Functional Study of Opsin Genes in Pardosa astrigera (Araneae: Lycosidae)
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
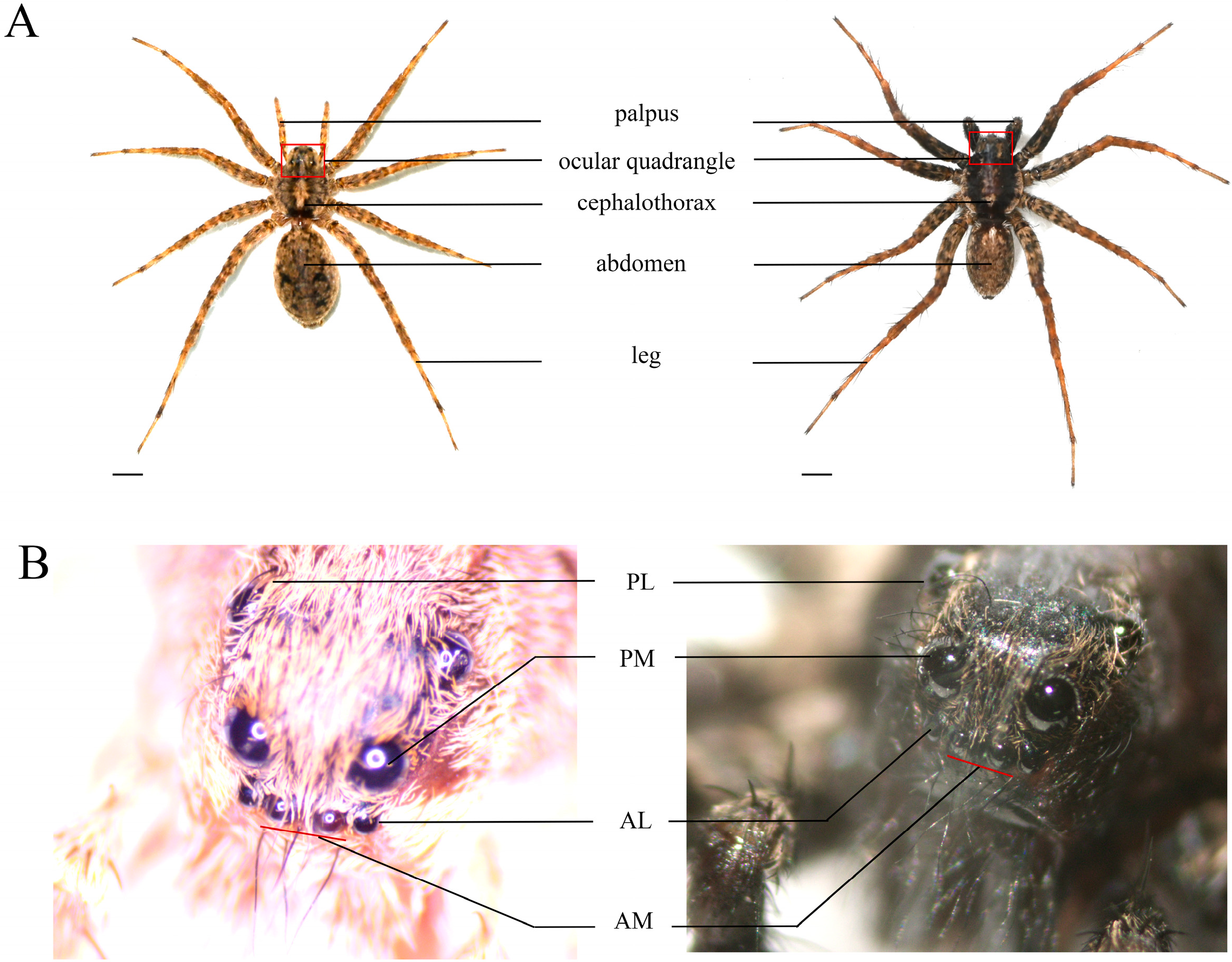
2.1. Spider Collection and Breeding
2.2. Identification, Cloning, and Analysis of Opsin Gene
2.3. Different Tissue, Development Stage and Light-Induced Expression of Opsin Genes
2.4. RNAi-Mediated Knockdown of Opsin Gene Expression
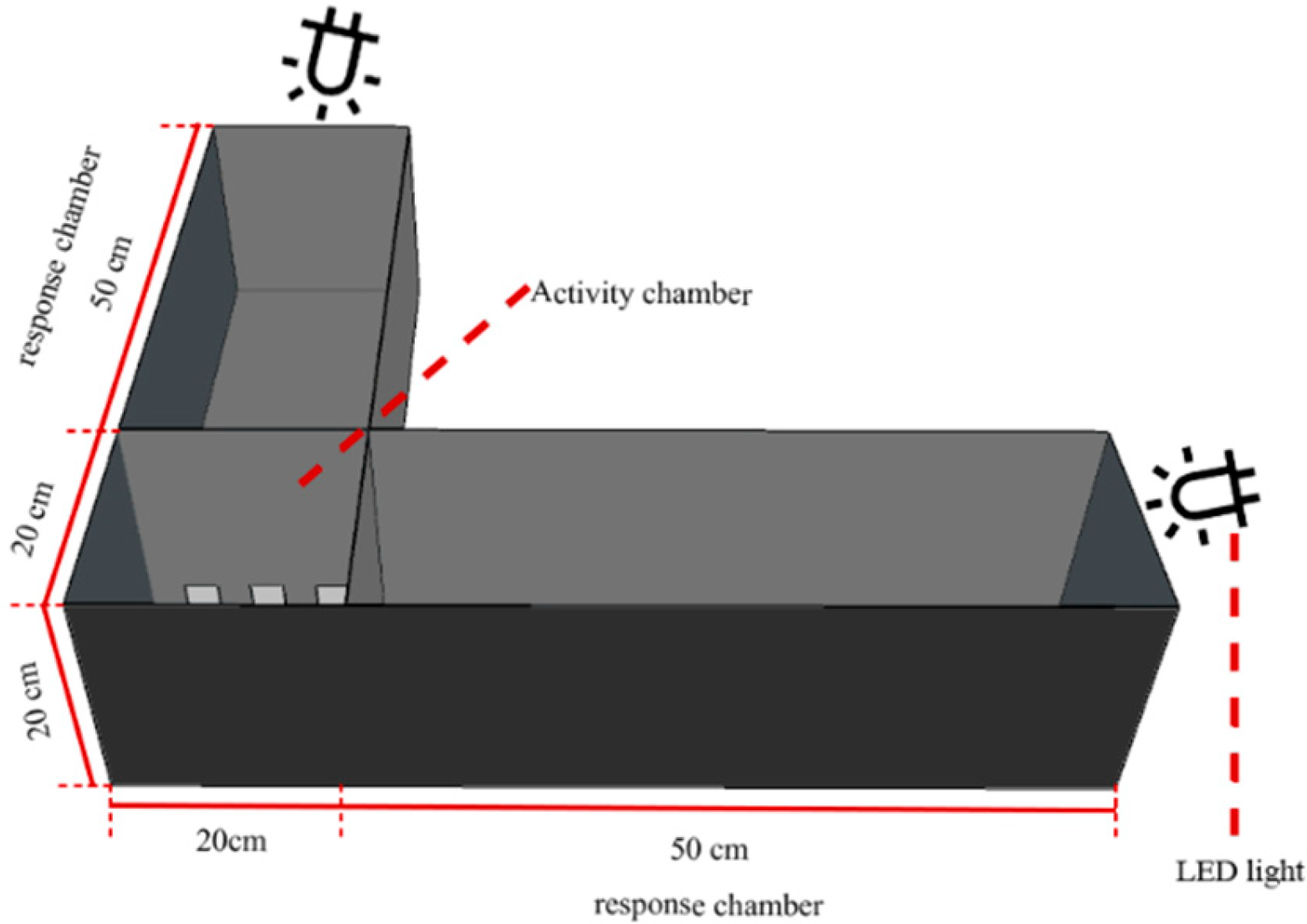
2.5. Behavioral Verification
2.6. Data Analysis
3. Results
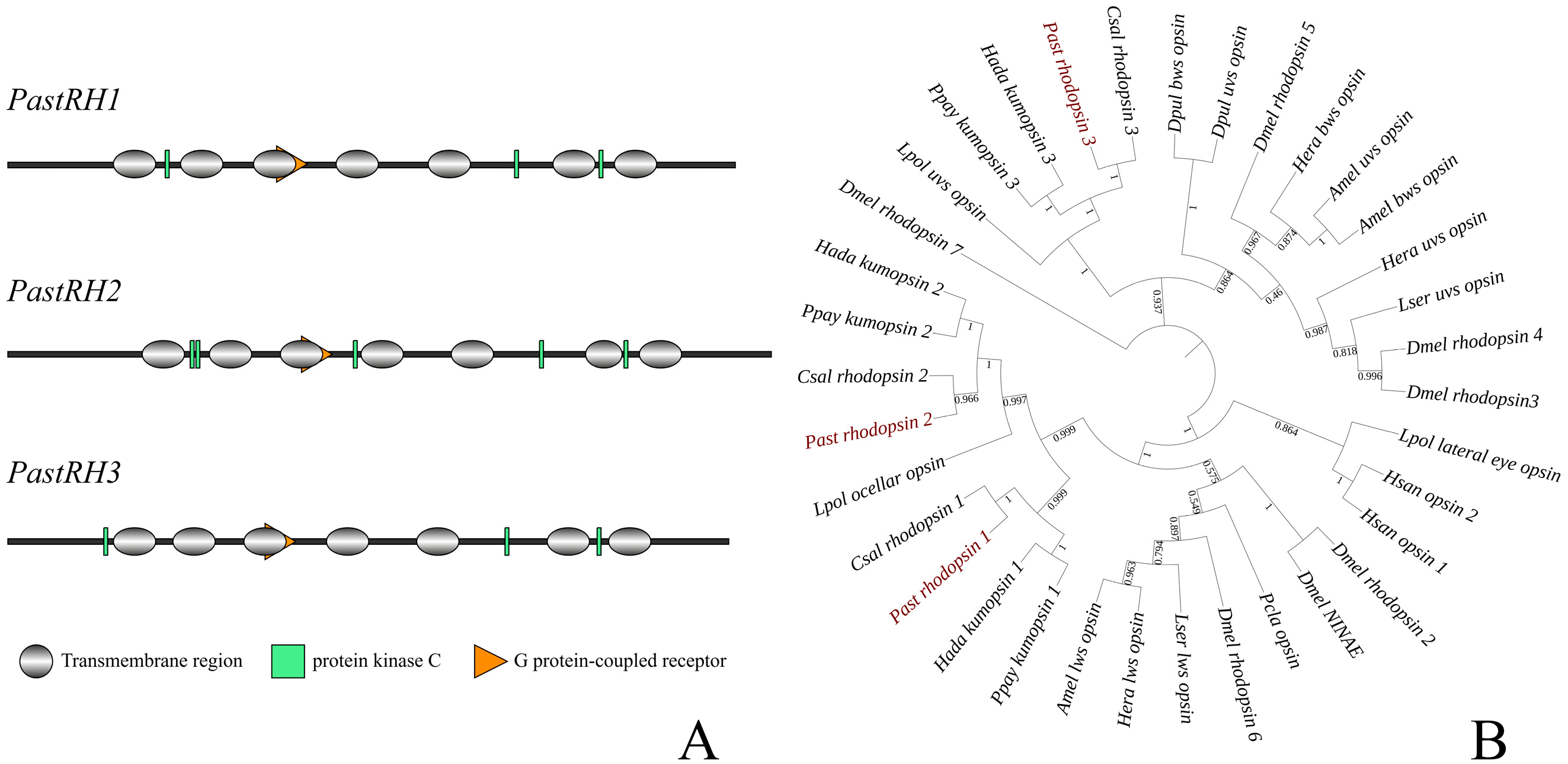
3.1. Identification of Opsin-Related Genes
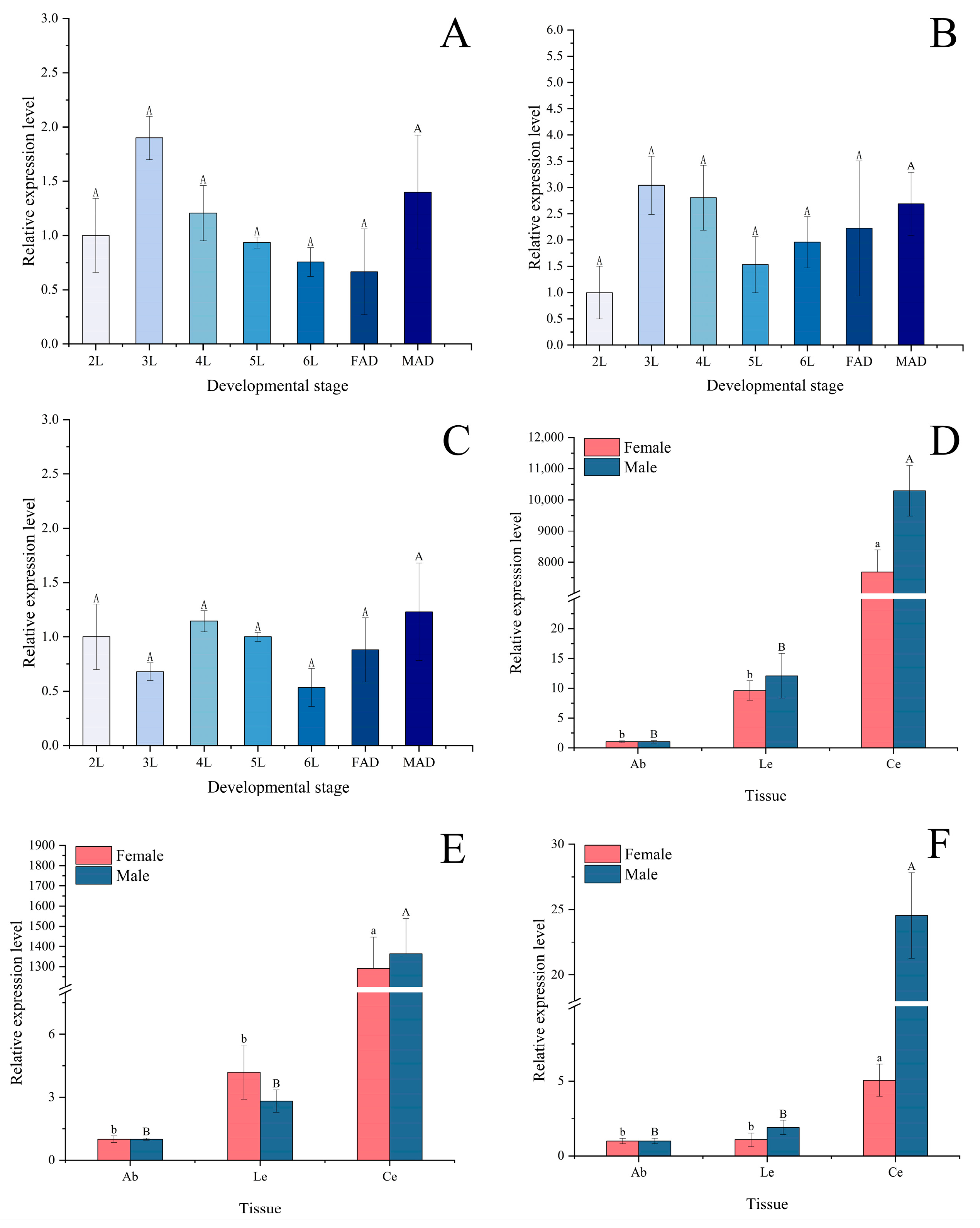
3.2. Expression Profiles Across Tissues and Developmental Stages
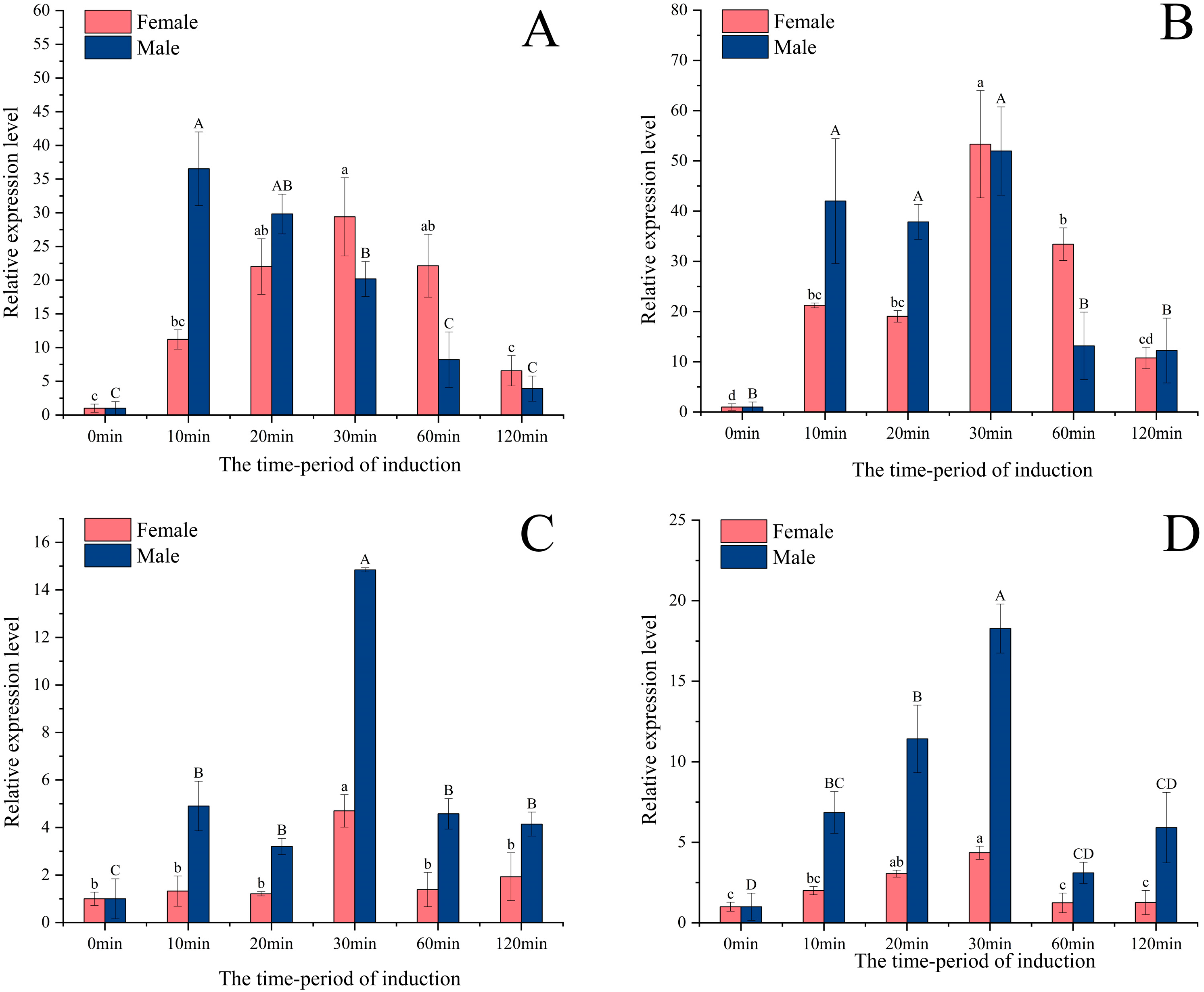
3.3. Expression Induced by Different Light Wavelengths
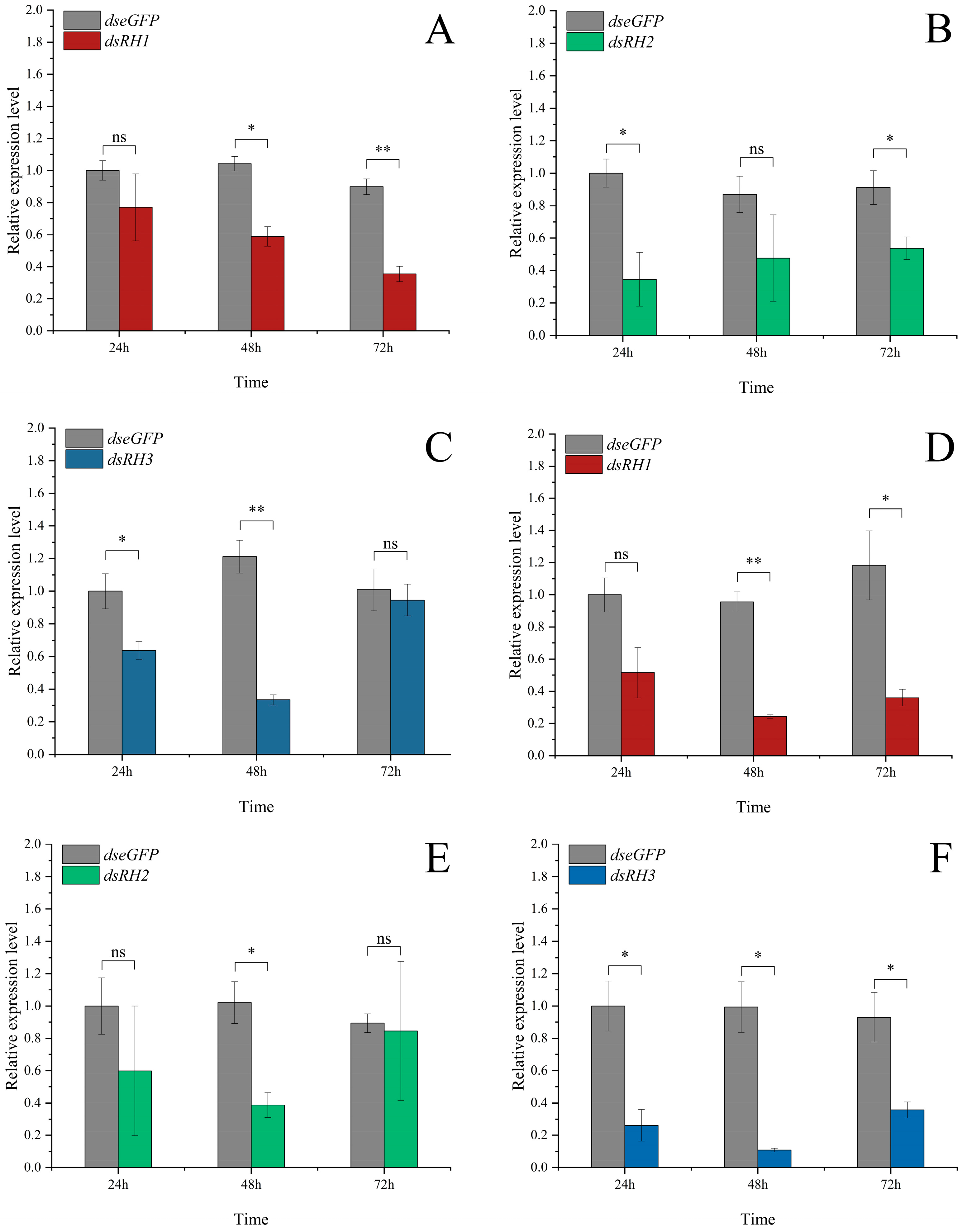
3.4. Functional Validation of Opsin Genes via RNAi
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Spider Catalog. World Spider Catalog. Version 25.0. Natural History Museum Bern. 2025. Available online: http://wsc.nmbe.ch (accessed on 21 March 2025).
- Nyffeler, M.; Birkhofer, K. An estimated 400−800 million tons of prey are annually killed by the global spider community. Sci. Nat. 2017, 104, 30. [Google Scholar] [CrossRef] [PubMed]
