Filling the Spring Gap in Southern Australia: Seasonal Activity of Four Dung Beetle Species Selected to Be Imported from Morocco
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Description of Studied Species
2.1.1. Euonthophagus crocatus
2.1.2. Onthophagus marginalis subsp. andalusicus
2.1.3. Onthophagus vacca
2.1.4. Gymnopleurus sturmi
2.2. Site Descriptions
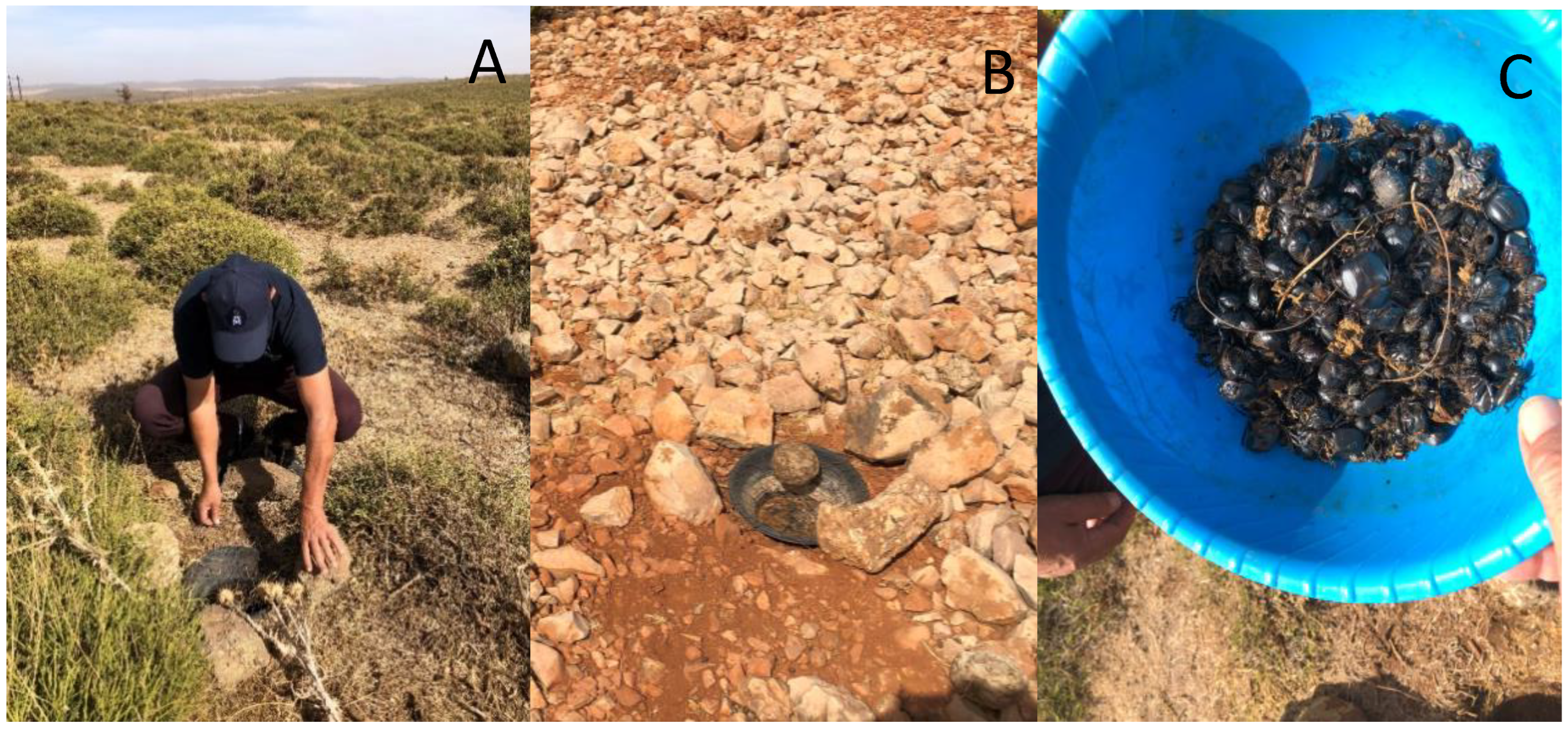
2.3. Sampling
2.4. Analyses
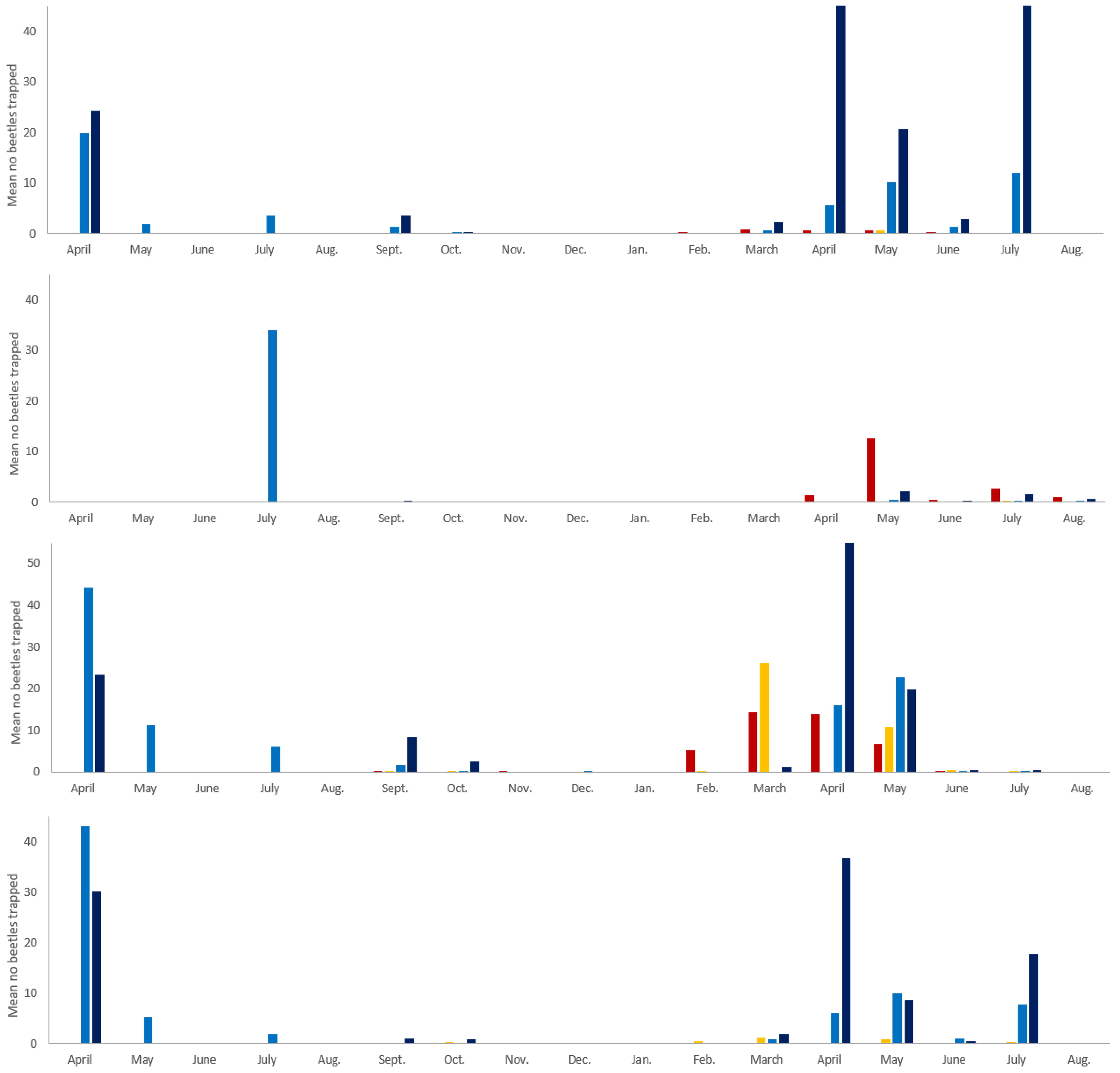
3. Results
4. Discussion
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ridsdill-Smith, T.J.; Edwards, P.B. Biological control: Ecosystem functions provided by dung beetles. In Ecology and Evolution of Dung Beetles; Simmons, L.W., Ridsdill-Smith, T.J., Eds.; Blackwell Publishing Ltd.: Hoboken, NJ, USA, 2011. [Google Scholar]
- Nichols, E.; Spector, S.; Louzada, J.; Larsen, T.; Amezquita, S.; Favila, M.E. Ecological functions and ecosystem services provided by Scarabaeinae dung beetles. Biol. Conserv. 2008, 141, 1461–1474. [Google Scholar] [CrossRef]
- Doube, B.; Macqueen, A.; Ridsdill-Smith, T.J.; Weir, T.A. Native and introduced dung beetles in Australia. In Dung Beetle Ecology; Hanski, I., Cambefort, Y., Eds.; Princeton University Press: Princeton, NJ, USA, 1991; pp. 255–278. [Google Scholar]
