 38 °C, 40 °C, or 42 °C for four hours in a controlled environment chamber (Binder KBWS 240, Tuttlingen, Germany). The temperature range and relatively short exposure period were selected based on upper temperature limits reported in the literature [30,31,32] and because heat waves are more likely to occur during the middle of day, both in greenhouses and fields, for a single day or for a few days [8]. Previous studies on the effect of heat stress on phytoseiid mites, e.g., [20,23,24], used the same or similar temperatures and a duration of 2–6 h. Following exposure, all of the cells were stored and cared for in the environmentally controlled cabinet at 25 ± 0.5 °C, 75 ± 5% RH, and under a 16:8 (L:D) photoperiod. In the control group, the eggs were kept under the same conditions; i.e., they were not exposed to any heat stress. Fifty eggs were used in each heat stress treatment and fifty eggs were used in the control condition, and the growth and survival of P. persimilis individuals were monitored every 12 h. When mites reached adulthood, they were additionally exposed to 36 °C, 38 °C, and 40 °C for four hours. Males and females were then paired, provided with 75 prey eggs, and allowed to copulate in the same cell until the first egg was laid. When the females began to oviposit, the males were removed and the duration of the pre-oviposition period was recorded. For the heat stress-free control group, the same method was used. Survival rates and the number of eggs laid were recorded daily until all the females had died.
2.4. Statistical Analysis
The computer program TWOSEX MS-Chart Version 2020.06.16 available at https://lifetablechi.com/software/, accessed on 3 June 2025, was applied to analyze the collected data using the age-stage, two-sex life table theory [39]. The standard errors and variances of biological characteristics and population parameters were evaluated using the bootstrap resampling approach with 100,000 iterations [40]. The paired bootstrap test was used to assess treatment differences. The calculated population parameters, along with their definitions and equations, are listed in Table A1.
3. Results
The eggs exposed to 42 °C did not hatch. The development time for each stage of P. persimilis under different temperature conditions are listed in Table 1. At the egg stage, the development time decreased significantly with increasing temperatures. At 40 °C, the shortest development time was observed (1.39 ± 0.10 days), which was significantly different to that of the control group (1.83 ± 0.05 days). In the larva and protonymph stage, there were no significant differences in development time between different temperature conditions. The shortest development time for the deutonymph stage was seen at 40 °C (1.59 ± 0.08 days), with significant differences found between the control and 38 °C conditions (1.56 ± 0.07 days). Significant differences were observed in the length of the total pre-adult period between the control and the heat-stress treatments, particularly at 38 °C and 40 °C. The pre-adult survival rate was highest at 36 °C (0.70 ± 0.06) and lowest at 40 °C (0.62 ± 0.07). Both the female and male adult durations decreased significantly as the temperature increased, with the longest lifespan found in the control group and the shortest found under 40 °C conditions. Significant differences were observed across all treatments, with reductions becoming more pronounced at 38 °C and 40 °C.

Table 1.
Mean (±SE) development time, survival rate, and adult duration of Phytoseiulus persimilis exposed to different heat stress.
Female and male longevity significantly differed across all treatments, with reductions becoming more pronounced at 38 °C and 40 °C (Table 2). Fecundity decreased significantly with an increase in temperature. The control group exhibited the highest fecundity (39 ± 1.39 eggs), while the lowest fecundity was found under 40 °C conditions (21.82 ± 0.75 eggs). The APOP remained consistent across most temperatures, with no significant differences between the control, 36 °C, and 38 °C conditions. The TPOP and the number of oviposition days were highest in the control group, while the lowest values were found at 40 °C. The percentage of females did not vary significantly across treatments, ranging from 0.44 ± 0.07 at 40 °C to 0.50 ± 0.07 at 36 °C (Table 2).

Table 2.
Mean (±SE) longevity, fecundity, pre-oviposition periods, and oviposition days of Phytoseiulus persimilis exposed to different heat stress.
Comparing the population parameters of P. persimilis under different temperature conditions (Table 3) revealed that r and λ were highest at 36 °C, significantly higher than in the control. The control group exhibited the highest R0 value (18.72 ± 2.84 offspring/individual), while the lowest value was found at 40 °C (9.60 ± 1.55 offspring/individual), showing a drastic decline under severe heat stress. Significant differences were also observed in the mean generation time (T), with the duration becoming progressively shorter as the temperature increased (Table 3).

Table 3.
Mean (±SE) of population parameters of Phytoseiulus persimilis exposed to different heat stress.
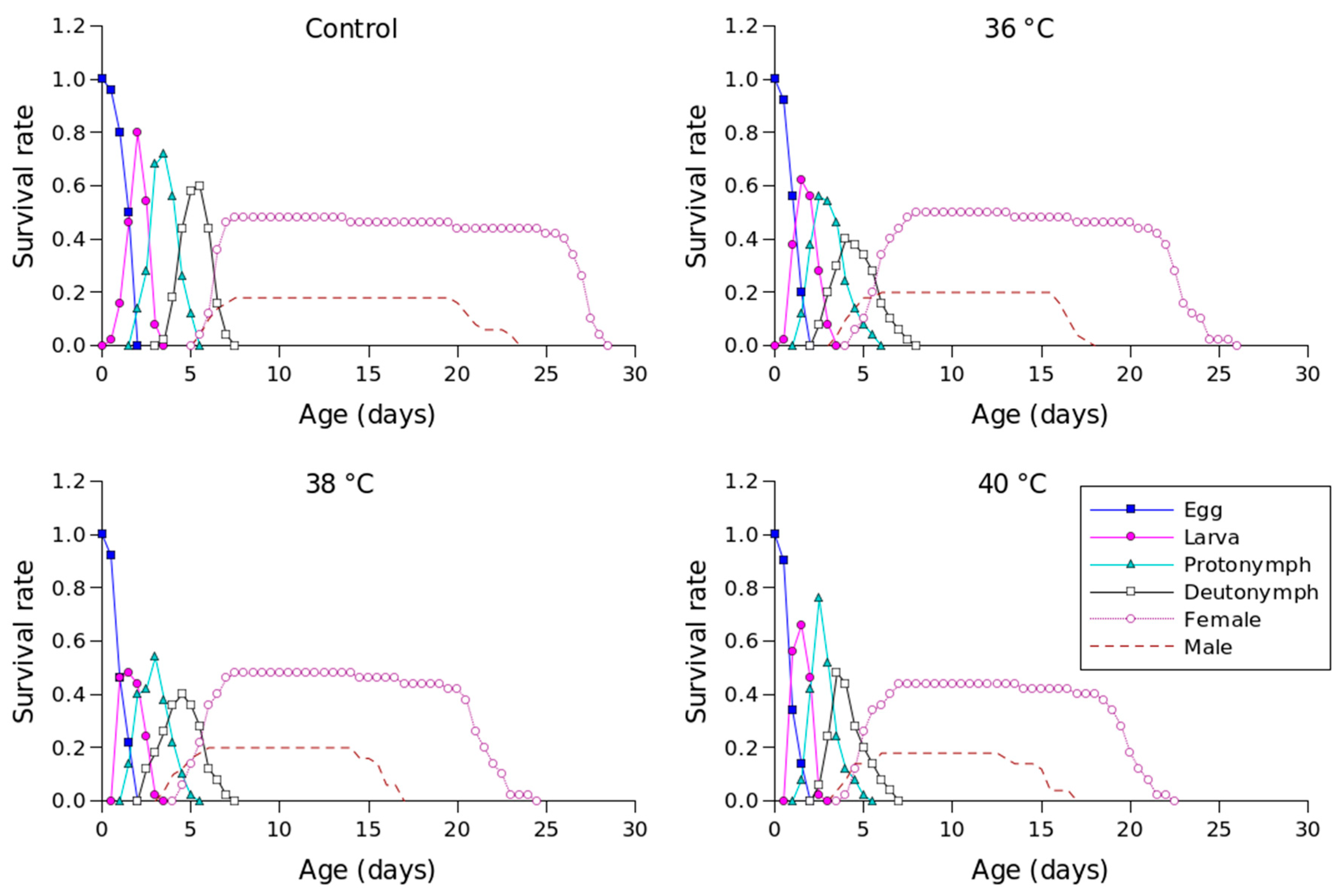
The sxj value for P. persimilis, which represents the likelihood that an individual would live to age x and stage j, allowed us to correctly determine stage overlap and distinction due to variations in development rates (Figure 1). The control group had the highest chance of a newly laid egg surviving to adulthood, with a probability of 0.51 for females and 0.21 for males. At 36 °C, the sxj value was also 0.51 and 0.21 for females and males, 0.49 for females and 0.21 for males at 38 °C, and 0.45 for females and 0.18 for males at 40 °C. Male and female individuals in the control group survived until days 28 and 18, respectively, while females and males survived until days 26 and 18 at 36 °C, days 25 and 17 at 38 °C, and days 23 and 17 at 40 °C (Figure 1).
