Simple Summary
Chelonus formosanus (Sonan) is an egg–larval parasitoid wasp with enormous potential for the biological control of various Noctuidae pests. However, little is known about its ontogenetic development and the associated microbiota. In this study, we examined the ontogenetic characteristics of C. formosanus. We performed 16S bacterial amplicon sequencing at different developmental stages. This study elucidates the morphological and developmental dynamics of C. formosanus and reveals its microbiota abundance at different developmental stages. Our findings lay a solid foundation for understanding the complexity of core microorganisms colonizing C. formosanus.
Abstract
Chelonus formosanus is a parasitic wasp capable of parasitizing various Noctuidae pests, including the highly invasive Spodoptera frugiperda, and it demonstrates strong pest control potential. Both egg and larval stages primarily occur within the host organism, and the total developmental time from egg to adult is approximately 19.62 days. To investigate the microbial communities at different stages, we performed 16S rDNA sequencing (V1–V9 region) using PacBio sequencing and identified 404 bacterial species belonging to 61 classes, 116 orders, 182 families, and 308 genera across larval, pupal, female, and male adult stages. Bacterial diversity and richness varied across the stages, with Enterobacter and Enterococcus dominating in larvae and pupae and Pseudomonas emerging as dominant in female adults. In contrast, male adults predominantly resided with Ralstonia and Achromobacter. The predicted functions of bacteria within C. formosanus at different developmental stages are predominantly marked by the high abundance of metabolic pathways. This study provides a comprehensive understanding of the morphology of C. formosanus and contributes to the practical control of host pests. Additionally, our findings preliminarily characterized the microbial community of various developmental stages, laying the groundwork for its functional study.
1. Introduction
Chelonus formosanus (Sonan) (Hymenoptera: Braconidae) is an egg–larval parasitoid wasp and serves as a natural enemy to various pest species within the Noctuidae family [1]. Recent reports have highlighted its role as a significant biological control agent against Spodoptera frugiperda, a globally invasive pest, in regions such as China [2], India [3], the Philippines [4], and Japan. Notably, in New Delhi, C. formosanus is the dominant parasitic wasp attacking S. frugiperda [5]. Parasitism rates of C. formosanus on S. frugiperda eggs ranged from 6.8% to 17.65% under natural conditions [2,4]. Moreover, large-scale rearing of C. formosanus is feasible when S. frugiperda is used as a host [3]. The highest parasitism rate of C. formosanus on S. frugiperda eggs could reach 100% under both indoor and field cage conditions, demonstrating its considerable potential for biocontrol. Although previous studies have primarily revealed the morphological traits of C. formosanus adults, there is a dearth of information regarding its developmental stages, particularly the larval and pupal phases.
Insects host a diverse and abundant array of microorganisms, including bacteria, archaea, and eukaryotes. These microbes are widely distributed across various insect tissues, including the body wall, intestine, hemolymph, and cells [6,7,8]. Recent advancements in sequencing technologies have significantly enhanced the study of insect-associated microbiota, drawing increasing attention from the scientific community. Although insects are associated with a variety of microbes, bacteria are the most widespread and common category. The impact of bacteria on insect physiology is multifaceted. For example, bacterial communities can promote the growth and development of larvae in species such as Bactrocera dorsalis and Plagiodera versicora [9,10], motivate the foraging decision and promote fecundity and survival of B. dorsalis [11], alter oviposition preferences of Encarsia pergandiella [12], and protect hosts from pathogenic threats and environmental stressors [6]. Furthermore, bacterial supplementation has been shown to optimize body weight gain in Hermetia illucens larvae with reduced food intake [13] and aid poikilothermic insects in adapting to climate change by boosting host immune responses [14].
Microbial diversity in insects is shaped by various factors, including species, diet, and geographic origin. Previous research has shown that the number of symbiotic bacteria in the four Sclerodermus parasitoids did not differ significantly; however, the composition of their bacterial communities was significantly different [15]. In other insects, such as Grapholita molesta [16], Harmonia axyridis [17], and S. frugiperda [18], the diversity and composition of microbial flora was affected by host species. For instance, the bacterial diversity and richness index in three different Rhyzopertha dominica populations sampled from different grain storage locations were significantly higher compared to the populations from laboratory settings [19]. Additionally, bacterial community composition in Diaphorina citri exhibited notable geographic variation [20]. However, some insects show minimal sensitivity to distinct diets and geographic locations and no significant differences were found in the gut microbiota diversity of Apriona germari larvae collected from Populus tomentosa and Malus pumila [21]. In the case of Riptortus pedestris, the gut microbiome composition of 12 populations across distinct geographic regions was largely defined by geographic or soil factors [22]. Furthermore, no significant variation in microbial richness and diversity was observed in Chironomus circumdatus sampled at three different locations [23].
Throughout the life cycle of insects, the composition of symbiotic bacterial communities undergoes dynamic changes. These changes may involve the loss, recombination, or acquisition of new symbiotic bacteria, with such fluctuations being most pronounced in holometabolous insects [24]. For example, the bacterial composition in Brithys crini shows significant variation among different developmental stages [25]. Similarly, the gut microbiomes of dragonfly nymphs differ from those of adults [26], and Chironomus transvaalensis exhibits a reduction in bacterial diversity with its development from egg to adult [27]. There are remarkable differences in the gut bacteria community between different instar larvae and diapause prepupae of Colleles gigas. The bacterial diversity decreases gradually from early instar to late instar and then to diapause prepupa [28]. Moreover, the bacterial abundance and diversity in Propylaea japonica and Osmia excavata also vary among different developmental stages [29,30]. However, some studies have found that the gut microbiome of Aldrichina grahami is not significantly influenced by diet or developmental stage [31]. To date, there are no reports relevant to the ontogenetic process and associated bacterial communities of C. formosanus at different developmental stages. Given the crucial role of bacteria in insect physiology, understanding the microbial dynamics of C. formosanus is essential for its potential utilization in controlling Noctuidae pests.
To gain a deeper understanding of C. formosanus biological characteristics and deploy itin practical pest management, it is crucial to investigate the developmental characteristics of C. formosanus within the host. This study aims to fill this knowledge gap, thereby providing a solid theoretical foundation for the enhanced use of C. formosanus in the control of S. frugiperda. This study aims to investigate the ontogenetic development of C. formosanus using ultra-depth-of-field three-dimensional microscopy and to analyze its microbiota community at various developmental stages by 16S rDNA sequencing. Clarifying the morphological and developmental changes of C. formosanus and characterizing the bacterial species and their dynamics at different stages will lay a solid foundation for further investigation into its role in modulating the growth, reproduction, and parasitism of host species.
2. Materials and Methods
2.1. Insect Rearing
The S. frugiperda and C. formosanus colonies used in this study were collected from Daoyu Village, Xiuying District, Haikou City, Hainan Province, China (19°52′22.47″ N, 110°9′56.48″ E). The specimens were transported to the laboratory for multi-generational breeding and are currently maintained at the Institute of Plant Protection, Hainan Academy of Agricultural Sciences. S. frugiperda was reared on maize leaves, while C. formosanus was maintained on S. frugiperda eggs for over 30 generations. The insects were housed in an intelligent artificial climate chamber with a temperature of 26 ± 1 °C, a relative humidity of 70 ± 10%, and a photoperiod of 12 h of light and 12 h of darkness (SAFE-RGQHS-2, Ningbo Saifu experimental instrument Co., Ltd., Ningbo, China). Additionally, the insects were provided with a nutritional supplement consisting of 10% honey water.
2.2. Experimental Method
2.2.1. Determination of Individual Development of Chelonus formosanus
Fresh eggs of S. frugiperda were collected and subsequently exposed to mated female C. formosanus adults in a sealed rearing apparatus. After being parasitized by C. formosanus, the parasitized S. frugiperda eggs were immediately removed and dissected to examine the initial oviposition of C. formosanus. Additional eggs were kept for subsequent observations, with the host being dissected every 24 h. The developmental stages of parasitic wasps were visualized using a Keyence VHX-7000 digital microscope, and the length was measured (eggs and larvae aged from one to seven days were preserved in PBS for imaging), and 30 larvae were dissected per session. The average developmental stage of multiple replicated measurements was recorded to represent the wasp’s developmental status at each time point. The wasp’s developmental duration was monitored at different stages, and each measurement was repeated 100 times.
2.2.2. Samples Collection
The larvae (L) were collected 10 days after parasitization and development in the host body, while the pupae (P) were collected on the first day of pupation. Newly emerged, unmated female (AF) and male (AM) adults were also collected accordingly. After disinfecting the surfaces of the collected S. frugiperda larvae and C. formosanus cocoons, the surfaces were opened using tweezers to extract larvae and pupae of C. formosanus. Then, all specimens were disinfected with 75% ethanol for three minutes under a laminar flow hood, followed by three washes with PBS buffer (pH 7.2–7.4) each for one minute. After washing, the specimens were immediately frozen in liquid nitrogen and stored at –80 °C. The sample sizes were as follows: 50 larvae, 30 pupae, 20 female adults, and 20 male adults per treatment group. Each treatment was repeated three times.