- Edwards, P. Introduced Dung Beetles in Australia 1967–2007: Current Status and Future Directions; Dung Beetles for Landcare Farming Committee; Landcare Australia: Sydney, Australia, 2007. [Google Scholar]
- Edwards, P.; Wilson, P.; Wright, J. Introduced Dung Beetles in Australia; CSIRO Publishing: Clayton, Australia, 2015. [Google Scholar]
- Wright, J.; Gleeson, P.; Robinson, F. Importation of 2 Winter-Spring Active Dung Beetles for Southern Australia: B.ERM.0213 Final. Report; CSIRO: Sydney, Australia, 2015; Available online: https://www.mla.com.au/research-and-development/reports/2015/importation-and-rearing-of-2-winter---spring-active-dung-beetles-for-southern-australia/ (accessed on 20 January 2025).
- Doube, B.M. Ecosystem services provided by dung beetles in Australia. Basic Appl. Ecol. 2018, 26, 35–49. [Google Scholar] [CrossRef]
- Sheppard, A.W.; Raghu, S. Working at the interface of art and science: How best to select an agent for classical biological control? Biol. Control 2005, 34, 233–235. [Google Scholar] [CrossRef]
- Sheppard, A.W.; Heard, T.A.; Briese, D.T. What Is Needed to Improve the Selection, Testing and Evaluation of Weed Biological Control Agents: Workshop Synthesis and Recommendations; CRC for Australian Weed Management: Canberra, Australia, 2003; pp. 87–98. [Google Scholar]
