Sec61s and Sec62/Sec63 Genes Are Essential for Survival by Regulating the Gut and Cuticle Development in Locusta migratoria
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Insects
2.2. Identification of LmSec61s and LmSec62/Sec63 and Bioinformatics Analysis
2.3. The Spatiotemporal Expression Analysis of LmSec61s and LmSec62/Sec63
2.4. Synthesis of Different dsRNAs
2.5. Body Weight and Survival Rate Assay After LmSec61s RNAi
2.6. The Gut Changes After LmSec61s RNAi
2.7. The Survival Rate and Cuticle Development Analysis After LmSec62/Sec63 RNAi
2.8. Transmission Electron Microscopy (TEM) of the Integument
2.9. Statistical Analysis
3. Results
3.1. Identification of LmSec61s and LmSec62/Sec63 and Bioinformatic Analysis
3.2. Tissue and Developmental Expression Patterns of LmSec61s and LmSec62/Sec63
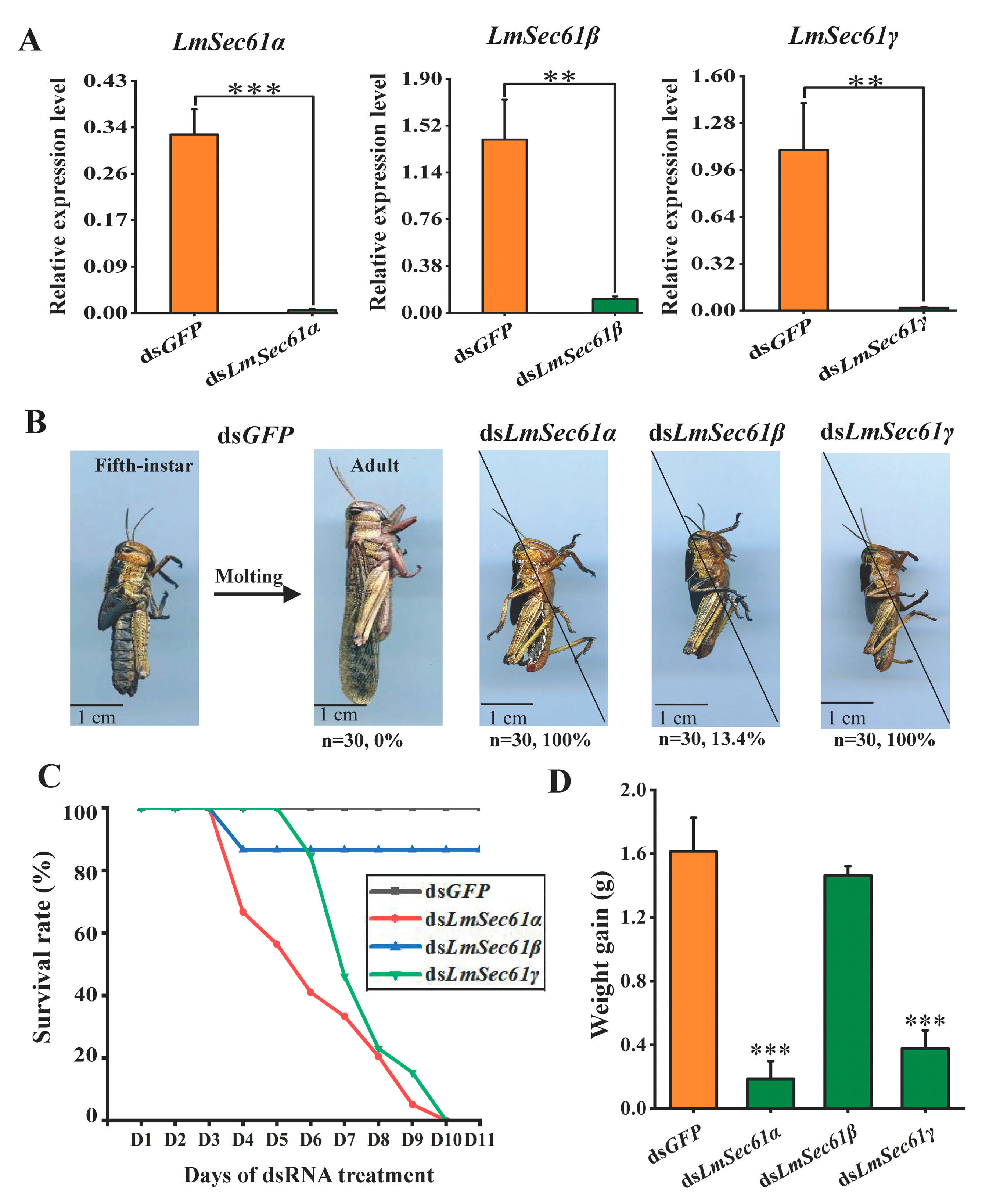
3.3. Effect on Nymphal Survival and Body Weight After LmSec61s RNAis
3.4. LmSec61α and LmSec61γ Knockdown Resulted in Gut Atrophy
3.5. The Histological Changes in the Midguts and Gastric Cecum of dsLmSec61s-Treated Locusts
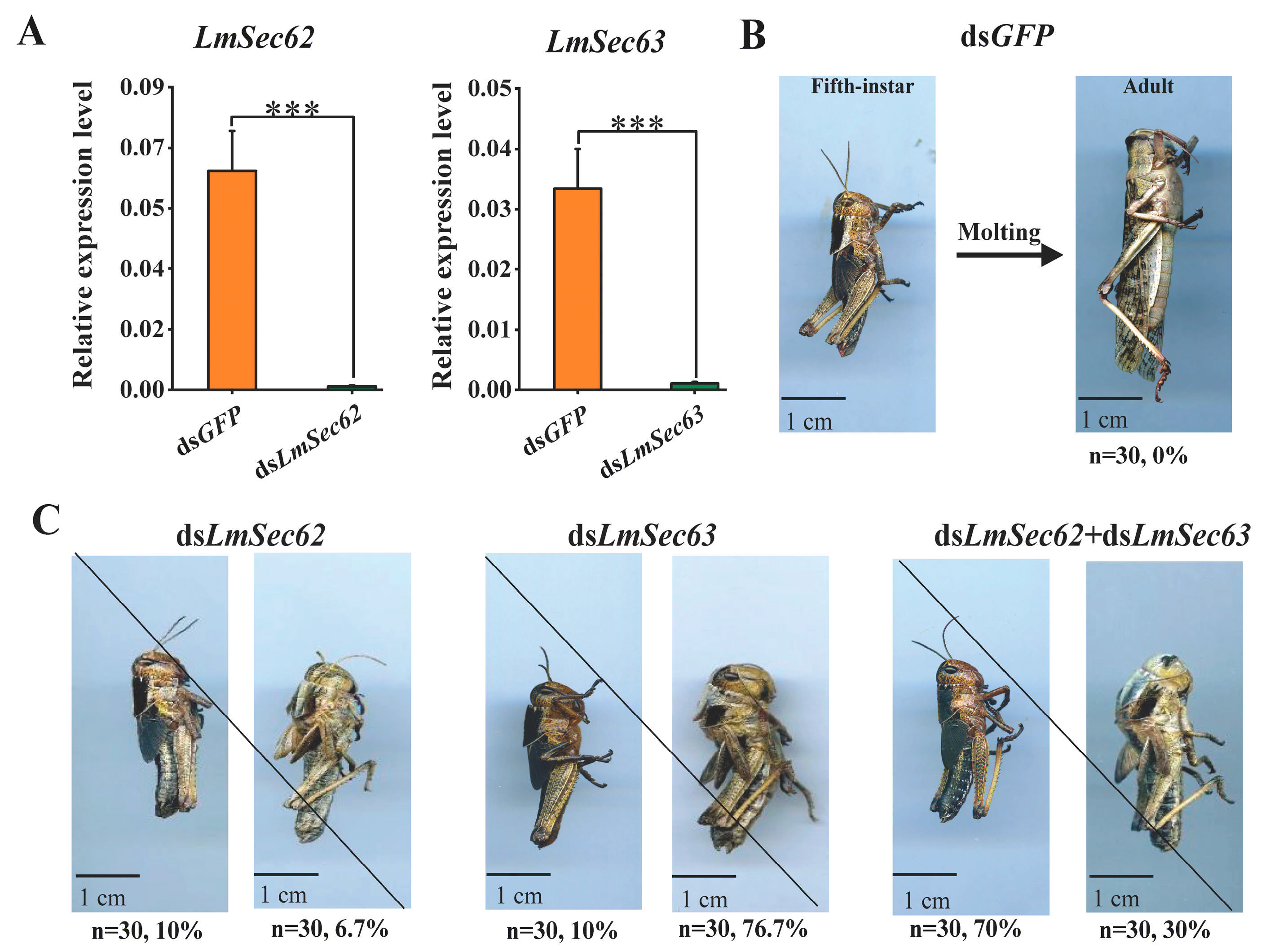
3.6. Effect on Nymphal Survival After Knockdown of LmSec62/Sec63
3.7. Effect on the Cuticle Development After Knockdown of LmSec63
4. Discussion
4.1. Sec Proteins Are Highly Conserved in Organisms
4.2. LmSec61s Are Required for the Gut Development
4.3. LmSec62/Sec63 Are Essential for the Cuticle Development
4.4. The Possibility of LmSec61 and LmSec63 in Pest Control
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Keenan, R.J.; Freymann, D.M.; Stroud, R.M.; Walter, P. The signal recognition particle. Annu. Rev. Biochem. 2001, 70, 55–775. [Google Scholar] [CrossRef] [PubMed]
- Linxweiler, M.; Schick, B.; Zimmermann, R. Let’s talk about Secs: Sec61, Sec62 and Sec63 in signal transduction, oncology and personalized medicine. Signal Transduct. Target. Ther. 2017, 2, 17002. [Google Scholar] [CrossRef]
- Itskanov, S.; Park, E. Mechanism of protein translocation by the Sec61 translocon complex. Cold Spring Harb. Perspect. Biol. 2023, 15, a041250. [Google Scholar] [CrossRef]
