2. Materials and Methods
Identification of samples
Workers were identified following the detailed keys published in [
3]. In addition, images from AntWeb (
https://www.antweb.org/, accessed on 1 March 2025) were used as references, as well as specimens deposited in various collections. Other additional information comes from [
7].
Photos were taken using a JSM 6300 Scanning Electron Microscope (JEOL Ltd., Tokyo, Japan) (morphometric measurements as well). SEM microscopes provide a depth of field that cannot be matched by conventional optical microscopy. This microscope model allows the movement of the sample observation platform in the spatial axes (X, Y, Z). Between 70 and 500 magnifications were used. This microscope belongs to the central research support services (SCAI) of the University of Córdoba. Additional observation and photography of the specimens were performed with the Leica S6D stereomicroscope (Leica Microsystems, Wetzlar, Germany) (×80). The photographs taken by Paco Alarcón required focus stacking (see the details of his technique at
https://pacoalarcon-hormigas.blogspot.com/, accessed on 1 March 2025).
To quantify some of the variables, the first step was to obtain a photograph. Subsequently, specific software, such as ImageJ 1.53e [
8], had to be used.
Measurements marked with a (*) are new, and I believe that they have never been used before.
HL—head length: measured in full-face view in a straight line from the mid-point of the anterior clypeal margin to the mid-point of the posterior margin.
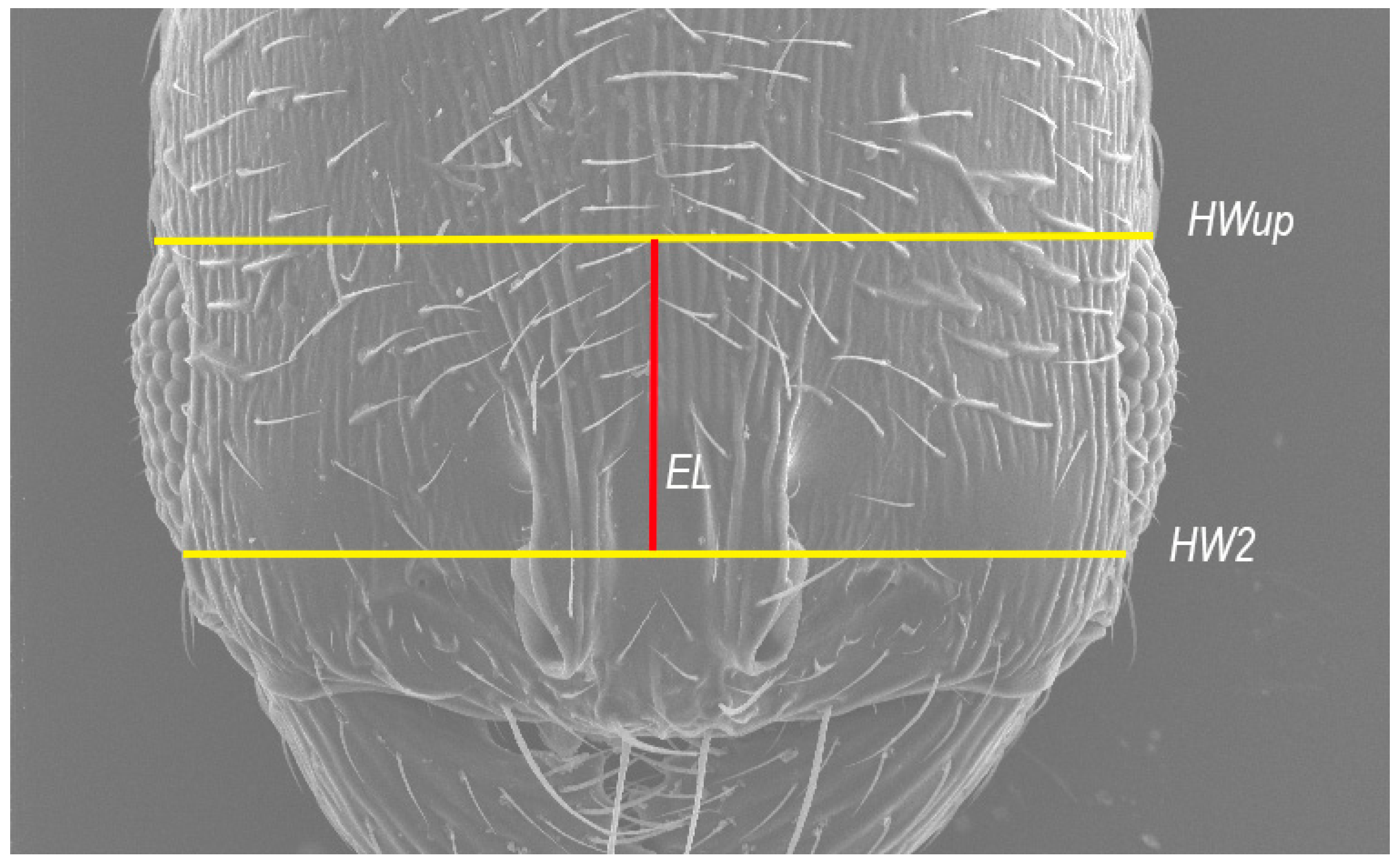
HWup—upper head width: measured in full-face view in a straight line directly above the eyes (
Figure 1).