- Lumaret, J.-P.; Kirk, A.A. Ecology of dung beetles in the French Mediterranean Region (Coleoptera: Scarabaeidae). Acta Zool. Mex. (N.S.) 1987, 24, 1–55. [Google Scholar] [CrossRef]
- Martínez, I.; Dellacasa, M.; Lumaret, J.P.; Dellacasa, G. Phenology and reproductive cycles in Mexican aphodiine dung beetles (Coleoptera: Scarabaeidae: Aphodiinae: Aphodiini). Ann. Soc. Entomol. Fr. 2022, 58, 173–185. [Google Scholar] [CrossRef]
- Sowig, P. Habitat selection and offspring survival rate in three paracoprid dung beetles: The influence of soil type and soil moisture. Ecography 1995, 18, 147–154. [Google Scholar] [CrossRef]
- Daniel, G.M.; Noriega, J.A.; da Silva, P.G.; Deschodt, C.M.; Sole, C.L.; Scholtz, C.H.; Davis, A.L.V. Soil type, vegetation cover and temperature determinants of the diversity and structure of dung beetle assemblages in a South African open woodland and closed canopy mosaic. Austral Ecol. 2022, 47, 79–91. [Google Scholar] [CrossRef]
- Leandro, C.; Jones, M.; Perrin, W.; Jay-Robert, P.; Ovaskainen, O. Dung beetle community patterns in Western Europe: Responses of Scarabaeinae to landscape and environmental filtering. Landsc. Ecol. 2023, 38, 2323–2338. [Google Scholar] [CrossRef]
- IUCN. The IUCN Red List of Threatened Species. Version 2022-2. 2023. Available online: https://www.iucnredlist.org/ (accessed on 20 January 2025).
