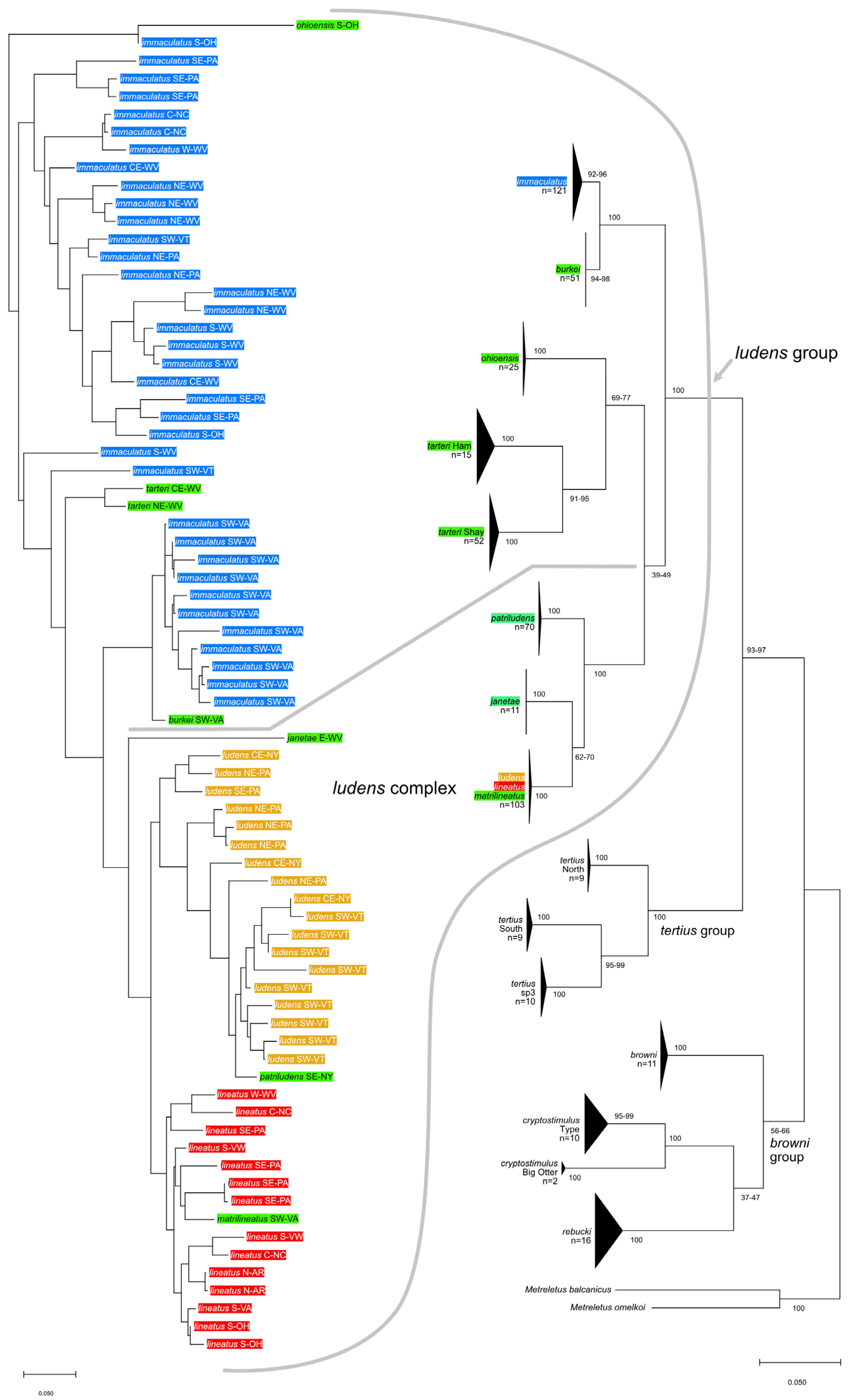
3.3. Key to Full-Grown Larvae (Walleyi Unknown)
- 1.
Mandible without a gap between the molar brush and the row of fine setae extending to the prostheca and incisor (i.e., no setal gap; Figure 14I); anal rib of gill 4 not inset from margin and with robust spines along only the costal margin ( Figure 14H).............................................................. tertius
|
- —
Mandible with a distinct setal gap that is at least 0.2× the length of the setal row (as in Figure 7C); anal rib of gill 4 inset or not inset from anal margin, but with spines on both the costal and anal margins.................................................................................................................................................2
|
- 2.
Setal gap on mandible wide, usually >0.8× the length of the setal row (as in Figure 13G); anal rib of gill 4 inset far from anal margin ( Figure 13F) or not inset at all (as in Figure 3G)...............................................................................................................................................................................................3
|
- —
Setal gap narrow, <0.5× length of the setal row (as in Figure 12F); anal rib of gill 4 narrowly inset on full-grown larvae (as in Figure 5E)............................................................................................................................................................................................................................ ludens group 6
|
- 3.
Caudal filaments with dark band usually extending from base to about the distal ¾, with every 4th segment pale giving them a speckled appearance ( Figure 13H); anal rib of gill 4 inset far from anal margin ( Figure 13F); northern transcontinental species................................... subnotatus
|
- —
Caudal filaments with dark band either extending from base, or restricted to middle 2/3, but with pigmentation uniform within the band ( Figure 1F and Figure 3E); anal rib of gill 4 not inset from anal margin ( Figure 3G); range New England south to South Carolina................... browni group 4
|
- 4.
Spinules present on posterior margins of tergites 1–10; dorsum of abdomen usually with a distinct median pale stripe ( Figure 1F); caudal filaments usually dark from base, with middle 2/3 only slightly darker; Quebec and Maritimes south to northern Pennsylvania....................... browni
|
- —
Spinules absent from posterior margins of tergites 1 and 2; dorsum of abdomen without a pale stripe; caudal filaments usually with a distinct dark band in middle 1/3 (basal 1/3 may be darkened somewhat; Figure 3E); New England south to South Carolina....................................................5
|
- 5.
Spinules present on posterior margins of tergites 5– or 6–10 in late instar larvae; range Virginia, North Carolina, eastern Tennessee, and South Carolina.....................................................................................................................................................................................................................cryptostimulus
|
- —
Spinules present on posterior margins of tergites 3– or 4–10 in late instar larvae; range from Maine south to West Virginia..............................rebucki
|
- 6.
Caudal filaments with a distinct dark band in the middle 1/3, with every 4th segment paler, giving the band a speckled appearance (as in Figure 9F); full-grown larvae with 3 dark stripes on the venter of the abdomen ( Figure 16B,C,E and Figure 6H)............................................. ludens complex 7
|
- —
Caudal filaments with dark band uniformly pigmented; venter of abdomen may have dark maculae but usually without stripes (approaching stripes in some ohioensis; see Figure 10F)....................................................................................................................................................... tarteri complex 11
|
- 7.
Spinules present on posterior margins of tergites 4–10 in late instars (absent from 1–2, sometimes scattered minute spinules on 3); sternal stripes not coalescing on sternite 9 ( Figure 6H); known distribution limited to a small area in eastern West Virginia........................................................ janetae
|
- —
Spinules present on posterior margins of tergites 1–10 in late instars; sternal stripes coalescing on sternite 9 ( Figure 16B,C,E); widely distributed, from Quebec and Newfoundland south to South Carolina and Georgia, west to Arkansas................................................................................................8
|
- 8.
Sternal stripes in last instar larvae with margins distinct; width of median stripe subequal to or only slightly wider than lateral stripes; stripes not coalescing until distal 1/3 of sternite 9 (right side of Figure 16E).............................................................................................................................................9