2.2.3. DNA Extraction and 16S rDNA Sequencing
Total DNA was extracted using the TGuide S96 magnetic bead DNA extraction kit (DP812, Tiangen Biotech Co., Ltd., Beijing, China). The DNA concentration was measured by combining 1X dsDNA HS Working Solution with an enzyme labeler for detection. Subsequently, detection and amplification were carried out on a Bailing 1000 automated platform (Revvity Biomedical Co., Ltd., Shanghai, China), depending on the determined DNA concentration and the target amplification region. The V1–V9 region of the 16S rRNA gene was amplified using the primers 27F (16S-F) (5′-AGRGTTTGATYNTGGCTCAG-3′) and 1492R (16S-R) (5′-TASGGHTACCTTGTTASGACTT-3′). The PCR master mix consisted of 2 µL genomic DNA, 6.5 µL NFW, 10 µL KOD ONE MM, and 1.5 µL of barcode primer pairs. The amplification was as follows: pre-denaturation at 95 °C for 2 min, denaturation at 98 °C for 10 s, annealing at 55 °C for 30 s, extension at 72 °C for one minute and 30 s, followed by 22 cycles. A final extension was carried out at 72 °C for two minutes. The integrity of the PCR products was assessed by electrophoresis on a 1.8% agarose gel. For library construction of the amplified products, the SMRTBELLS PREP KIT 3.0 (PacBio, Menlo Park, CA, USA) was used for damage repair, terminal repair, and adapter ligation of the amplified products. The reaction was carried out on a PCR instrument, and the resulting library was purified using AMpure PB magnetic beads (Beckman Coulter Trading Co., Ltd., Shanghai, China). The purified library was quantified using Qubit to confirm its suitability for sequencing. The library was combined with the Revio polymerase kit prior to sequencing to enable binding of the primer and polymerase. PCR products were purified with cleanup beads before being sequenced on a PacBio Revio platform. The sequencing depth was assessed by examining the rarefaction curve, which leveled off at higher sequencing depths, indicating that increasing the number of sequences would result in negligible gains in species detection. The database construction and sequencing were outsourced to Beijing Baimaike Biotechnology Co., Ltd. (Beijing, China).
2.3. Data Analysis
CCS sequences were identified using the Lima (version 1.7.0) with barcode-based detection. The raw CCS sequence data were filtered to remove primer sequences and perform length filtering using Cutadapt (version 1.9.1). This process yielded clean CCS sequences that were devoid of primer residues. Chimera sequences were identified and removed using UCHIME (version 4.2), resulting in effective CCS sequences. These sequences were clustered at a 97% similarity threshold using Usearch (version 10), resulting in operational taxonomic units (OTUs) [32]. The SILVA reference database was used for taxonomic classification, employing the naive Bayes classifier in combination with the comparison method to assign taxonomic annotations to the featured sequences. Species classification data were obtained for each feature, and the sample community composition was analyzed at various taxonomic levels. Species abundance tables were generated at different classification levels using QIIME2 (version 2020.6). Community structure maps at different taxonomic levels were visualized using R language tools. The alpha diversity index at the OTU level was evaluated using QIIME2 (version 2020.6), and differences in alpha diversity between treatments were compared using independent-sample t-test. Principal coordinate analysis (PCoA) [33] and non-metric multidimensional scaling (NMDS) [34] were performed using the binary Jaccard algorithm, followed by statistical analysis using ANOSIM. Functional prediction of microbial communities was based on the KEGG database (level 3) using PICRUSt2 [35]. Statistical analysis of the experimental data was conducted using SPSS (version 20.0, IBM, Armonk, NY, USA), image processing was carried out using Adobe Photoshop (2022 version, Adobe Systems Incorporated, San Jose, CA, USA), and visualizations were generated using Excel (2021 version, Microsoft, Redmond, DC, USA).
3. Results
3.1. The Life Cycle of Chelonus formosanus
Egg to Larvae: The eggs dissected from ovaries of newly emerged female wasps are predominantly elongated and oval in shape, with a length of 252.80 ± 25.14 μm and a width of 34.56 ± 5.62 μm. Upon oviposition, these eggs undergo noticeable morphological changes, becoming shorter and wider, with a length of 180.49 ± 2.17 μm and a width of 64.59 ± 1.43 μm. Most of the eggs hatch within 24 h. After hatching, the body of wasp larvae immediately forms distinct segmentations, with a noticeably broader head compared to the abdomen and the posterior becoming more pointed. Within four to seven days of parasitism, the larvae lengthen, and the head width becomes considerably smaller relative to the thorax and abdomen. By days 8–11, larval segmentation becomes less distinct, the head nearly disappears, and the anal vesicle gradually diminishes in size. Concurrently, the body color of the larvae turns yellow. On day 11 post-parasitism, the digestive tract becomes visible with a clearly light green color, and on day 12, mature larvae emerge from the host. At this stage, white fat globules are visible, and the mouthparts and ocelli are discernible on the head (Figure 1).

Figure 1.
Egg-to-larval development of Chelonus formosanus. (a) Eggs dissected from the ovaries of newly emerged C. formosanus females. (b) Newly laid eggs in host hemocoel by C. formosanus. (c) Eggs shortly before hatching. (d–n) Larvae from days 1 to 11 post-parasitism. (o) The process by which the larvae of C. formosanus crawl out of S. frugiperda larva. (p) C. formosanus larvae fully detached from the host.
Pupa to Adult: Before pupation, C. formosanus larvae expel silk to construct a white cocoon, a process that lasts approximately one day. During the pupal stage, both the internal organs and external morphology of parasitoids undergo significant changes. On the second day of pupation, the differentiation of various organs begins. Between day three and day four, the compound eyes and ocelli gradually darken in color. By day five and day six, the body of C. formosanus gradually turns black, and the wings start to take shape. At this point, the pupal development is complete, and C. formosanus is about to eclose. On the 19th day post-parasitization, adult C. formosanus begin to emerge, mostly within one day. After eclosion, male and female wasps can be distinguished primarily by their genitalia (Figure 2).

Figure 2.
Developmental stages of Chelonus formosanus from pupa to adult. (a–f) C. formosanus pupal stages from day 1 to day 6. (g) Newly emerged C. formosanus adult. (h) The female genitalia of an adult wasp. (i) The male genitalia of an adult wasp. The orientations of images are as follows: 1, 2, and 3 represent frontal, lateral, and dorsal views at each developmental stage, respectively.
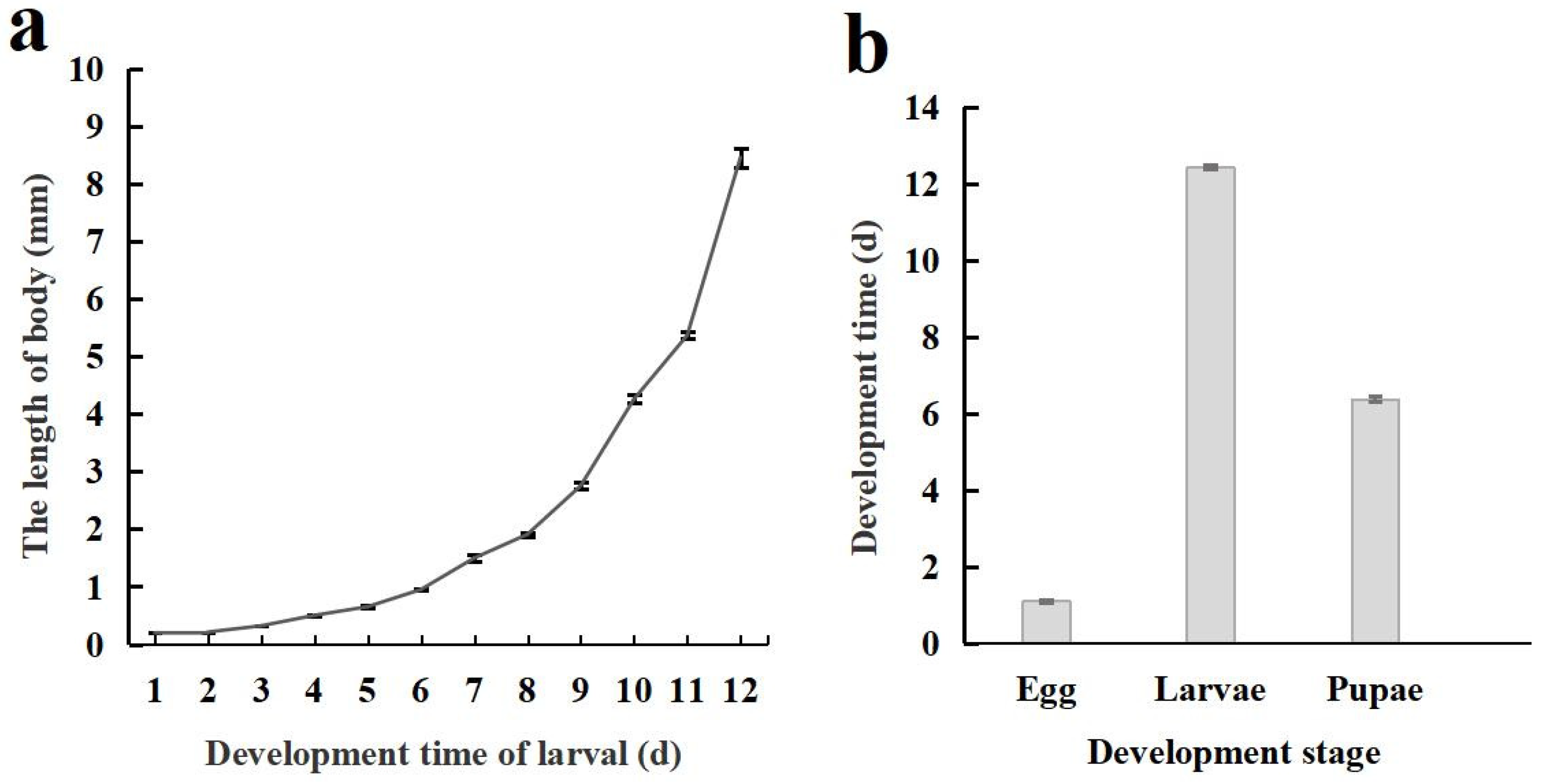
The life cycle of C. formosanu comprises four distinct stages: egg, larva, pupa, and adult. The body length of larvae increases in a curvilinear fashion with its development, and a notably faster growth rate was observed during later stages (Figure 3a). The duration of each developmental stage is illustrated in Figure 3b, with the total time required for development from egg to adult being 19.62 ± 0.07 days.
