Effects of Periodic Short-Term Heat Stress on Biological Characteristics and Gut Bacteria of Spodoptera frugiperda
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Insect Rearing
2.2. Experimental Method
2.2.1. Effects of Periodic Short-Term Heat Stress on the Biological Characteristics of Spodoptera frugiperda
2.2.2. Gut Sample Collection from Spodoptera frugiperda Exposed to Periodic Short-Term Heat Stress
2.2.3. Gut Sample DNA Extraction, PCR Amplification, and Sequencing Library Construction
2.3. Data Processing and Statistical Analysis
3. Results
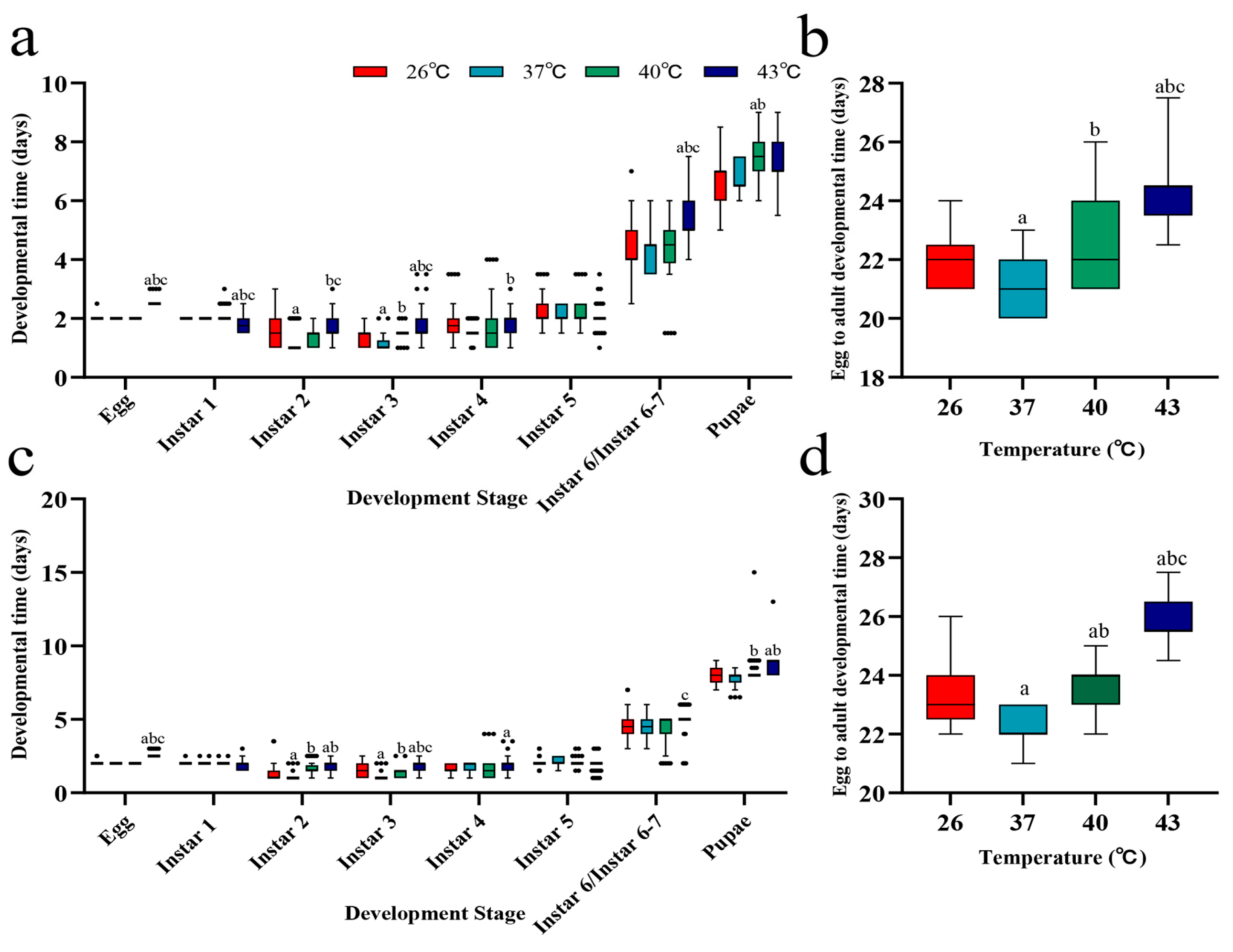
3.1. Effects of Periodic Short-Term Heat Stress on the Developmental Duration of Spodoptera frugiperda
3.2. Effects of Periodic Short-Term Heat Stress on Larval Survival Rate, Pupation Rate, and Eclosion Rate of Spodoptera frugiperda
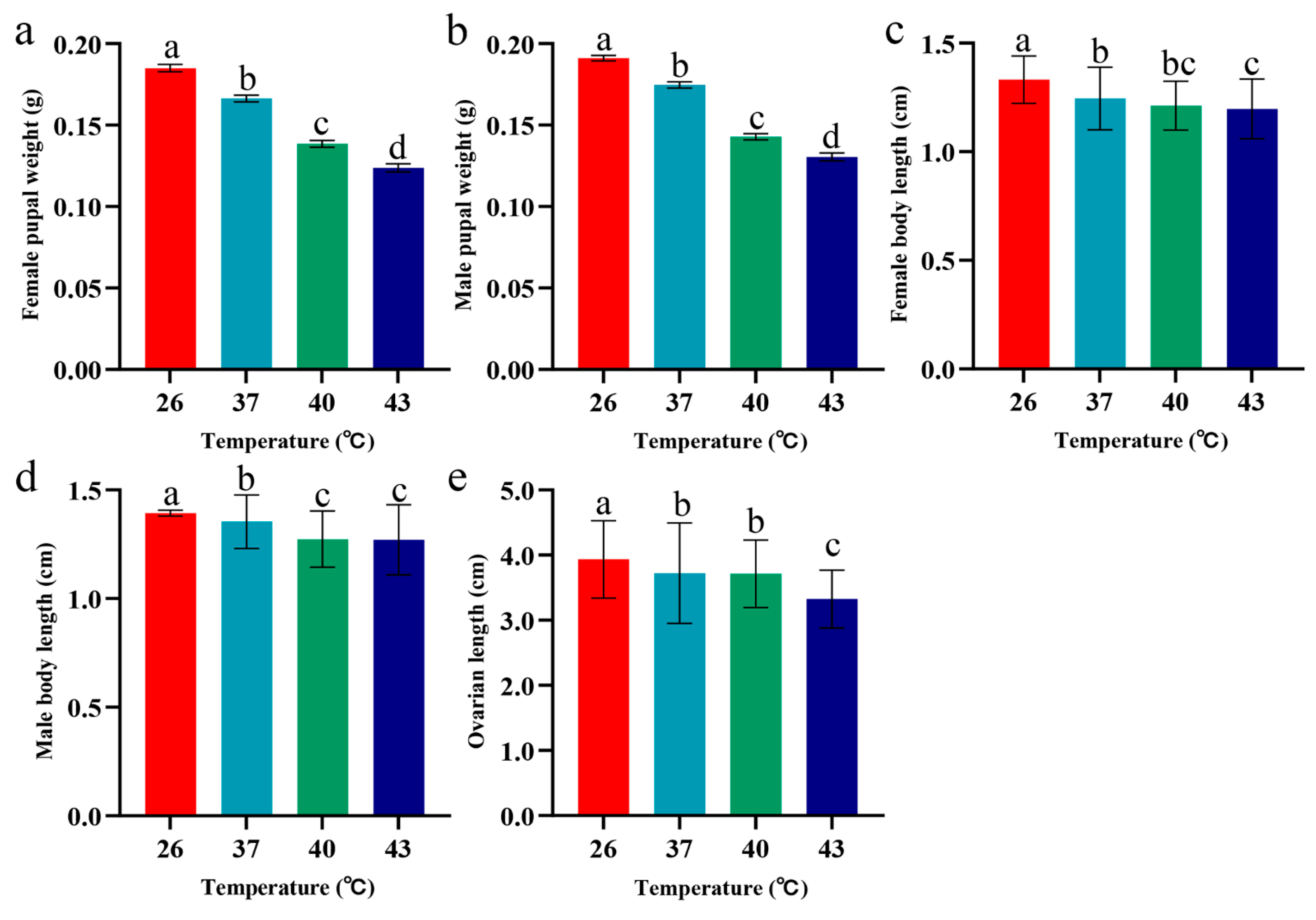
3.3. Effects of Periodic Short-Term Heat Stress on Pupal Weight, Adult Body Length, and Ovarian Length of Spodoptera frugiperda
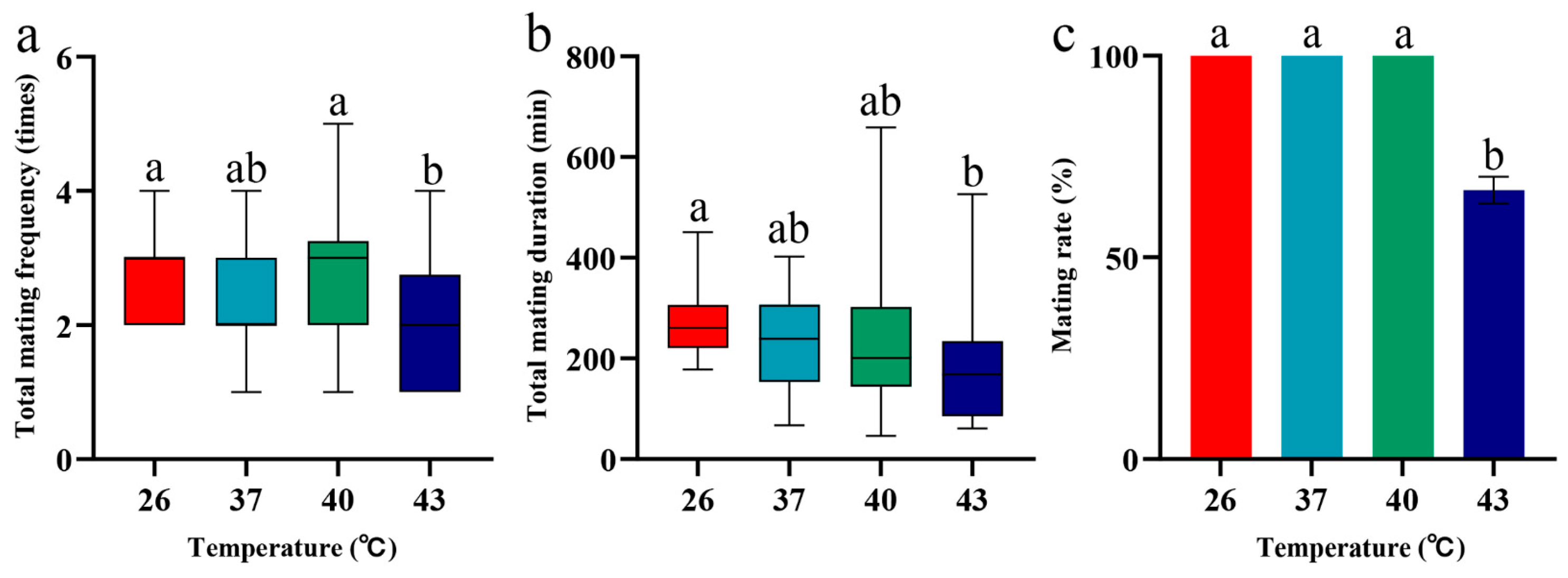
3.4. Effects of Periodic Short-Term Heat Stress on Mating of Spodoptera frugiperda
3.5. Effects of Periodic Short-Term Heat Stress on Adult Fecundity and Longevity of Spodoptera frugiperda
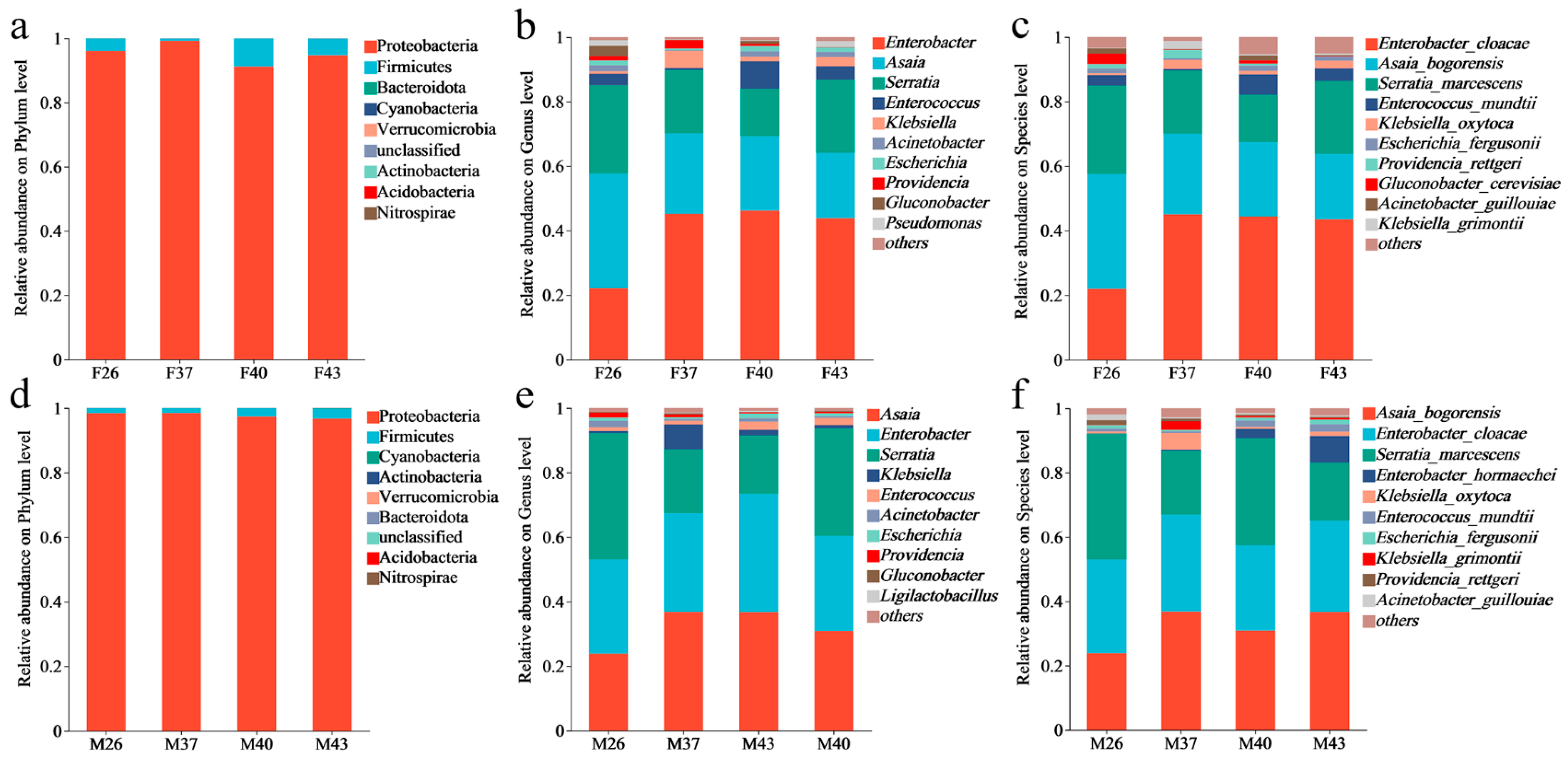
3.6. Analysis of Gut Bacterial Community Sequencing Data and Species Composition in Female and Male Adults of Spodoptera frugiperda Under Periodic Short-Term Heat Stress
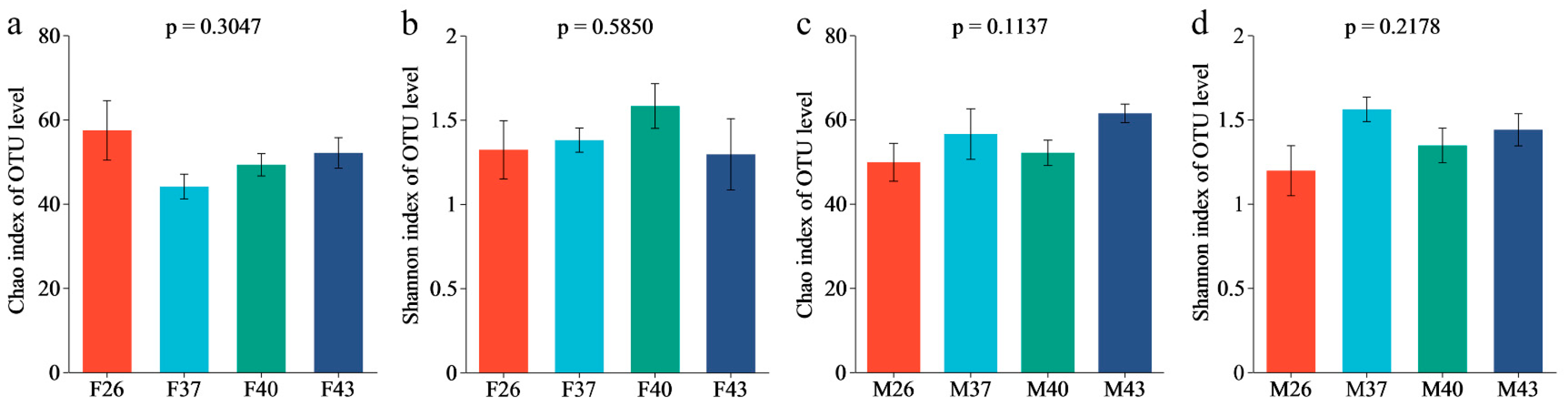
3.7. Analysis of Gut Bacterial Communities Diversity and Richness in Female and Male Adults of Spodoptera frugiperda Under Periodic Short-Term Heat Stress
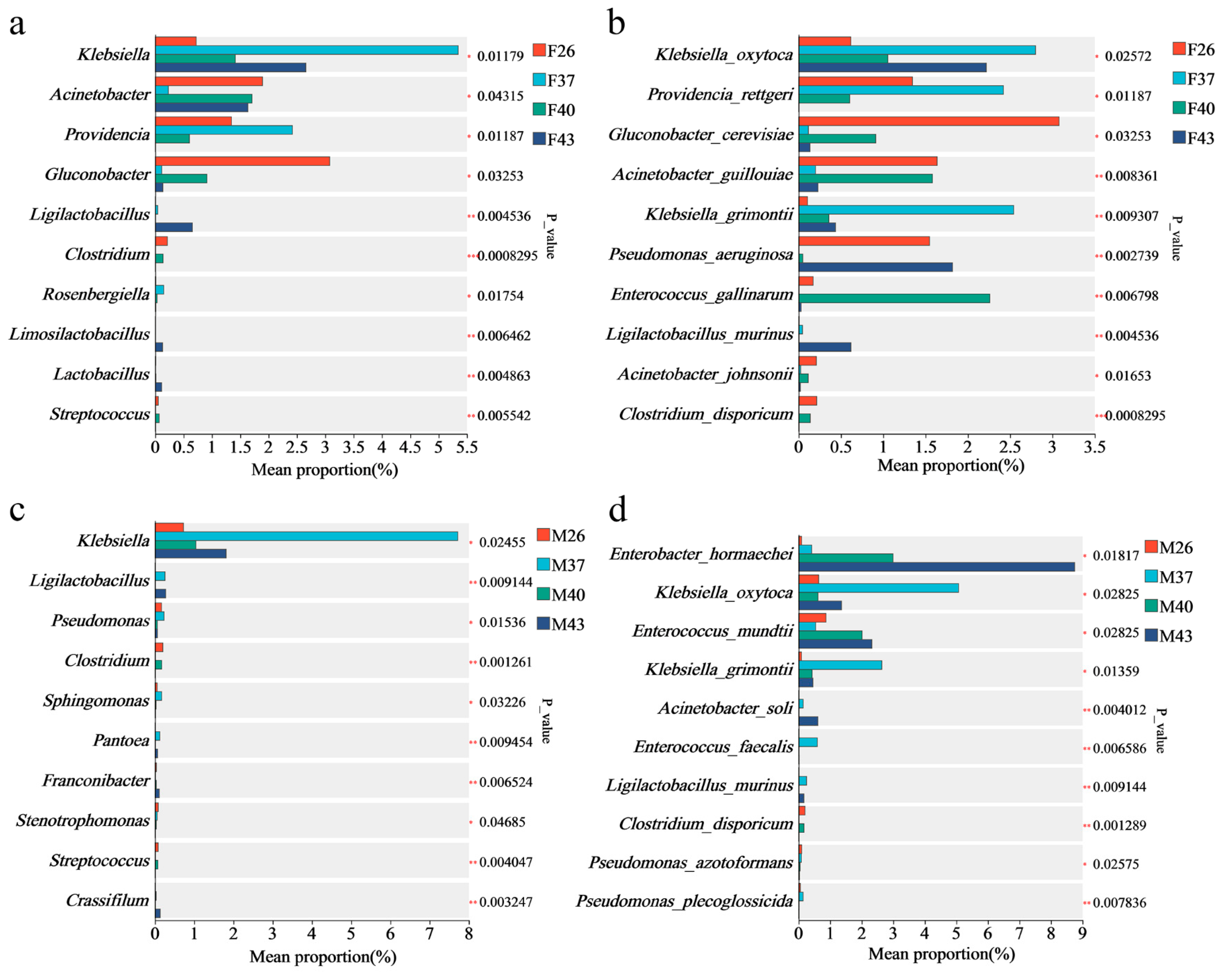
3.8. Differential Analysis of Gut Bacterial Composition in Female and Male Adults of Spodoptera frugiperda Under Periodic Short-Term Heat Stress
3.9. Functional Prediction of Gut Bacterial Communities in Female and Male Adults of Spodoptera frugiperda Under Periodic Short-Term Heat Stress
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wu, K.M. Management strategies of fall armyworm (Spodoptera frugiperda) in China. Plant Prot. 2020, 46, 1–5. [Google Scholar] [CrossRef]