- Numa, C.; Tonelli, M.; Lobo, J.M.; Verdú, J.R.; Lumaret, J.-P.; Sánchez-Piñero, F.; Ruiz, J.L.; Dellacasa, M.; Ziani, S.; Arriaga, A.; et al. The Conservation Status and Distribution of Mediterranean Dung Beetles; IUCN: Gland, Switzerland; Málaga, Spain, 2020. [Google Scholar]
- Climate-Data.org. Climate: Morocco. 2023. Available online: https://en.climate-data.org/africa/morocco-181/ (accessed on 30 October 2024).
- Errouissi, F.; Labidi, I.; Nouira, S. Seasonal occurrence and local coexistence within scarabaeid dung beetle guilds (Coleoptera: Scarabaeoidea) in Tunisian pasture. Eur. J. Entomol. 2009, 106, 85–94. [Google Scholar] [CrossRef]
- Hajji, H.; Janati-Idrissi, A.; Taybi, A.F.; Caron, V.; Lumaret, J.-P.; Mabrouki, Y. Seasonal Variation in the Organization of Dung Beetle Communities in the Moroccan Middle Atlas (Coleoptera: Scarabaeoidea). Diversity 2023, 15, 1138. [Google Scholar] [CrossRef]
- Lumaret, J.-P. Recommendations for Future Dung Beetle Research in Australia; Internal Report; CSIRO: Canberra, Australia, 2016. [Google Scholar]
- Skrijka, P. Investigations of the fertilizer value of sheep excrements left on pasture. In International Symposium of the European Grassland Federation; Van Der Meer, H.G., Unwin, R.J., Van Dijk, T.A., Ennik, G.C., Eds.; Springer: Wageningen, The Netherlands, 1987. [Google Scholar]
- Ferrar, P. The immature stages of dung-breeding muscoid flies in Australia, with notes on the species and keys to larvae and puparia. Aust. J. Zool. 1979, 27, 671. [Google Scholar] [CrossRef]
- ABS. Agricultural Commodities Australia; ABS: Canberra, Australia, 2022. Available online: https://www.abs.gov.au/statistics/industry/agriculture/agricultural-commodities-australia/latest-release (accessed on 5 December 2024).
- Romero-Samper, J. Las Comunidades de Coleópteros Escarabeido Coprófagos (Coleoptera, Scarabaeoidea) del Medio Atlas (Marruecos): Influencia del tipo de Hábitat, Altitud y Estacionalidad: Análisis Comparado de su Estructura; Universidad Complutense de Madrid: Madrid, Spain, 2008. [Google Scholar]
- Hajji, H.; Janati-Idrissi, A.; el Fattouhi, Y.; el Ouaryaghli, A.; Caron, V.; Lumaret, J.P. Light orientation in the ball-rolling dung beetle, (MacLeay, 1821), in Morocco (Coleoptera: Scarabaeinae: Gymnopleurini). Ann. Société Entomol. Fr. 2023, 59, 406–416. [Google Scholar] [CrossRef]
- Zamprogna, A.; Hajji, H.; Janati-Idrissi, A. Sexual dimorphism in Gymnopleurus sturmi (MacLeay, 1821) (Coleoptera: Scarabaeidae), a Palaearctic dung beetle being imported to Australia. Aust. Entomol. 2022, 49, 15–22. [Google Scholar]
- Hajji, H.; Rehali, M.; Taybi, A.F.; Lumaret, J.-P.; Mabrouki, Y. Dung beetles, dung burial, and plant growth: Four Scarabaeoid species and Sorghum. Insects 2024, 15, 1002. [Google Scholar] [CrossRef]
- Hajji, H.; Janati-Idrissi, A.; Taybi, A.F.; Lumaret, J.-P.; Mabrouki, Y. Contribution of dung beetles to the enrichment of soil with organic matter and nutrients under controlled conditions. Diversity 2024, 16, 462. [Google Scholar] [CrossRef]
- Baraud, J. Coleoptères Scarabaeoidea, Faune de l’Europe Occidentale; Fédération Fançaise des Sociétés de Sciences Naturelles: Paris, France; Société Linnéenne de Lyon: Lyon, France, 1992. [Google Scholar]