- Birkhofer, K.; Djoudi, E.A.; Schnerch, B.; Michalko, R. Climatic conditions and functional traits affect spider diets in agricultural and non-agricultural habitats worldwide. Ecography 2022, 2022, e06090. [Google Scholar] [CrossRef]
- Petráková, L.; Michalko, R.; Loverre, P.; Sentenská, L.; Korenko, S.; Pekar, S. Intraguild predation among spiders and their effect on the pear psylla during winter. Agric. Ecosyst. Environ. 2016, 233, 67–74. [Google Scholar] [CrossRef]
- Wyss, E.; Niggli, U.; Nentwig, W. The impact of spiders on aphid populations in a strip-managed apple orchard. J. Appl. Entomol. 1995, 119, 473–478. [Google Scholar] [CrossRef]
- Murata, K.; Tanaka, K. Spatial interaction between spiders and prey insects: Horizontal and vertical distribution in a paddy field. Acta Arachnol. 2004, 53, 75–86. [Google Scholar] [CrossRef]
- Matevski, D.; Sagolla, V.; Beule, L.; Schuldt, A. Temperate alley-cropping agroforestry improves pest control potential by promoting spider abundance and functional diversity. J. Appl. Ecol. 2024, 61, 3079–3091. [Google Scholar] [CrossRef]
- Cotes, B.; González, M.; Benítez, E.; De Mas, E.; Clemente-Orta, G.; Campos, M.; Rodríguez, E. Spider communities and biological control in native habitats surrounding greenhouses. Insects 2018, 9, 33. [Google Scholar] [CrossRef]