- Sun, X.X.; Hu, C.X.; Jia, H.R.; Wu, Q.L.; Shen, X.J.; Zhao, S.Y.; Jiang, Y.Y.; Wu, K.M. Case study on the first immigration of fall armyworm, Spodoptera frugiperda invading into China. J. Integr. Agric. 2021, 20, 664–672. [Google Scholar] [CrossRef]
- Tay, W.T.; Meagher, R.L.; Czepak, C.; Groot, A.T. Spodoptera frugiperda: Ecology, Evolution, and Management Options of an Invasive Species. Annu. Rev. Entomol. 2023, 68, 299–317. [Google Scholar] [CrossRef]
- Lu, Z.H.; He, S.Q.; Yan, N.S.; Zhao, W.J.; Yao, W.F.; Chen, Y.P.; Yang, T.; Jiang, Y.Y.; Gui, F.R. Effects of temperatures on the development and reproduction of fall armyworm (Spodoptera frugiperda Smith). Plant Prot. 2019, 45, 27–31. [Google Scholar] [CrossRef]
- Ge, X.Y. Study on the Effects of Temperature on the Development and Control Method to Spodoptera frugiperda. Master’s Thesis, Foshan University, Foshan, China, 2020. [Google Scholar]
- Du Plessis, H.; Schlemmer, M.L.; Van Den Berg, J. The effect of temperature on the development of Spodoptera frugiperda (Lepidoptera: Noctuidae). Insects 2020, 11, 228. [Google Scholar] [CrossRef]
- Zhang, H.M.; Yin, Y.Q.; Zhao, X.Q.; Li, X.Y.; Wang, Y.; Liu, Y.; Chen, F.S.; Chen, A.D. The growth and development characteristics of Spodoptera frugipetda under different temperature conditions. J. Environ. Entomol. 2020, 42, 52–59. [Google Scholar]
- Wan, P.; Lv, B.Q.; Lu, H.; Tang, J.H.; Qiu, H.Y.; Zhang, Q.K.; Li, J.H. Comparison of the life table of Spodoptera frugiperda populations kept under either a constant indoor, or a variable outdoor, temperature. Chin. J. Appl. Entomol. 2023, 60, 1141–1147. [Google Scholar] [CrossRef]
- Ma, C.S.; Ma, G. The Impacts of Extreme High Temperature on Insect Populations Under Climate Change: A Review. Sci. Sin. Vitae 2016, 46, 556–564. [Google Scholar] [CrossRef]
- Zhang, H.M.; Wang, Y.; Yin, Y.Q.; Li, X.Y.; Chen, F.S.; Chen, A.D. Effects of short-term high temperature in egg and pupal stages on the growth and development of fall armyworm Spodoptera frugiperda. J. Plant Prot. 2020, 47, 831–838. [Google Scholar] [CrossRef]
- Tao, Y.D.; Liu, Y.; Wan, X.S.; Xu, J.; Fu, D.Y.; Zhang, J.Z. High and low temperatures differentially affect survival, reproduction, and gene transcription in male and female moths of Spodoptera frugiperda. Insects 2023, 14, 958. [Google Scholar] [CrossRef]