|
- —
Sternal stripes in last instar larvae with margins less distinct (especially the median stripe); median stripe distinctly wider than lateral stripes; stripes coalescing midway on sternite 8 and in the basal 1/3 or sternite 9 (left side of Figure 16E)..................................................................................10
|
- 9.
Bisexual, with males and females present in approximately equal proportion (see Figure 17A–E for determination of sex in larvae); known only from southwest Virginia............................................................................................................................................................................................ matrilineatus
|
- —
Triploid parthenogen, with a complete absence of males; widespread from northern Pennsylvania south to South Carolina and Georgia, west to Arkansas..............................................................................................................................................................................................................................lineatus
|
- 10.
Bisexual, with males and females present in approximately equal proportion (see Figure 17A–E for determination of sex in larvae); known only from southern New York and northern Pennsylvania............................................................................................................................................. patriludens
|
- —
Triploid parthenogen, with a complete absence of males; widespread from Quebec and Newfoundland south to West Virginia.......................ludens
|
- 11.
Sternites with variable pattern of dark mottling ( Figure 10E–F); bisexual, with males and females in approximately equal proportions; known from southeastern Ohio and adjacent West Virginia................................................................................................................................................... ohioensis
|
- —
Without distinct color pattern on sternites (although there may be some darkening, especially near lateral margins); bisexual or parthenogenetic; widespread from northern New England to South Carolina, west to at least West Virginia and Ohio............................................................................12
|
- 12.
Triploid parthenogen, with males completely absent (see Figure 17A–E for determining sex of larvae)....................................................... immaculatus
|
- —
Bisexual, with males and females in approximately equal proportion.................................................................................................................................13
|
- 13.
Range eastern West Virginia and western Maryland; with minimal dark shading on sternites; often (but not exclusively) found in streams with very low pH (<5)..................................................................................................................................................................................................................tarteri
|
- —
Known only from southwest Virginia; some specimens with more extensive dark shading on sternites; probably intolerant of low pH............burkei
|
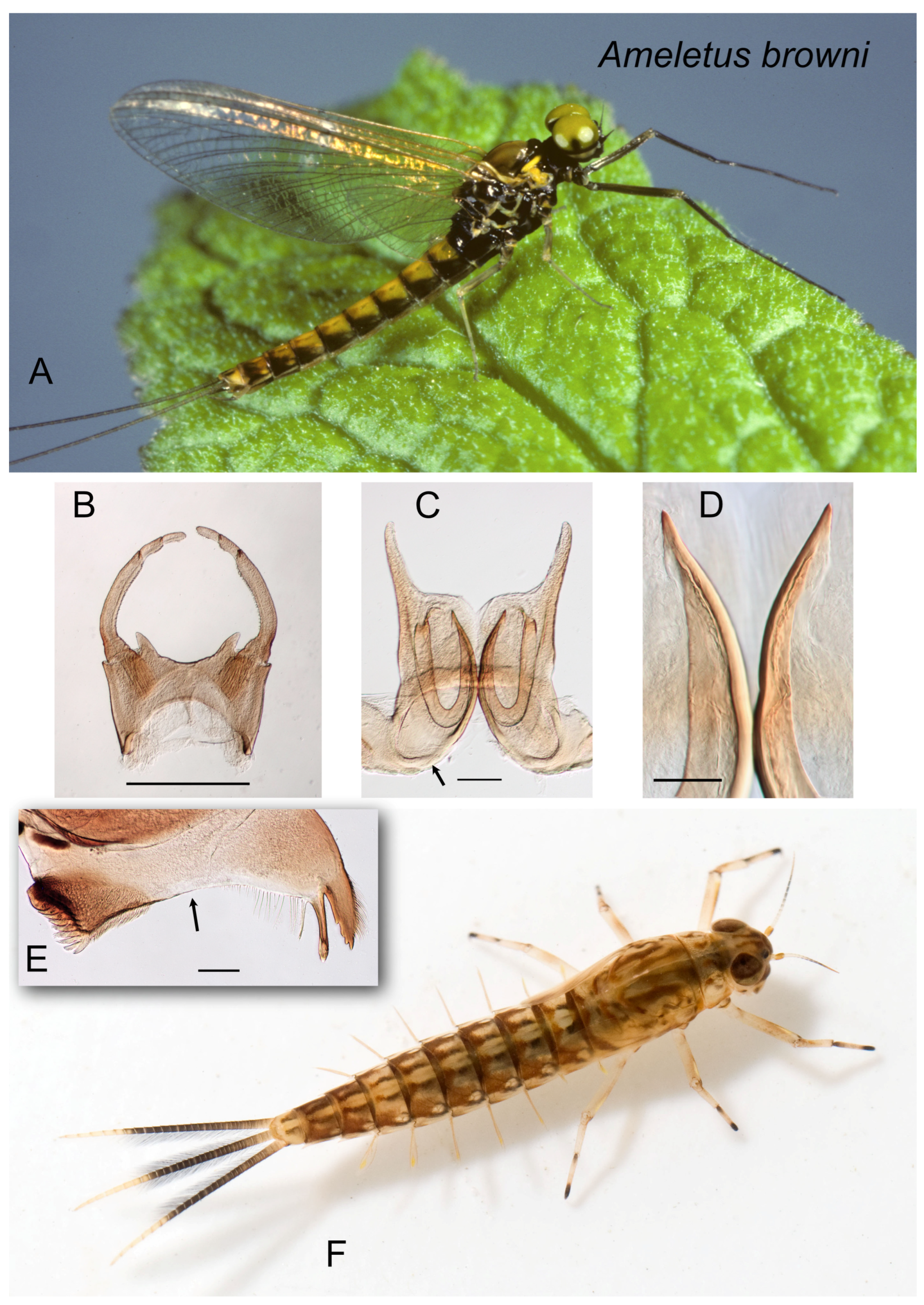
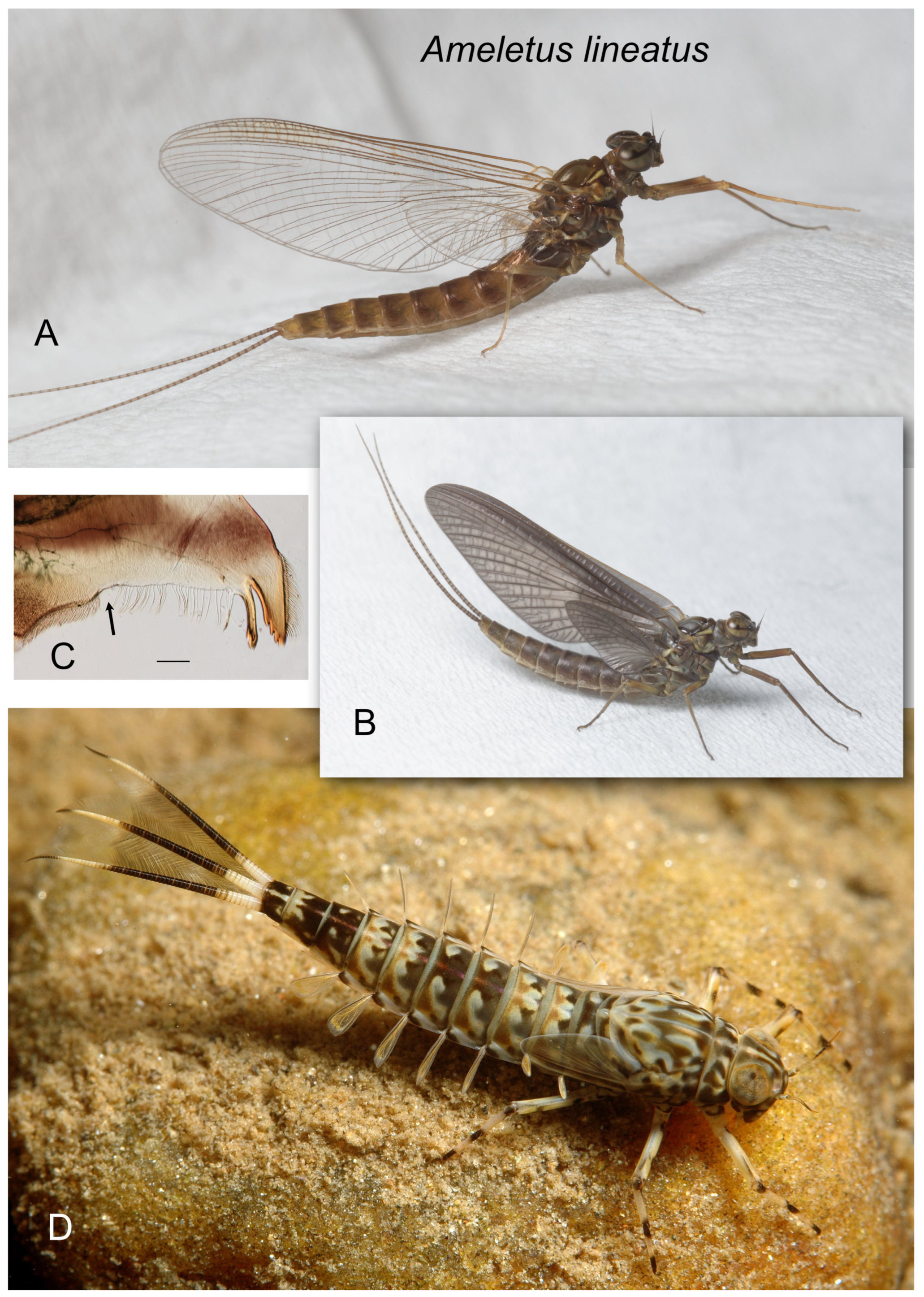
Figure 1.
Ameletus browni. (A). Living male imago, lateral view. (B). Male styliger plate and forceps, dorsal view. Scale = 0.5 mm. (C). Penes of male, ventral view. Arrow indicates basal sclerite. Scale = 0.1 mm. (D). Ventral plates of penes, ventral view. Scale = 0.05 mm. (E). Left mandible of larva, dorsal view. Arrow indicates setal gap. Scale = 0.1 mm. (F). Living larva, dorsal view.
Figure 1.
Ameletus browni. (A). Living male imago, lateral view. (B). Male styliger plate and forceps, dorsal view. Scale = 0.5 mm. (C). Penes of male, ventral view. Arrow indicates basal sclerite. Scale = 0.1 mm. (D). Ventral plates of penes, ventral view. Scale = 0.05 mm. (E). Left mandible of larva, dorsal view. Arrow indicates setal gap. Scale = 0.1 mm. (F). Living larva, dorsal view.
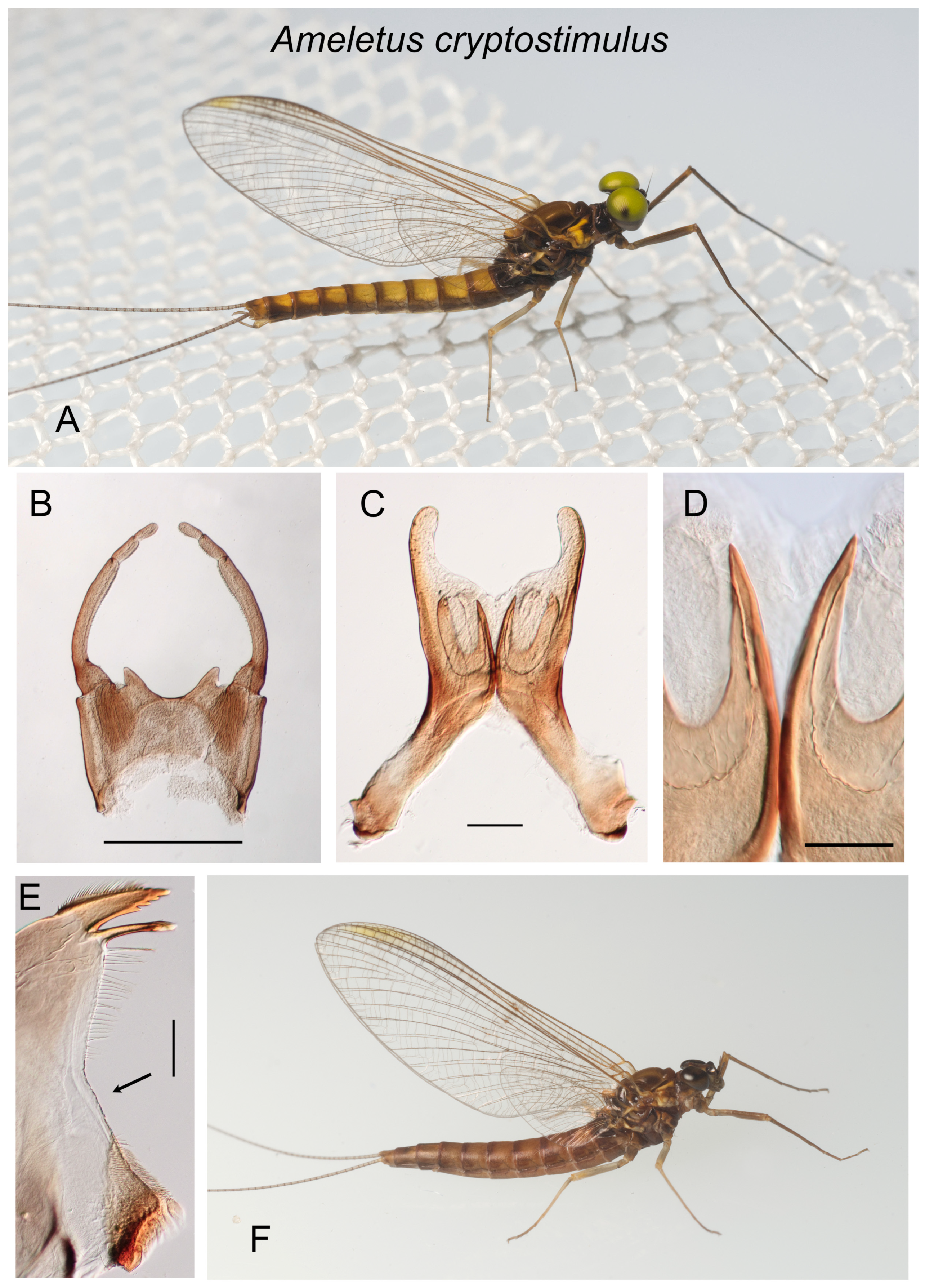
Figure 2.
Ameletus cryptostimulus. (A). Living male imago, lateral view. (B). Male styliger plate and forceps, dorsal view. Scale = 0.5 mm. (C). Penes of male, ventral view. Scale = 0.1 mm. (D). Ventral plates of penes, ventral view. Scale = 0.05 mm. (E). Left mandible of larva, dorsal view. Arrow indicates setal gap. Scale = 0.1 mm. (F) Living female imago, lateral view.
Figure 2.
Ameletus cryptostimulus. (A). Living male imago, lateral view. (B). Male styliger plate and forceps, dorsal view. Scale = 0.5 mm. (C). Penes of male, ventral view. Scale = 0.1 mm. (D). Ventral plates of penes, ventral view. Scale = 0.05 mm. (E). Left mandible of larva, dorsal view. Arrow indicates setal gap. Scale = 0.1 mm. (F) Living female imago, lateral view.
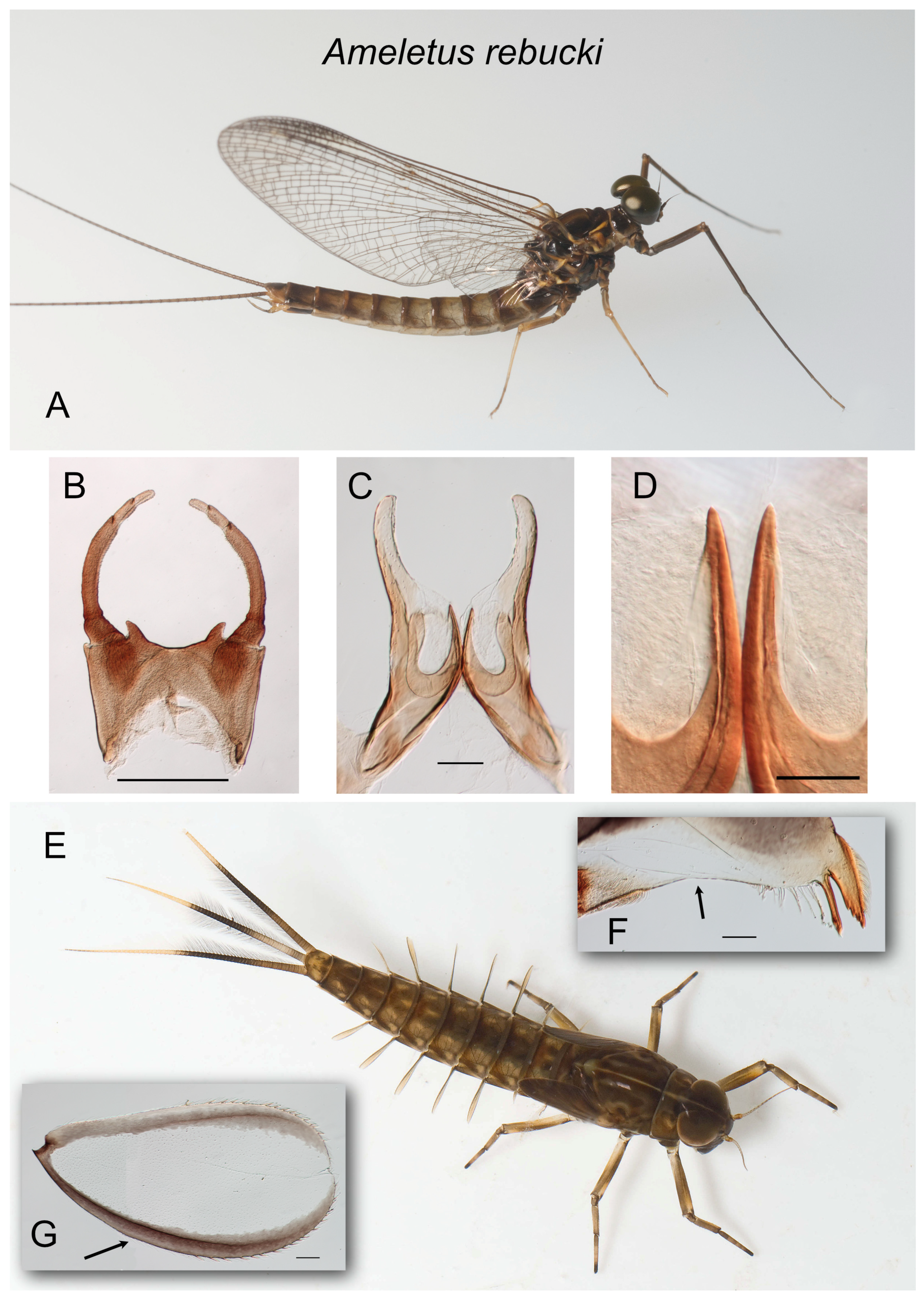
Figure 3.