HW2—head width: measured in full-face view directly under the eyes.
EL (*)—average between the maximum visible eye length, in full-face view. Graphically, EL represents the straight line connecting the centers of the lines corresponding to the HWup and HW measurements (see
Figure 1 for a better understanding).
SL—scape length: maximum straight-line length of the scape.
ML—mesosoma length: measured as diagonal length from the anterior end of the neck shield to the posterior margin of the propodeal lobe.
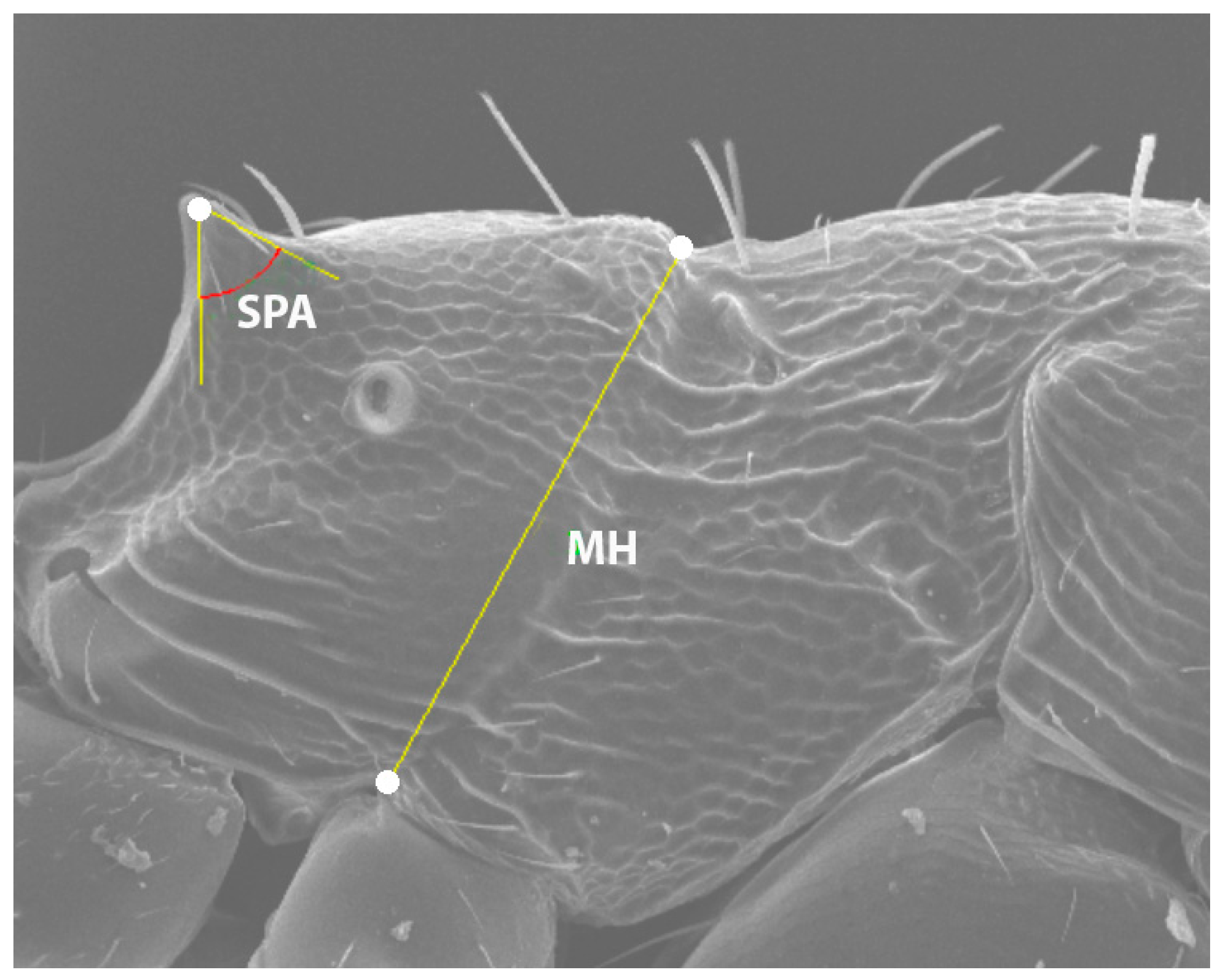
MH (*)—metanotal height: measured from the upper edge of the metanotal groove to the upper point of the mesopleural–coxal excavation in lateral view.