- Jung, S.J.; Kim, H. Emerging view on the molecular functions of Sec62 and Sec63 in protein translocation. Int. J. Mol. Sci. 2021, 22, 12757. [Google Scholar] [CrossRef]
- Itskanov, S.; Park, E. Structure of the posttranslational Sec protein-translocation channel complex from yeast. Science 2019, 363, 84–87. [Google Scholar] [CrossRef]
- Itskanov, S.; Kuo, K.M.; Gumbart, J.C.; Park, E. Stepwise gating of the Sec61 protein-conducting channel by Sec63 and Sec62. Nat. Struct. Mol. Biol. 2021, 28, 162–172. [Google Scholar] [CrossRef]
- Wu, X.; Cabanos, C.; Rapoport, T.A. Structure of the post-translational protein translocation machinery of the ER membrane. Nature 2019, 566, 136–139. [Google Scholar] [CrossRef]
- Valcárcel, R.; Weber, U.; Jackson, D.B.; Benes, V.; Ansorge, W.; Bohmann, D.; Mlodzik, M.J. Sec61β, a subunit of the protein translocation channel, is required during Drosophila development. J. Cell Sci. 1999, 112 Pt 23, 4389–4396. [Google Scholar] [CrossRef]
- Kelkar, A.; Dobberstein, B. Sec61β, a subunit of the Sec61 protein translocation channel at the endoplasmic reticulum, is involved in the transport of Gurken to the plasma membrane. BMC Cell Biol. 2009, 10, 11. [Google Scholar] [CrossRef]
- Minami, R.; Sato, C.; Yamahama, Y.; Kubo, H.; Hariyama, T.; Kimura, K.I. An RNAi screen for genes involved in nanoscale protrusion formation on corneal lens in Drosophila melanogaster. Zool. Sci. 2016, 33, 583–591. [Google Scholar] [CrossRef][Green Version]
- Kanuka, H.; Hiratou, T.; Igaki, T.; Kanda, H.; Kuranaga, E.; Sawamoto, K.; Aigaki, T.; Okano, H.; Miura, M. Gain-of-function screen identifies a role of the Sec61α translocon in Drosophila postmitotic neurotoxicity. Biochim. Biophys. Acta 2005, 1726, 225–237. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Ma, G.; Ren, F.; Awais, M.M.; Sun, J. Potential proteins interactions with Bombyx mori nucleopolyhedrovirus revealed by co-immunoprecipitation. Insects 2022, 13, 575. [Google Scholar] [CrossRef]