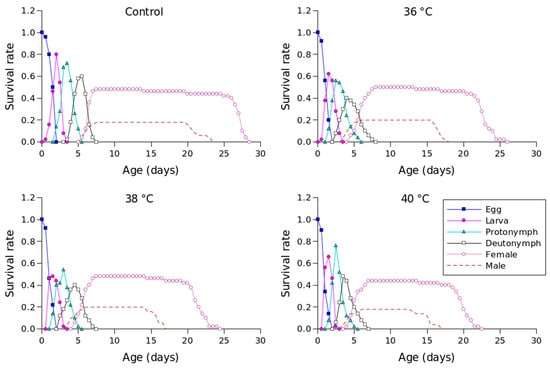
Figure 1.
Age-stage-specific survival rates (sxj) of Phytoseiulus persimilis exposed to different heat stress conditions.
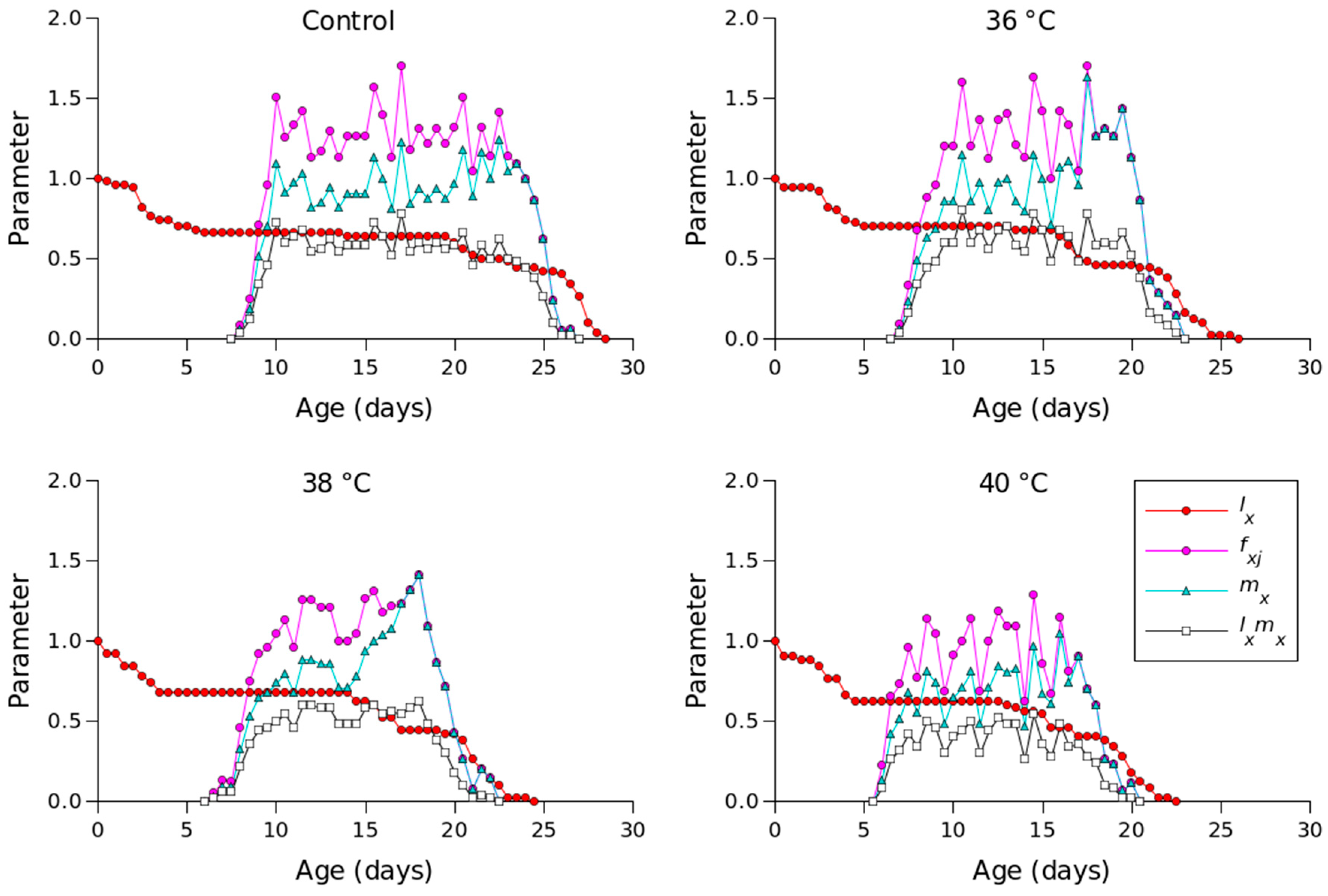
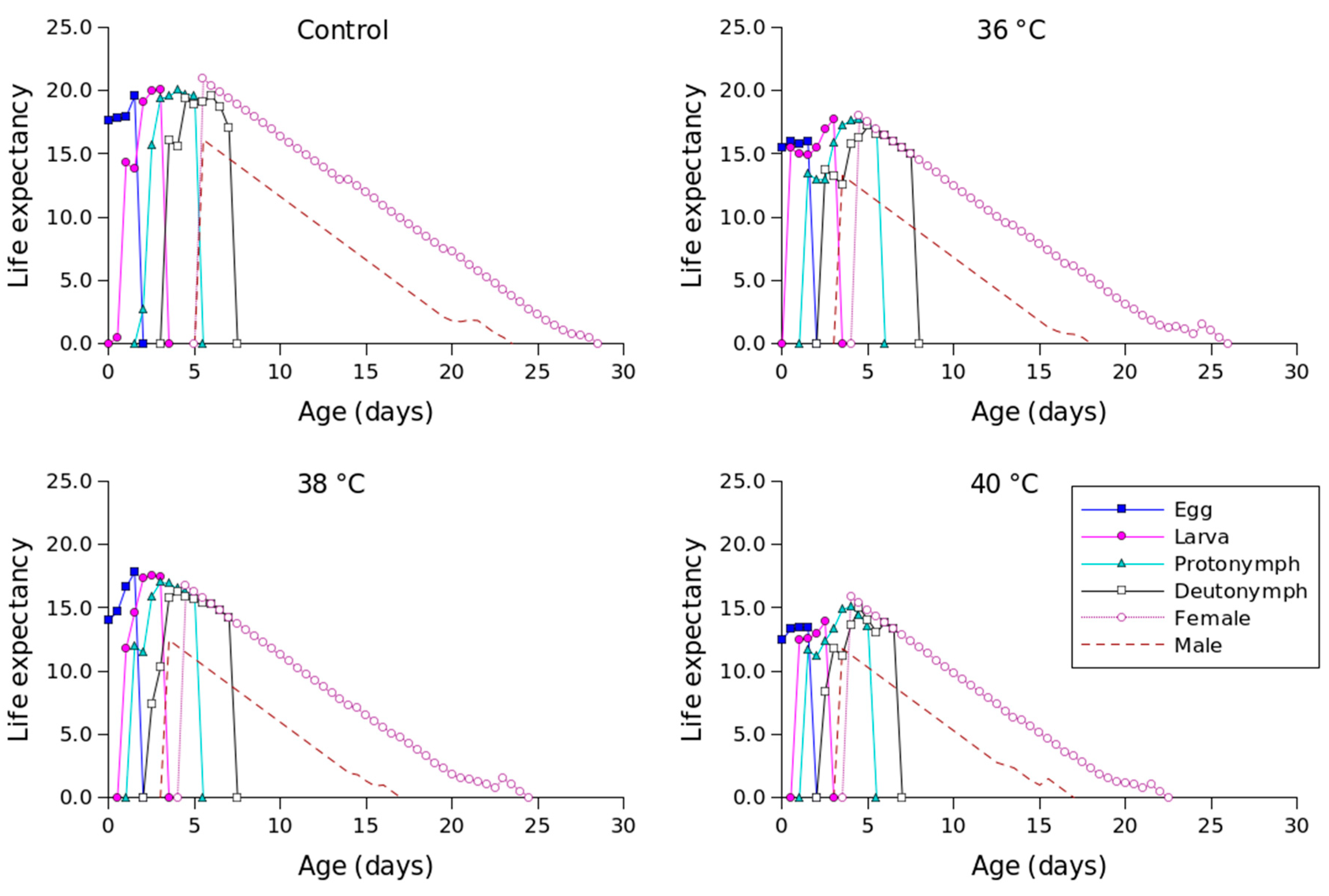
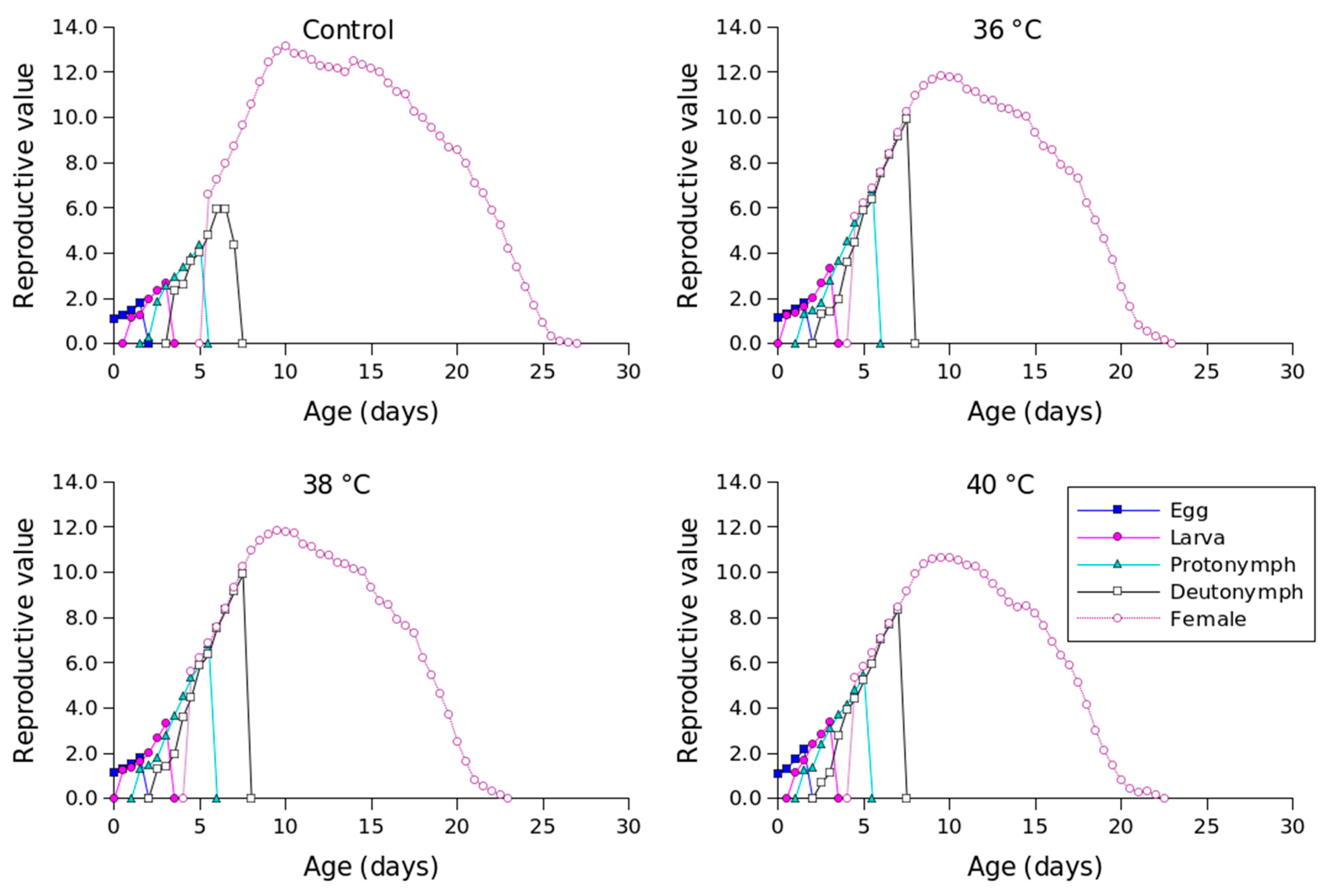
Figure 2 shows the lx, mx, lxmx, and fxj values for different temperature conditions. Compared to the other temperature conditions, 36 °C produced the longest oviposition period and the highest mx value. The highest female age-stage-specific fecundity values for the control (day 23), 36 °C (day 18), 38 °C (day 18), and 40 °C (day 16) were 1.23, 1.63, 1.41, and 1.04 eggs, respectively. The exj values of P. persimilis, which represent an individual’s expected remaining lifetime at age x and stage j, were predicted to differ across treatments. The control group had an age-stage life expectancy (exj) of 6 days. For female adults at 36, 38, and 40 °C, the values were 5, 5, and 4 days, respectively (Figure 3). The maximum reproductive values (vxj), which reflect the individual’s potential for reproduction based on its current age and stage, as well as the probability of survival to reproductive maturity, were observed at ages of 10 days (13.16 d−1), 10 days (11.85 d−1), 9 days (10.61 d−1), and 8 days (8.79 d−1) under control, 36 °C, 38 °C, and 40 °C conditions, respectively (Figure 4).