- Chong, H.; Zhu, Y.; Lai, Q.; Wu, S.; Jiang, T.; Zhang, D.; Xiao, H. Response of spider and epigaeic beetle assemblages to overwinter planting regimes and surrounding landscape compositions. Insects 2023, 14, 951. [Google Scholar] [CrossRef]
- Raška, J.; Štys, P.; Exnerová, A. Perception of olfactory aposematic signals by jumping spiders. Ethology 2018, 124, 773–776. [Google Scholar] [CrossRef]
- Liu, J.; Sun, L.; Fu, D.; Zhu, J.; Liu, M.; Xiao, F.; Xiao, R. Herbivore-induced rice volatiles attract and affect the predation ability of the wolf spiders, Pirata subpiraticus and Pardosa pseudoannulata. Insects 2022, 13, 90. [Google Scholar] [CrossRef]
- Barth, F.G. How to catch the wind: Spider hairs specialized for sensing the movement of air. Die Naturwissenschaften 2000, 87, 51. [Google Scholar] [CrossRef] [PubMed]
- Shamble, P.S.; Menda, G.; Golden, J.R.; Nitzany, E.I.; Walden, K.; Beatus, T.; Elias, D.O.; Cohen, I.; Miles, R.N.; Hoy, R.R. Airborne acoustic perception by a jumping spider. Curr. Biol. 2016, 26, 2913–2920. [Google Scholar] [CrossRef] [PubMed]
- Mortimer, B. A spider’s vibration landscape: Adaptations to promote vibrational information transfer in orb webs. Integr. Comp. Biol. 2019, 59, 1636–1645. [Google Scholar] [CrossRef] [PubMed]
- Ganske, A.S.; Uhl, G. The sensory equipment of a spider–A morphological survey of different types of sensillum in both sexes of Argiope bruennichi (Araneae, Araneidae). Arthropod Struct. Dev. 2018, 47, 144–161. [Google Scholar] [CrossRef]