Ameletus rebucki. (A). Living male imago, lateral view. (B). Male styliger plate and forceps, dorsal view. Scale = 0.5 mm. (C). Penes of male, ventral view. Scale = 0.1 mm. (D). Ventral plates of penes, ventral view. Scale = 0.05 mm. (E). Living larva, dorsal view. (F). Left mandible of larva, dorsal view. Arrow indicates setal gap. Scale = 0.1 mm. (G). Larval gill 4 (right). Arrow indicates anal rib, which is not inset. Scale = 0.1 mm.
Figure 3.
Ameletus rebucki. (A). Living male imago, lateral view. (B). Male styliger plate and forceps, dorsal view. Scale = 0.5 mm. (C). Penes of male, ventral view. Scale = 0.1 mm. (D). Ventral plates of penes, ventral view. Scale = 0.05 mm. (E). Living larva, dorsal view. (F). Left mandible of larva, dorsal view. Arrow indicates setal gap. Scale = 0.1 mm. (G). Larval gill 4 (right). Arrow indicates anal rib, which is not inset. Scale = 0.1 mm.
Figure 4.
Ameletus burkei. (A). Living male imago, lateral view. (B). Male styliger plate and forceps, dorsal view. Scale = 0.5 mm. (C). Penes of male, ventral view. Scale = 0.1 mm. (D). Ventral plates of penes, ventral view. Scale = 0.05 mm. (E). Living larva, dorsolateral view. (F). Right mandible of larva, dorsal view. Arrow indicates setal gap. Scale = 0.1 mm. (G). Female (above) and male imagos during laboratory-induced copulation.
Figure 4.
Ameletus burkei. (A). Living male imago, lateral view. (B). Male styliger plate and forceps, dorsal view. Scale = 0.5 mm. (C). Penes of male, ventral view. Scale = 0.1 mm. (D). Ventral plates of penes, ventral view. Scale = 0.05 mm. (E). Living larva, dorsolateral view. (F). Right mandible of larva, dorsal view. Arrow indicates setal gap. Scale = 0.1 mm. (G). Female (above) and male imagos during laboratory-induced copulation.
Figure 5.
Ameletus immaculatus. (A). Living female imago, lateral view. (B). Group of living female larvae on the stream bottom. (C). Living female larva, dorsal view. (D). Left mandible of larva, dorsal view. Arrow indicates setal gap. Scale = 0.1 mm. (E). Larval gill 4 (right), dorsal view. Arrow indicates where anal rib is inset. Scale = 0.1 mm.
Figure 5.
Ameletus immaculatus. (A). Living female imago, lateral view. (B). Group of living female larvae on the stream bottom. (C). Living female larva, dorsal view. (D). Left mandible of larva, dorsal view. Arrow indicates setal gap. Scale = 0.1 mm. (E). Larval gill 4 (right), dorsal view. Arrow indicates where anal rib is inset. Scale = 0.1 mm.
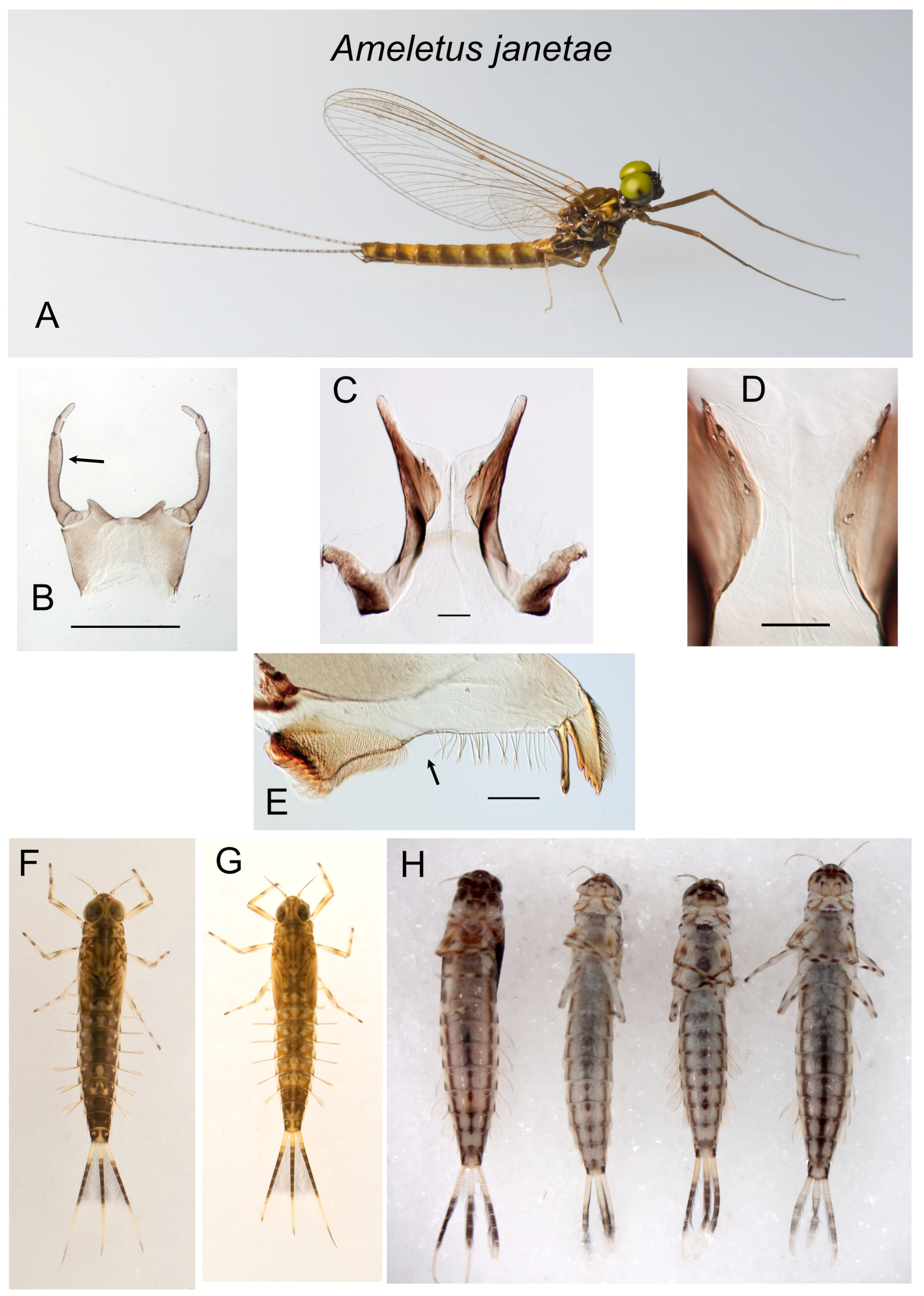
Figure 6.
Ameletus janetae. (A). Living male imago, lateral view. (B). Male styliger plate and forceps, dorsal view. Arrow indicates dilation of long joint. Scale = 0.5 mm. (C). Penes of male, ventral view. Scale = 0.1 mm. (D). Ventral plates of penes, ventral view. Scale = 0.05 mm. (E). Left mandible of larva, dorsal view. Arrow indicates setal gap. Scale = 0.1 mm. (F). Living larva, dorsal view. (G). Living larva, dorsal view. (H). Larvae, ventral view, showing variation in ventral abdominal striping.
Figure 6.
Ameletus janetae. (A). Living male imago, lateral view. (B). Male styliger plate and forceps, dorsal view. Arrow indicates dilation of long joint. Scale = 0.5 mm. (C). Penes of male, ventral view. Scale = 0.1 mm. (D). Ventral plates of penes, ventral view. Scale = 0.05 mm. (E). Left mandible of larva, dorsal view. Arrow indicates setal gap. Scale = 0.1 mm. (F). Living larva, dorsal view. (G). Living larva, dorsal view. (H). Larvae, ventral view, showing variation in ventral abdominal striping.
Figure 7.
Ameletus lineatus. (A). Living female imago, lateral view. (B). Living female subimago, lateral view (C). Left mandible of larva, dorsal view. Arrow indicates setal gap. Scale = 0.1 mm. (D). Living female larva, dorsolateral view.
Figure 7.
Ameletus lineatus. (A). Living female imago, lateral view. (B). Living female subimago, lateral view (C). Left mandible of larva, dorsal view. Arrow indicates setal gap. Scale = 0.1 mm. (D). Living female larva, dorsolateral view.
Figure 8.
Ameletus ludens. (A). Living female imago, lateral view. (B). Living female subimago, lateral view (C). Living female larva, dorsolateral view.
Figure 8.
Ameletus ludens. (A). Living female imago, lateral view. (B). Living female subimago, lateral view (C). Living female larva, dorsolateral view.
Figure 9.
Ameletus matrilineatus. (A). Living male imago, lateral view. (B). Male styliger plate and forceps, dorsal view. Arrow indicates dilation of the long joint. Scale = 0.5 mm. (C). Penes of male, ventral view. Scale = 0.1 mm. (D). Ventral plates of penes, ventral view. Scale = 0.05 mm. (E). Living female imago, lateral view. (F). Caudal filaments of larva showing light speckling within the dark band on the middle third.
Figure 9.
Ameletus matrilineatus. (A). Living male imago, lateral view. (B). Male styliger plate and forceps, dorsal view. Arrow indicates dilation of the long joint. Scale = 0.5 mm. (C). Penes of male, ventral view. Scale = 0.1 mm. (D). Ventral plates of penes, ventral view. Scale = 0.05 mm. (E). Living female imago, lateral view. (F). Caudal filaments of larva showing light speckling within the dark band on the middle third.
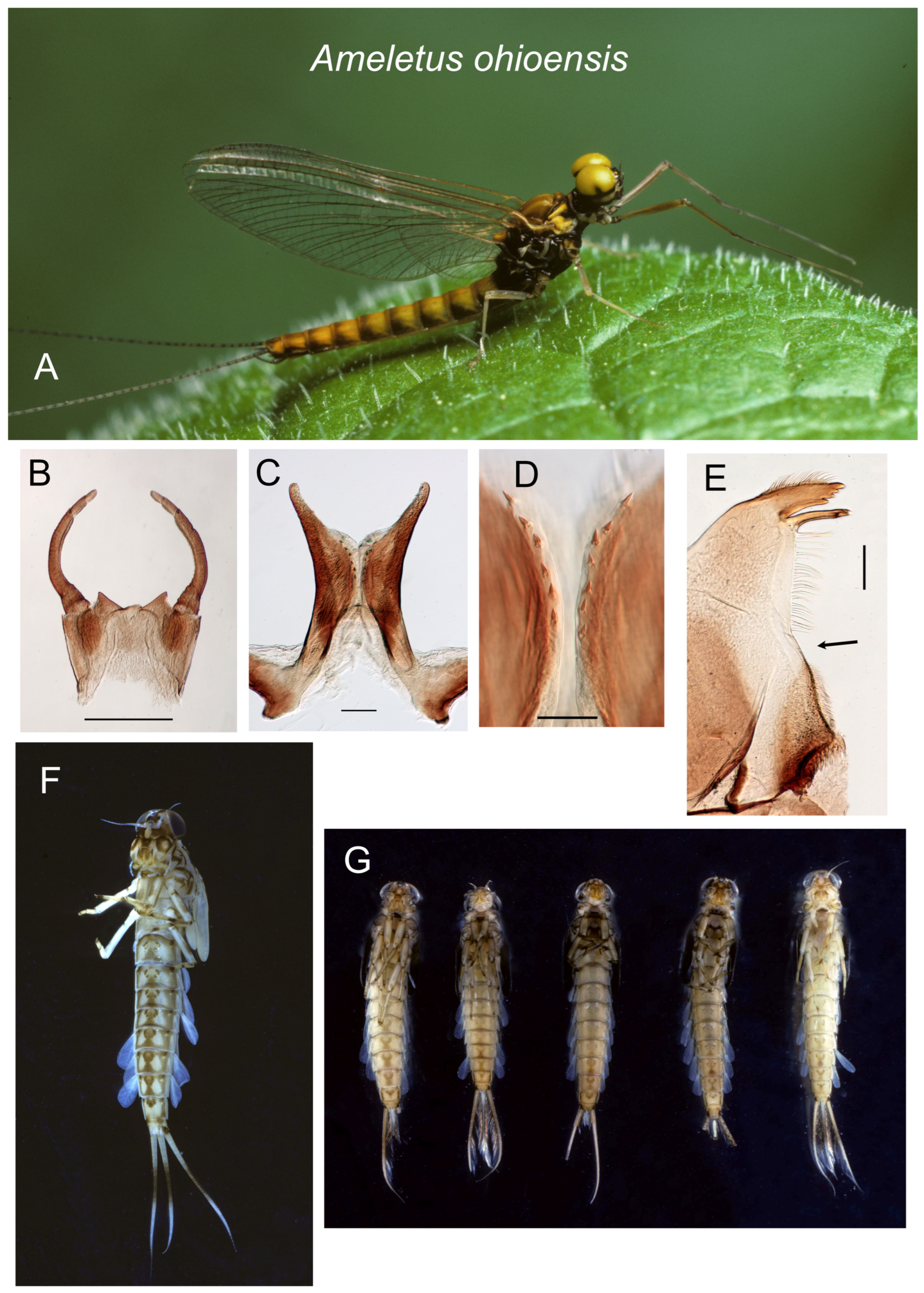
Figure 10.