- Li, X.R.; Chen, Z.Y.; Ba, T.X.; Yin, X.T.; Zhu, X.; Zhang, Y.H. Effects of short-term heat stress on the survival and fecundity of Spodoptera frugiperda (Lepidoptera: Noctuidae). Plant Prot. 2022, 48, 90–96. [Google Scholar] [CrossRef]
- Yang, Y.W.; Yuan, D.Z.; Xie, F.Z.; Wang, J.Z.; Yang, J.; Wei, P.Y.; Chen, Z.J.; Hong, B. Impact of short-term low or high temperature on pupa and adult survival, development and fecundity of Spodoptera frugiperda. Plant Prot. 2022, 48, 246–250. [Google Scholar] [CrossRef]
- Ma, L.; Cao, J.Y.; Bai, J.Y.; Xu, Z.; Li, L.; Zhang, Y.; Min, M.R. Research progress in insect gut microbes and the methods for studying their functions. Acta Entomol. Sin. 2023, 66, 1415–1424. [Google Scholar]
- Fu, J.R.; Feng, Q.L.; Deng, H.M. Advances in understanding the effects of gut microbiota on insect reproduction. Chin. J. Appl. Entomol. 2024, 61, 237–245. [Google Scholar] [CrossRef]
- Cai, Z.H. The Effects of Gut Microbiota on the Growth and the Repair of Irradiated Damage in Bactrocera dorsalis. Ph.D. Thesis, Huazhong Agricultural University, Wuhan, China, 2020. [Google Scholar]
- Ma, M.Q.; Luo, J.; Chen, X.T.; Li, C.; Li, S.Q.; Sun, J.H.; Xu, L.T. Gut Bacteria Facilitate Leaf Beetles in Adapting to Dietary Specialization by Enhancing Larval Fitness. Npj Biofilms Microbiomes 2024, 10, 110. [Google Scholar] [CrossRef]
- Shan, H.W.; Xia, X.J.; Feng, Y.L.; Wu, W.; Li, H.J.; Sun, Z.T.; Li, J.M.; Chen, J.P. The plant-sucking insect selects assembly of the gut microbiota from environment to enhance host reproduction. Npj Biofilms Microbiomes 2024, 10, 64. [Google Scholar] [CrossRef]
- Yu, Y.Q.; Zhang, J.; Zhu, F.L.; Fan, M.X.; Zheng, J.S.; Cai, M.M.; Zheng, Y.L.; Huang, F.; Yu, Z.N.; Zhang, J.B. Enhanced protein degradation by black soldier fly larvae (Hermetia illucens L.) and its gut microbes. Front. Microbiol. 2023, 13, 1095025. [Google Scholar] [CrossRef]
- Fu, Z.Y. Response of Different Geographical of Laodelphax striatellus to Short-Term High Temperature Stress and Its Symbiotic Bacteria. Master’s Thesis, Shenyang Agricultural University, Liaoning, China, 2023. [Google Scholar]