- Lin, S.W.; Lopardo, L.; Uhl, G. Diversification through gustatory courtship: An X-ray micro-computed tomography study on dwarf spiders. Front. Zool. 2021, 18, 1–33. [Google Scholar] [CrossRef]
- Winsor, A.M.; Remage-Healey, L.; Hoy, R.R.; Jakob, E.M. Visual attention and processing in jumping spiders. Trends Neurosci. 2024, 47, 6–8. [Google Scholar] [CrossRef]
- De Voe, R.D. Ultraviolet and green receptors in principal eyes of jumping spiders. J. Gen. Physiol. 1975, 66, 193–207. [Google Scholar] [CrossRef]
- Barth, F.G.; Nakagawa, T.; Eguchi, E. Vision in the ctenid spider Cupiennius salei: Spectral range and absolute sensitivity. J. Exp. Biol. 1993, 181, 63–80. [Google Scholar] [CrossRef]
- Walla, P.; Barth, F.G.; Eguchi, E. Spectral sensitivity of single photoreceptor cells in the eyes of the ctenid spicier Cupiennius salei keys. Zool. Sci. 1996, 13, 199–202. [Google Scholar] [CrossRef]
- Koyanagi, M.; Nagata, T.; Katoh, K.; Yamashita, S.; Tokunaga, F. Molecular evolution of arthropod color vision deduced from multiple opsin genes of jumping spiders. J. Mol. Evol. 2008, 66, 130–137. [Google Scholar] [CrossRef]