SPA (*)—spine angle: Maximum angle included within the spine (lateral view), with the vertex at the apex of the spine. The sides of the angle progressively diverge until each intersects the lateral contour of the spine at a single point, forming a tangent line (see
Figure 2).
SDL—spiracle-to-declivity length: minimum distance from the center of the propodeal spiracle to the propodeal declivity in lateral view.
PSL—propodeal spine length: measured from the center of the propodeal spiracle to the tip of the propodeal spine in lateral view.
PH—petiole height: maximum height of the petiole in lateral view.
PL—petiole length: measured from the spiracle to the most distal point in lateral view.
Indexes
HI—cephalic index: HWup/HL × 100.
SI—scape index: SL/HL × 100.
SPI—propodeal spine index: PSL/SDL × 100.
The format used for the measurements is µm: 178 ± 15 (163–211) = the average measurement ± standard error (min.–max.).
Examined specimens are housed in the following collections:
MNCN—Madrid Natural Science Museum. Spain.
CCZ-UGR—University of Granada. Spain.
RLC—Reyes-López collection, University of Córdoba. Spain.
Statistical analysis
For the biometric study, four taxonomic groups were used. The two species of this genus considered valid for Spain were used, and the other two groups were used to constitute potential new species (
Table 1).
The nests were classified into these four groups based on an initial visual inspection. The differences between them were so evident that no prior exploratory statistical analysis was required to determine the possible number of groups. Therefore, the analyses were conducted solely for confirmatory purposes, which simplifies the process.
A linear discriminant analysis (LDA) was used based on morphometric variables and using the species as classifier variables. Each row of the database represents the measurements of one worker. Discriminant analysis is used for dimensionality reduction, as it identifies the most important variables for distinguishing between groups (species).
LDA was conducted in R (ver. 4.1.1) (R Core Team (2021)) using the “lda” function of the package MASS (version 7.3–64) [
8]. It is based on finding the linear combinations of features that best separate the classes in the data set. All variables may not be equally important in separating taxonomic groups. To this end, a stepwise selection of variables can be performed using Wilk’s lambda criterion [
9]. The “greedy.wilks” function, package klaR (version 1.7-3), was used. The initial model is defined by starting with the variable that most separates the groups. The model is subsequently expanded to include more variables according to Wilk’s lambda criterion; the one that minimizes the Wilk’s lambda of the model that includes the variable is selected if its
p-value is still statistically significant.
The results of the LDA were plotted using the “ggplot2” package (version 3.5.0) and the “geom_polygon” function, to draw a convex hull polygon around the points (=workers) of each group.
Collinearity and its impact
In morphological studies, multicollinearity refers to a situation where two or more morphological variables are highly correlated with each other [
10]. Multicollinearity can inflate standard errors and make it difficult to interpret the effects of individual variables. Stepwise discriminant analysis may sometimes eliminate collinear variables, but its primary objective is to identify the most informative subset of predictors. The level of multicollinearity in the data can be assessed using various statistical techniques, such as the determinant of the correlation matrix among variables or the Variance Inflation Factor (VIF). When the determinant approaches zero or the VIF exceeds 10, the degree of multicollinearity is considered problematic [
10,
11,
12].