Ameletus ohioensis. (A). Living male imago, lateral view. (B). Male styliger plate and forceps, dorsal view. Scale = 0.5 mm. (C). Penes of male, ventral view. Scale = 0.1 mm. (D). Ventral plates of penes, ventral view. Scale = 0.05 mm. (E). Left mandible of larva, dorsal view. Arrow indicates setal gap. Scale = 0.1 mm. (F). Full-grown male larva, ventral view. (G). Full-grown male larvae, ventral view, showing variation in sternal color pattern.
Figure 10.
Ameletus ohioensis. (A). Living male imago, lateral view. (B). Male styliger plate and forceps, dorsal view. Scale = 0.5 mm. (C). Penes of male, ventral view. Scale = 0.1 mm. (D). Ventral plates of penes, ventral view. Scale = 0.05 mm. (E). Left mandible of larva, dorsal view. Arrow indicates setal gap. Scale = 0.1 mm. (F). Full-grown male larva, ventral view. (G). Full-grown male larvae, ventral view, showing variation in sternal color pattern.
Figure 11.
Ameletus patriludens. (A). Living male imago, lateral view. (B). Male styliger plate and forceps, dorsal view. Arrow indicates dilation of long joint. Scale = 0.5 mm. (C). Penes of male, ventral view. Scale = 0.1 mm. (D). Ventral plates of penes, ventral view. Scale = 0.05 mm. (E). Male larva, dorsal (left) and ventral views (F). Full-grown male larva, ventral view. (G). Full-grown male larva, ventral view. Color variant with ventral stripes that do not coalesce on sternite 9 (see Remarks in species account).
Figure 11.
Ameletus patriludens. (A). Living male imago, lateral view. (B). Male styliger plate and forceps, dorsal view. Arrow indicates dilation of long joint. Scale = 0.5 mm. (C). Penes of male, ventral view. Scale = 0.1 mm. (D). Ventral plates of penes, ventral view. Scale = 0.05 mm. (E). Male larva, dorsal (left) and ventral views (F). Full-grown male larva, ventral view. (G). Full-grown male larva, ventral view. Color variant with ventral stripes that do not coalesce on sternite 9 (see Remarks in species account).
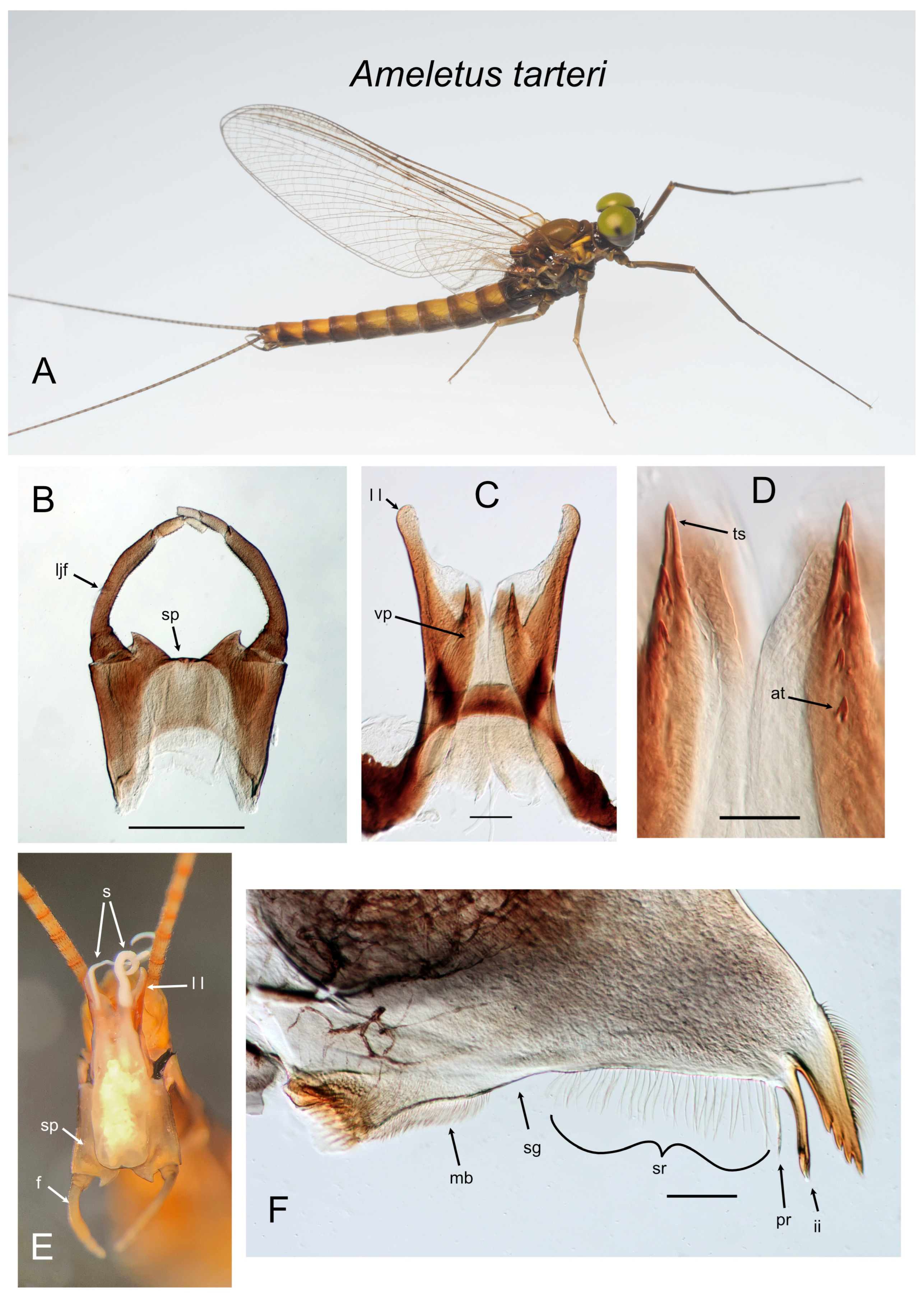
Figure 12.
Ameletus tarteri. (A). Living male imago, lateral view. (B). Male styliger plate and forceps, dorsal view. Scale = 0.5 mm. (C). Penes of male, ventral view. Scale = 0.1 mm. (D). Ventral plates of penes, ventral view. Scale = 0.05 mm. (E). Male imago, distal end of abdomen in copulatory position, caudal view (F). Left mandible of larva, dorsal view. Scale = 0.1 mm.. Abbreviations: at—accessory tooth; f—forceps; ii—inner incisor; ljf—long joint of forceps; ll—lateral lobe of penes; mb—molar brush; pr—prostheca; s—sperm; sg—setal gap; sp—styliger plate; sr—setal row; ts—terminal spine of ventral plate; vp—ventral plate of penes.
Figure 12.
Ameletus tarteri. (A). Living male imago, lateral view. (B). Male styliger plate and forceps, dorsal view. Scale = 0.5 mm. (C). Penes of male, ventral view. Scale = 0.1 mm. (D). Ventral plates of penes, ventral view. Scale = 0.05 mm. (E). Male imago, distal end of abdomen in copulatory position, caudal view (F). Left mandible of larva, dorsal view. Scale = 0.1 mm.. Abbreviations: at—accessory tooth; f—forceps; ii—inner incisor; ljf—long joint of forceps; ll—lateral lobe of penes; mb—molar brush; pr—prostheca; s—sperm; sg—setal gap; sp—styliger plate; sr—setal row; ts—terminal spine of ventral plate; vp—ventral plate of penes.
Figure 13.
Ameletus subnotatus (except E: burkei). (A). Living male imago, lateral view. (B). Penes of male, ventral view. Arrow indicates tooth at base of lateral lobe. Scale = 0.1 mm. (C). Living male subimago, lateral view. (D). Male imago segment 9, penes, styliger plate and forceps (10th segment removed). Scale = 0.5 mm. (E). A. burkei male imago segment 9, penes, styliger plate and forceps (10th segment removed). Scale = 0.5 mm. (F). Larval gill 4 (right), dorsal view. Arrow indicates anal rib which is inset far from anal margin. Scale = 0.1 mm. (G). Left mandible of larva, dorsal view. Arrow indicates setal gap. Scale = 0.1 mm. (H). Caudal filaments of final larval instar (exuviae), dorsal view.
Figure 13.
Ameletus subnotatus (except E: burkei). (A). Living male imago, lateral view. (B). Penes of male, ventral view. Arrow indicates tooth at base of lateral lobe. Scale = 0.1 mm. (C). Living male subimago, lateral view. (D). Male imago segment 9, penes, styliger plate and forceps (10th segment removed). Scale = 0.5 mm. (E). A. burkei male imago segment 9, penes, styliger plate and forceps (10th segment removed). Scale = 0.5 mm. (F). Larval gill 4 (right), dorsal view. Arrow indicates anal rib which is inset far from anal margin. Scale = 0.1 mm. (G). Left mandible of larva, dorsal view. Arrow indicates setal gap. Scale = 0.1 mm. (H). Caudal filaments of final larval instar (exuviae), dorsal view.
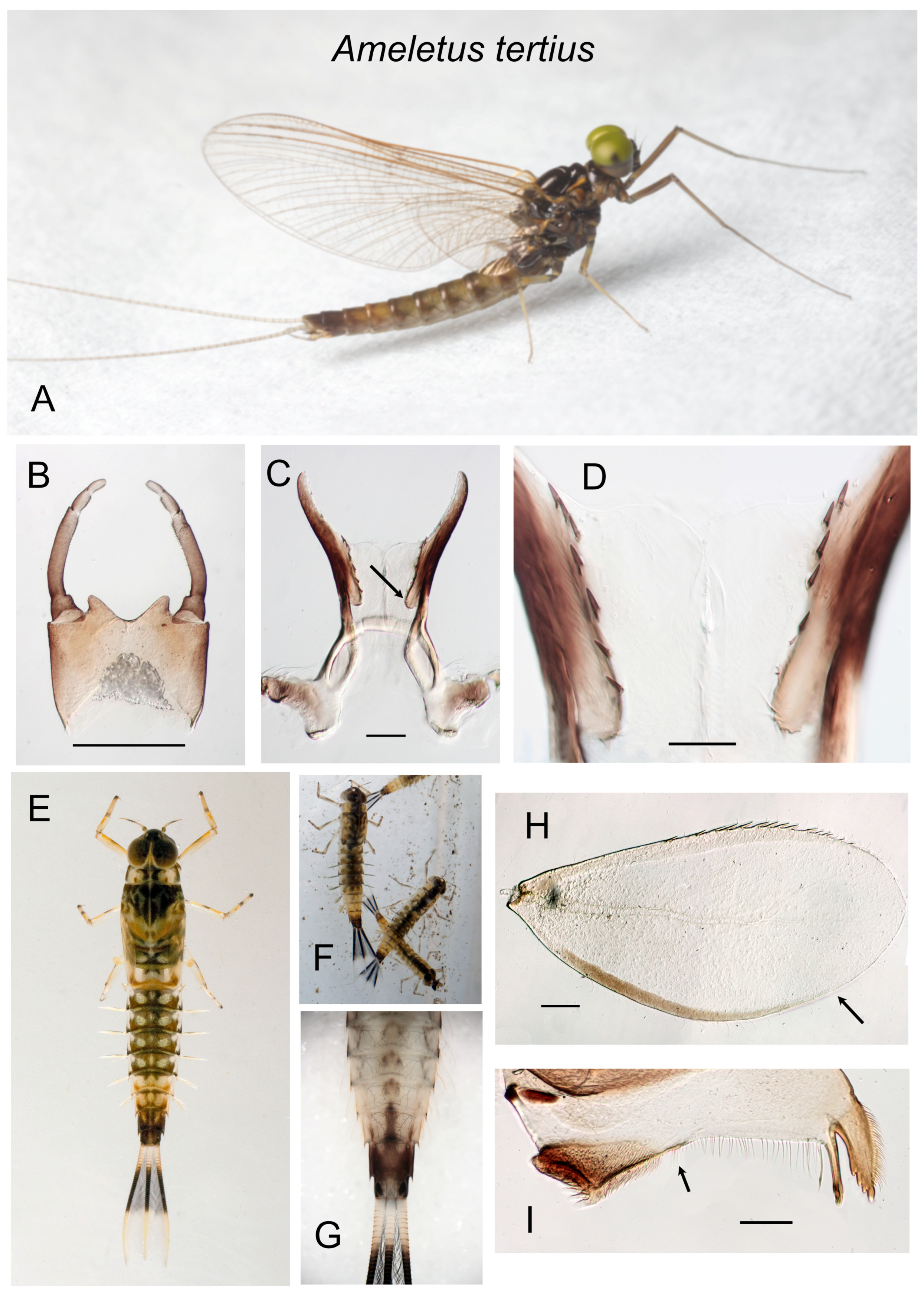
Figure 14.