- Zopf, L.M.; Schmid, A.; Fredman, D.; Eriksson, B.J. Spectral sensitivity of the ctenid spider Cupiennius salei. J. Exp. Biol. 2013, 216, 4103–4108. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, T.; Yamashita, S. Learning and discrimination of colored papers in jumping spiders (Araneae, Salticidae). J. Comp. Physiol. A 2000, 186, 897–901. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.; Wang, B.; Zheng, A.N.; Yan, H.M. Vision Distance and Color Selection of Pardosa pseudoannulata. Chin. J. Zool. 2014, 49, 772–777. [Google Scholar]
- Chen, Z. The Phototactic Characteristics of Insects and the Ecological Impact of the Application of Sticky Color Boards for Pest Control. Ph.D. Dissertation, Yunnan University, Kunming, China, 2016. [Google Scholar]
- Saitou, N.; Nei, M. The neighbor-joining method: A new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 1987, 4, 406–425. [Google Scholar]
- Felsenstein, J. Confidence limits on phylogenies: An approach using the bootstrap. Evolution 1985, 39, 783–791. [Google Scholar] [CrossRef]
- Jones, D.T.; Taylor, W.R.; Thornton, J.M. The rapid generation of mutation data matrices from protein sequences. Comput. Appl. Biosci. 1992, 8, 275–282. [Google Scholar] [CrossRef]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA 11: Molecular Evolutionary Genetics Analysis Version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, Z.X.; Lei, C.L.; Zhu, F. Effects of wavelength, density, and light intensity on the phototactic behavior of the armyworm (Mythimna separata). J. Plant Protection 2021, 48, 855–861. (In Chinese) [Google Scholar]