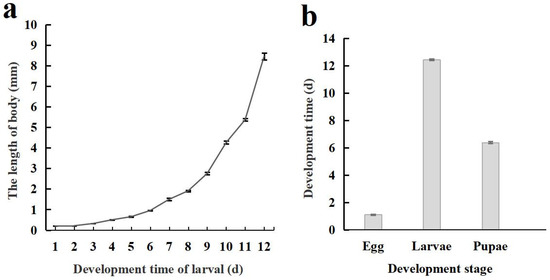
Figure 3.
Body length changes and developmental stages of Chelonus formosanus larvae. (a) Accumulative changes in larval body length of C. formosanus. (b) Developmental stages of C. formosanus.
3.2. Bacterial Composition and Diversity in Chelonus formosanus
3.2.1. Overview of 16S rDNA Sequencing Associated with Taxonomic Annotation
A total of 126,235 raw reads were generated by 16S rDNA high-throughput sequencing across all samples. After filtering and de-chimerization, 107,412 valid reads remained, with individual sample valid read counts ranging from 6885 to 11,286 and an average sequence length of 1459 bp. The effective range of 72.42–90.74% represents the proportion of high-quality data in the Raw CCS dataset after filtering and optimization, indicating the sequencing data’s reliability. The sequencing coverage for all samples surpassed 99.3%, ensuring that the majority of the bacterial species present at different developmental stages of C. formosanus coccinellid wasps were captured. Clustering the reads at a 97% similarity level resulted in the identification of 721 identified OTUs (Table 1).

Table 1.
The 16S RNA sequencing data of in vivo bacteria at different developmental stages of Chelonus formosanus.
A total of 404 bacterial species spanning 182 families and 308 genera were annotated across various taxonomic levels at different developmental stages of C. formosanus. Notably, the bacterial compositions of male wasps differed significantly from those of female wasps, larvae, and pupae at all taxonomic levels (p < 0.05). In contrast, the bacterial composition of larvae differed significantly from that of female wasps only at the genus level, with no significant differences observed at other taxonomic levels (p > 0.05) (Table 2).

Table 2.
Annotated taxonomic units at different stages of Chelonus formosanus.
3.2.2. Composition of Bacterial Communities at Different Developmental Stages of Chelonus formosanus
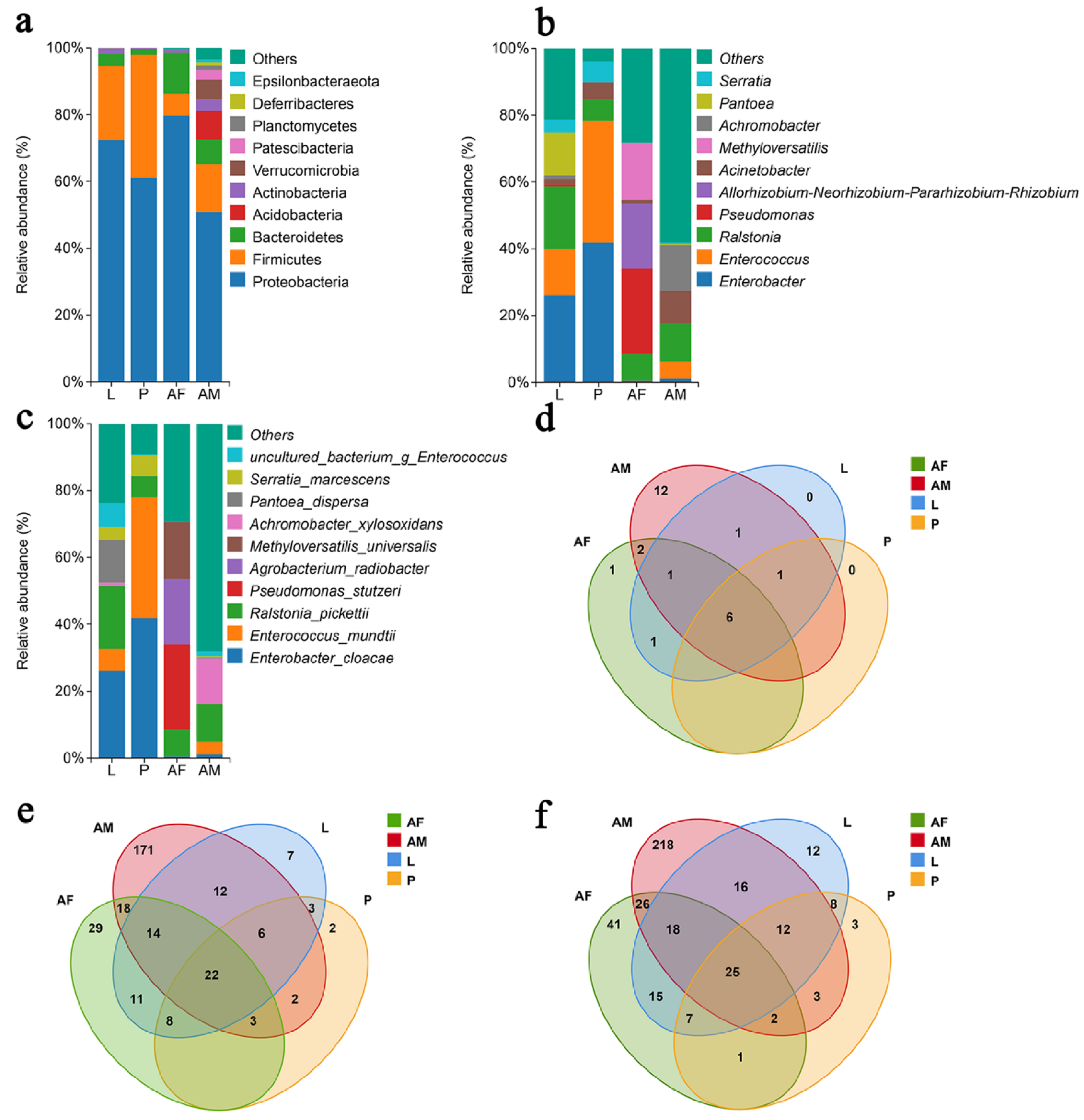
Proteobacteria is the dominant bacterial phylum across the different developmental stages of C. formosanus. The relative abundance of Proteobacteria in larvae, pupae, females, and males was 72.47%, 61.16%, 79.73%, and 50.91%, respectively, followed by Firmicutes and Bacteroidetes as the second most prevalent taxa (Figure 4a). At the genus level, bacterial composition and relative abundance varied considerably across developmental stages. In larvae and pupae, the predominant genera are Enterobacter and Enterococcus, while in male wasps, Achromobacter, Ralstonia, and Achromobacter were the most abundant genera with relative abundances of 13.70%, 13.70%, 11.30%, and 9.75%, respectively. Female wasps, on the other hand, are dominated by Pseudomonas, Allorhizobium-Neorhizobium-Pararhizobium-Rhizobium, and Methyloversatilis, with relative abundances of 25.48%, 19.41%, and 17.09%, respectively (Figure 4b). At the species level, Enterobacter cloacae, Ralstonia pickettii, and Pantoea dispersa are the dominant species in larvae, with relative abundances of 26.18%, 18.67%, and 12.86%, although P. dispersa was only identified in one sample. In pupae, E. cloacae and Enterococcus mundtii dominated, with relative abundances of 41.83% and 36.09%, while adult females exhibited a dominance of Pseudomonas stutzeri, Agrobacterium radiobacter, and Methyloversatilis universalis, with relative abundances of 25.46%, 19.41%, and 17.08%. Male wasps are primarily dominated by Ralstonia pickettii and Achromobacter xylosoxidans, with relative abundances of 11.30% and 13.63% (Figure 4c). Overall, the composition of the bacterial community in C. formosanus exhibited a dynamic change across developmental stages. The comparison between commonly colonized and stage-specific species revealed that 25 species are shared across the stages. The largest number of stage-specific species was observed in male wasps, followed by female wasps, with the pupal stage showing the fewest endemic species.