- Liu, Y.P. Study on the Mechanism of Gut Bacteria-Mediated Gluttony of Spodoptera frugiperda, a Major Invasive Pest in China. Ph.D. Thesis, Hubei University, Wuhan, China, 2024. [Google Scholar]
- Yang, J.; Liu, W.Q.; Han, X.Z.; Hao, X.; Yao, Q.B.; Du, W.G. Gut microbiota modulation enhances the immune capacity of lizards under climate warming. Microbiome 2024, 12, 37. [Google Scholar] [CrossRef]
- Liang, Y.T.; Liu, Y.C.; Gao, Y.; Wang, H.B.; Xu, Y.S. Diversity analysis on gut microbiota of Glyphodes pyloalis Walker under high temperature. J. Zhejiang Univ. (Agric. Life Sci.) 2018, 44, 215–222. [Google Scholar] [CrossRef]
- Jaramillo, A.; Castañeda, L.E. Gut Microbiota of Drosophila subobscura Contributes to Its Heat Tolerance and Is Sensitive to Transient Thermal Stress. Front. Microbiol. 2021, 12, 654108. [Google Scholar] [CrossRef]
- Shan, H.W.; Deng, W.H.; Luan, J.B.; Zhang, M.J.; Zhang, Z.; Liu, S.S.; Liu, Y.Q. Thermal sensitivity of bacteriocytes constrains the persistence of intracellular bacteria in whitefly symbiosis under heat stress. Environ. Microbiol. Rep. 2017, 9, 706–716. [Google Scholar] [CrossRef] [PubMed]
- Nie, S.Q.; Liu, Y.J.; Ge, Y. The host phylogeny and climate determine the gut bacteria of global insects. Sci. Total Environ. 2025, 966, 178812. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Ma, C.S. Impacts of periodic repeated heat events on ecological performance in insects. Chin. J. Appl. Entomol. 2013, 50, 1499–1508. [Google Scholar] [CrossRef]
- Weisburg, W.G.; Barns, S.M.; Pelletier, D.A.; Lane, D.J. 16S Ribosomal DNA Amplification for Phylogenetic Study. J. Bacteriol. 1991, 173, 697–703. [Google Scholar] [CrossRef]
- Edgar, R.C. UPARSE: Highly accurate OTU sequences from microbial ampliconreads. Nat. Methods 2013, 10, 996–998. [Google Scholar] [CrossRef]
- Stackebrandt, E.; Goebel, B.M. Taxonomic Note: A Place for DNA-DNA Reassociation and 16S rRNA Sequence Analysis in the Present Species Definition in Bacteriology. Int. J. Syst. Evol. Microbiol. 1994, 44, 846–849. [Google Scholar] [CrossRef]
- Wang, Q.; Garrity, G.M.; Tiedje, J.M.; Cole, J.R. Naive Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl. Environ. Microbiol. 2007, 73, 5261–5267. [Google Scholar] [CrossRef]