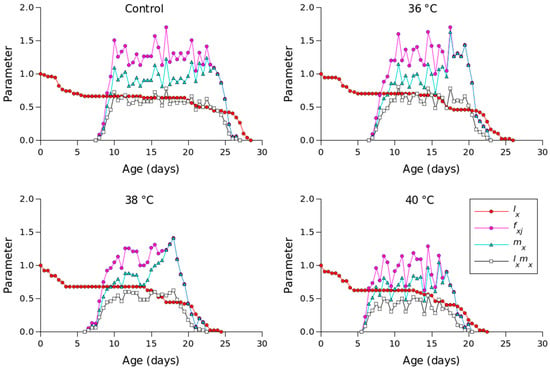
Figure 2.
Age-specific survival rate (lx), age-specific fecundity (mx), age-specific maternity (lxmx), and age-stage-specific fecundity (fxj) of Phytoseiulus persimilis exposed to different heat stress conditions.
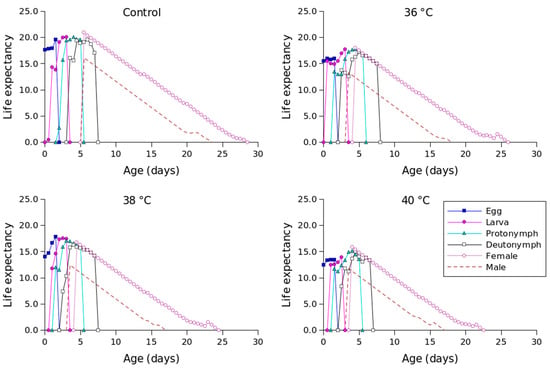
Figure 3.
Age-stage-specific life expectancy (exj) of Phytoseiulus persimilis exposed to different heat stress conditions.

Figure 4.
Age-stage-specific reproductive values (vxj) of Phytoseiulus persimilis exposed to different heat stress.
4. Discussion
Global warming is expected to affect both pests [41] and biological control agents [42], as well as their interactions [43]. Consequently, climate change will result in new challenges for the pest management of various crops [44,45]. In this work, short-term heat stress, lasting for four hours, was administered to both eggs and adult females of the species P. persimilis, to determine the effect of heat stress on the development time, reproduction and population parameters of P. persimilis, thereby assessing how the species responds to high temperatures. We observed significant changes in these parameters, indicating that short-term heat stress had a negative impact on P. persimilis. Furthermore, similar impacts have been found in previous research [9,11,20,23,37,46].
A short period of heat stress is unlikely to cause direct mortality but may modify population dynamics via impacting life history traits, e.g., fertility [20]. The short-term exposure of the eggs of the predatory mite Neoseiulus barkeri (Acari: Phytoseiidae) to temperatures of 38 °C and 40 °C reduced its fitness [9,47], and the daily fecundity of female adults exposed to 40 °C was significantly lower that of female adults exposed to 38 °C [23]. In our investigation, predatory mites were able to develop and lay eggs properly after being exposed to temperatures of 36–40 °C for 4 h during the egg stage, although their development, oviposition, and longevity were significantly affected. On the other hand, P. persimilis eggs exposed to extreme temperatures of 42 °C did not hatch. This is in accordance with previous findings regarding N. barkeri eggs, which did not hatch when exposed to 42 °C for 2, 4, and 6 h [20,24]. However, when the newly emerged female and male N. barkeri adults were exposed to 42 °C for 4 h, the egg hatchability of the progeny generations was not affected; however, the females exhibited a markedly extended pre-oviposition period, a shortened oviposition period, and reduced fecundity and longevity [20]. It, thus, seems that phytoseiid eggs are more sensitive to heat stress than adults; nevertheless, more studies are required to confirm this hypothesis.
The results of the present paper showed the shortest development time of the juvenile stage when eggs were exposed to 40 °C (5.3 days), compared to the control group (6.5 days). Although this difference might seem to be modest, such changes can accumulate and impact population growth rates and synchrony with prey populations. However, the heat stress conditions in our experiments simultaneously reduced the longevity and fecundity of P. persimilis. When taken together, these opposing effects illustrate a life-history trade-off: faster development may not translate into higher fitness under stressful temperatures, which is demonstrated by the estimated values of an intrinsic rate of increase. This shows that predatory mites may choose between survival and reproduction in an adverse environment [9,23]. The fact that P. persimilis has the lowest daily fecundity and the shortest life span under unfavorable conditions might be attributed to energy being prioritized for reproduction. High temperatures can speed mite growth and eventually reduce adult individuals’ size [11]. According to Yuan et al. [48], damage induced by heat stress frequently comes at the expense of longevity and reproduction. It has been found that Neoseiulus californicus McGregor (Acari: Phytoseiidae) has a considerably reduced total fecundity at 30 °C than at 20 °C and 25 °C, with the lowest daily reproduction rates found at 20 °C. As the temperature increases, the pre-oviposition period, oviposition period, and overall longevity of adults decrease [49]. Adult females of Mononychellus tanajoa (Bondar) (Acari: Tetranychidae) did not deposit eggs after being exposed to 42 °C for 4 h [50]. The fertility of N. barkeri reduced by more than 50% after 2 h of exposure to 40 °C, demonstrating that high temperatures over a short duration could inhibit population increase [23]. Temperature has a greater impact on reproduction than on development and survival. The longer an adult female is exposed to high temperatures, the more likely their fecundity will be influenced [20,51]. The injury induced by high temperatures at the egg stage can be corrected later in its development, but the damage to reproduction is indirect. On the other hand, the damage caused by high temperature at the adult stage is more direct, resulting in a decrease in fecundity. High temperatures are necessary to limit sustained growth in a population. The acceleration of development and the reduction in reproduction might be attributed to long-term high-temperature adaptation for improved survival [23].
Our study was conducted at a constant temperature of 25 °C, except for the 4 h of exposure to heat stress. In real conditions, the temperature naturally goes up and down and such fluctuations have huge impacts on arthropods’ development, survival, and reproduction, as demonstrated by many studies. For example, parasitoids reared under fluctuating temperatures but at high average temperatures exhibited longer development times and reduced longevity compared to those reared under constant temperature regimes with corresponding means [52]. In T. urticae, the effect of temperature fluctuation depended on the range: egg-to-female adult development was faster under fluctuating temperatures from 12.5 to 27.5 °C than under constant temperatures, whereas the opposite trend was observed for temperatures above 30 °C [53]. Prey consumption by two types of phytoseiid mites and their oviposition rates were also affected by an alternating temperature regimen [54]. Therefore, temperature fluctuations should be considered in future heat stress experiments.