3. Results
Biometric analysis
Biometric comparisons were made with a sample of 15 nests (
Table 1): 4 nests of
Oxyopomyrmex saulcyi, 4 nests of
Oxyopomyrmex magnus and 7 nests of the new species. Therefore, comparisons were made with the species present in Spain. Between four and five workers were measured in each nest. The total sample was 57 workers.
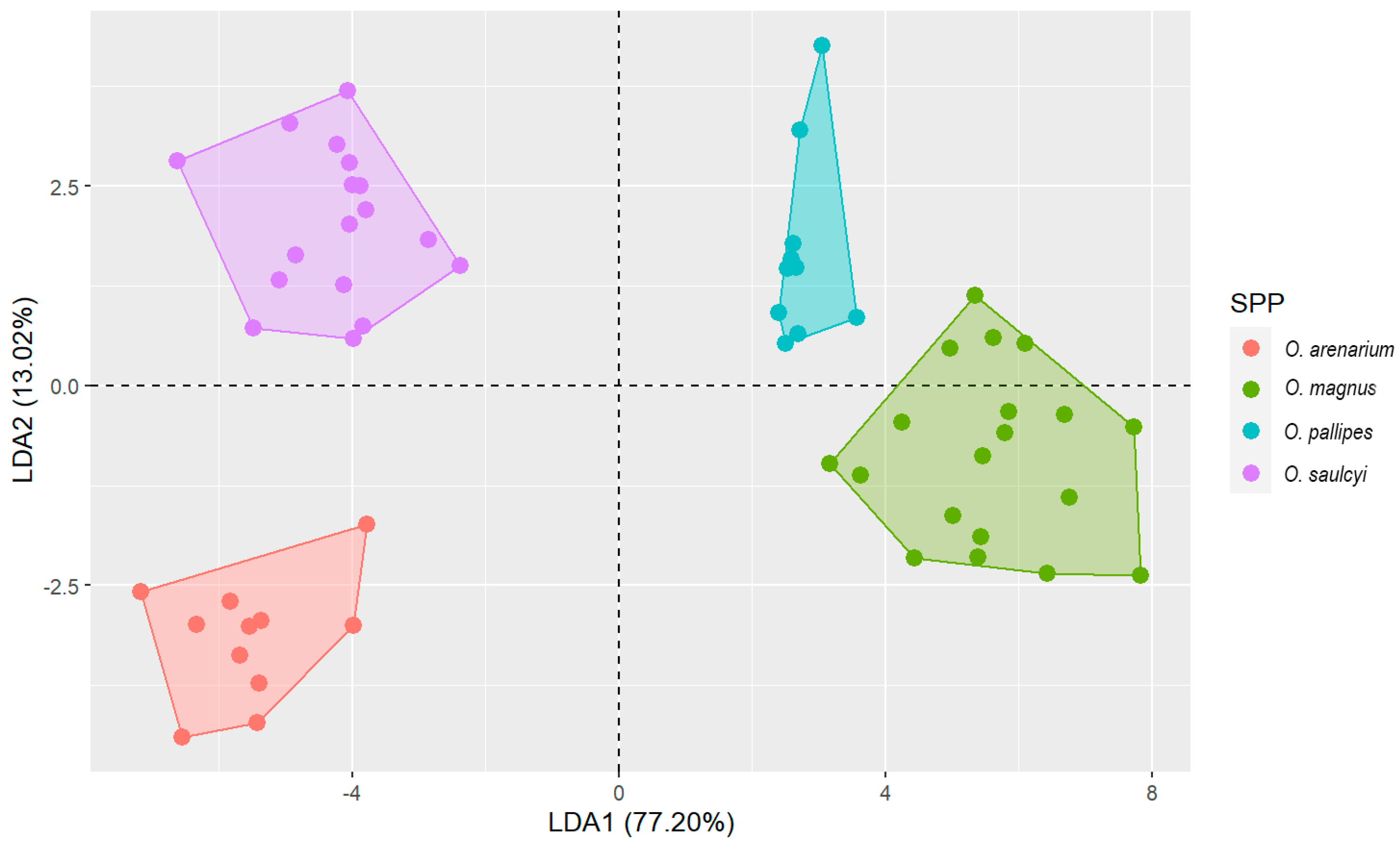
The LDA showed a significant result (Wilks’ lambda: 0.005, F (15,135) = 53.052,
p < 0.0001), using only five morphological variables: HI, EL, SPA, SPI and PH (order resulting from the step-by-step analysis). The first eigenvalue accounted for 77% of the variance. All pairwise comparisons across all four species were significant (
p < 0.001). Finally, 100% of the original grouped cases were correctly classified (see
Figure 3). One of the variables with the greatest weight in the LDA was SPA (internal angle of the propodeal spines). This highlights the value of using this variable in future revisions.