Ameletus tertius. (A). Living male imago, lateral view. (B). Male styliger plate and forceps, dorsal view. Scale = 0.5 mm. (C). Penes of male, ventral view. Arrow indicates the basal lobe of the ventral plate. Scale = 0.1 mm. (D). Ventral plates of penes, ventral view. Scale = 0.05 mm. (E). Living larva, dorsal view. (F). Living larvae of color morph with a dark band that extends to the base of the caudal filaments. (G). Larval abdomen, ventral view. (H). Larval gill 4 (right), dorsal view. Arrow indicates anal margin which lacks spines. Scale = 0.1 mm. (I). Left mandible of larva, dorsal view. Arrow indicates where the setal row is continuous with the molar brush, i.e., lack of setal gap. Scale = 0.1 mm.
Figure 14.
Ameletus tertius. (A). Living male imago, lateral view. (B). Male styliger plate and forceps, dorsal view. Scale = 0.5 mm. (C). Penes of male, ventral view. Arrow indicates the basal lobe of the ventral plate. Scale = 0.1 mm. (D). Ventral plates of penes, ventral view. Scale = 0.05 mm. (E). Living larva, dorsal view. (F). Living larvae of color morph with a dark band that extends to the base of the caudal filaments. (G). Larval abdomen, ventral view. (H). Larval gill 4 (right), dorsal view. Arrow indicates anal margin which lacks spines. Scale = 0.1 mm. (I). Left mandible of larva, dorsal view. Arrow indicates where the setal row is continuous with the molar brush, i.e., lack of setal gap. Scale = 0.1 mm.
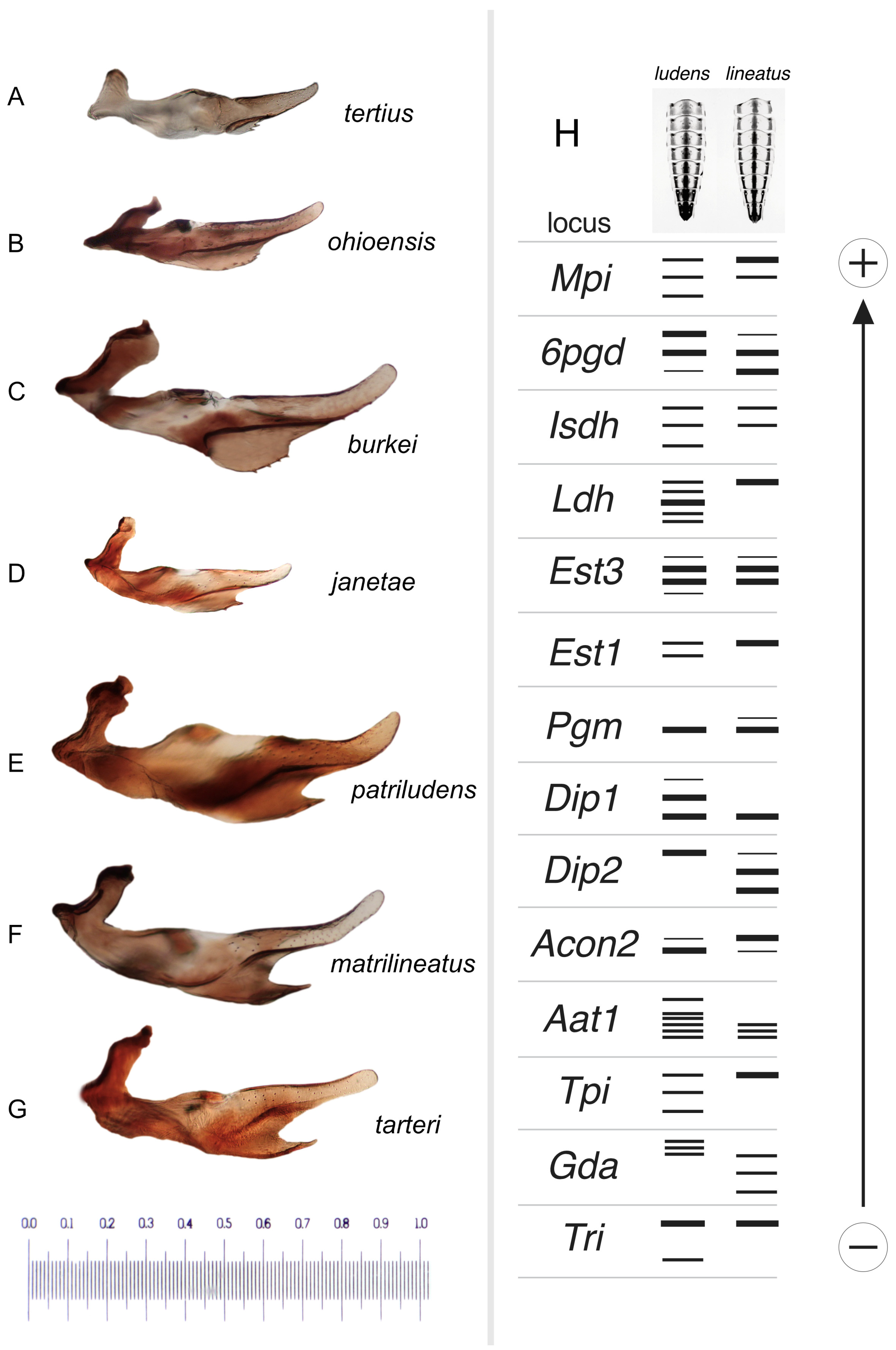
Figure 15.
(A–G). Male penes, left lateral view, with species labeled. Scale in mm. (H). Graphic representation of allozyme banding patterns at 14 loci that distinguish the dominant Ameletus ludens and A. lineatus clones present in White Clay Creek, Pennsylvania, USA. Larval sternite color patterns above.
Figure 15.
(A–G). Male penes, left lateral view, with species labeled. Scale in mm. (H). Graphic representation of allozyme banding patterns at 14 loci that distinguish the dominant Ameletus ludens and A. lineatus clones present in White Clay Creek, Pennsylvania, USA. Larval sternite color patterns above.
Figure 16.
(A). Ameletus ludens larvae, clonal siblings, developmental series, dorsal view. Scale = 5 mm. (B). Same individuals as (A), ventral view. (C). Ameletus matrilineatus larvae, siblings, developmental series, ventral view. Scale = 5 mm. (D). Same individuals as (C), right lateral view. (E). Ameletus ludens and lineatus, sternites from final larval exuviae. (F). Ameletus immaculatus egg in post-diapause stage, with embryo visible. Scale = 0.1 mm.
Figure 16.
(A). Ameletus ludens larvae, clonal siblings, developmental series, dorsal view. Scale = 5 mm. (B). Same individuals as (A), ventral view. (C). Ameletus matrilineatus larvae, siblings, developmental series, ventral view. Scale = 5 mm. (D). Same individuals as (C), right lateral view. (E). Ameletus ludens and lineatus, sternites from final larval exuviae. (F). Ameletus immaculatus egg in post-diapause stage, with embryo visible. Scale = 0.1 mm.
Figure 17.
(A–C). Determining the gender of half- to full-grown larvae by the presence of developing styliger plate and forceps at the posterior margin of sternite 9 in male Ameletus burkei (larval exuviae with 10th segment removed for clarity). Body length (A) 6.5 mm; (B): 8.5 mm; (C): 12 mm (final larval instar). (D,E). Female larvae of A. immaculatus for comparison. Body length (D): 7 mm; (E): 12 mm. (F). Photograph of a starch gel stained for glucose phosphate isomerase (Gpi; E.C. 5.3.2.9). Homogenates from 30 individual adult mayflies (11 A. tarteri and 19 A. immaculatus) were electrophoresed (origins along the horizontal gap visible at the lower third of image). The five alleles present among these individuals (designated C, D, G, J, and K) are represented by gray elliptical spots on the right. Six distinct genotypes are labeled at the top. Nine individuals of A. tarteri were homozygous for allele J, resulting in a single band of activity. Two of the A. tarteri and all 19 of the A. immaculatus were heterozygous. Gpi is dimeric (composed of two subunits) such that a diploid individual, which is heterozygous for two alleles, will stain with three bands: a slower band corresponding to one allele, a faster band from the other allele, and a hybrid band with intermediate mobility. If the polypeptides produced by the two alleles contribute equally and the subunits combine randomly, the relative intensity of the three bands will be 1:2:1 (slow:hybrid:fast, e.g., genotype GJ). In the case of triploids, if an individual is heterozygous with three different alleles, there will be 6 bands (e.g., genotype CGK), although depending on the relative mobilities of the subunits, hybrid bands may superimpose on primary bands. Triploids with two copies of one allele and one of the second allele will show three bands (e.g., CJJ) with relative intensities of 4:4:1.
Figure 17.
(A–C). Determining the gender of half- to full-grown larvae by the presence of developing styliger plate and forceps at the posterior margin of sternite 9 in male Ameletus burkei (larval exuviae with 10th segment removed for clarity). Body length (A) 6.5 mm; (B): 8.5 mm; (C): 12 mm (final larval instar). (D,E). Female larvae of A. immaculatus for comparison. Body length (D): 7 mm; (E): 12 mm. (F). Photograph of a starch gel stained for glucose phosphate isomerase (Gpi; E.C. 5.3.2.9). Homogenates from 30 individual adult mayflies (11 A. tarteri and 19 A. immaculatus) were electrophoresed (origins along the horizontal gap visible at the lower third of image). The five alleles present among these individuals (designated C, D, G, J, and K) are represented by gray elliptical spots on the right. Six distinct genotypes are labeled at the top. Nine individuals of A. tarteri were homozygous for allele J, resulting in a single band of activity. Two of the A. tarteri and all 19 of the A. immaculatus were heterozygous. Gpi is dimeric (composed of two subunits) such that a diploid individual, which is heterozygous for two alleles, will stain with three bands: a slower band corresponding to one allele, a faster band from the other allele, and a hybrid band with intermediate mobility. If the polypeptides produced by the two alleles contribute equally and the subunits combine randomly, the relative intensity of the three bands will be 1:2:1 (slow:hybrid:fast, e.g., genotype GJ). In the case of triploids, if an individual is heterozygous with three different alleles, there will be 6 bands (e.g., genotype CGK), although depending on the relative mobilities of the subunits, hybrid bands may superimpose on primary bands. Triploids with two copies of one allele and one of the second allele will show three bands (e.g., CJJ) with relative intensities of 4:4:1.
![Insects 16 00530 g017]()
3.4. Taxonomic Treatment
| |
| Ameletus browni species group |
| |
The
A. browni group is proposed here and includes
Ameletus browni,
A. rebucki, and
A. cryptostimulus. This group is endemic to the eastern Nearctic. Adult males share a distinctive recurved (U-shaped) structure on the ventral plates of the penes (
Figure 1C,
Figure 2C and
Figure 3C) unique to this group. Larvae can be distinguished from other eastern Nearctic
Ameletus by gill 4, which has the anal rib on its margin (i.e., not inset) and both the costal and anal margins have robust spines.
Ameletus browni McDunnough [
12]: 278; Needham et al. [
13]: 450; Zloty [
1]: 303.
Type Material. Male holotype and female allotype: Mt.Lyall, Gaspe County, Quebec, Canada 11 August 1933; No. 3652 in CNC (male paratype 3652 examined).
Diagnosis.
Ameletus browni is a diploid bisexual species. Male imagos (
Figure 1A) are clear-winged with a slight amber tinge and light brown veins and cross-veins. Upper portion of eyes yellow-green in life. They can be distinguished from most other eastern Nearctic species by the ventral plates of penes which form a distinctive U-shaped structure, the central portion consisting of a single large spine without accessory teeth or spinules (
Figure 1C,D).
Ameletus cryptostimulus and
A. rebucki are similar in this regard, but
A. browni can be distinguished from those by the shape of the basal sclerite of the penes (arrow in
Figure 1C) and the relatively short and slender lateral lobes.
Larvae can be distinguished from all other eastern Nearctic species by the following combination: wide setal gap on the mandible (
Figure 1E); gill 4 with spines on the costal and anal margins and with anal rib on the margin (i.e., not inset; as in
Figure 3G); dark band on caudal filaments evenly colored and extending from near base to about 2/3 out to tips (
Figure 1F); dorsum of abdomen with color pattern forming a pale median longitudinal stripe; spines present on posterior margins of all tergites.
Distribution. Quebec and New England south to northern Pennsylvania.
Phenology. Apparently univoltine, with emergence in mid-May in the southern portion of its range to mid-August in the north. Emergence begins later in the season than species with which it is commonly found (A. rebucki, A. ludens, A. immaculatus). Egg development has not been documented.
Remarks. Ameletus browni larvae can usually be spotted easily in the field by the distinctive pale median stripe running down the terga. Similarities to both A. cryptostimulus and A. rebucki with regard to the gills of larvae and the ventral plate of the penes in adult males, as well as genetic data (both COI and allozymes), show the close relationship among these three. Ameletus browni can often be found together with A. rebucki. The latter lacks the dorsal stripe in the larva, is larger, and emerges earlier in the year.
| |
| Ameletus cryptostimulus Carle |
| (Figure 2A–F) |
| |
Ameletus cryptostimulus Carle [
14]: 581; Zloty [
1]: 305.