- Douglas, G.M.; Maffei, V.J.; Zaneveld, J.R.; Yurgel, S.N.; Brown, J.R.; Taylor, C.M.; Huttenhower, C.; Langille, M.G.I. PICRUSt2 for prediction of metagenome functions. Nat. Biotechnol. 2020, 38, 685–688. [Google Scholar] [CrossRef]
- Schloss, P.D.; Westcott, S.L.; Ryabin, T.; Hall, J.R.; Hartmann, M.; Hollister, E.B.; Lesniewski, R.A.; Oakley, B.B.; Parks, D.H.; Robinson, C.J.; et al. Introducing Mothur: Open-Source, Platform-Independent, Community-Supported Software for Describing and Comparing Microbial Communities. Appl. Environ. Microbiol. 2009, 75, 7537–7541. [Google Scholar] [CrossRef]
- Pan, M.Z.; Shen, R.C.; Fu, Z.X.; Lu, Z.Z.; Ma, B.B.; Liu, T.X. High-temperature responses of Myzus persicae and its parasitoid Aphidius gifuensis in relation to heat Level, duration and developmental Stage. Pest Manag. Sci. 2024, 80, 4628–4636. [Google Scholar] [CrossRef]
- Zhang, J.Q. Effects of Chlorantraniliprole on Growth, Development and Intestinal Microflora of Ostrinia furnacalis Under High Temperature Stress. Master’s Thesis, Shandong Agricultural University, Taian, China, 2023. [Google Scholar]
- Wang, J.; Mu, Y.L.; Yang, C.; Huang, X.D.; Wang, Y.C.; Yu, H.P.; Chen, X.S. Effects of High and Low Temperature Stress on Development and Cold Tolerance of Spodoptera frugiperda (Smith) (Lepidoptera:Noctuidae). J. Mt. Agric. Biol. 2022, 41, 18–25. [Google Scholar]
- Huang, L.L.; Xue, F.S.; Chen, C.; Guo, X.; Tang, J.J.; Zhong, L.; He, H.M. Effects of temperature on life-history traits of the newly invasive fall armyworm, Spodoptera frugiperda in Southeast China. Ecol. Evol. 2021, 11, 5255–5264. [Google Scholar] [CrossRef]
- Malekera, M.J.; Acharya, R.; Mostafiz, M.M.; Hwang, H.S.; Bhusal, N.; Lee, K.Y. Temperature-Dependent Development Models Describing the Effects of Temperature on the Development of the Fall Armyworm Spodoptera frugiperda (J. E. Smith) (Lepidoptera: Noctuidae). Insects 2022, 13, 1084. [Google Scholar] [CrossRef]
- Zhang, Y.X.; Shi, L.; Yuan, Y.; Zhang, T.W. Effect of temperature on body size, growth and reproduction of Acyrthosiphon pisum. J. Gansu Agric. Univ. 2024, 59, 165–170. [Google Scholar] [CrossRef]
- Wang, Y. Effect of High Temperature Stress on Ovary Development and Vitellogenin Genes Expression of Agasicles hygrophila. Master’s Thesis, Chinese Academy of Agricultural Sciences, Beijing, China, 2017. [Google Scholar]