The value of the determinant (D) was nearly zero, and the VIF values were below 10 for only three variables, indicating the presence of multicollinearity. After applying stepwise LDA, the determinant increased to 0.47, and the VIF values ranged from 1.50 to 2.08, well below the threshold of 10.
Description of the species
Oxyopomyrmex arenarius sp. nov.
Etymology. The term “arenarius” derives from the Latin {arenarius, -a, -um}, meaning “of sand” or “sandy”. So far, all nests have been detected in sandy soils (dune origin).
Type locality. Doñana Biological Station (EBD-CSIC). Spain.
Type material. Holotype worker (pin): SPAIN|Doñana|18-XI-2017|RLC; two paratype workers: the same data as holotype (MNCN). The rest of the paratypes are in the author’s collection.
Measurements and ratios (in microns, n = 11): HL: 595.5 ± 9.3 (561–655); HWup: 560.6 ± 6.3 (540–596); HW2: 536.2 ± 5.7 (518–572); EL: 175.6 ± 3.3 (162–200); ML: 684.8 ± 10.3 (628–745); PSL: 109.4 ± 2.9 (96–127); SDL: 80.8 ± 2.0 (72–91); MH: 242.8 ± 5.7 (210–269); SPA (in degrees): 60.7° ± 1.6° (51°–68°); PH: 184.3 ± 4.2 (151–205); PL: 179.3 ± 9.6 (90–208); SL: 430.8 ± 4.9 (404–452); HI: 91.2 ± 0.6 (88–94); SI: 130.6 ± 0.8 (127–135).
Coloration. Head and abdomen dark brown. Thorax and legs brown in color, lighter joints. Antennae dark brown, only apex of the scapes and first segments of funiculus paler (
Figure 4A–C).
Head. Head oval, more long than wide (
Figure 4A,
Supplementary Figure S1). Antennae with 11 segments; antennal club three-jointed. Mandibles striate, with 7–8 teeth, sometimes the apical tooth massive and long. Compound eyes large, elongated, narrowing downward, reaching the anteroventral margin of the head. Shiny front, with very soft longitudinal striae, sometimes barely perceptible. Gena with either striae and rugae sparser than on frons or smooth, without sculpture, often shinier. General distribution of the setae following the pattern of the genus. Ventral surface of the head with a long psammophore.
Mesosoma. Dorsal surface of the pronotum shiny, punctuate, with some very short and smooth longitudinal rugae in the posterior part, next to the promesonotal suture (
Figure 4B,C). Mesonotum alveolate on the entire surface, lateral surfaces with several transverse striae on the posterior surface, propodeum punctate, with 3–4 distinct longitudinal rugae under the spiracles. Propodeal spines always with a wide base, usually triangular and rising obliquely upward, clearly shorter than in other species of the genus (
Figure 5).
Petiolo/Postpetiolo. Petiole rounded with short peduncle, its anterior face slightly concave, node rounded in profile. Postpetiole in profile regularly rounded (
Figure 5).
Gaster. Gaster bright without micropunctation; with sparse, erect and semierect setae.
Legs. Legs with short, sparse, appressed setae, inner margin with a row of sparse, long, semierect setae. Tibiae covered with long, appressed-to-semierect setae on the entire surface, inner margins with a row of appressed setae. Very light-colored, yellowish joints.
Gyne and Male. Unknown.
Codes of all captured nests
B0370: 4w, 28-VIII-2016, Cañada del Pocito, Cartaya, Huelva (37.258800, −7.0668960, 38 m).
B0854: 10w, 19-XI-2022, Laguna del Ojillo, PN Doñana (37.007289, −6.507369°, 25 m).