- Salcedo, E.; Zheng, L.; Phistry, M.; Bagg, E.E.; Britt, S.G. Molecular basis for ultraviolet vision in invertebrates. J. Neurosci. 2003, 23, 10873–10878. [Google Scholar] [CrossRef]
- Friedrich, M. Parallel losses of blue opsin correlate with compensatory neofunctionalization of UV-opsin gene duplicates in aphids and planthoppers. Insects 2023, 14, 774. [Google Scholar] [CrossRef]
- Kirchner, S.M.; Döring, T.F.; Saucke, H. Evidence for Trichromacy in the Green Peach Aphid, Myzus Persicae (Sulz.) (Hemiptera:Aphididae). J. Insect Physiol. 2005, 51, 1255–1260. [Google Scholar] [CrossRef] [PubMed]
- Ning, H.; Wang, C.; Hao, W.; Jin, H.; Li, F.; Wu, S. Eye structure, phototactic behavior, and opsin gene expression patterns of Frankliniella intonsa. J. Plant Prot. 2024, 51, 1403–1412. [Google Scholar]
- Xu, M. Study on the Eye Structure and Visual Gene Expression Characteristics of Ectropis grisescens. Master’s Thesis, Henan Institute of Science and Technology, Xinxiang, China, 2022. [Google Scholar]
- Huang, M. Cloning and Functional Study of Opsin Genes in Bactrocera Cucurbitae. Master’s Thesis, Guizhou University, Guiyang, China, 2023. [Google Scholar]
- Xue, Y.; Peng, W.; Liu, F.; Wang, Y. Effects of different light conditions on opsin gene expression in Mythimna separata. Huazhong Insect Stud. 2018, 14, 282–287. [Google Scholar]