- Baraud, J. Coléoptères Scarabaeoidea. Faune du Nord de l’Afrique du Maroc au Sinaï. In Encyclopédie Entomologique; Lechevalier: Paris, France, 1985. [Google Scholar]
- Martín-Piera, F. Los Onthophagini íbero-baleares (Col., Scarabaeoidea); II. Corología y Autoecología. EOS 1984, 60, 101–173. [Google Scholar]
- Ruiz, J.L.; Labidi, I.; Verdú, J.R.; Lumaret, J.P.; Ziani, S.; Dellacasa, M. Euonthophagus crocatus. 2015. Available online: https://www.iucnredlist.org/species/47268384/48594696 (accessed on 13 December 2018). [CrossRef]
- Janati-Idrissi, A. Les Scarabéides Coprophages des Pelouses Sèches de Maroc Central: Structure des Communautés et Rôle Écologique (Coleoptera, Scarabaeoidea); Université Sidi Mohamed Ben Abdellah: Fez, Morocco, 2000. [Google Scholar]
- Labidi, I.; Errouissi, F.; Nouira, S. Spatial and temporal variation in species composition, diversity, and structure of Mediterranean dung beetle assemblages (Coleoptera: Scarabaeidae) across a bioclimatic gradient. Environ. Entomol. 2012, 41, 785–801. [Google Scholar] [CrossRef]
- Boucher, J.F. Captures intéressantes de Coléoptères Scarabaeoidea coprophages au Maroc. Bull. Mens. Société Linnéenne Lyon. 1990, 59, 49–55. [Google Scholar]
- Martín-Piera, F.; López-Colón, J.I. Fauna Iberica; Museo Nacional de Ciencias Naturales: Madrid, Spain, 2000. [Google Scholar]
- Verdú, J.R.; Ruiz, J.L.; Labidi, I. Onthophagus andalusicus. 2015. Available online: https://www.iucnredlist.org/species/47414308/48594731 (accessed on 13 December 2018). [CrossRef]
- Errouissi, F.; Haloti, S.; Jay-Robert, P.; Janati-Idrissi, A.; Lumaret, J.P. Effects of the attractiveness for dung beetles of dung pat origin and size along a climatic gradient. Environ. Entomol. 2004, 33, 45–53. [Google Scholar] [CrossRef]
- Janati-Idrissi, A.; Kadiri, N.; Lumaret, J.-P. Le partage du temps et de l’espace entre les guildes de coléoptères coprophages dans le Moyen-Atlas (Maroc). Ann. Société Entomol. Fr. (N.S.) 1999, 35, 213–221. [Google Scholar]
- Rössner, E.; Schönfeld, J.; Ahrens, D. Onthophagus (Palaeonthophagus) medius (Kugelann, 1792)—A good western palaearctic species in the Onthophagus vacca complex (Coleoptera: Scarabaeidae: Scarabaeinae: Onthophagini). Zootaxa 2010, 2629, 1–28. [Google Scholar] [CrossRef]
- Roy, L.; Bon, M.C.; Cesarini, C.; Serin, J.; Bonato, O. Pinpointing the level of isolation between two cryptic species sharing the same microhabitat: A case study with a scarabaeid species complex. Zool. Scr. 2016, 45, 407–420. [Google Scholar] [CrossRef]
- Sowig, P. Brood care in the dung beetle Onthophagus vacca (Coleoptera: Scarabaeidae): The effect of soil moisture on time budget, nest structure, and reproductive success. Ecography 1996, 19, 254–258. [Google Scholar] [CrossRef]
- Lumaret, J.P.; Arriaga, A.A.; Cabrero Sañudo, F.J.; Sanchez Piñero, F.; Dellacasa, M.; Ziani, S. Gymnopleurus sturmi; The IUCN Red List of Threatened Species 2015. e.T47268005A48594706. Available online: https://www.iucnredlist.org/species/47268005/48594706 (accessed on 10 December 2023).