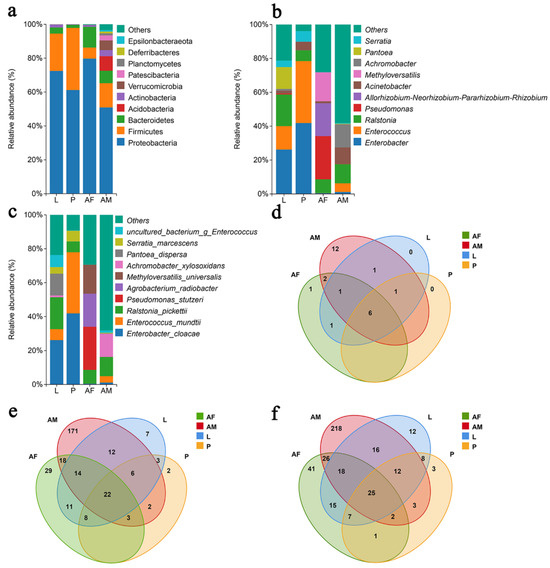
Figure 4.
Panels (a–c) depict the relative abundance of bacteria at the phylum, genus, and species levels, respectively, across different developmental stages of Chelonus formosanus. Panels (d–f) show the Venn diagrams illustrating the overlap of bacterial taxa at the phylum, genus, and species levels at the same stages.
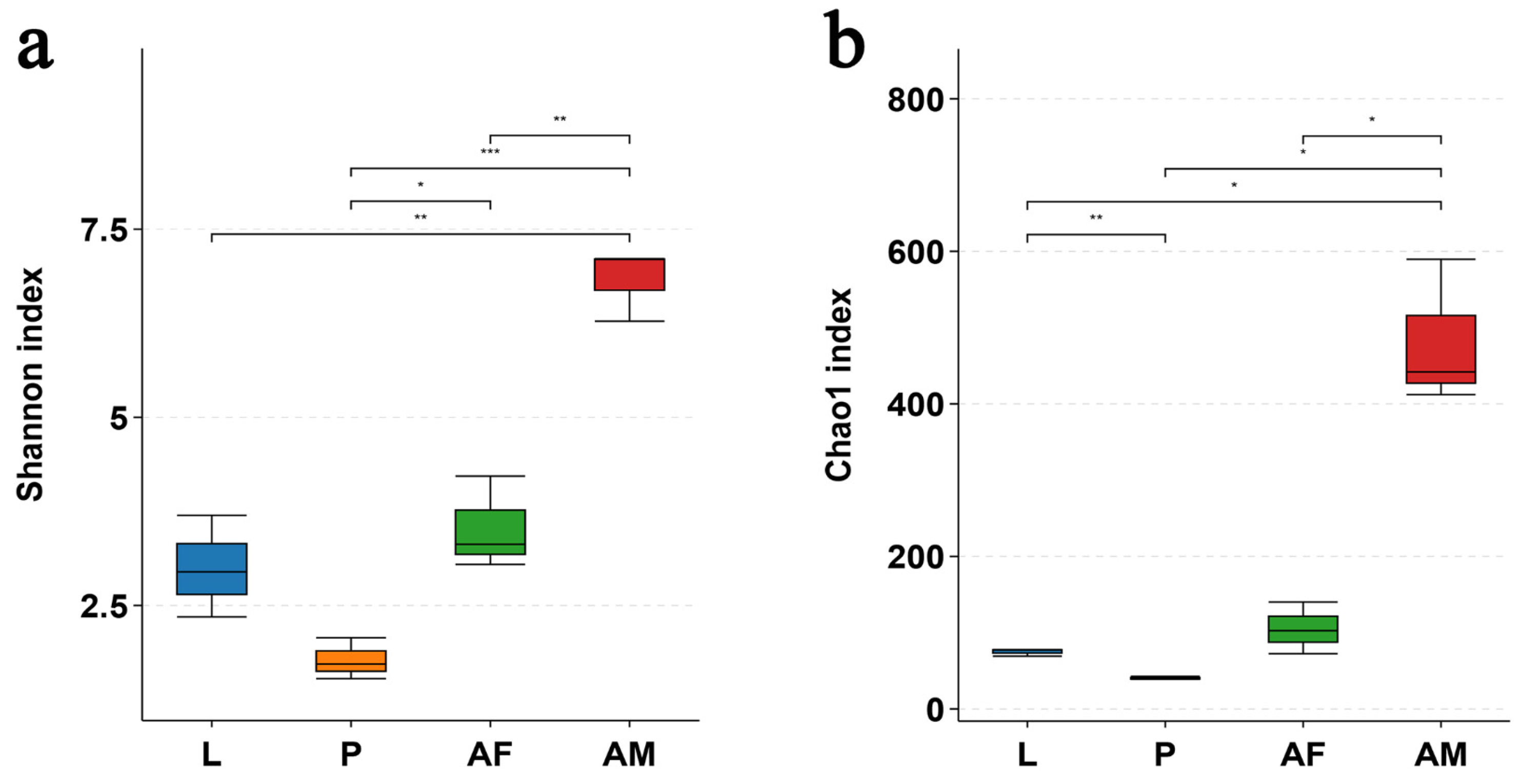
The bacterial diversity, as assessed by the Shannon index, and the bacterial richness, as measured by the Chao1 index, were significantly higher in male C. formosanus compared to larvae, pupae, and females. Among these developmental stages, pupae exhibited the lowest bacterial diversity and richness (Figure 5).
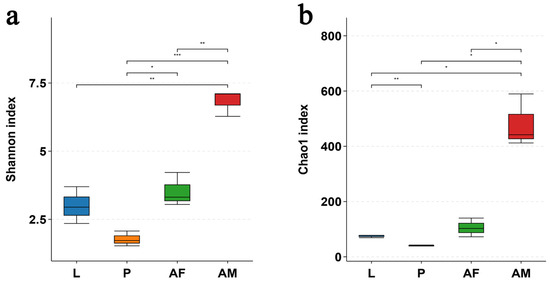
Figure 5.
Alpha diversity of in vivo bacteria at the OTU level at different developmental stages of Chelonus formosanus. (a) Shannon index and (b) Chao1 index. Statistical analyses were performed using independent-sample Student’s t-test. Asterisks indicate significance levels: * p < 0.05, ** p < 0.01, and *** p < 0.001.
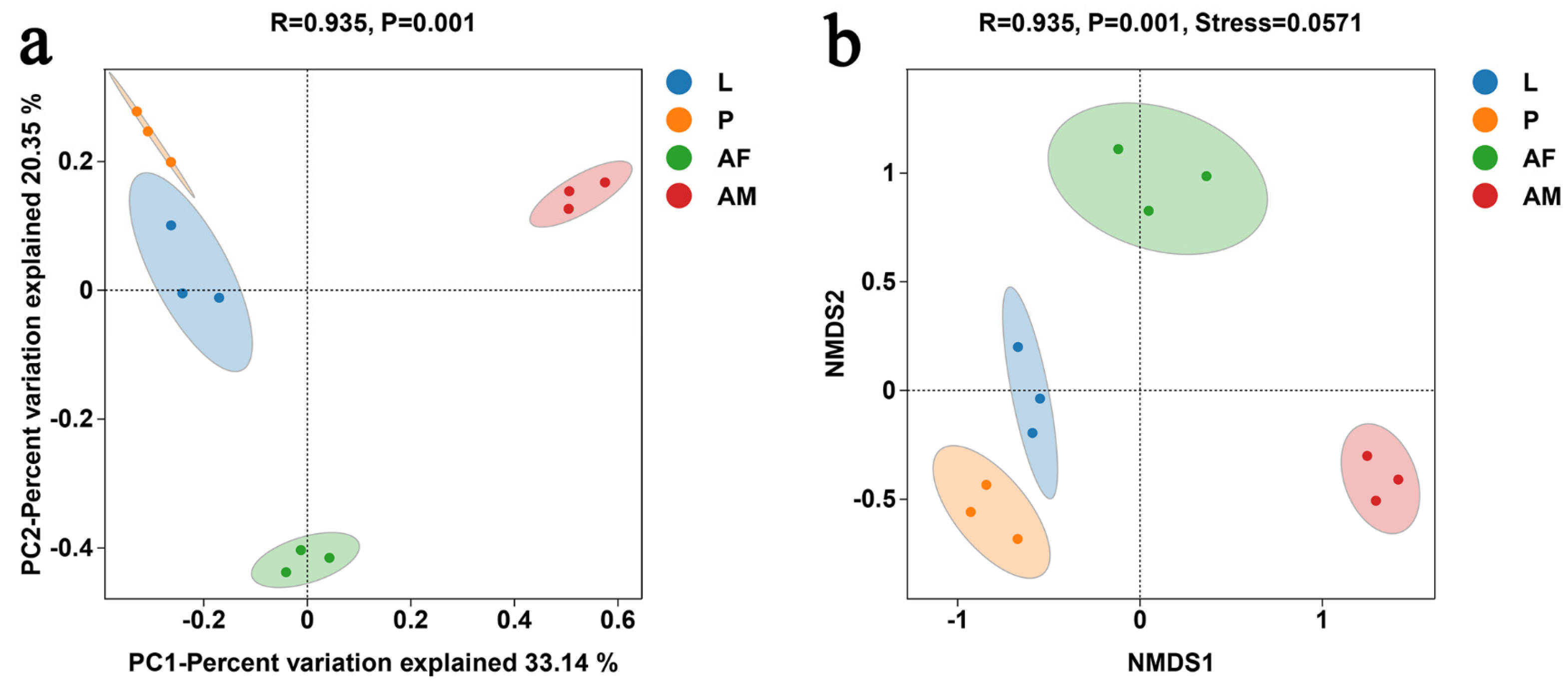
PCoA revealed significant differences in bacterial communities across different developmental stages of C. formosanus (ANOISM, R = 0.935, P = 0.001). The bacterial composition was more similar between larvae and pupae, while males were the most distinct from the other stages, indicating that males had the least similarity in bacterial species relative to other developmental stages (Figure 6a). The NMDS analysis, with a stress value below 0.2, demonstrated the reliability of the analysis (Figure 6b). The NMDS results were consistent with that of PCoA, further confirming that bacterial communities varied across different developmental stages of C. formosanus.