Most researchers found that relative humidity (RH) was the most critical factor influencing the egg hatching rate, functional responses, and reproduction of phytoseiid mites [55,56]. As a result, Walzer et al. [57] employed egg hatchability as a drought resistance indicator, effectively applied it to the screening of resistant strains, and conducted appropriate verification experiments. Nonetheless, the leaf boundary layer’s relative humidity in the canopy microclimate was greater than that of the surrounding air. In contrast, some researchers thought that some phytoseiid species were only marginally affected by relative humidity or an environment with low humidity [58]. Therefore, the primary factor influencing the field population and biological control efficacy of these phytoseiid mites was a high-temperature environment. On the other hand, P. persimilis was reported to be very sensitive to low humidity and to not hatch when the RH was below 55% [59]. Because humidity usually decreases when the temperature increases [34], it should also be taken into account when the effects of heat waves are studied, at least in the field, where, contrary to greenhouse conditions, humidity cannot be controlled. A short decrease in humidity during the day, however, does not necessary harm P. persimilis; the results of a study by Le Hesran et al. [60] suggested that P. persimilis eggs are able to adapt to variable humidity conditions and that even short periods of high humidity can mitigate the effects of drought.
High temperatures can also cause increased locomotory and dispersal abilities [61], faster respiration and metabolic rates [62], or even mortality [63]. According to Chen et al. [64] and Colinet et al. [65], they may also cause oxidative stress and increase the expression of heat shock proteins. While temperatures above 41 °C are lethal for adult females of P. persimilis, at 40 °C, females ceased movement but could recover [31]. It is, therefore, likely that even a short exposure to such extremely high temperatures could have negative effects on the foraging behavior of P. persimilis. Li et al. [24] reported that, with an increasing temperature, the attack rate of N. barkeri decreased, while its handling time increased, indicating the negative effects of short-term heat stress on the predation ability of a phytoseiid mite.
Heat stress will ultimately limit population expansion and continuation; this is because, while these physiological or behavioral responses may help the organism to survive the adverse environment, they also consume energy at the expense of the potential reproduction or survival rate [20,37,66]. In accordance with the findings of earlier research [11], we discovered that P. persimilis could grow, mate, and reproduce after short exposures to temperatures of 36, 38, and 40 °C, but its fitness was much lower than those exposed to 25 °C. This suggests that high temperature is a significant factor limiting sustainable population growth. The lower reproductive rate and faster development rate were most likely caused by a long-term adaptation mechanism to high temperatures in order to increase the probability of survival.
5. Conclusions
This study underscores the vulnerability of P. persimilis to heat stress, emphasizing its detrimental effects on development and demographic parameters. Addressing these challenges through targeted research and adaptive management is essential to sustaining the efficacy of P. persimilis in biological control programs, particularly in the context of global climate change. However, because environmental complexity, many plants, and other natural qualities cannot be fully replicated in small areas, most similar research, including ours, conducted in a laboratory setting might not be entirely representative of outdoor areas. Therefore, more research is required to study the effects of heat waves on predatory mites under greenhouse or field conditions.
Based on the results of the present study, we can recommend that both commercial transportation and field releases need to avoid high temperature exposure, even for short periods. In order to maximize P. persimilis’s effectiveness in bio-controlling T. urticae in both indoor and outdoor environments, growers must abstain from releasing the predatory mite in temperatures higher than 36 °C. Future research on the effects of heat stress on phytoseiid mites should include fluctuating temperatures, various humidity conditions, and the measurement of predation efficacy, e.g., functional response.
Author Contributions
Conceptualization, H.P.; methodology, H.P.; investigation, H.P.; data curation, H.P.; writing—original draft preparation, H.P. and R.Z.; writing—review and editing, H.P. and R.Z.; visualization, H.P. and R.Z.; funding acquisition, H.P. and R.Z. All authors have read and agreed to the published version of the manuscript.
Funding
The publication of this research was funded by Biology Centre CAS, Institute of Entomology (RVO 60077344).
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors on request.
Acknowledgments
The authors express their gratitude for the support received from the Islamic Azad University, Takestan Branch, Iran, in carrying out this research.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| APOP | Adult pre-oviposition period |
| TPOP | Total pre-oviposition period |
| L:D | Light–dark |
| RH | Relative humidity |
Appendix A

Table A1.
Population parameters, their definitions, and equations used in their calculations.
Table A1.
Population parameters, their definitions, and equations used in their calculations.
| Parameter and Equation | Definition |
|---|---|
| Adult pre-ovipositional period (APOP) | The period between the adult emergence and first oviposition. |
| Total pre-ovipositional period (TPOP) | The period from birth to first oviposition. |
| Age-stage-specific survival rate (sxj) | The probability that a newly laid egg will survive to age x and stage j. nxj is the number of individuals to survive to age x and stage j, and n01 is the number of newborn offspring used at the beginning of the life table study [67]. |