- Pflüger, H.J.; Bräunig, P. One hundred years of phase polymorphism research in locusts. J. Comp. Physiol. A 2021, 207, 321–326. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.Y.; Zeng, B.J.; Shang, T.T.; Zhou, S.T. Identification of G protein-coupled receptors required for vitellogenesis and egg development in an insect with panoisticovary. Insect Sci. 2021, 28, 1005–1017. [Google Scholar] [CrossRef] [PubMed]
- Niu, J.; Chen, R.; Wang, J.J. RNA interference in insects: Link between antiviral defense and pest control. Insect Sci. 2024, 31, 2–12. [Google Scholar] [CrossRef]
- Zhao, X.M.; Gou, X.; Qin, Z.Y.; Li, D.Q.; Wang, Y.; Ma, E.B.; Li, S.; Zhang, J.Z. Identification and expression of cuticular protein genes based on Locusta migratoria transcriptomes. Sci. Rep. 2017, 7, 45462. [Google Scholar] [CrossRef]
- Liu, X.J.; Li, F.; Zhang, W.Q.; Ma, E.B.; Zhu, K.Y.; Zhang, J.Z. Molecular and functional analysis of UDP-N-acetylglucosamine pyrophosphorylases from the migratory locust Locusta migratoria. PLoS ONE 2013, 8, e71970. [Google Scholar] [CrossRef]
- Liu, W.M.; Xie, Y.P.; Xue, J.L.; Gao, Y.; Zhang, Y.F.; Zhang, X.M.; Tan, J.S. Histopathological changes in Ceroplastes japonicus infected with Lecanicillium lecanii. J. Invertebr. Pathol. 2009, 101, 96–105. [Google Scholar] [CrossRef]
- Zhao, X.M.; Qin, Z.Y.; Liu, W.M.; Liu, X.J.; Moussian, B.; Ma, E.B.; Li, S.; Zhang, J.Z. Nuclear receptor HR3 controls locust molt by regulating chitin synthesis and degradation genes of Locusta migratoria. Insect Biochem. Mol. Biol. 2018, 92, 1–11. [Google Scholar] [CrossRef]
- Park, E.; Rapoport, T.A. Mechanisms of Sec61/SecY-mediated protein translocation across membranes. Annu. Rev. Biophys. 2012, 41, 21–40. [Google Scholar] [CrossRef]
- Voorhees, R.M.; Hegde, R.S. Structure of the Sec61 channel opened by a signal sequence. Science 2016, 351, 88–91. [Google Scholar] [CrossRef]
- Matlack, K.E.; Misselwitz, B.; Plath, K.; Rapoport, T.A. BiP acts as a molecular ratchet during posttranslational transport of proteins across the ER membrane. Cell 1999, 97, 553–564. [Google Scholar] [CrossRef] [PubMed]