Type Material. Male holotype and female allotype: Little Stony Creek, Giles County, Virginia, USA 10 April 1977, F. Carle; in USNM (not examined).
Diagnosis.
Ameletus cryptostimulus is a diploid bisexual species. Male imagos (
Figure 2A) are clear-winged with a very slight amber tinge and light brown veins and cross-veins. The body size ranges from 9 to 11 mm, forewing length 9–11 mm. Upper portion of eyes yellow to green in life. They can be distinguished from most other eastern Nearctic species by the ventral plates of penes, which form a distinctive U-shaped structure, the central portion consisting of a single large spine without accessory teeth or spinules (
Figure 2C,D).
Ameletus browni and
A. rebucki are similar in this regard, but
A. cryptostimulus can be distinguished from
A. browni by the shape of the basal sclerite of the penes (compare the arrow in
Figure 1C with
Figure 2C) and the relatively long and wide lateral lobes. Compared to
A. rebucki, the lateral lobes of the penes in
A. cryptostimulus are shorter relative to the U-shaped ventral plates (
Figure 2C vs.
Figure 3C), and
A. cryptostimulus males are more brightly colored (see
Figure 2A vs.
Figure 3A).
Larvae can be distinguished from all other eastern Nearctic species by the following combination: wide setal gap on the mandible that is about as long as setal row (
Figure 2E); gill 4 with spines on the costal and anal margins and anal rib on margin (i.e., not inset; as in
Figure 3G); dark band on caudal filaments evenly colored and extending from near base to about 2/3 out to tips; dorsum of abdomen without a pale median longitudinal stripe; spines present on posterior margins of tergites 5– or 6–10 (missing on 1–4).
Distribution. Mountains of Virginia, North Carolina, South Carolina, and eastern Tennessee.
Phenology. Univoltine, with emergence from March through June (Huryn and Wallace [
15]; predominantly April and May). Eggs begin hatching in summer (without a diapause), but hatching continues over an extended period into autumn.
Remarks. Ameletus cryptostimulus, A. rebucki, and A. browni form a distinct group (the browni group) based on male genitalia and larval gills. Morphologically, A. cryptostimulus is most similar to A. rebucki, but the two appear to have non-overlapping ranges.
Ameletus cryptostimulus Carle; Zloty [
1]: 305 (in part).
Description. Male holotype imago (fresh, in alcohol) (
Figure 3A).
Body length 9 mm, forewing length 9.5 mm. Head blackish-brown; compound eyes dark greenish-brown dorsally, black laterally. Prothoracic tergum dark brown; mesotergum brown with slightly paler submedian streaks, scutellum blackish-brown; metatergum dark brown; thoracic pleuron dark brown with paler areas at the base of wings, legs, and surrounding mesothoracic spiracle. Fore legs dark brown; mid- and hind legs light brown. Wings transparent with dark infuscation in stigmatic area. Longitudinal veins mostly dark brown (lighter toward costal region), cross-veins dark brown. Abdominal tergite 1 dark brown; tergites 2–8 dark creamy with dark brown shading on posterior margin that extends medially and laterally to form triangular posterior lateral patches; tergite 9–10 dark brown. Abdominal sternite 1 brown; sternites 2–8 creamy with brown submedian spots and distinct ganglionic spots; 9 pale creamy with dark brown laterally. Styliger plate creamy; forceps brown. Caudal filaments uniformly light brown. Long joint of forceps with uniform thickness through its length (
Figure 3B). Penes (
Figure 3C) with ventral plates produced into a distinctive U-shaped structure, with a large median spine and no accessory teeth (
Figure 3D).
Larva: Body length 10–13 mm [holotype 10 mm]. Dorsally with pale median and lateral spots on a dark brown base (
Figure 3E). Sternal coloration mostly pale, often distinctly darkened on the lateral ¼ of each sternite. Mandible with a wide setal gap as long or longer than setal row (
Figure 3F). Legs mostly brown, not distinctly patterned except for black-tipped tarsi. Small spines present on posterior margins of terga 3– or 4–10. Gill 4 of mature larvae with anal rib on anal margin and spines present on both costal and anal margins (
Figure 3G). Caudal filaments usually dark from base but with a darker band in middle 1/3 (with all articulations similarly colored) and with distal 1/3 pale.
Type Material. Male imago holotype. Reared from larva, tributary of Wyalusing Creek, 4.8 km W of Montrose, Susquehanna County, Pennsylvania, USA (41.81972° N, 75.93333° W, elev. 372 m), 24 April 1985, D.I.Rebuck and D.H.Funk. One female allotype and two male paratypes, reared from larvae 26 April 1984, same data. Types will be deposited at PERC.
Etymology. Named for David I. Rebuck, whose meticulous field and laboratory work helped make this study possible.
Diagnosis.
Ameletus rebucki is a diploid bisexual species. Adult males can be distinguished from most other eastern Nearctic species by the ventral plates of penes which form a distinctive U-shaped structure, the central portion consisting of a single large spine without accessory teeth or spinules (
Figure 3C,D).
Ameletus browni and
A. cryptostimulus have similar ventral plates, but
A. rebucki can be distinguished from
A. browni by the shape of the basal sclerite of the penes (see arrow in
Figure 1C) and the relatively long and wide lateral lobes. Compared to
A. cryptostimulus, the lateral lobes of the penes in
A. rebucki are longer relative to the U-shaped ventral plates (
Figure 3C vs.
Figure 2C), and
A. rebucki males are darker than
A. cryptostimulus (see
Figure 3A). Also, the upper portion of the compound eyes is dark brown in life, with usually only a hint of greenish.
Larvae can be distinguished from all other eastern Nearctic species by the following combination of characters: wide setal gap on the mandible that is subequal to the length of the setal row (
Figure 3F); gill 4 with spines on the costal and anal margins and with anal rib on margin (i.e., not inset;
Figure 3G); caudal filaments usually dark from base, with a darker, evenly colored band in middle 2/3 and paler beyond (
Figure 3E); dorsum of abdomen dark with paler spots, but without a pale median longitudinal stripe; spines present on posterior margins of tergites 3– or 4–10 (missing on 1–2 or –3).
Distribution. Vermont and New Hampshire south to West Virginia.
Phenology. Ameletus rebucki is univoltine but does not have a summer egg diapause. Emergence is early, beginning in late March in West Virginia and Pennsylvania to late April in Vermont.
Remarks. Previous records of A. cryptostimulus from areas north of Virginia are attributable to A. rebucki. See remarks under A. cryptostimulus.
| |
| Ameletus ludens species group |
| |
The
A. ludens group is proposed here and includes
A. burkei,
A. immaculatus,
A. janetae,
A. lineatus,
A. ludens,
A. matrilineatus,
A. patriludens,
A. ohioensis, and
A. tarteri. It includes the three known polyploid parthenogens and their bisexual progenitors. Adult males can be distinguished from other eastern Nearctic
Ameletus (except for
A. tertius) by their long lateral lobes (reaching beyond the forceps base;
Figure 13E vs.
Figure 13D) and by the ventral plate not being recurved (as in
A. browni group). Larvae are the only eastern Nearctic species with the anal rib of gill 4 narrowly inset (as in
Figure 5E).
Description. Male holotype imago (fresh, in alcohol) (
Figure 4A). Body length 13 mm, forewing length 12 mm. Head blackish-brown with paler patches on face and between compound eyes and antennae; compound eyes pale yellow-green dorsally with a black line laterally, light gray immediately below, then dark gray below that. Prothoracic tergum blackish-brown; mesotergum light brown, scutellum light brown changing to blackish at the posterior lateral areas; metatergum blackish; thoracic pleuron dark brown with paler areas at the base of wings, legs, and surrounding mesothoracic spiracle. Forelegs gray-brown; mid- and hind legs pale creamy. Wings transparent. Longitudinal veins amber brown, cross-veins light brown. Abdominal tergite 1 dark brown; tergites 2–9 yellowish with brown shading on posterior margin that extends and forms triangular posterior lateral patches; tergite 10 yellowish with brown submedian streaks. Abdominal sternites 3–7 with conspicuous ganglionic markings; sternite 1 blackish; sternites 2–4 creamy yellowish with brown submedian spots; 5–8 yellowish; 9 yellowish with dark brown along lateral margin. Styliger plate creamy yellowish; basal half of forceps long joint gray brown, distally yellow to creamy. Caudal filaments light brown with a reddish-brown ring near the base of each segment. Long joint of forceps of uniform thickness through its length (
Figure 4B). Penes (
Figure 4B and
Figure 15C) with broad ventral plates, not produced into a spine posteriorly, with a row of 3–6 teeth along the ventral margin (
Figure 4D).
Larva: (
Figure 4E). Body length 10–16 mm (holotype 13). Dorsal color pattern subtle and variable. Sternal coloration also variable, but usually pale creamy with two pairs of small submedian dots on most sternites, frequently with dark shading near lateral margins on 1–9, which sometimes extends across the sternite. Mandible with distinct setal gap, 20–40% length of setal row (
Figure 4F). Legs not distinctly patterned except for apical black band on tarsi and white patch on the distal 1/3 of the anterior face of the femora, the latter visible in living specimens. Small spines always present on the posterior margins of terga 4–10; most individuals also have smaller spines on tergum 3, and often traces on tergum 2. Gill 4 of mature larvae with anal rib narrowly inset, with spines present on both costal and anal margins (as in
Figure 5E). Caudal filaments pale at base with a distinct dark band in middle 1/3 (with all articulations similarly colored) and with distal tips darkened.
Type Material. Male imago holotype. Reared from larva, headwaters of Station Spring Creek, Tazewell County, Virginia, USA (37.087180° N, 81.403560° W, elev. 1100 m), 15 April 2024, D.H.Funk and A.C.Graham. Three male, one female imago paratypes, reared from larvae, same locality, 22 April 1990, D.H.Funk. Types will be deposited at PERC.
Etymology. Named for the type locality in Burke’s Garden, Virginia.
Diagnosis. Ameletus burkei is a diploid bisexual species. Adult males can be distinguished from all other eastern Nearctic
Ameletus by the following combination of characters: long lateral lobes of the penes extending beyond the base of the forceps; long joint of forceps with uniform thickness (i.e., without dilation in apical 1/3); ventral plates of the penes neither produced into a spine distally, nor with a prominent rounded lobe projecting anteriorly, but extending ventrally into a broad plate with 2–6 teeth along its rounded ventral margin (
Figure 15C). Larvae can be distinguished from most other eastern Nearctic species by the following combination: distinct but narrow setal gap on the mandible; gill 4 with spines on both the costal and anal margins, with anal rib narrowly inset (as in
Figure 5E); dark band in middle 1/3 of caudal filaments with segments uniformly colored; venter of abdomen without distinct stripes (
Figure 4E).
Ameletus burkei larvae are not distinguishable from
A. immaculatus other than by the presence of males, and from
A. tarteri or
A. ohioensis other than known geographic range or COI haplotype.
Distribution. Presently known only from the type locality in southwest Virginia.
Phenology. Ameletus burkei is univoltine, with an obligate summer egg diapause. Adults are present in mid-April and emergence likely extends through May.
Remarks. Genetic data indicate A. burkei is the closest known relative to the maternal ancestor of the obligate thelytokous parthenogen A. immaculatus, with which it coexists at the type locality in Station Spring Creek. There, A. burkei (and A. immaculatus) are found in the headwaters and are replaced by A. matrilineatus downstream in the same pattern evident in much of the eastern U.S., where the unstriped parthenogen A. immaculatus is found in headwaters and is replaced by one or both of the striped parthenogens A. ludens and A. lineatus downstream.
Ameletus burkei is presently known only from the type locality. Future collections may reveal that the species is more widespread. However, as discussed under A. matrilineatus (below), the apparent uniqueness of the Station Spring Creek fauna may be due to its physical isolation.
Description. Female holotype imago (fresh, in alcohol) (
Figure 5A). Body length 10 mm, forewing length 10.5 mm. Head brown with paler patches on face and between compound eyes and antennae, and on vertex; compound eyes dark gray dorsally with a black line laterally, light gray in middle 1/3, black again below. Prothoracic tergum brown; mesotergum yellowish brown, scutellum light brown changing to black at the posterior lateral areas; metatergum blackish; thoracic pleuron dark brown with paler areas at the base of wings, legs, and surrounding mesothoracic spiracle. All legs light brown. Wings transparent. Longitudinal veins and cross-veins brown. Abdominal tergite 1 dark brown; tergites 2–9 light brown with darker shading on posterior margin that extends and forms triangular posterior lateral patches; tergite 10 light brown with dark brown submedian streaks. Abdominal sternites without conspicuous ganglionic markings; sternite 1 brown; sternites 2–9 light red-brown. Caudal filaments light brown with a dark ring near the base of each segment.