| Age-stage-specific fecundity (fxj) | The mean number of offspring produced by individuals at age x and stage j [39]. |
| Age-specific fecundity (mx) | The mean number of offspring produced by individuals at age x [39]. |
| Net reproductive rate (R0) | The total number of offspring that an average individual (including females, males, and those that died in the immature stage) can produce during its lifetime [68]. |
| The intrinsic rate of increase (r) | The population growth rate as time approaches infinity and the population reaches a stable age-stage distribution (SASD). The population size will increase at the rate of er per time unit. It is calculated using the Euler–Lotka equation with age indexed from 0 [68]. |
| The finite rate of increase (λ) | The population growth rate as time approaches infinity and the population reaches a stable age-stage distribution. The population size will increase at the rate of λ per time unit [68]. |
| Mean generation time (T) | The period that a population requires to increase to R0-fold of its size as the time approaches infinity and the population settles down to a stable age-stage distribution [68]. |
| Age-stage-specific life expectancy (exj) | The duration for which an individual of age x and stage j is expected to survive. s’iy is the probability that an individual of age x and stage j will survive to age i and stage y and it is calculated by assuming sxj = 1 [69]. |
| Age-stage-specific reproductive value (vxj) | The reproductive value is described as the contribution of an individual of age x and stage j to the future population. For immature stages such as the larva stage, the reproductive value is not an actual reproductive output, but rather a projection of their expected future reproductive contribution if they survive to adulthood [40]. |
References
- Jerbi-Elayed, M.; Lebdi-Grissa, K.; Foray, V.; Muratori, F.; Hance, T. Using Multiple Traits to Estimate the Effects of Heat Shock on the Fitness of Aphidius colemani. Entomol. Exp. Appl. 2015, 155, 18–27. [Google Scholar] [CrossRef]
- Jin, H.-F.; Fu, B.-L.; Qiu, H.-Y.; Yang, S.-Y.; Zhou, S.-H.; Ma, X.-T.; Li, S.-G.; Zhang, F.-P.; Tang, L.-D.; Liu, K. Effects of High Temperature Stress on the Biology and Predatory Behavior of Menochilus sexmaculates. Chin. J. Appl. Entomol. 2020, 57, 700–707. [Google Scholar] [CrossRef]
- Huey, R.B.; Kingsolver, J.G. Climate Warming, Resource Availability, and the Metabolic Meltdown of Ectotherms. Am. Nat. 2019, 194, E140–E150. [Google Scholar] [CrossRef] [PubMed]
- Seneviratne, S.I.; Donat, M.G.; Mueller, B.; Alexander, L.V. No Pause in the Increase of Hot Temperature Extremes. Nat. Clim. Change 2014, 4, 161–163. [Google Scholar] [CrossRef]
- Basarin, B.; Lukić, T.; Matzarakis, A. Review of Biometeorology of Heatwaves and Warm Extremes in Europe. Atmosphere 2020, 11, 1276. [Google Scholar] [CrossRef]
- Barriopedro, D.; García-Herrera, R.; Ordóñez, C.; Miralles, D.G.; Salcedo-Sanz, S. Heat Waves: Physical Understanding and Scientific Challenges. Rev. Geophys. 2023, 61, e2022RG000780. [Google Scholar] [CrossRef]
- Singh, D.; Yadav, A.; Singh, S.; Yadav, R.K.; Sen, M.; Yadav, A.K.; Gehlot, T.; Mishra, A.; Pandey, V.; Singh, A.K. Heat Waves and Its Impact on Crop Production and Mitigation Techniques: A Review. Int. J. Enviorn. Clim. Change 2023, 13, 377–382. [Google Scholar] [CrossRef]
- Robinson, P.J. On the Definition of a Heat Wave. J. Appl. Meteor. 2001, 40, 762–775. [Google Scholar] [CrossRef]
- Hao, X.; Wang, E.; Yan, H.; Zhao, P.; Sheng, F.; Ren, Q.; Liu, M.; Zhang, B.; Xu, X. Effects of Heat Stresses on Fitness of Three Commercial Predatory Mites. Syst. Appl. Acarol. 2024, 29, 1673–1684. [Google Scholar] [CrossRef]
- Sentis, A.; Morisson, J.; Boukal, D.S. Thermal Acclimation Modulates the Impacts of Temperature and Enrichment on Trophic Interaction Strengths and Population Dynamics. Glob. Change Biol. 2015, 21, 3290–3298. [Google Scholar] [CrossRef]
- Tscholl, T.; Nachman, G.; Spangl, B.; Walzer, A. Heat Waves Affect Prey and Predators Differently via Developmental Plasticity: Who May Benefit Most from Global Warming? Pest Manag. Sci. 2022, 78, 1099–1108. [Google Scholar] [CrossRef] [PubMed]
- Acosta, N.C.; Zehr, L.N.; Snook, J.S.; Szendrei, Z.; Kalwajtys, M.; Wetzel, W.C. Heat Wave Impacts on Crop-pest Dynamics Are Dependent upon Insect Ontogeny and Plant Resistance. Ecosphere 2024, 15, e70028. [Google Scholar] [CrossRef]
- Harvey, J.A.; Heinen, R.; Gols, R.; Thakur, M.P. Climate Change-mediated Temperature Extremes and Insects: From Outbreaks to Breakdowns. Glob. Change Biol. 2020, 26, 6685–6701. [Google Scholar] [CrossRef]
- Lesk, C.; Rowhani, P.; Ramankutty, N. Influence of Extreme Weather Disasters on Global Crop Production. Nature 2016, 529, 84–87. [Google Scholar] [CrossRef]
- Hart, A.J.; Bale, J.S.; Tullett, A.G.; Worland, M.R.; Walters, K.F.A. Effects of Temperature on the Establishment Potential of the Predatory Mite Amblyseius californicus McGregor (Acari: Phytoseiidae) in the UK. J. Insect Physiol. 2002, 48, 593–599. [Google Scholar] [CrossRef] [PubMed]
- Ghazy, N.A.; Suzuki, T.; Amano, H.; Ohyama, K. Effects of Air Temperature and Water Vapor Pressure Deficit on Storage of the Predatory Mite Neoseiulus californicus (Acari: Phytoseiidae). Exp. Appl. Acarol. 2012, 58, 111–120. [Google Scholar] [CrossRef]
- Skirvin, D.J.; Fenlon, J.S. The Effect of Temperature on the Functional Response of Phytoseiulus persimilis (Acari: Phytoseiidae). Exp. Appl. Acarol. 2003, 31, 37–49. [Google Scholar] [CrossRef]
- Sabelis, M.W. Modelling the Predator-Prey Interaction at the Individual Level; Biological control of two-spotted spider mites using phytoseiid predators; PUDOC: Wageningen, The Netherlands, 1982; ISBN 978-90-220-0776-1. [Google Scholar]
- Zhao, Y.; Ducharne, A.; Sultan, B.; Braconnot, P.; Vautard, R. Estimating Heat Stress from Climate-Based Indicators: Present-Day Biases and Future Spreads in the CMIP5 Global Climate Model Ensemble. Environ. Res. Lett. 2015, 10, 084013. [Google Scholar] [CrossRef]