- Hu, J.R.; Medison, R.G.; Zhang, S.; Ma, P.F.; Shi, C.H. Impacts of Non-Lethal High-Temperature Stress on the Development and Reproductive Organs of Bradysia odoriphaga. Insects 2022, 13, 74. [Google Scholar] [CrossRef]
- Yu, W.Y. Effects of High Temperature Stress in Pupa Stage on the Growth, Physiological Metabolism and Ovarian Development of Cotesia vestalis. Master’s Thesis, Northwest A&F University, Yangling, China, 2021. [Google Scholar]
- Katsuki, M.; Miyatake, T. Effects of temperature on mating duration, sperm transfer and remating frequency in Callosobruchus chinensis. J. Insect Physiol. 2009, 55, 113–116. [Google Scholar] [CrossRef]
- Stazione, L.; Norry, F.M.; Sambucetti, P. Heat-hardening effects on mating success at high temperature in Drosophila melanogaster. J. Therm. Biol. 2019, 80, 172–177. [Google Scholar] [CrossRef]
- Yi, X.L. Study on the Mating Ability and Reproductive Fitness of Bactrocera dorsalis. Master’s Thesis, Guangxi University, Nanning, China, 2021. [Google Scholar]
- Augustin, J.; Bourgeois, G.; Brodeur, J.; Boivin, G. Low and high temperatures decrease the mating success of an egg parasitoid and the proportion of females in the population. J. Therm. Biol. 2022, 110, 103382. [Google Scholar] [CrossRef]
- Ren, Q.L. Effects of Brief High-Temperature Stress on the Survival and Reproduction of Fall Armyworm (Spodoptera frugiperda). Master’s Thesis, Guizhou University, Guiyang, China, 2021. [Google Scholar]
- Chen, L.; Cai, D.C.; Cheng, Q.; Tang, C.; Peng, Z.Q.; Jing, Q.A.; Wen, H.B. Effect of short-term high temperature on survival and fecundity of Agasicles hygrophila. Chin. Bull. Entomol. 2010, 47, 308–312. [Google Scholar]
- Chen, H.; Fang, C.; Xu, X.L.; Wu, J.X. Impact of shortterm high temperature on adult survival and fitness of the oriental. Fruit moth, Grapholita molesta (Lepidoptera: Tortricidae). Acta Entomol. Sin. 2014, 57, 696–702. [Google Scholar] [CrossRef]
- LI, D.D. Diversity of Gut Bacteria and Their Sensitivity to Chlorpyrifos in Spodoptera frugiperda. Ph.D. Thesis, Northwest A&F University, Yangling, China, 2023. [Google Scholar]