Larva: Body length 8–16 mm. Dorsal color pattern variable but subtle (
Figure 5B,C). Sterna unpatterned except for two pairs of small submedian dots on most sternites, and sometimes dark shading near lateral margins of sternum 9, which may extend anteriorly to 8 and 7. Mandible with distinct setal gap, 20–40% length of setal row (
Figure 5D). Legs not distinctly patterned except for apical black band on tarsi and white patch on the distal 1/3 of the anterior face of the femora, the latter visible in living specimens. Small spines always present on posterior margins of terga 4–10 in full-grown larvae; larger individuals also have smaller spines on tergum 3, and often traces on tergum 2. Gill 4 of full-grown larvae with anal rib narrowly inset, and spines present on both costal and anal margins (
Figure 5E). Caudal filaments pale at base with a distinct dark band in middle 1/3 (with all articulations similarly colored) and with distal tips darkened.
Type Material. Female imago holotype. Reared from larva, headwaters of Puncheoncamp Branch, Browns Creek, McDowell County, West Virginia, USA (37.486243° N, 81.565984° W, elev. 563 m), 22 April 1990, D.H.Funk. Two female imago paratypes, same data. Types will be deposited at PERC.
Etymology. immaculatus = unmarked (Latin), in reference to the lack of ventral stripes in the larva, which are present in the other eastern Nearctic parthenogens.
Diagnosis. Ameletus immaculatus is a clonal, obligate thelytokous parthenogen. All clones that have been tested are triploid. Adults are variable in size, with body length up to 14 mm and wing length up to 15 mm. Size not only decreases through the emergence period but also varies considerably by population. The female imago is predominantly dark brown or reddish brown, with clear wings and brown venation, and aside from the absence of ganglionic spots on the venter of the abdomen (which may be present to varying degrees in its close relatives),
A. immaculatus is not distinguishable morphologically from females of other clear-winged species. The subimago looks similar but with a dull gray overcast and slate gray wings (as in
Figure 7B). Full grown larvae range up to 16 mm body length but may be as small as 8 mm.
Ameletus immaculatus larvae can be distinguished from most other eastern Nearctic species by the combination of the presence of a narrow but distinct setal gap in the mandible; a distinct (but uniformly colored, i.e., unspeckled) dark band in the middle 1/3 of the caudal filaments; anal rib narrowly inset on gill 4; the lack of dark patterning on the venter of the abdomen (other than small submedian dots and some shading on the lateral margins of sternite 8 and 9); and the absence of males. However, the only sure way to distinguish
A. immaculatus from female larvae of the bisexual species
A. burkei,
A. tarteri, and
A. ohioensis, with which it may co-occur, is by ploidy level, parthenogenetic reproductive mode, and COI haplotype.
Distribution. From northern New England south to North Carolina, west to Ohio.
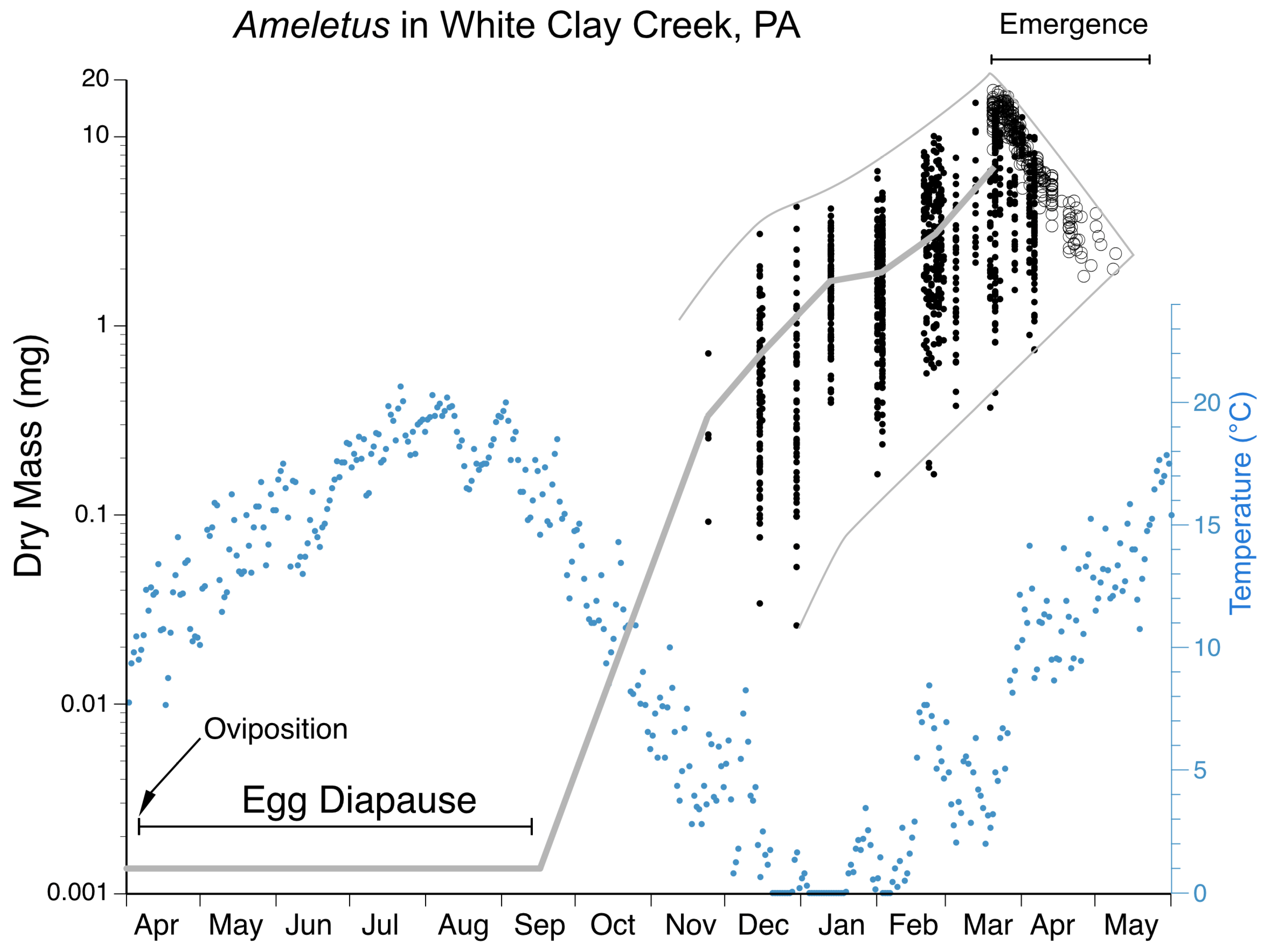
Phenology. Ameletus immaculatus is univoltine, with an obligate summer egg diapause. Young larvae first appear in autumn and grow through winter and early spring. Adults are present from mid-March in the south and at lower elevations, through June in more northern areas and higher elevations.
Remarks. Ameletus immaculatus is a widespread, but previously unrecognized, obligately parthenogenetic species. It inhabits small headwater streams and springs and is nearly always found in proximity to one or both of the other two eastern Nearctic parthenogens
A. ludens and
A. lineatus (in fact, Traver [
16] may have been looking at an
A. immaculatus imago when she determined that
A. lineatus imagos lack ganglionic spots). In a given stream, the three parthenogens have an upstream-downstream distributional relationship, with
A. immaculatus being the upstream member but mixing with one or both of the others in the middle reaches, and
A. immaculatus being absent downstream. As is the case with
A. ludens and
A. lineatus,
A. immaculatus has a much broader geographic distribution than any of its close bisexual relatives (viz.,
A. burkei,
A. ohioensis, or
A. tarteri). At sites where these bisexuals are found,
A. immaculatus is usually also present, and larvae are difficult or impossible to distinguish from those except by their sexuality, ploidy level, or COI haplotype. Published larval records of
A. tarteri from localities other than eastern West Virginia likely represent misidentified
A. immaculatus.
The type locality of A. immaculatus in southern West Virginia is now, like many first-order streams in that region, buried beneath valley fill from a nearby coal mining operation.
Ameletus janetae Kondratieff and Meyer [
2]: 526.
Type Material. Male imago holotype. Hardy County, West Virginia, USA 3mi NE [sic] Mathias, 38.917° N, 78.883° W, 8–29 May 2008, D. R. Smith, Malaise Trap (USNM). Paratype: 1 male, same data as holotype (CSUC).
Diagnosis. Ameletus janetae is a diploid bisexual species. Male imagos (
Figure 6A) can be distinguished from other eastern Nearctic
Ameletus species by the following combination of characters: long lateral lobes of the penes extending beyond the base of the forceps; long joint of forceps with slight dilation in the apical 1/2 (Kondratieff and Meyer [
2]:
Figure 1;
Figure 6B); ventral plates of the penes produced into a very small spine distally (Kondratieff and Meyer [
2]:
Figure 3;
Figure 15D), with 3–5 teeth along their ventral margins (
Figure 6D).
Larvae can be distinguished from all other eastern Nearctic species by the following combination: distinct but narrow setal gap on the mandible (
Figure 6E); gill 4 with spines on both the costal and anal margins, with anal rib narrowly inset (as in
Figure 5E); dark band in middle portion of caudal filaments with every 4th segment pale, giving it a speckled appearance (
Figure 6F–H); venter of abdomen with three dark stripes (median stripe often broken into a series of spots) that do not coalesce on sternite 9 (
Figure 6H); spines present on posterior margins of tergites 4–9 in late instars (sometimes small and scattered on 3, but absent from 1 and 2).
Distribution. Known only from a few streams in the vicinity of the type locality in eastern West Virginia.
Phenology. Presumably univoltine, with a summer egg diapause, but the latter is undocumented. Emergence in May. All streams where the larvae have been collected had no surface flow in late summer and early autumn.
Remarks. Kondratieff and Meyer [
2] described
A. janetae from two male imagos captured in a malaise trap near Lost River State Park, NW of Mathias, West Virginia. I was able to rear larvae from three first-order tributaries to Howard’s Lick Creek near the type locality in 2022 through 2024. All three of those streams were dry at the surface from late July through early October. No
A. janetae larvae were found in the mainstem of Howard’s Lick Creek, which retained surface flow throughout the year.
Ameletus immaculatus was abundant in the streams where
A. janetae was found and vastly outnumbered the latter.
Ameletus ludens and
A. lineatus were common just downstream of these sites.
Ameletus lineatus Traver [
16]: 194; Traver [
13]: 453; Burks [
17]:102.
Type Material. Female imago holotype. Reared from nymph, Big Alamance Creek, North Carolina, USA, Feb. 20, 1930. No. 1079.1 in CUIC. Two female subimago paratypes, same data Feb. 28, 1930. No. 1079.2-3 CUIC.
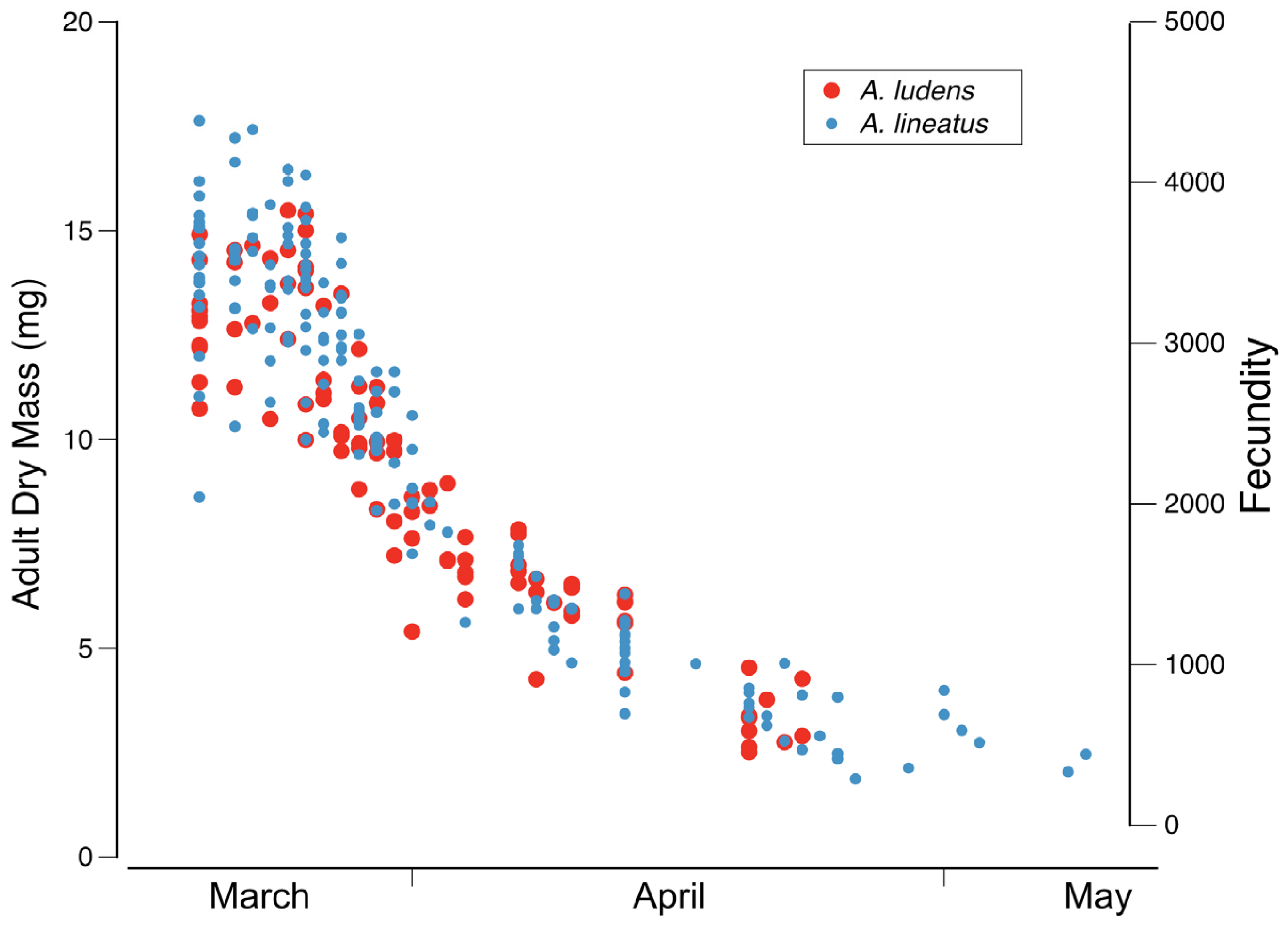
Diagnosis. Ameletus lineatus is a clonal, obligate thelytokous parthenogen. All clones that have been tested are triploid. Adults are quite variable in size; wing length of large individuals 13 mm, but the size drops off dramatically through the emergence period (see
Figure 21). The female imago is predominantly dark brown or reddish brown, with clear wings and brown venation (
Figure 7A), and like
A. ludens, is not distinguishable morphologically from females of other clear-winged species. The subimago looks similar but with a dull gray overcast and slate gray wings (
Figure 7B). Full grown larvae range up to 14.5 mm body length and are distinguished from all eastern Nearctic species (except for
A. matrilineatus) by the following combination of characters: the presence of a narrow setal gap on the mandible (
Figure 7C); caudal filaments with a distinct dark band in middle 1/2, with every 4th segment within band lighter, giving the band a speckled appearance (as in
Figure 9F); small spines present on posterior margins of terga 1–10 (may be quite small on tergum 1, but always distinct on 2–10 in full-grown larvae); abdomen ventrally with three narrow dark stripes with distinct margins that coalesce at the posterior margin of sternum 9 (
Figure 16E). Distinguishable from
matrilineatus only by the absence of males (see
Figure 17A–E) and ploidy level.