- Zhang, G.H.; Li, Y.Y.; Zhang, K.J.; Wang, J.J.; Liu, Y.Q.; Liu, H. Effects of Heat Stress on Copulation, Fecunditiy and Longevity in Newly-Emerged Adults of the Predatory Mite Neoseiulus barkeri (Acari: Phytoseiidae). Syst. Appl. Acarol. 2016, 21, 295–306. [Google Scholar] [CrossRef]
- Yao, F.; Zheng, Y.; Ding, X.; Zhao, J.; Lu, X.; Desneux, N.; He, Y.; Weng, Q. Effects of Heat Shock on Survival and Predation of an Important Whitefly Predator, Serangium japonicum. Entomol. Exp. Appl. 2019, 167, 476–489. [Google Scholar] [CrossRef]
- Zhao, C.; Chen, H.; Guo, J.; Zhou, Z. Effects of Fluctuating Thermal Regimes on Life History Parameters and Body Size of Ophraella communa. Insects 2022, 13, 821. [Google Scholar] [CrossRef]
- Li, W.Z.; Li, H.L.; Guo, Z.K.; Shang, S.Q. Effects of Short-Term Heat Stress on the Development and Reproduction of Predatory Mite Neoseiulus barkeri (Acari, Phytoseiidae). Syst. Appl. Acarol. 2021, 26, 713–723. [Google Scholar] [CrossRef]
- Li, W.; Zhu, T.; Li, H.; Shang, S. The Effects of Short-term Heat Stress on Functional Response of Neoseiulus barkeri to Tetranychus urticae. J. Appl. Entomol. 2022, 146, 310–318. [Google Scholar] [CrossRef]
- Walzer, A.; Formayer, H.; Tixier, M.-S. Evidence of Trans-Generational Developmental Modifications Induced by Simulated Heat Waves in an Arthropod. Sci. Rep. 2020, 10, 4098. [Google Scholar] [CrossRef] [PubMed]
- Mcmurtry, J.A.; Moraes, G.J.D.; Sourassou, N.F. Revision of the Lifestyles of Phytoseiid Mites (Acari: Phytoseiidae) and Implications for Biological Control Strategies. Syst. Appl. Acarol. 2013, 18, 297–320. [Google Scholar] [CrossRef]
- Pakyari, H.; Zemek, R. The Effect of Light Cycles on the Predation Characteristics of Phytoseiulus persimilis (Acari: Phytoseiidae) Feeding on Tetranychus urticae (Acari: Tetranychidae). Plants 2025, 14, 687. [Google Scholar] [CrossRef]
- Helle, W.; Sabelis, M.W. (Eds.) Spider Mites: Their Biology, Natural Enemies, and Control; World crop pests; Elsevier Science Pub. Co.: Amsterdam, The Netherlands; New York, NY, USA, 1985; ISBN 978-0-444-42372-6. [Google Scholar]
- Tixier, M.-S. Predatory Mites (Acari: Phytoseiidae) in Agro-Ecosystems and Conservation Biological Control: A Review and Explorative Approach for Forecasting Plant-Predatory Mite Interactions and Mite Dispersal. Front. Ecol. Evol. 2018, 6, 192. [Google Scholar] [CrossRef]
- Vangansbeke, D.; Audenaert, J.; Nguyen, D.T.; Verhoeven, R.; Gobin, B.; Tirry, L.; De Clercq, P. Diurnal Temperature Variations Affect Development of a Herbivorous Arthropod Pest and Its Predators. PLoS ONE 2015, 10, e0124898. [Google Scholar] [CrossRef] [PubMed]
- Coombs, M.R.; Bale, J.S. Comparison of Thermal Activity Thresholds of the Spider Mite Predators Phytoseiulus macropilis and Phytoseiulus persimilis (Acari: Phytoseiidae). Exp. Appl. Acarol. 2013, 59, 435–445. [Google Scholar] [CrossRef]
- Voroshilov, N.V. Breeding of Thermo-Resistant Lines of Phytoseiulus (Phytoseiulus persimilis A-H). Genetika 1979, 15, 70–76. [Google Scholar]
- Walzer, A.; Nachman, G.; Spangl, B.; Stijak, M.; Tscholl, T. Trans- and within-Generational Developmental Plasticity May Benefit the Prey but Not Its Predator during Heat Waves. Biology 2022, 11, 1123. [Google Scholar] [CrossRef] [PubMed]
- Tscholl, T.; Nachman, G.; Spangl, B.; Serve, H.C.; Walzer, A. Reproducing during Heat Waves: Influence of Juvenile and Adult Environment on Fecundity of a Pest Mite and Its Predator. Biology 2023, 12, 554. [Google Scholar] [CrossRef] [PubMed]
- Tscholl, T.; Nachman, G.; Spangl, B.; Scalmani, I.; Walzer, A. Parental Exposure to Heat Waves Improves Offspring Reproductive Investment in Tetranychus urticae (Acari: Tetranychidae), but Not in Its Predator, Phytoseiulus persimilis (Acari: Phytoseiidae). Ecol. Evol. 2023, 13, e10748. [Google Scholar] [CrossRef]
- Zhu, G.; Xue, M.; Luo, Y.; Ji, G.; Liu, F.; Zhao, H.; Sun, X. Effects of Short-Term Heat Shock and Physiological Responses to Heat Stress in Two Bradysia Adults, Bradysia odoriphaga and Bradysia difformis. Sci. Rep. 2017, 7, 13381. [Google Scholar] [CrossRef]
- Sun, Y.; Yang, M.; Ye, Z.; Zhu, J.; Fu, Y.; Chen, J.; Zhang, F. Effects of High-Temperature Stress on Biological Characteristics of Coccophagus japonicus Compere. Insects 2024, 15, 801. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Shen, X.-X.; Yu, X.-F.; Gou, J.-Y.; Huang, C.-Y.; Yang, M.-F. Effects of Short-Term Heat Stress on the Performance of the Predatory Gall Midge Aphidoletes aphidimyza (Diptera: Cecidomyiidae). Biocontrol Sci. Technol. 2023, 33, 190–203. [Google Scholar] [CrossRef]
- Chi, H.; Liu, H. Two New Methods for the Study of Insect Population Ecology. Bull. Inst. Zool. Acad. Sin. 1985, 24, 225–240. [Google Scholar]
- Huang, Y.; Chi, H. Age-stage, Two-sex Life Tables of Bactrocera cucurbitae (Coquillett) (Diptera: Tephritidae) with a Discussion on the Problem of Applying Female Age-specific Life Tables to Insect Populations. Insect Sci. 2012, 19, 263–273. [Google Scholar] [CrossRef]
- Lehmann, P.; Ammunét, T.; Barton, M.; Battisti, A.; Eigenbrode, S.D.; Jepsen, J.U.; Kalinkat, G.; Neuvonen, S.; Niemelä, P.; Terblanche, J.S.; et al. Complex Responses of Global Insect Pests to Climate Warming. Front. Ecol. Environ. 2020, 18, 141–150. [Google Scholar] [CrossRef]
- Aguilar-Fenollosa, E.; Jacas, J.A. Can We Forecast the Effects of Climate Change on Entomophagous Biological Control Agents? Pest. Manag. Sci. 2014, 70, 853–859. [Google Scholar] [CrossRef]
- Yao, F.-L.; You, M.-S. Impacts of Global Warming on the Interaction between Host Plants, Insect Pests and Their Natural Enemies. Chin. J. Appl. Entomol. 2012, 49, 563–572. [Google Scholar]
- Sharma, H.C.; Dhillon, M.K. Climate Change Effects on Arthropod Diversity and Its Implications for Pest Management and Sustainable Crop Production. In Agronomy Monographs; Hatfield, J.L., Sivakumar, M.V.K., Prueger, J.H., Eds.; American Society of Agronomy, Crop Science Society of America, and Soil Science Society of America, Inc.: Madison, WI, USA, 2018; pp. 595–619. ISBN 978-0-89118-358-7. [Google Scholar]
- Peñalver-Cruz, A.; Escudero-Colomar, L.A.; Alins, G.; Franco, M.E.E.; Bosch-Serra, D. Consequences of Global Warming on Apple Orchards in the Mediterranean Basin: Challenges of Insect Pest Management. Entomol. Gen. 2025, 45, 53–68. [Google Scholar] [CrossRef]