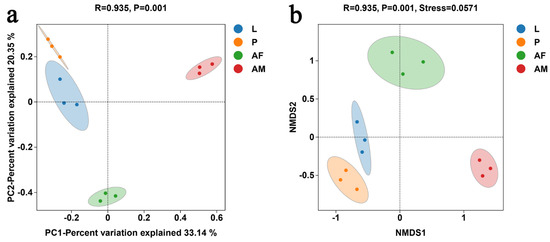
Figure 6.
Principal coordinate analysis (PCoA) (a) and non-metric multidimensional scaling (NMDS) (b) of bacterial communities at different developmental stages of C. formosanus, based on the binary–Jaccard algorithm.
3.2.3. Functional Predictions of Bacterial Community of Chelonus formosanus
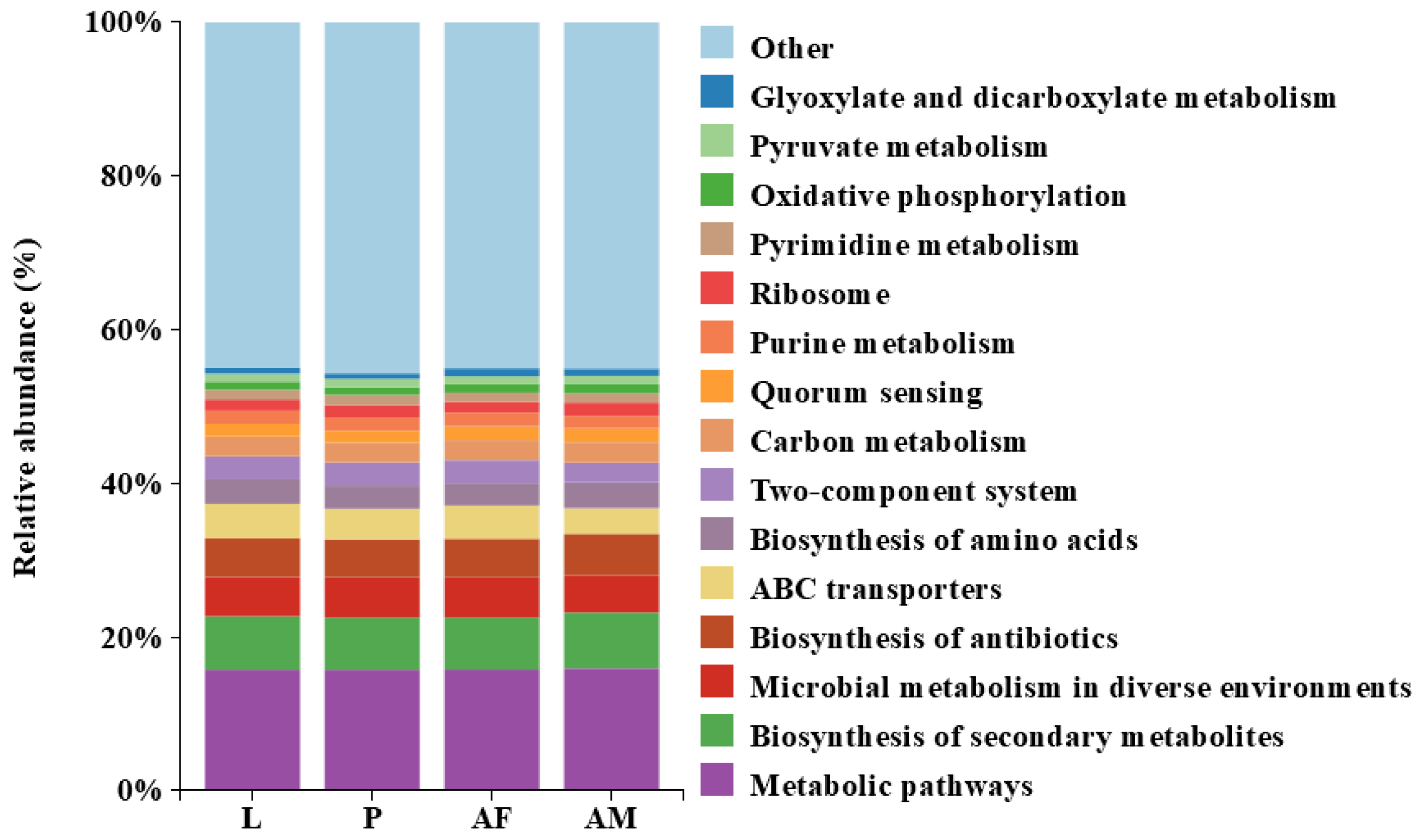
Bacterial profiling of C. formosanus revealed a high degree of similarity in the functional abundance across different developmental stages. Notably, metabolic pathways were the most abundant, highlighting their critical role in the insect’s physiological processes and their potential impact on host development and health. Additionally, the biosynthesis of antibiotics and quorum sensing were present in significant proportions at all stages, which may indicate bacterial defense mechanisms and communication. The biosynthesis of amino acids and ribosome functions were also consistently represented, emphasizing the importance of protein synthesis. In contrast, the abundance of glyoxylate and dicarboxylate metabolism, as well as pyruvate metabolism, was relatively low, suggesting that these pathways may not be central to the metabolic activities during C. formosanus development (Figure 7).
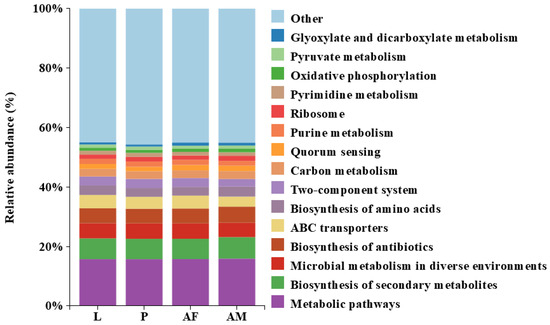
Figure 7.
Predicted results of the Top 15 bacterial functional abundances at different developmental stages of Chelonus formosanus.
4. Discussion
C. formosanus is an egg-larval parasitoid wasp that includes egg, larva, pupa, and adult developmental stages. Its eggs and larvae primarily grow and develop within the host. In the present study, we observed that the parasitic capacity of C. formosanus ranged from 1 to 15 eggs per S. frugiperda egg and 6.48 ± 0.62 eggs on average. However, only one parasitoid successfully survived on the third day of post-parasitism, with occasional instances of two larvae surviving. Whether the cause of the survived parasitoid larvae killing others is by competitive interactions or by host immunity remains unclear and warrants further investigation. Previous studies have shown that Cotesia plutellae typically develop into wasps with mostly one individual, even if they lay multiple eggs [36]. Female wasps of mono-parasitic species engage in self-regulating parasitism when intra-specific competition is intense, thereby increasing the chances of their offspring’s survival in competition with those of other females [37]. However, only one parasitoid larva typically completes its development within each host, with excess larvae being eliminated during the physical or physiological competition [38]. Consequently, the ratio of parasitoid wasps to hosts must be carefully controlled to minimize hyperparasitism and prevent resource waste when utilizing C. formosanus for the biological control of S. frugiperda in the field.
C. formosanus eggs typically hatch into larvae within approximately one day. In contrast, Psyttalia incisi eggs take 40 to 48 h to hatch at 25 ± 1 °C [39], while Cotesia ruficrus eggs develop into larvae within about three days at 26 °C [40]. These findings indicate significant interspecific variation in the egg developmental duration among parasitoid wasp species. When C. formosanus parasitizes the host S. frugiperda, completing a generation takes approximately 19.62 days. However, when Spodoptera exigua served as the host, the development period of this wasp extends to 21 days at 27 °C [41]. This suggests that distinct host species can significantly influence the development rate of C. formosanus. Regarding adult emergence, C. formosanus exhibits diurnal eclosion, with males generally emerging earlier than females. In comparison, P. incisi adults emerge over a 24 h period [39]. Zele chlorophthalmus adults, both male and female, predominantly emerge between 06:00 and 14:00, with fewer individuals emerging between 14:00 and 22:00. Males tend to emerge 2–3 days earlier than females [42]. In Cotesia marginiventris, there is a clear sexual dimorphism in eclosion timing, with males emerging primarily in the morning (comprising over 90% of the total emergence), while females predominantly emerge in the evening (approximately 75%), between 20:30 and 01:30. Males typically emerge about 12 h earlier than females [43].