- Liu, F.; Mao, Y.; Li, Q.S.; Xue, Y.Y.; Peng, W.; Wang, Y. Cloning of the opsin gene in Tribolium castaneum and its response to different light sources. Huazhong Insect Stud. 2018, 14, 291–299. [Google Scholar]
- Liu, S.; Wang, Y.; Tang, J.; Zhang, Y.; Fu, X.; Liang, G. Effects of different wavelengths of light on phototactic behavior and opsin expression in Spodoptera frugiperda adults. Plant Prot. 2023, 49, 176–183. [Google Scholar]
- Wang, B.; Xiao, J.H.; Bian, S.N.; Niu, L.M.; Murphy, R.W.; Huang, D.W. Evolution and expression plasticity of opsin genes in a fig pollinator, Ceratosolen solmsi. PLoS ONE 2013, 8, e53907. [Google Scholar] [CrossRef]
- Sasagawa, H.; Narita, R.; Kitagawa, Y.; Kadowaki, T. The expression of genes encoding visual components is regulated by a circadian clock, light environment and age in the honeybee (Apis mellifera). Eur. J. Neurosci. 2003, 17, 963–970. [Google Scholar] [CrossRef]
- Yan, S.; Zhu, J.; Zhu, W.; Zhang, X.; Li, Z.; Liu, X.; Zhang, Q. The expression of three opsin genes from the compound eye of Helicoverpa armigera (Lepidoptera: Noctuidae) is regulated by a circadian clock, light conditions and nutritional status. PLoS ONE 2014, 9, e111683. [Google Scholar] [CrossRef]