- Colinet, H.; Sinclair, B.J.; Vernon, P.; Renault, D. Insects in Fluctuating Thermal Environments. Annu. Rev. Entomol. 2015, 60, 123–140. [Google Scholar] [CrossRef] [PubMed]
- Jafari, S.; Fathipour, Y.; Faraji, F. Temperature-dependent Development of Neoseiulus barkeri (Acari: Phytoseiidae) on Tetranychus urticae (Acari: Tetranychidae) at Seven Constant Temperatures. Insect Sci. 2012, 19, 220–228. [Google Scholar] [CrossRef]
- Yuan, X.; Wang, X.; Wang, J.; Zhao, Y. Effects of brief exposure to high temperature on Neoseiulus californicus. Ying Yong Sheng Tai Xue Bao 2015, 26, 853–858. [Google Scholar]
- Xu, Y.; Zhang, K.; Zhang, Z.-Q. Development, Survival, and Reproduction of Phytoseiulus persimilis Athias-Henriot (Acari: Phytoseiidae) Feeding on Fresh versus Frozen Eggs of Tetranychus urticae Koch (Acari: Tetranychidae). Acarologia 2023, 63, 24–30. [Google Scholar] [CrossRef]
- Lu, F.; Chen, Q.; Chen, Z.; Lu, H.; Xu, X.; Jing, F. Effects of Heat Stress on Development, Reproduction and Activities of Protective Enzymes in Mononychellus mcgregori. Exp. Appl. Acarol. 2014, 63, 267–284. [Google Scholar] [CrossRef] [PubMed]
- Ma, G.; Rudolf, V.H.W.; Ma, C. Extreme Temperature Events Alter Demographic Rates, Relative Fitness, and Community Structure. Global Change Biol. 2015, 21, 1794–1808. [Google Scholar] [CrossRef]
- McCalla, K.A.; Keçeci, M.; Milosavljević, I.; Ratkowsky, D.A.; Hoddle, M.S. The Influence of Temperature Variation on Life History Parameters and Thermal Performance Curves of Tamarixia radiata (Hymenoptera: Eulophidae), a Parasitoid of the Asian Citrus Psyllid (Hemiptera: Liviidae). J. Econ. Entomol. 2019, 112, 1560–1574. [Google Scholar] [CrossRef]
- Bayu, M.S.Y.I.; Ullah, M.S.; Takano, Y.; Gotoh, T. Impact of Constant versus Fluctuating Temperatures on the Development and Life History Parameters of Tetranychus urticae (Acari: Tetranychidae). Exp. Appl. Acarol. 2017, 72, 205–227. [Google Scholar] [CrossRef]
- Vangansbeke, D.; Nguyen, D.T.; Audenaert, J.; Verhoeven, R.; Gobin, B.; Tirry, L.; De Clercq, P. Prey Consumption by Phytoseiid Spider Mite Predators as Affected by Diurnal Temperature Variations. BioControl 2015, 60, 595–603. [Google Scholar] [CrossRef]
- Doker, I.; Kazak, C.; Karut, K. Functional Response and Fecundity of a Native Neoseiulus californicus Population to Tetranychus urticae (Acari: Phytoseiidae, Tetranychidae) at Extreme Humidity Conditions. Syst. Appl. Acarol. 2016, 21, 1463. [Google Scholar] [CrossRef]
- Ferrero, M.; Gigot, C.; Tixier, M.-S.; Van Houten, Y.M.; Kreiter, S. Egg Hatching Response to a Range of Air Humidities for Six Species of Predatory Mites. Entomol. Exp. Appl. 2010, 135, 237–244. [Google Scholar] [CrossRef]
- Walzer, A.; Castagnoli, M.; Simoni, S.; Liguori, M.; Palevsky, E.; Schausberger, P. Intraspecific Variation in Humidity Susceptibility of the Predatory Mite Neoseiulus californicus: Survival, Development and Reproduction. Biol. Control 2007, 41, 42–52. [Google Scholar] [CrossRef]
- Zundel, C.; Hanna, R.; Scheidegger, U.; Nagel, P. Living at the Threshold: Where Does the Neotropical Phytoseiid Mite Typhlodromalus aripo Survive the Dry Season? Exp. Appl. Acarol. 2007, 41, 11–26. [Google Scholar] [CrossRef]
- De Courcy Williams, M.E.; Kravar-garde, L.; Fenlon, J.S.; Sunderland, K.D. Phytoseiid Mites in Protected Crops: The Effect of Humidity and Food Availability on Egg Hatch and Adult Life Span of Iphiseius degenerans, Neoseiulus cucumeris, N. californicus and Phytoseiulus persimilis (Acari: Phytoseiidae). Exp. Appl. Acarol. 2004, 32, 1–13. [Google Scholar] [CrossRef]
- Le Hesran, S.; Groot, T.; Knapp, M.; Bukovinszky, T.; Forestier, T.; Dicke, M. Phenotypic Variation in Egg Survival in the Predatory Mite Phytoseiulus persimilis under Dry Conditions. Biol. Control 2019, 130, 88–94. [Google Scholar] [CrossRef]
- Kjærsgaard, A.; Blanckenhorn, W.U.; Pertoldi, C.; Loeschcke, V.; Kaufmann, C.; Hald, B.; Pagès, N.; Bahrndorff, S. Plasticity in Behavioural Responses and Resistance to Temperature Stress in Musca domestica. Animal Beh. 2015, 99, 123–130. [Google Scholar] [CrossRef]
- Dingha, B.N.; Appel, A.G.; Vogt, J.T. Effects of Temperature on the Metabolic Rates of Insecticide Resistant and Susceptible German Cockroaches, Blattella germanica (L.) (Dictyoptera: Blattellidae). Midsouth Entomol. 2009, 2, 17–27. [Google Scholar]
- Pakyari, H.; Fathipour, Y.; Enkegaard, A. Effect of Temperature on Life Table Parameters of Predatory Thrips Scolothrips longicornis (Thysanoptera: Thripidae) Fed on Twospotted Spider Mites (Acari: Tetranychidae). J. Econ. Entom. 2011, 104, 799–805. [Google Scholar] [CrossRef]
- Chen, W.; Li, D.; Zhang, M.; Zhao, Y.; Wu, W.; Zhang, G. Cloning and Differential Expression of Five Heat Shock Protein Genes Associated with Thermal Stress and Development in the Polyphagous Predatory Mite Neoseiulus cucumeris (Acari: Phytoseiidae). Exp. Appl. Acarol. 2015, 67, 65–85. [Google Scholar] [CrossRef] [PubMed]
- Colinet, H.; Overgaard, J.; Com, E.; Sørensen, J.G. Proteomic Profiling of Thermal Acclimation in Drosophila melanogaster. Insect Biochem. Mol. Biol. 2013, 43, 352–365. [Google Scholar] [CrossRef] [PubMed]
- Xia, B.; Zou, Z.; Li, P.; Lin, P. Effect of Temperature on Development and Reproduction of Neoseiulus barkeri (Acari: Phytoseiidae) Fed on Aleuroglyphus ovatus. Exp. Appl. Acarol. 2012, 56, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Chi, H. Life-Table Analysis Incorporating Both Sexes and Variable Development Rates among Individuals. Environ. Entomol. 1988, 17, 26–34. [Google Scholar] [CrossRef]
- Goodman, D. Optimal Life Histories, Optimal Notation, and the Value of Reproductive Value. Am. Nat. 1982, 119, 803–823. [Google Scholar] [CrossRef]
- Chi, H.; Su, H.-Y. Age-Stage, Two-Sex Life Tables of Aphidius gifuensis (Ashmead) (Hymenoptera: Braconidae) and Its Host Myzus persicae (Sulzer) (Homoptera: Aphididae) with Mathematical Proof of the Relationship between Female Fecundity and the Net Reproductive Rate. Environ. Entomol. 2006, 35, 10–21. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).