- Toni, A.S.B.; Fialho, V.S.; Cossolin, J.F.S.; Serrão, J.E. Larval and adult digestive tract of the carrion beetle Oxelytrum discicolle (Brullé, 1840) (Coleoptera: Silphidae). Arthropod Struct. Dev. 2022, 71, 101213. [Google Scholar] [CrossRef]
- Liu, X.J.; Cooper, A.M.W.; Zhang, J.Z.; Zhu, K.Y. Biosynthesis, modification, and degradation of chitin during the formation and turnover of the peritrophic matrix in insects. J. Insect Physiol. 2019, 114, 109–115. [Google Scholar] [CrossRef] [PubMed]
- Noriega, F.G.; Edgar, K.A.; Bechet, R.; Wells, M.A. Midgut exopeptidase activity in Aedes aegypti was induced by blood feeding. J. Insect Physiol. 2002, 48, 205–212. [Google Scholar] [CrossRef]
- Li, Y.L.; Hou, M.Z.; Shen, G.M.; Lu, X.P.; Wang, Z.; Jia, F.X.; Wang, J.J.; Dou, W. Functional analysis of five trypsin-like protease genes in the oriental fruit fly, Bactrocera dorsalis (Diptera: Tephritidae). Pestic. Biochem. Physiol. 2017, 136, 52–57. [Google Scholar] [CrossRef]
- Isoe, J.; Rascón, A.A., Jr.; Kunz, S.; Miesfeld, R.L. Molecular genetic analysis of midgut serine proteases in Aedes aegypti mosquitoes. Insect Biochem. Mol. Biol. 2009, 39, 903–912. [Google Scholar] [CrossRef]
- Liu, X.J.; Zhang, H.H.; Li, S.; Zhu, K.Y.; Ma, E.B.; Zhang, J.Z. Characterization of a midgut-specific chitin synthase gene (LmCHS2) responsiblefor biosynthesis of chitin of peritrophic matrix in Locusta migratoria. Insect Biochem. Mol. Biol. 2012, 42, 902–910. [Google Scholar] [CrossRef]
- Zhao, X.; Zhang, J.; Yang, J.; Niu, N.; Zhang, J.; Yang, Q. Mucin family genes are essential for the growth and development of the migratory locust, Locusta migratoria. Insect Biochem. Mol. Biol. 2020, 123, 103404. [Google Scholar] [CrossRef]
- Muthukrishnan, S.; Mun, S.; Noh, M.Y.; Geisbrecht, E.R.; Arakane, Y. Insect cuticular chitin contributes to form and function. Curr. Pharm. Des. 2020, 26, 3530–3545. [Google Scholar] [CrossRef]