- Collantes-Alegre, J.M.; Mattenberger, F.; Barberà, M.; Martínez-Torres, D. Characterisation, analysis of expression and localisation of the opsin gene repertoire from the perspective of photoperiodism in the aphid Acyrthosiphon pisum. J. Insect Physiol. 2018, 104, 48–59. [Google Scholar] [CrossRef]
- Persons, M.H.; Uetz, G.W. Age and sex-based differences in the use of prey sensory cues in wolf spiders (Araneae: Lycosidae). J. Insect Behav. 1999, 12, 723–736. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhai, S.; Ren, B.; Zhang, X.; Shen, F.; Ma, M.; Li, X.; Li, R. Functional Study of Opsin Genes in Pardosa astrigera (Araneae: Lycosidae). Insects 2025, 16, 595. https://doi.org/10.3390/insects16060595
Zhai S, Ren B, Zhang X, Shen F, Ma M, Li X, Li R. Functional Study of Opsin Genes in Pardosa astrigera (Araneae: Lycosidae). Insects. 2025; 16(6):595. https://doi.org/10.3390/insects16060595
Chicago/Turabian StyleZhai, Shuxin, Boqi Ren, Xinghua Zhang, Fangyu Shen, Min Ma, Xinmin Li, and Rui Li. 2025. "Functional Study of Opsin Genes in Pardosa astrigera (Araneae: Lycosidae)" Insects 16, no. 6: 595. https://doi.org/10.3390/insects16060595
APA StyleZhai, S., Ren, B., Zhang, X., Shen, F., Ma, M., Li, X., & Li, R. (2025). Functional Study of Opsin Genes in Pardosa astrigera (Araneae: Lycosidae). Insects, 16(6), 595. https://doi.org/10.3390/insects16060595