A4871: 8w, 18-XI-/2017. Laguna de Santa Olaya, PN Doñana (36.981655°, −6.483920°, 8 m).
A5002: 9w, 27-IV-2018, Laguna de Santa Olaya, PN Doñana (36.981655°, −6.483920°, 8 m).
A5003: 8w, 27-IV-2018. Laguna de Santa Olaya, PN Doñana (36.981572°, −6.483313°, 8 m).
Biological data
This species has been captured in the Doñana National Park and in the pine forests of Cartaya, always in areas with sandy soil. After rain, the nests form a very characteristic cone, 2–3 cm wide at the base and the same height. Without recent rain, the nest entrance is a simple 2–3 mm hole in the ground. Without workers entering or exiting it, it is impossible to locate, given its small diameter. Some workers were observed transporting seeds individually, without forming trails.
The stabilized dune vegetation of Doñana is dominated by halimium rockrose (Halimium halimifolium (L.)), whose light-gray foliage gives rise to the name of this plant community (“Monte Blanco”). Other aromatic plants such as rosemary (Salvia Rosmarinus L.), thyme (Thymus mastichina L.) and lavender (Lavandula stoechas L.) are abundant.
In Doñana, the species
O. saulcyi is also cited [
13,
14], although with low frequency.
Distribution
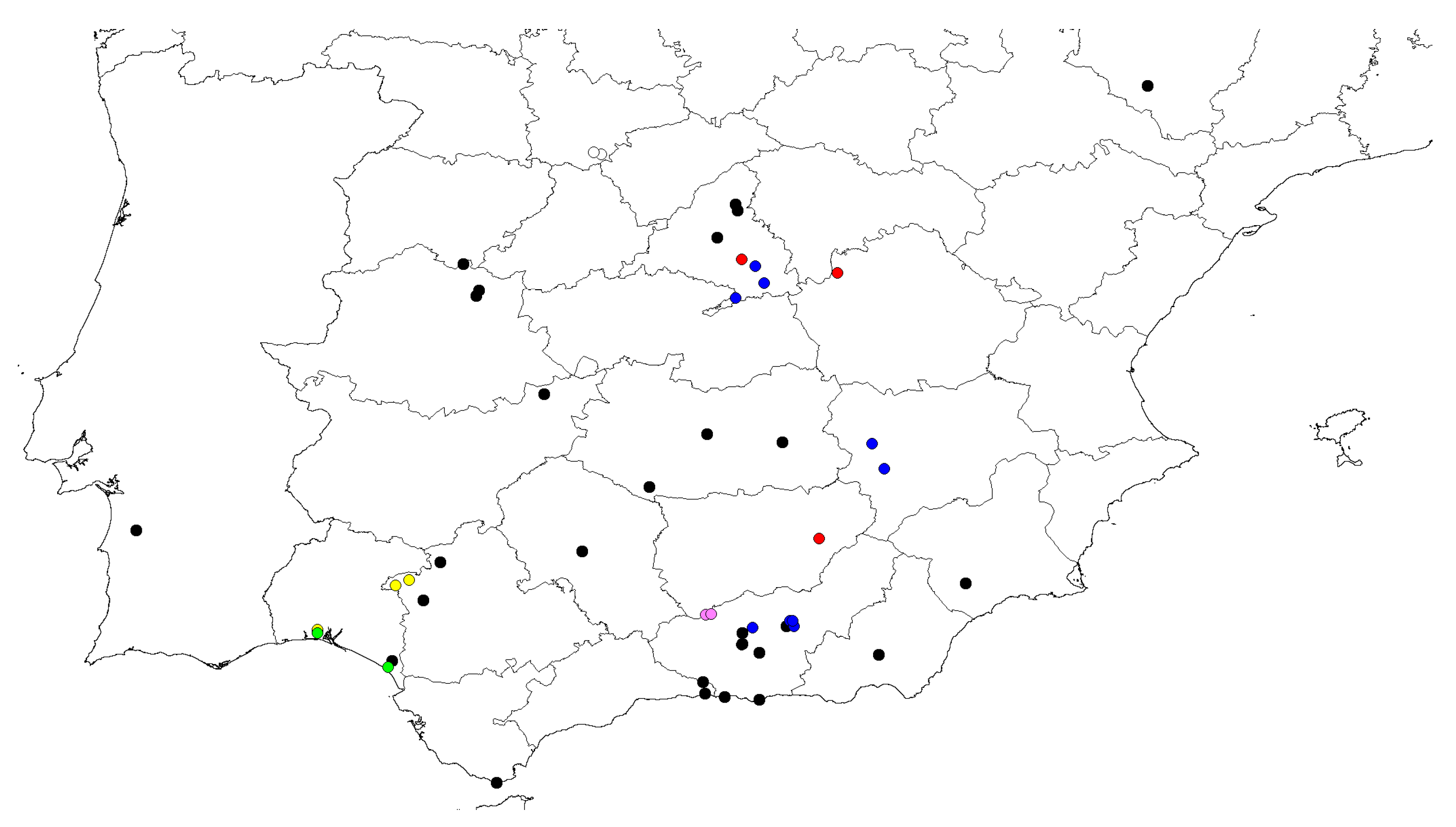
So far, all the captures have been made in the province of Huelva (southern Spain; see
Figure 6).