- Li, X.; Jia, J.J.; An, J.L.; Meng, F.X.; Liu, T.X.; Zhang, S.Z. Effect of Cotesia Ruficrus parasitization on diversity and community composition of intestinal bacteria in Spodoptera Frugiperda. Insects 2024, 15, 570. [Google Scholar] [CrossRef] [PubMed]
- Fan, Z.Y.; Khan, M.M.; Wang, K.; Li, Y.H.; Jin, F.L.; Peng, J.; Chen, X.Y.; Kong, W.Z.; Lv, X.L.; Chen, X.Y.; et al. Disruption of midgut homeostasis by microplastics in Spodoptera frugiperda: Insights into inflammatory and oxidative mechanisms. J. Hazard. Mater. 2025, 487, 137262. [Google Scholar] [CrossRef] [PubMed]
- Hafsi, A.; Moquet, L.; Hendrycks, W.; De Meyer, M.; Virgilio, M.; Delatte, H. Evidence for a gut microbial community conferring adaptability to diet quality and temperature stressors in phytophagous insects: The melon fruit fly Zeugodacus cucurbitae (Diptera: Tephritidae) as a case study. BMC Microbiol. 2024, 24, 514. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y. Effects of Developmental Stages, Diets and High Temperature Stress on Bacterial Diversity in Propylaea japonica. Master’s Thesis, Northwest A&F University, Yangling, China, 2023. [Google Scholar]
- Sun, X.N.; Yuan, Q.; Du, B.B.; Jin, X.Y.; Huang, X.Y.; Li, Q.Y.; Zhong, Y.Q.; Pan, Z.H.; Xu, S.Q.; Sima, Y.H. Relationship between Changes in Intestinal Microorganisms and Effect of High Temperature on the Growth and Development of Bombyx Mori Larvae. Int. J. Mol. Sci. 2022, 23, 10289. [Google Scholar] [CrossRef]
- Ren, L.P.; Zhang, X.Y.; Yang, F.Q.; Jocelin, N.F.; Shang, Y.J.; Wang, Q.; Liu, Z.Y.; Guo, Y.D. Effects of heat tolerance on the gut microbiota of Sarcophaga peregrina (Diptera: Sarcophagidae) and impacts on the life history traits. Parasites Vectors 2023, 16, 364. [Google Scholar] [CrossRef]
- O’Donnell, M.P.; Fox, B.W.; Chao, P.H.; Schroeder, F.C.; Sengupta, P. A neurotransmitter produced by gut bacteria modulates host sensory behaviour. Nature 2020, 583, 415–420. [Google Scholar] [CrossRef]
- Msaad Guerfali, M.; Djobbi, W.; Charaabi, K.; Hamden, H.; Fadhl, S.; Marzouki, W.; Dhaouedi, F.; Chevrier, C. Evaluation of Providencia rettgeri pathogenicity against laboratory Mediterranean fruit fly strain (Ceratitis capitata). PLoS ONE 2018, 13, e0196343. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, S.M.; Zhang, X.Y.; Zhang, K.X.; Liu, W.J.; Zhang, R.L.; Zhang, Z. Enterobacter hormaechei in the intestines of housefly larvae promotes host growth by inhibiting harmful intestinal bacteria. Parasites Vectors 2021, 14, 598. [Google Scholar] [CrossRef]