A total of 404 bacterial species were identified and annotated across various developmental stages of C. formosanus. At the phylum level, Proteobacteria was the predominant group, followed by Firmicutes in larvae, pupae, and both male and female adults. This finding is consistent with previous studies reporting that Proteobacteria and Firmicutes are the dominant bacterial phyla in the microbiota of parasitoid wasps [44]. Similar dominance of Proteobacteria has been observed in the gut microbiota of other parasitoid wasps, including Aphelinus mali [45], Lepidoptoea boulardi, Leptopilina heterotoma [46], and Cotesia vestalis [47]. Furthermore, Proteobacteria has also been identified as the predominant bacterial flora in adults of other insect species, such as Laodelphax striatellus [48], Propylaea japonica [49], and Tessaratoma papillosa [50]. In larvae and pupae of C. formosanus, the dominant bacterial genera were Enterobacter and Enterococcus. Previous studies have similarly reported that Enterococcus is the predominant genus of C. vestalis larvae [47], suggesting its commonly dominant taxon in parasitoid larvae within the family of coccinellidae. Among the dominant bacteria, E. cloacae were found in high abundance in both the larva and pupa of C. formosanus. Prior research has shown that E. cloacae can promote the growth and development of B. dorsalis larvae [9], produce insecticidal proteins toxic to pests, and aggravate host insect mortality [51]. Additionally, E. mundtii, the dominant bacterium of C. formosanus pupae, has been shown to confer host resistance to entomopathogens, although it may result in reduced fecundity in Tribolium castaneum following exposure to E. mundtii isolates [52]. Moreover, the injection of E. mundtii into corn has been reported to significantly promote the growth of early-stage peach borer larvae with increasing length and weight [53]. The bacterium Pseudomonas, dominant in female C. formosanus, has a potential for pest biocontrol [54]. Studies showed that the gut bacterium of Hypothenemus hampei, Pseudomonas, relies on caffeine as a source of carbon and nitrogen sources to survive [55]. Another dominant bacteria in female wasps, P. stutzeri, can degrade chlorpyrifos [56]. In males, the dominant bacterium, Acinetobacter, is capable of degrading toxic phenolic glycosides secreted by host plants [57]. Furthermore, R. pickettii, another highly abundant and dominant bacterium found at various developmental stages of C. formosanus, is a versatile pathogen that can inhabit diverse environments, including aquatic habitats, sediments, plants, and drinking water [58]. These results indicate that distinct bacterial groups dominate at different developmental stages of C. formosanus, with each group potentially playing a critical role at specific development stages. However, further research is required to investigate their specific functions.
In this study, we revealed that the bacterial abundance was significantly lower in C. formosanus larvae compared to those in male and female adults, consistent with the findings reported in Nasonia [59]. In contrast, the intestine microbial diversity of L. boulardi and L. heterotoma larvae was markedly higher than their counterparts in adult females [46]. This disparity is attributed to their microbial communities heavily influenced by the host’s gut microbiota. Previous studies have shown that larval parasitoids depend on host gut microbes for nutrition and survival [60]. For example, the microbial communities in three Nasonia larvae closely resembled those of their hosts, suggesting that larvae acquire the predominant microbial taxa directly from their hosts [59]. Furthermore, significant differences in bacterial composition were observed between male and female C. formosanus adults, with male wasps harboring a higher number of bacterial species than females. This pattern is consistent with observations in other insect species, such as Chrysoperla sinica [61], Leptocybe invasa [62], and Helicoverpa armigera [63], where males also exhibited higher microbial diversity. Conversely, P. japonica females had greater gut bacterial abundance and diversity than males, while in Ericerus pela, females displayed higher bacterial diversity than males, likely due to the fact that male wasps develop under a protective ash layer and experience a more simplified environment compared to females [64]. These findings suggest that variations in the bacterial communities between wasp male and female adults may be related to the differences in their physiological metabolism and life history traits. Picrust2-based functional prediction of bacterial KEGG pathways at different developmental stages of C. formosanus revealed that metabolic pathways were the most abundant functional category across all different development stages of C. formosanus. The presence of genes associated with metabolic pathways is likely crucial for nutrient acquisition and host growth [28]. The biosynthesis of secondary metabolites was also prominently represented. Although the abundance of bacterial functional categories appeared similar across developmental stages, given that the functional predictions from PICRUSt2 are somewhat speculative, it is necessary to further verify through metagenomic studies whether these functions truly exist and play specific roles in the growth and development of C. formosanus.
This study represents the first comprehensive examination of developmental dynamics in C. formosanus. However, the interspecies competition between multiple individuals within the host and the underlying mechanism of the eventual sole surviving wasp remains to be further elucidated. Additionally, the species composition and abundance of intestinal bacteria were unveiled across different developmental stages of C. formosanus, thereby broadening the existing knowledge of its resided microbiota. These findings provide valuable insights into the core microorganisms in C. formosanus and shed light on their potential biological functions, which may serve as a foundation for exploring their application for pest control.
5. Conclusions
In summary, this study provides a comprehensive analysis of the developmental and morphological dynamics of Chelonus formosanus, establishing a morphological foundation on this wasp and enabling deeper investigations. The dominant bacterial taxa were characterized at different developmental stages, revealing significant differences in bacterial composition and abundance across developmental stages, as well as between female and male adults. Notably, males harbored the greatest number of bacterial species and endemics, while pupae exhibited the lowest bacterial abundance and diversity. Additionally, the functional profiling of the most abundant category of bacterial communities was annotated as metabolic pathways across various developmental stages. However, the specific physiological roles of these microbial functions warrant further investigation.
Author Contributions
Conceptualization, J.J. and X.J.; investigation, J.J. and Q.F.; formal analysis, J.J. and W.H.; software, J.J. and W.H.; methodology, J.J.; data curation, J.J.; writing—original draft, J.J.; writing—review and editing, J.J. and Q.F.; recourses, X.J. and Z.L.; project administration, X.J. and Z.L.; funding acquisition, X.J. and Z.L. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the innovative team project of monitoring and integrated prevention and control of agricultural harmful invasive organisms of the Hainan Academy of Agricultural Sciences (HAAS2023TDYD11) and the technical innovation project of the provincial scientific research institute of the Hainan Academy of Agricultural Sciences (FW20230002).