Differential diagnosis
Workers are somewhat smaller than O. saulcyi and are never bicolored like the latter. They have much smoother, sometimes almost indistinguishable striations on the head and mesosoma, giving them a nearly smooth and shiny appearance. The propodeal spines are shorter and triangular (SPI = 135.6 ± 2.8 (119.1–154.2); SPA = 60.7° ± 1.61° (51.0°–68.0°)). The prodopeum, viewed laterally, has a very straight back.
Oxyopomyrmex pallens sp. nov.
Etymology. The epithet “pallens” derives from the Latin {pallens, -entis} and means “pale, colorless”.
Type locality. Mures (Jaen). Spain.
Type material. Holotype worker (top on the pin): SPAIN|Mures|28-VI-2019|(RLC); two paratype workers: the same data as holotype (MNCN). The rest of the paratypes in the author’s collection.
Worker description
Measurements and ratios (in microns, n = 10): HL: 580.8 ± 10.0 (538–626); HWup: 601.7 ± 9.0 (561–644); HW2: 573.2 ± 9.0 (530–608); EL: 178.7 ± 1.7 (170–185); ML: 715.3 ± 15.7 (639–782); PSL: 144.7 ± 4.4 (123–166); SDL: 86.2 ± 3.8 (63–105); MH: 262.9 ± 7.3 (221–287); SPA (in degrees): 40.5° ± 1.3° (35°-46°); PH: 195.9 ± 3.2 (183–207); PL: 197.5 ± 5.7 (170–212); SL: 421.3 ± 8.5 (377–460); HI: 103.6 ± 0.3 (102–105); SI: 143.0 ± 1.1 (137–150).
Coloration. Head, thorax and abdomen pale brown (
Figure 7A–C,
Supplementary Figure S2). Antennal scapes pale yellow, apex of the scapes, pedicels and first segments of the funiculus yellowish brown. Mandibles brown to pale brown. Femora pale yellow, tibiae and tarsi yellowish brown. In some specimens, coloration may be darker, approaching black.
Head. Head quadrangular, more wide than long, with the lateral surfaces below the eyes straight and gently rounded at the posterior edges (as in
O. magnus). Frons area smooth and shiny. Typical compound eyes of the genus: longitudinal, strongly narrowed downward, reaching the anteroventral margin. Scape short, 0.7 times as long as the width of the head. Surface of the scape without microsculpture, shiny, covered with short, dense and semierect setae. Clypeus shiny, with some very faint longitudinal rugae. Frontal carinae short, extending to the upper edge of the antennal fossa; antennal fossae smooth without rounded striae, frontal lobes with coarse longitudinal striae, area between striae shiny. Vertex shiny, with the entire surface with coarse longitudinal rugae. Area above eyes rough with thinner longitudinal rugae. Ventral surface of the head with distinctive striations and roughened, gena shiny with longitudinal striations and roughness. Entire head bearing semierect setae, the posterior margin with sparse erect setae directed forward, lateral surfaces of the head with setae directed toward the anterior margin, the frontal area with dense semierect setae placed transversely, directed to the center of the head, the ventral surface of the head with a psammophore.