- Grau, T.; Vilcinskas, A.; Joop, G. Probiotic Enterococcus mundtii Isolate Protects the Model Insect Tribolium castaneum against Bacillus thuringiensis. Front. Microbiol. 2017, 8, 1261. [Google Scholar] [CrossRef]
- Zhang, Y.; Zheng, R.W.; Yao, L.; L, Q.Q.; Lu, S.H.; Li, G.T.; Tang, Q.F. Analysis of the diversity of intestinal microbiomes in fall armyworm Spodoptera frugiperda fed on different host plants. J. Plant Prot. 2022, 49, 1712–1723. [Google Scholar] [CrossRef]
- Wu, L.H. Effects of Host Plants on the Development, Reproduction, Mating Behavior and Gut Microbiota of Fall Armyworm (Spodoptera frugiperda). Master’s Thesis, Guizhou University, Guiyang, China, 2022. [Google Scholar]
- Li, C. Effects of Graphene Oxide on the Growth, Development, Reproduction, and Gut Microbiota of Spodoptera frugiperda. Master’s Thesis, Guizhou University, Guiyang, China, 2023. [Google Scholar]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jia, J.; Liang, M.; Zhao, Z.; Huang, W.; Feng, Q.; Lin, Z.; Ji, X. Effects of Periodic Short-Term Heat Stress on Biological Characteristics and Gut Bacteria of Spodoptera frugiperda. Insects 2025, 16, 584. https://doi.org/10.3390/insects16060584
Jia J, Liang M, Zhao Z, Huang W, Feng Q, Lin Z, Ji X. Effects of Periodic Short-Term Heat Stress on Biological Characteristics and Gut Bacteria of Spodoptera frugiperda. Insects. 2025; 16(6):584. https://doi.org/10.3390/insects16060584
Chicago/Turabian StyleJia, Jingjing, Min Liang, Zhitao Zhao, Weikang Huang, Qing Feng, Zhufeng Lin, and Xuncong Ji. 2025. "Effects of Periodic Short-Term Heat Stress on Biological Characteristics and Gut Bacteria of Spodoptera frugiperda" Insects 16, no. 6: 584. https://doi.org/10.3390/insects16060584
APA StyleJia, J., Liang, M., Zhao, Z., Huang, W., Feng, Q., Lin, Z., & Ji, X. (2025). Effects of Periodic Short-Term Heat Stress on Biological Characteristics and Gut Bacteria of Spodoptera frugiperda. Insects, 16(6), 584. https://doi.org/10.3390/insects16060584