Data Availability Statement
Raw sequencing data were deposited in the NCBI Short Read Archive (SRA) BioProject PRJNA1206106.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
References
- Zhang, H.Y. A Taxonomic Study on Genus Chelonus Panzer, 1806 (Hvmenoptera: Braconidae: Cheloninae) from China. Ph.D. Thesis, Zhejiang University, Hangzhou, China, 2008. [Google Scholar]
- Tang, J.H.; Lv, B.Q.; Lu, H.; Ji, X.C.; Yang, P.Y.; Su, H.; Cai, B. Investigation and preliminary study of biological characteristic of parasitic wasps of Spodoptera frugiperda in Hainan. Chin. J. Trop. Crops. 2020, 41, 1189–1195. [Google Scholar]
- Gupta, A.; Lalitha, Y.; Varshney, R.; Shylesha, A.; Van, C. Chelonus formosanus Sonan (Hymenoptera: Braconidae) an egg-larval parasitoid of the invasive pest Spodoptera frugiperda (J.E. Smith) (Lepidoptera: Noctuidae) amenable to laboratory mass production in India. J. Entomol. Zool. Stud. 2020, 8, 1521–1524. [Google Scholar]
- Calcetas, O.A.; Joshi, R.C.; Gupta, A.; Ranjith, A.P.; Madrid, M.A.; Fameronag, J. New record of the egg-larval parasitoid, Chelonus Formosanus Sonan of Fall Armyworm, Spodoptera frugiperda (J.E. Smith) in the Philippines. JPT J. Prot. Tanam. (J. Plant Prot.) 2023, 7, 103–114. [Google Scholar] [CrossRef]
- Keerthi, M.C.; Suroshe, S.S.; Doddachowdappa, S.; Shivakumara, K.T.; Mahesha, H.S.; Rana, V.S.; Gupta, A.; Murukesan, A.; Casini, R.; Elansary, H.O.; et al. Bio-Intensive tactics for the management of invasive Fall Armyworm for organic maize production. Plants 2023, 12, 685. [Google Scholar] [CrossRef]
- Douglas, A.E. Multiorganismal Insects: Diversity and function of resident microorganisms. Annu. Rev. Entomol. 2015, 60, 17–34. [Google Scholar] [CrossRef]
- Mondal, S.; Somani, J.; Roy, S.; Babu, A.; Pandey, A.K. Insect Microbial Symbionts: Ecology, Interactions, and Biological Significance. Microorganisms 2023, 11, 2665. [Google Scholar] [CrossRef] [PubMed]
- Holt, J.R.; Cavichiolli de Oliveira, N.; Medina, R.F.; Malacrinò, A.; Lindsey, A.R.I. Insect–Microbe Interactions and Their Influence on Organisms and Ecosystems. Ecol. Evol. 2024, 14, e11699. [Google Scholar] [CrossRef] [PubMed]
- Cai, Z.H. The Effects of Gut Microbiota on the Growth and the Repair of Irradiated Damage in Bactrocera dorsalis. Ph.D. Thesis, Huazhong Agricultural University, Wuhan, China, 2020. [Google Scholar]
- Ma, M.Q.; Luo, J.; Chen, X.T.; Li, C.; Li, S.Q.; Sun, J.H.; Xu, L.T. Gut bacteria facilitate leaf beetles in adapting to dietary specialization by enhancing larval fitness. NPJ Biofilms Microbiomes 2024, 10, 110. [Google Scholar] [CrossRef]
- Akami, M.; Ren, X.M.; Qi, X.W.; Mansour, A.; Gao, B.L.; Cao, S.; Niu, C.Y. Symbiotic bacteria motivate the foraging decision and Promote fecundity and survival of Bactrocera dorsalis (Diptera: Tephritidae). BMC Microbiol. 2019, 19, 229. [Google Scholar] [CrossRef] [PubMed]
- Kenyon, S.G.; Hunter, M.S. Manipulation of oviposition choice of the parasitoid wasp, Encarsia pergandiella, by the endosymbiotic bacterium Cardinium. J. Evol. Biol. 2007, 20, 707–716. [Google Scholar] [CrossRef]
- Franks, K.; Kooienga, E.; Sanders, M.; Pendarvis, K.; Yang, F.; Tomberlin, J.K.; Jordan, H.R. The effect of Rhodococcus rhodochrous supplementation on black soldier fly (Diptera: Stratiomyidae) development, nutrition, and waste conversion. J. Insects Food Feed 2021, 7, 397–408. [Google Scholar] [CrossRef]
- Yang, J.; Liu, W.Q.; Han, X.Z.; Hao, X.; Yao, Q.B.; Du, W.G. Gut microbiota modulation enhances the immune capacity of lizards under climate warming. Microbiome 2024, 12, 37. [Google Scholar] [CrossRef]
- Kang, K.; Wang, L.N.; Gong, J.; Tang, Y.L.; Wei, K. Diversity analyses of bacterial symbionts in four Sclerodermus (Hymenoptera: Bethylidae) parasitic wasps, the dominant biological cntrol agents of wood-boring beetles in China. Front. Cell. Infect. Microbiol. 2024, 14, 1439476. [Google Scholar] [CrossRef]
- Yuan, X.Q.; Zhang, X.; Liu, X.Y.; Dong, Y.L.; Yan, Z.Z.; Lv, D.B.; Wang, P.; Li, Y.P. Comparison of gut bacterial communities of Grapholita molesta (Lepidoptera: Tortricidae) reared on different host plants. Int. J. Mol. Sci. 2021, 22, 6843. [Google Scholar] [CrossRef] [PubMed]
- Gao, P. Effects of Consumption of Different Types of Prey on Diversity and Composition of Gut Microbial Communities in Harmonia axyridis (Coleoptera: Coccinellidae). Master’s Thesis, Nanjing Agricultural University, Nanjing, China, 2020. [Google Scholar]
- Li, Y.N.; Liu, L.Y.; Cai, X.M.; Yang, X.M.; Lin, J.T.; Shu, B.S. The bacterial and fungal communities of the larval midgut of Spodoptera frugiperda (Lepidoptera: Noctuidae) varied by feeding on two cruciferous vegetables. Sci. Rep. 2022, 12, 13063. [Google Scholar] [CrossRef]
- Cheng, T.T. Differences in Microbial Diversity and the Effects of Intracellular Symbiont on the Growth of Rhyzopertha dominica. Master’s Thesis, Henan of Technology University, Zhengzhou, China, 2023. [Google Scholar]
- Guo, G.S. The Research on Composition of Symbiotie Microbial Communitiesof Diaphorina citri and the Factors Influencing Composition. Master’s Thesis, Nanchang University, Nanchang, China, 2024. [Google Scholar]
- Zhang, J.; Wang, H.L.; Su, X.Y.; Wang, X.F.; Yang, M.; Bai, J.H.; Zeng, J.Y.; Li, H.P. Similar gut bacteria composition in Apriona Germari on two preferred host plants. Arch. Insect Biochem. Physiol. 2022, 110, e21899. [Google Scholar] [CrossRef]
- Shan, H.W.; Xia, X.J.; Feng, Y.L.; Wu, W.; Li, H.J.; Sun, Z.T.; Li, J.M.; Chen, J.P. The Plant-sucking insect selects assembly of the gut microbiota from environment to enhance host reproduction. NPJ Biofilms Microbiomes 2024, 10, 64. [Google Scholar] [CrossRef] [PubMed]
- Laviad-Shitrit, S.; Sela, R.; Sharaby, Y.; Thorat, L.; Nath, B.B.; Halpern, M. Comparative microbiota composition across developmental stages of natural and laboratory-reared Chironomus circumdatus populations from India. Front. Microbiol. 2021, 12, 746830. [Google Scholar] [CrossRef]
- Wang, X.Y.; Huangfu, N.; Chen, L.L.; Zhang, K.X.; Li, D.Y.; Gao, X.K.; Li, B.B.; Wang, L.; Zhu, X.Z.; Ji, J.C.; et al. Effects of developmental stages, sex difference, and diet types of the host marmalade hoverfly (Episyrphus balteatus) on symbiotic bacteria. Front. Microbiol. 2024, 15, 1433909. [Google Scholar] [CrossRef]
- González-Serrano, F.; Pérez-Cobas, A.E.; Rosas, T.; Baixeras, J.; Latorre, A.; Moya, A. The gut microbiota composition of the moth Brithys crini reflects insect metamorphosis. Microb. Ecol. 2020, 79, 960–970. [Google Scholar] [CrossRef] [PubMed]
- Nobles, S.; Jackson, C.R. Effects of life stage, site, and species on the dragonfly gut microbiome. Microorganisms 2020, 8, 183. [Google Scholar] [CrossRef]
- Sela, R.; Laviad-Shitrit, S.; Halpern, M. Changes in microbiota composition along the metamorphosis developmental stages of Chironomus transvaalensis. Front. Microbiol. 2020, 11, 586678. [Google Scholar] [CrossRef]
- Kou, R.M.; Li, Y.; Dou, F.Y.; Zhou, Z.Y.; Huang, D.Y. Diversity and differences of gut bacterial communities in different instarlarvae and diapause prepupae of Colletes gigas (Hymenoptera:Colletidae). Acta Entomol. Sin. 2021, 64, 682–693. [Google Scholar] [CrossRef]
- Zhang, Y. Effects of Developmental Stages, Diets and High Temperature Stress on Bacterial Diversity in Propylaea japonica. Master’s Thesis, Northwest Agricultural & Forestry University, Yang Ling, China, 2023. [Google Scholar]
- Wang, G.Z.; Wang, G.P.; Ma, Y.X.; Lv, Z.Y.; You, Y.W.; Ma, P.T.; Yu, Y. Composition and diversity of gut bacterial community in different life stages of Osmia excavata Alfken (Hymenoptera: Megachilidae). Microorganisms 2024, 12, 1709. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Yue, C.; Ma, N.; Yan, G.J. Effects of diet on the gut bacterial community of AldriChina grahami (Diptera: Calliphoridae) across developmental stages. Insects 2024, 15, 181. [Google Scholar] [CrossRef]