Mesosoma. Promesonotum clearly convex in profile. Promesonotal suture distinct. Metanotal groove is well marked. Propodeal spines triangular, rising obliquely upward (
Figure 5). Pronotum shiny, rugose with longitudinal striae, lateral surfaces finely rugulose with longitudinal striae. Dorsal surface of pronotum rugose, the area between rugae shiny, lateral surfaces with longitudinal striae and finely rugulose, shiny. Mesonotum rugose and shiny on the dorsum, lateral surfaces rugose with longitudinal striae, the dorsal surface of the propodeum with longitudinal striae or rugose, punctate with longitudinal striae and rugose below spiracles.
Petiolo/postpetiolo. Petiole node rounded with a short peduncle, its anterior surface concave, clearly rounded node on the dorsal surface when seen in profile. Posterior surface slightly convex. Postpetiole rounded in profile. Base of the petiole and postpetiole on the entire surface punctate, nodes punctate, sparse punctation or micropunctae on the top, shiny. Sides punctate without longitudinal striae, covered with several setae.
Gaster. Gaster shiny without microreticulation, with erect setae.
Legs. In some of the workers examined, the legs (and joints) are very light in color, yellowish white.
Differential diagnosis
This species is characterized by a head that is wider than it is long (HI > 100). It belongs to the magnus group, which also includes
Oxyopomyrmex magnus,
O. emeryi Santschi, 1908, and
O. nitidior Santschi, 1910 [
3]. Within this group, the new species is clearly distinguishable from
O. nitidior by its light coloration (light brown), as well as by the presence of pronounced striations covering the entire surface of the head, with no smooth areas on the frons or occipital region. This trait is consistent across all examined specimens.
It differs from
O. magnus primarily in coloration and in the absence of prominent rugae on the mesosoma. Additionally,
O. magnus is significantly larger. Compared to
O. emeryi, the new species can be distinguished by the lack of a shiny, finely punctate pronotum; in
O. emeryi, the entire surface of the pronotum is smooth or micropunctate, particularly on the lateral areas. The maximum size measured so far for ML is 782 microns. For
O. magnus, the minimum value is 799 µm according to our data (844 µm in [
3]). There appears to be a clear size separation between the two species. The size range of
O. nitidior is between 704 and 894 µm [
3].
Gyne and Male. Unknown.
Biological data. Nothing is known about the biology of this species. The nests were in secondary pastures used for livestock farming. There were scattered holm oaks (Quercus rotundifolia).
Distribution. Spain: mainland (
Figure 6).
Codes of captured nests
A5289, 17w, 14-VI-2019, Mures, Jaén (37.415170, –3.847481, 840 m).
A5293, 12w, 14-VI-2019, Mures, Jaén (idem).
A5309, 34w, 28-VI-2019, Mures, Jaén (idem).
A5310, 21w, 10-VII-2021, Gumiel, Granada, (37.418762, –3.805206, 848 m).
Geographical distribution of continental Spanish species
In the last work of a compilation of citations on this species [
6], after the taxonomic revision [
3], it was said to be distributed throughout 17 Spanish provinces, Andorra and Portugal, covering almost the entire peninsula (except the northwest). All collected nests are geographically located in the southern part of Spain.
O. saulcyi exhibits a broader distribution [
6], covering nearly all of Spain, and our data contribute several new locality records. In contrast, for
O. magnus, this study provides new occurrence data for a species about which very little geographical information is available (see
Table 1,
Figure 6).
O. magnus continues to show a tendency to nest at high altitudes, exhibiting a marked orophilous character (590–1411 m). We consistently found the excavated nests of this species in open areas, away from forested zones.
Key to known species of Oxyopomyrmex in continental Spain (workers)
- -
Head longer than wide (HI < 100)...................................................................................................................................................................................................................................3
- 2.
Center of frons rugulose with longitudinal, uniformly distributed striae. Dark brown or black color ...............................................................................................O. magnus
- -
Softer striae on the forehead. Body color is paler, never black......................................................................................................................................................O. pallipes sp. nov.
- 3.
Propodeum with a very straight profile in lateral view. Propodeal spines are triangular in appearance (SPA: 60.7° ± 1.6° (51°–68°)), very short. Dorsal surface of pronotum at least partly punctate with or without striation .........................................................................O. arenarius sp. nov.
- -
Propodeum with convex profile. Propodeal spines longer and thinner (SPA: 44.1° ± 1.07° (37°–53°)). Dorsal surface of pronotum distinctly rugulose ................O. saulcyi