- Ren, Y.; Li, Y.; Ju, Y.; Zhang, W.; Wang, Y. Insect cuticle and insecticide development. Arch. Insect Biochem. Physiol. 2023, 114, e22057. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.j.; Sun, Y.w.; Li, D.Q.; Li, S.; Ma, E.B.; Zhang, J.Z. Identification of LmUAP1 as a 20-hydroxyecdysone response gene in the chitin biosynthesis pathway from the migratory locust, Locusta migratoria. Insect sci. 2018, 25, 211–221. [Google Scholar] [CrossRef]
- Yang, M.; Wang, Y.; Jiang, F.; Song, T.; Wang, H.; Liu, Q.; Zhang, J.; Zhang, J.; Kang, L. miR-71 and miR-263 jointly regulate target genes chitin synthase and chitinase to control locust molting. PLoS Genet. 2016, 12, e1006257. [Google Scholar] [CrossRef]
- Sviben, S.; Spaeker, O.; Bennet, M.; Albéric, M.; Dirks, J.H.; Moussian, B.; Fratzl, P.; Bertinetti, L.; Politi, Y. Epidermal cell surface structure and chitin-protein co-assembly determine fiber architecture in the locust cuticle. ACS Appl. Mater. Interfaces 2020, 12, 25581–25590. [Google Scholar] [CrossRef]
- Bachman, P.M.; Bolognesi, R.; Moar, W.J.; Mueller, G.M.; Paradise, M.S.; Ramaseshadri, P.; Tan, J.G.; Uffman, J.P.; Warren, J.; Wiggins, E.; et al. Characterization of the spectrum of insecticidal activity of a double-stranded RNA with targeted activity against Western corn rootworm (Diabrotica virgifera virgifera LeConte). Transgenic Res. 2013, 22, 1207–1222. [Google Scholar] [CrossRef]
- Bachmann, P.M.; Huizinga, K.M.; Jensen, P.D.; Mueller, G.; Tan, J.G.; Uffman, J.P.; Levine, S.L. Ecological risk assessment for DvSnf7 RNA: A plantincorporated protectant with targeted activity against western corn rootworm. Regul. Toxicol. Pharm. 2016, 81, 77–88. [Google Scholar] [CrossRef]
- Chen, Y.; Shi, Y.; Wang, Z.; An, X.; Wei, S.; Andronis, C.; Vontas, J.; Wang, J.J.; Niu, J. dsRNAEngineer: A web-based tool of comprehensive dsRNA design for pest control. Trends Biotechnol. 2025, 43, 969–983. [Google Scholar] [CrossRef]
- Song, H.F.; Fan, Y.H.; Zhang, J.Q.; Cooper, A.M.; Silver, K.; Li, D.Q.; Li, T.; Ma, E.B.; Zhu, K.Y.; Zhang, J.Z. Contribution of dsRNases to differential RNAi efficiencies between injection and oral delivery of dsRNA in Locusta migratoria. Pest Manag. Sci. 2019, 75, 1707–1717. [Google Scholar] [CrossRef]