- Haloti, S.; Janati-Idrissi, A.; Chergui, H.; Lumaret, J.-P. Structure des communautés de Scarabéides coprophages du Maroc nord-occidental (Coleoptera, Scarabaeoidea). Bull. L’institut Sci. Rabat Sect. Sci. Vie 2006, 28, 25–34. [Google Scholar]
- Ruiz, J.L. Los Scarabaeoidea (Coleoptera) coprófagos de la región de Ceuta (Norte de África). Aproximación faunística. Transfretana Monogr. 1995, 2, 11–114. [Google Scholar]
- Lumaret, J.P. Atlas des Coléoptères Scarabéides Laparosticti de France; Secrétariat Faune-Flore, Muséum National d’Histoire Naturelle: Paris, France, 1990. [Google Scholar]
- Mehdi, E.A. Composition et Organisation du Peuplement de Scarabéidés Coprophages Dans le Nord-Est Algérien: Occupation de L’espace et Rôle Écologique; Université Badji Mokhtar: Annaba, Algeria, 2014. [Google Scholar]
- Beck, H.E.; Zimmermann, N.E.; McVicar, T.R.; Vergopolan, N.; Berg, A.; Wood, E.F. Data Descriptor: Present and future Köppen-Geiger climate classification maps at 1-km resolution. Sci. Data 2018, 5, 180214. [Google Scholar] [CrossRef]
- Lobo, J.M.; Martinpiera, F.; Veiga, C.M. Dung-Baited Pitfall Traps for Studying Coprophagous Scarabaeoidea (Col) Communities.1. Characteristics Determining Capacity of Capture. Rev. Ecol. Biol. Sol. 1988, 25, 77–100. [Google Scholar]
- Larsen, T.H.; Forsyth, A. Trap spacing and transect design for dung beetle biodiversity studies. Biotropica J. Biol. Conserv. 2005, 37, 322–325. [Google Scholar]
- Osberg, D.C.; Doube, B.M.; Hanrahan, S.A. Habitat specificity in African dung beetles: The effect of soil type on the survival of dung beetle immatures (Coleoptera Scarabaeidae). Trop. Zool. 1994, 7, 1–10. [Google Scholar] [CrossRef]
- Andresen, E. Effects of season and vegetation type on community organization of dung beetles in a tropical dry forest. Biotropica 2005, 37, 291–300. [Google Scholar] [CrossRef]
- Labidi, I.; Nouira, S.; Errouissi, F. Diversity and structure of dung beetle assemblages under two contrasted habitats in Tunisia: Oases vs. humid pastures. Austral Entomol. 2017, 56, 54–63. [Google Scholar] [CrossRef]
- Carpaneto, G.M.; Mazziotta, A.; Piattella, E. Changes in food resources and conservation of scarab beetles: From sheep to dog dung in a green urban area of Rome (Coleoptera, Scarabaeoidea). Biol. Conserv. 2005, 123, 547–556. [Google Scholar] [CrossRef]
- Sánchez Piñero, F.; Avila, J.M. Dung-insect community composition in arid zones of south-eastern Spain. J. Arid. Environ. 2004, 56, 303–327. [Google Scholar] [CrossRef]
- Dewhurst, C.F. Notes on some dung beetles collected in Morocco (Coleoptera, Scarabaeidae). Bull. L’institut Sci. Rabat 1981, 4, 53–68. [Google Scholar]
- Hanski, I.; Cambefort, Y. Competition in Dung Beetles. In Dung Beetle Ecology; Hanski, I., Cambefort, Y., Eds.; Princeton University Press: Princeton, NJ, USA, 1991; pp. 305–329. [Google Scholar]