- Edgar, R.C. UPARSE: Highly accurate OTU sequences from microbial amplicon reads. Nat. Methods 2013, 10, 996–998. [Google Scholar] [CrossRef] [PubMed]
- Dubois, P.C.A.; Trynka, G.; Franke, L.; Hunt, K.A.; Romanos, J.; Curtotti, A.; Zhernakova, A.; Heap, G.A.R.; Ádány, R.; Aromaa, A.; et al. Multiple common variants for celiac disease influencing immune gene expression. Nat. Genet. 2010, 42, 295–302. [Google Scholar] [CrossRef]
- Looft, T.; Johnson, T.A.; Allen, H.K.; Bayles, D.O.; Alt, D.P.; Stedtfeld, R.D.; Sul, W.J.; Stedtfeld, T.M.; Chai, B.; Cole, J.R.; et al. In-feed antibiotic effects on the swine intestinal microbiome. Proc. Natl. Acad. Sci. USA 2012, 109, 1691–1696. [Google Scholar] [CrossRef]
- Parks, D.H.; Tyson, G.W.; Hugenholtz, P.; Beiko, R.G. STAMP: Statistical analysis of taxonomic and functional profiles. Bioinformatics 2014, 30, 3123–3124. [Google Scholar] [CrossRef]
- Li, Y.X.; Liu, Y.Q.; Liu, S.S. Effect of superparasitism on bionomics of Cotesia plutellae. Chin. J. Biol. Control 2001, 17, 151–154. [Google Scholar] [CrossRef]
- Chen, J.N. The Study on Molecular Mechanisms of Leptopilina Wasps Manipulating Host Behavior to Avoid Superparasitism. Ph.D. Thesis, Zhejiang University, Hangzhou, China, 2021. [Google Scholar]
- Tillman, P.G.; Powell, J.E. Intraspecific host discrimination and larval competition in Microplitis croceipes, Microplitis demolitor, Cotesia kazak (Hym.: Braconidae) and Hyposoter didymator (Hym.: Ichneumonidae), parasitoids of Heliothis virescens (Lep.: Noctuidae). Entomophaga 1992, 37, 229–237. [Google Scholar] [CrossRef]
- Liang, H.G.; Yang, J.Q.; Ji, Q.E.; Huang, J.C.; Cheng, J.H. Characteristics of individual development of Psyttalia incisi (Silvestri). J. Hunan Agric. Univ. (Nat. Sci.) 2007, 3, 321–323. [Google Scholar]
- He, P.Y.; Li, X.; Liu, T.X.; Zhang, S.Z. Ontogeny of Cotesia ruficrus, a Parasitoid of Spodoptera frugiperda. Chin. J. Biol. Control 2023, 39, 1488–1494. [Google Scholar]
- Ji, X.C.; Yue, J.J.; Qing, S.; Chen, H.Y.; Liang, Y.P. A preliminary study on the biological characteristics of Chelonus formosanus Sonan. Chin. J. Biol. Control 2013, 29, 153–156. [Google Scholar] [CrossRef]
- Li, Q.; Cheng, Y.X.; Luo, L.Z.; Du, Q.; Jiang, X.F.; Zhang, L. Biology and bio-control potential of Zele chlorophthalmus (Hymenoptera: Braconidae), a parasitoid of beet webworm, Loxostege sticticalis (Lepidoptera: Crambidae). Chin. J. Biol. Control 2017, 33, 803–810. [Google Scholar] [CrossRef]
- Zhang, H.Y.; Zhang, J.J.; Wang, S.S.; Wang, Y.L.; Wang, Q.Q.; Wang, X.P. Parasitic efficacy and development performance of Cotesia marginiventris (Cresson) on Spodoptera frugiperda (Smith). J. Henan Agric. Sci. 2021, 50, 86–91. [Google Scholar] [CrossRef]
- Dicke, M.; Cusumano, A.; Poelman, E.H. Microbial Symbionts of Parasitoids. Annu. Rev. Entomol. 2020, 65, 171–190. [Google Scholar] [CrossRef]
- Du, M.; Yu, J.N.; Zhou, Y.J.; Wang, X.Y.; Ma, T.T.; Tan, X.M.; Wan, F.H.; Zhou, H.X. Differentiation of symbiotic bacteria is a new evidence for two genetic clades of Aphelinus mali (Hymenoptera: Aphelinidae) in China. Orient. Insects 2020, 54, 447–464. [Google Scholar] [CrossRef]
- Pan, Z.Q. Preliminary Studies on the Compositions and Functions of Microbiota from Adult and Larval Gut of Two Leptopilina Wasps. Master’s Thesis, Zhejiang University, Hangzhou, China, 2020. [Google Scholar]
- Hu, N.N.; Wang, Z.Q.; Zhang, S.J.; Wang, Z.Z.; Chen, X.X. Characterization of larval gut microbiota of two endoparasitoid Wasps associated with their common host, Plutella xylostella (Linnaeus) (Lepidoptera: Plutellidae). Microbiol. Spectr. 2024, 12, e01208-24. [Google Scholar] [CrossRef]
- Yang, X.Q.; Wang, Z.L.; Wang, T.Z.; Yu, X.P. Analysis of the bacterial community structure and diversity in the small brown planthopper, Laodelphax striatellus (Hemiptera: Delphacidae) by16S rRNA high-throughput sequencing. Acta Entomol. Sin. 2018, 61, 200–208. [Google Scholar] [CrossRef]
- Wu, L.K.; Zhang, S.; Luo, J.Y.; Zhang, L.J.; Lv, L.M.; Zhu, X.Z.; Wang, L.; Cui, J.J. Analysis of symbiotic bacteria of Propylea japonica larvae. Chin. J. Biol. Control 2018, 34, 317–323. [Google Scholar] [CrossRef]
- Han, S.C.; Jiang, J.T.; Cheng, L.; Liu, J.S.; Li, T.; Peng, L.F. Bacterial composition and diversity in the litchi stink bug, Tessaratoma papillosa (Hemiptera: Tessaratomidae) from six provinces of China. J. Plant Prot. 2022, 49, 749–757. [Google Scholar] [CrossRef]
- Liao, C.L.; Huang, R.; Yang, Y.; Huang, Y.P.; Zhang, K.; Ma, L.; Li, T.T.; Wang, L.Z.; Zhang, H.M.; Li, B.B. Effects of insecticidal proteins of Enterobacter cloacae NK on cellular immunity of Galleria mellonella larvae. Front. Microbiol. 2023, 14, 1154811. [Google Scholar] [CrossRef] [PubMed]
- Grau, T.; Vilcinskas, A.; Joop, G. Probiotic Enterococcus mundtii isolate rotects the model insect Tribolium castaneum against Bacillus thuringiensis. Front. Microbiol. 2017, 8, 1261. [Google Scholar] [CrossRef] [PubMed]
- Li, J.Y.; Ni, B.Q.; Wu, Y.N.; Yang, Y.Y.; Mu, D.L.; Wu, K.N.; Zhang, A.H.; Du, Y.L.; Li, Q. The cultivable gut bacteria Enterococcus mundtii promotes early-instar larval growth of Congethes punctiferalis via enhancing digestive enzyme activity. Pest Manag. Sci. 2024, 80, 61796188. [Google Scholar] [CrossRef]
- Teoh, M.C.; Furusawa, G.; Veera Singham, G. Multifaceted interactions between the pseudomonads and Insects: Mechanisms and prospects. Arch. Microbiol. 2021, 203, 1891–1915. [Google Scholar] [CrossRef] [PubMed]
- Ceja-Navarro, J.A.; Vega, F.E.; Karaoz, U.; Hao, Z.; Jenkins, S.; Lim, H.C.; Kosina, P.; Infante, F.; Northen, T.R.; Brodie, E.L. Gut microbiota mediate caffeine detoxification in the primary insect pest of coffee. Nat. Commun. 2015, 6, 7618. [Google Scholar] [CrossRef]
- Sasikala, C.; Jiwal, S.; Rout, P.; Ramya, M. Biodegradation of chlorpyrifos by bacterial consortium isolated from agriculture soil. World J. Microbiol. Biotechnol. 2012, 28, 1301–1308. [Google Scholar] [CrossRef]
- Mason, C.J.; Lowe-Power, T.M.; Rubert-Nason, K.F.; Lindroth, R.L.; Raffa, K.F. Interactions between bacteria and aspen defense chemicals at the phyllosphere–herbivore interface. J. Chem. Ecol. 2016, 42, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Yuan, C.; An, T.F.; Li, X.L.; Zou, J.; Lin, Z.; Gu, J.L.; Hu, R.X.; Fang, Z.Z. Genomic analysis of Ralstonia pickettii reveals the genetic features for potential pathogenicity and adaptive evolution in drinking water. Front. Microbiol. 2024, 14, 1272636. [Google Scholar] [CrossRef] [PubMed]
- Brucker, R.M.; Bordenstein, S.R. The roles of host evolutionary relationships (Genus: Nasonia) and development in structuring microbial communities. Evolution 2012, 66, 349–362. [Google Scholar] [CrossRef]
- Zhou, S.C.; Lu, Y.Q.; Chen, J.N.; Pan, Z.Q.; Pang, L.; Wang, Y.; Zhang, Q.C.; Strand, M.R.; Chen, X.X.; Huang, J.H. Parasite reliance on its host gut microbiota for ntrition and survival. ISME J. 2022, 16, 2574–2586. [Google Scholar] [CrossRef]
- Zhao, H.; Zhang, S.; Luo, J.Y.; Zhang, L.J.; Wang, A.Y.; Cui, J.J. Analysis of endophytic symbiotic bacterial composition in Chrysoperla sinica (Tjeder) Adults with 16S rDNA clone library. Chin. J. Biol. Control 2017, 33, 849–856. [Google Scholar] [CrossRef]
- Guo, C.H.; Peng, X.; Zheng, X.L.; Wang, X.Y.; Wang, R.R.; Huang, Z.Y.; Yang, Z.D. Comparison of bacterial diversity and abundance between sexes of Leptocybe Invasa Fisher & La Salle (Hymenoptera: Eulophidae) from China. PeerJ 2020, 8, e8411. [Google Scholar] [CrossRef]
- Zhao, C.C. The Biodiversity of Helicoverpa armigera Symbiotic Bacteria and Its Regulatory Effects on the Defense Response in Contton. Ph.D. Thesis, Huazhong Agricultural University, Wuhan, China, 2020. [Google Scholar]
- Lv, P. Diversity and Dynamic Changes of Symbiotic Bacteriain the Development of Ericerus pela. Master’s Thesis, Chinese Academy of Forestry, Beijing, China, 2020. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).