- Lu, Q.; Cui, H.Y.; Li, W.D.; Liu, T.; Chen, Q.X.; Yang, Q. Synthetic nanoscale RNAi constructs as pesticides for the control of Locust migratoria. J. Agric. Food Chem. 2022, 70, 10762–10770. [Google Scholar] [CrossRef]
- Kong, X.; Tan, S.Q.; Guan, M.; Lin, X.X.; Shen, J.; Shi, W.P.; Wang, D. Nanocarrier-mediated transdermal delivery of Lmidgf4 dsRNA expedites biological control of locusts by Beauveria bassiana. J. Nanobiotechnol. 2025, 23, 272. [Google Scholar] [CrossRef]






| Name | CDS (bp) | Amino Acids | Mw (KDa) | pI | Transmembrane Domains (TM) (Position; aa) |
|---|---|---|---|---|---|
| LmSec61α | 1431 | 476 | 55.33 | 8.50 | 34–53; 77–96; 118–138; 145–165; 173–193; 241–261; 289–309; 354–375; 421–441; 446–462 |
| LmSec61β | 297 | 98 | 10.14 | 11.08 | 72–92 |
| LmSec61γ | 207 | 68 | 7.69 | 9.92 | 36–61 |
| LmSec62 | 1104 | 367 | 42.77 | 6.23 | 193–213; 224–245 |
| LmSec63 | 2277 | 758 | 88.05 | 5.54 | 13–35; 74–94; 192–212 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, X.; Ji, M.; Zhang, J. Sec61s and Sec62/Sec63 Genes Are Essential for Survival by Regulating the Gut and Cuticle Development in Locusta migratoria. Insects 2025, 16, 550. https://doi.org/10.3390/insects16060550
Liu X, Ji M, Zhang J. Sec61s and Sec62/Sec63 Genes Are Essential for Survival by Regulating the Gut and Cuticle Development in Locusta migratoria. Insects. 2025; 16(6):550. https://doi.org/10.3390/insects16060550
Chicago/Turabian StyleLiu, Xiaojian, Mingzhu Ji, and Jianzhen Zhang. 2025. "Sec61s and Sec62/Sec63 Genes Are Essential for Survival by Regulating the Gut and Cuticle Development in Locusta migratoria" Insects 16, no. 6: 550. https://doi.org/10.3390/insects16060550
APA StyleLiu, X., Ji, M., & Zhang, J. (2025). Sec61s and Sec62/Sec63 Genes Are Essential for Survival by Regulating the Gut and Cuticle Development in Locusta migratoria. Insects, 16(6), 550. https://doi.org/10.3390/insects16060550