- Berson, J.D.; Edwards, P.B.; Ridsdill-Smith, T.J.; Taylor, C.K.; Anderson, D.J.; Andrew, N.R.; Barrow, R.A.; Cousins, D.A.; Emery, R.N.; Fagan, L.L.; et al. Deliberately introduced dung beetles in Australia: 12 years of occurrence and abundance records from 2001 to 2022. Ecology 2024, 105, e4328. [Google Scholar] [CrossRef]
- Englmeier, J.; von Hoermann, C.; Rieker, D.; Benbow, M.E.; Benjamin, C.; Fricke, U.; Ganuza, C.; Haensel, M.; Lackner, T.; Mitesser, O.; et al. Dung-visiting beetle diversity is mainly affected by land use, while community specialization is driven by climate. Ecol. Evol. 2022, 12, e9386. [Google Scholar] [CrossRef]
- Denoth, M.; Frid, L.; Myers, J.H. Multiple agents in biological control: Improving the odds? Biol. Control 2002, 24, 20–30. [Google Scholar] [CrossRef]
- Kadiri, N.; Lumaret, J.-P.; Martínez, M.I. Funciones ecológicas y servicios ecosistémicos brindados por los escarabajos del estiércol. In Escarabajos Estercoleros. Biología Reproductiva y su Regulación; Martínez, M.I., Lumaret, J.-P., Eds.; Asociación Española de Entomología: Alicante, Spain, 2022; pp. 313–371. [Google Scholar]
- Romero-Samper, J.; Lobo, J.M. Datos ecológicos y biogeográficos sobre las comunidades de coleópteros escarabeidos paracópridos (Coleoptera: Scarabaeidae y Geotrupidae) del Medio Atlas (Marruecos). Boletín Soc. Entomológica Aragonesa 2008, 43, 121–144. [Google Scholar]



| Site | Location | Altitude | Site Characteristics | Main Grazers |
|---|---|---|---|---|
| Fez-Sais (Ain Cheggag) | 33°54′14″ N–4°59′55″ W | 609 m | Open, mostly flat environment. Herbaceous vegetation, with Asphodelus ramosus F. and sparse trees (Pistacia terebinthus L.), close to olive orchards. Karstic formation of lacustrine origin, composed mainly of massive limestone and travertine associated with conglomerates and marl, resulting in a hard and rocky soil. Semi-arid bioclimatic zone with temperate winters. Hot summer Mediterranean climate [17]. | Sheep Goats A few cattle |
| Imouzzer Kandar | 33°47′53″ N–4°59′21″ W | 898 m | Open, overgrazed scrubland environment. Herbaceous vegetation with a few patches of Chamaerops humilis L. (palm) (locally called doum) and more closed areas of holm oak (Quercus ilex L.) coppice. Gentle slope. Rocky area interspersed in clear patches of soil/vegetation. The substratum consists of superficial red clay soil, with outcrops of limestone blocks. The climate is classified as Csa according to the Köppen–Geiger classification, with hot, dry summers and mild, wet winters [48]. | Sheep Donkeys |
| Ifrane 1 | 33°32′42″ N–5°09′56″ W | 1631 m | Open, flat roadside site. Herbaceous vegetation with dense broom cover (Genista quadriflora Munby), numerous clumps of Chamaerops humilis, indicating intensive grazing by sheep. Rocky, with a superficial red soil made of silty clay. Many volcanic stones on the surface. Humid bioclimatic zone with cold winter and warm summer, Mediterranean climate [17]. | Sheep Goats Donkeys |
| Ifrane 2 | 33°33′03″ N–5°10′02″ W | 1613 m | Open environment, grasslands, with few brooms near forested areas (holm oak) and cropped fields. Superficial red soil made of silty clay. Volcanic stones on the surface. Humid bioclimatic zone with cold winter and warm summer, Mediterranean climate [17]. | Sheep Cattle Horses Donkeys |
| Euonthophagus crocatus | ||||
| Fez | Imouzzer | Ifrane 1 | Ifrane 2 | |
| Fez | - | |||
| Imouzzer | R2 = 0.21 | - | ||
| Ifrane 1 | R2 = 0.22 | R2 = 0.28 | - | |
| Ifrane 2 | R2 = 0.19 | R2 = 0.35 | R2 = 0.78 p < 0.001 y = 0.200 + 0.903x | - |
| Onthophagus vacca | ||||
| Fez | Imouzzer | Ifrane 1 | Ifrane 2 | |
| Fez | - | |||
| Imouzzer | R2 = 0.03 | - | ||
| Ifrane 1 | R2 = 0.001 | R2 = 0.18 | - | |
| Ifrane 2 | R2 = 0.004 | R2 = 0.30 | R2 = 0.67 p < 0.001 y = 0.236 + 0.882x | - |
| Onthophagus m. andalusicus | ||||
| Fez | Imouzzer | Ifrane 1 | Ifrane 2 | |
| Fez | - | |||
| Imouzzer | R2 = 0.01 | - | ||
| Ifrane 1 | R2 = 0.01 | R2 = 0.12 | - | |
| Ifrane 2 | R2 = 0.07 | R2 = 0.31 | R2 = 0.75 p < 0.001 y = 0.240 + 1.125x | - |
| Gymnopleurus sturmi | ||||
| Fez | Imouzzer | Ifrane 1 | Ifrane 2 | |
| Fez | - | |||
| Imouzzer | R2 = 0.003 | - | ||
| Ifrane 1 | R2 = 0.10 | R2 = 0.02 | - | |
| Ifrane 2 | R2 = 0.04 | R2 = 0.003 | R2 = 0.82 p < 0.001 y = 0.075 + 2.013x | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hajji, H.; Janati-Idrissi, A.; Zamprogna, A.; Serin, J.; Lumaret, J.-P.; Kadiri, N.; Pérez Vila, S.; Gleeson, P.V.; Wright, J.; Caron, V. Filling the Spring Gap in Southern Australia: Seasonal Activity of Four Dung Beetle Species Selected to Be Imported from Morocco. Insects 2025, 16, 538. https://doi.org/10.3390/insects16050538
Hajji H, Janati-Idrissi A, Zamprogna A, Serin J, Lumaret J-P, Kadiri N, Pérez Vila S, Gleeson PV, Wright J, Caron V. Filling the Spring Gap in Southern Australia: Seasonal Activity of Four Dung Beetle Species Selected to Be Imported from Morocco. Insects. 2025; 16(5):538. https://doi.org/10.3390/insects16050538
Chicago/Turabian StyleHajji, Hasnae, Abdellatif Janati-Idrissi, Alberto Zamprogna, José Serin, Jean-Pierre Lumaret, Nassera Kadiri, Saleta Pérez Vila, Patrick V. Gleeson, Jane Wright, and Valérie Caron. 2025. "Filling the Spring Gap in Southern Australia: Seasonal Activity of Four Dung Beetle Species Selected to Be Imported from Morocco" Insects 16, no. 5: 538. https://doi.org/10.3390/insects16050538
APA StyleHajji, H., Janati-Idrissi, A., Zamprogna, A., Serin, J., Lumaret, J.-P., Kadiri, N., Pérez Vila, S., Gleeson, P. V., Wright, J., & Caron, V. (2025). Filling the Spring Gap in Southern Australia: Seasonal Activity of Four Dung Beetle Species Selected to Be Imported from Morocco. Insects, 16(5), 538. https://doi.org/10.3390/insects16050538