Distribution. From Pennsylvania south to South Carolina, west to Missouri and Arkansas.
Phenology. Ameletus lineatus is univoltine, with an obligate summer egg diapause. Young larvae first appear in autumn and grow through winter and early spring. Adults are present from mid-March in the south and at lower elevations, through May in more northern areas and higher elevations.
Remarks. Traver [
16] described
A. lineatus from adult females reared from larvae collected from the piedmont of North Carolina.
See remarks under
A. ludens (below). Because ventral stripes on larvae do not completely develop until the larva is fully grown (see
Figure 17C–D), the distinction between
A. ludens and
A. lineatus (with which it commonly occurs) is not possible for younger larvae. The range of
A. lineatus extends further south and west than
A. ludens and because of the difficulty in distinguishing the two morphologically, I consider published records of
A. lineatus from north of Pennsylvania, Ohio, Indiana and Illinois to be questionable.
Ameletus ludens Needham [
18]: 36; Morgan [
19]: 117; Needham [
20]: 310; Traver [
18]: 454; Traver [
16]: 194; Burks [
17]:103.
Type Material. None designated but described from larvae and female subimagos collected at Newport, New York, USA (CUIC) (examined).
Diagnosis. Ameletus ludens is a clonal, obligate thelytokous parthenogen. All clones that have been tested are triploid. Adults are quite variable in size; wing length of large individuals 12.5 mm, but the size drops off dramatically through the emergence period (see
Figure 21). The female imago is predominantly dark brown or reddish brown, with clear wings and brown venation (
Figure 8A), and is not distinguishable morphologically from females of other clear-winged species. The subimago looks similar but with a dull gray overcast and slate gray wings (
Figure 8B). Full-grown larvae (
Figure 8C) range up to about 13 mm body length and are distinguished from all eastern Nearctic species (except for
A. patriludens) by the following combination of characters: the presence of a narrow setal gap on the mandible (as in
Figure 7C); caudal filaments with a distinct dark band in middle 1/2, with every 4th segment within band lighter, giving the band a speckled appearance (as in
Figure 9F); small spines present on posterior margins of terga 1–10 (may be quite small on tergum 1, but always distinct on 2–10 in full-grown larvae); abdomen ventrally with three dark stripes that coalesce on sternum 9 (and to varying degrees on 7 and 8), with the median stripe wider and its borders poorly defined compared with those of
A. lineatus and
A. matrilineatus (
Figure 16B,E). Distinguishable from
patriludens only by the absence of males (see
Figure 17A–E), ploidy level, and COI haplotype.
Distribution. From eastern Canada (southern Ontario to Newfoundland) to the southern Appalachians; south of Pennsylvania generally restricted to the mountains.
Phenology. Ameletus ludens is univoltine, with an obligate summer egg diapause. Young larvae first appear in autumn and continue to grow through winter and early spring. Adults are present from early spring in the south and at lower elevations, through early summer in the most northern areas and highest elevations. See
Figure 20 and
Figure 21.
Remarks. Needham [
18] described
A. ludens from larvae and female subimagos collected in the Hasenclever Hills of central New York. Much subsequent effort to obtain male imagos by Needham and others, including Morgan [
19] and Clemens [
21], was unsuccessful (although Clemens found one presumed male subimago which failed to transform). Morgan [
19] was the first to suggest parthenogenesis in
A. ludens, and Clemens [
21] demonstrated parthenogenetic hatching of eggs. In 1924, Needham [
20] described what he believed to be the male of
A. ludens, from a single imago captured in flight. The basis for its association with
A. ludens was the fact that no other
Ameletus species was known from the eastern Nearctic at the time. Genitalia from that specimen were illustrated by Traver in Needham et al. [
13] but have since been lost. Although Traver’s illustration lacks sufficient detail for the penes, the distinctive dilation in the long joint of the forceps makes it likely that the specimen was
A. patriludens.
The imagos of both
A. ludens and
A. lineatus have ganglionic markings on the venter of the abdomen that vary from barely perceptible to rather distinct on abdominal segments 2 or 3 to 8, and contrary to indications by Traver [
13,
16] and Burks [
17], the two species cannot be separated by their presence or absence (see Remarks under
A. immaculatus). Nor is the shape of the distal margin of sternum 9 consistently different, as illustrated by Figures 238 and 239 in Burks [
17]. And contrary to Traver’s ([
13]: 448) key, there is no “white line present between each lateral stripe and pleural fold” in
A. lineatus larvae. In both
A. ludens and
A. lineatus, the lateral edge of each lateral stripe borders the pleural fold. The difference between the two species is most evident in the median stripe (see description above and
Figure 16E). However, the ventral stripes on larvae do not fully develop until the last one or two instars (see
Figure 16C). At least partly due to this limitation, identifications in the literature are usually unreliable. The best way to assess larval developmental stage is by the extent of the developing wing pads: if the distal tip of the forewing pad does not reach at least as far as the posterior margin of tergite 2, the ventral stripes are likely not fully developed.
Description. Male imago (
Figure 9A) body length 14 mm, forewing length 12.5 mm. In life, head blackish with opaque white patches on face and between compound eyes and antennae; compound eyes greenish yellow dorsally and gray ventrally with dark line dividing in living individuals. Prothoracic tergum blackish; mesotergum yellowish and brown, with yellow streaks along anterolateral margin, scutellum yellow changing to dark brown and black at the posterior lateral areas; metatergum blackish; thoracic pleuron brown with pale at the base of wings and mesothoracic spiracle. Fore legs brown; middle and hind legs light brown. Wings transparent with brown longitudinal veins and cross-veins. Abdominal tergites with conspicuous tracheae; tergite 1 dark brown; tergites 2–9 yellowish with dark brown shading on posterior margin that extends and forms triangular posterior lateral patches; tergite 10 light brown with dark brown submedian streaks. Abdominal sternites with ganglionic markings on sternites 2–7; sternite 1 brown; sternites 2–8 largely pale yellowish, with conspicuous tracheae; sternite 9 and styliger plate yellow brown with brown spots on styliger plate near the base of each forcep. Long joint of forceps distinctly swollen in distal 2/3 (
Figure 9B). Penes (
Figure 9C) with ventral plates forming a prominent spine with a row of 4–7 teeth on the ventral margin (
Figure 9D). Caudal filaments brown with a narrow, darker ring at the base of each segment. Female imago (
Figure 9E) indistinguishable from most other
Ameletus females. However, female
A. matrilineatus imagos can be distinguished from those of
A. burkei or
A immaculatus, with which it may be found, by the presence of distinct ganglionic markings on the sternites. Larva: identical to
A. lineatus (see
Figure 7D and
Figure 16C–D) except for sexuality and ploidy level.
Type Material. Reared male holotype: Specimen AA_SSC1_265, Station Spring Creek, Burkes Garden, Tazewell County, Virginia, USA (37.095416° N, 81.383856° W, elev. 972 m) 15 April 2024, D.H.Funk and A.C.Graham. Allotype female and paratype male and female, same data, 13 May 1990, D.H.Funk. Types will be deposited at PERC.
Etymology. matri = mother (Latin), named for giving rise to the parthenogenetic A. lineatus.
Diagnosis. Ameletus matrilineatus is a diploid bisexual species. Male imagos can be distinguished from all other eastern Nearctic species except
A. patriludens by the following combination of characters: penes with long narrow lateral lobes extending beyond the base of the forceps; ventral plates forming a prominent spine with a row of about 6 teeth on ventral margin; long joint of forceps with distinct dilation in apical 2/3.
Ameletus matrilineatus and
A. patriludens males are very similar, but the pointed extension formed by the ventral plate is longer in
A. matrilineatus (
Figure 15F) and the dilation on the long joint of the forceps is more pronounced in
A. patriludens (compare
Figure 9B with
Figure 11B). COI sequences from the two differ by about 6%. Larvae of
A. matrilineatus are distinguished from other eastern Nearctic species by the same combination of characters used to distinguish
A. lineatus (see diagnosis under that species). From
A. lineatus, A. matrilineatus be distinguished only by ploidy level and the presence of males.
Distribution. Presently known only from southwestern Virginia.
Phenology. Ameletus matrilineatus is univoltine, with an obligate summer egg diapause. Young larvae first appear in autumn and grow through winter and early spring. Adults first appear in mid-April and emergence extends through May.
Remarks. Ameletus matrilineatus is the bisexual progenitor of the triploid parthenogen A. lineatus, and the maternal source of the hybrid triploid parthenogen A. ludens. Ameletus matrilineatus probably once had a broader geographic distribution but is now quite rare and local, possibly having been displaced by its parthenogenetic offshoots. The type locality at Station Spring Creek is just upstream from where that stream sinks into the limestone floor of the Appalachian cove known as Burke’s Garden. The upper reaches of Station Spring Creek have no surface connection with other streams or rivers, and are devoid of fish. (The latter was confirmed in 2024 using an eDNA kit from Jonah Ventures, Boulder Colorado.) Ameletus matrilineatus is abundant at that site, but neither of the parthenogens that arose from A. matrilineatus (A. lineatus and A. ludens) are present. In 1990 I collected and reared 261 specimens on 4 April (prior to the start of emergence) and the sex ratio was 53% male, 47% female. I incubated unfertilized eggs dissected from 25 of those females with the following results: 16 clutches with zero hatch, 3 clutches each yielded a single hatchling, for 2 clutches two hatchlings resulted, 1 clutch each at three and five hatchlings, and 2 clutches with nine hatchlings. Each of these clutches contained >1000 eggs, which means parthenogenetic hatch rates were all less than 1%. Parthenogenetic hatch rates for A. ludens and A. lineatus are >90%. A collection of 85 individuals on 15 April 2024 (emergence period was already underway) yielded 48% male and 52% female, indicating no change over the intervening 34 years.
There may be other extant populations of A. matrilineatus yet to be discovered. In the Virginia Tech collection there is a single male reared from Mill Creek, Montgomery Co., VA (37.261448° N, 80.340619° W), 12 April 1979 by Smith.
Description. Male holotype imago (fresh, in alcohol) (
Figure 10A).
Body length 8.8 mm, forewing length 8.7 mm. Head blackish-brown with paler patches on face and between compound eyes and antennae and on vertex; compound eyes pale yellow-green dorsally with a black line laterally, light gray immediately below, then dark gray below that. Prothoracic tergum dark brown; mesotergum light reddish-brown, scutellum light reddish-brown changing to blackish at the posterior lateral areas; metatergum dark brown; thoracic pleuron dark reddish-brown with paler areas at the base of wings, legs and surrounding mesothoracic spiracle. Fore legs light brown; mid- and hind legs pale creamy. Wings transparent. Longitudinal veins amber brown, cross-veins light brown. Abdominal tergite 1 dark brown; tergites 2–9 yellowish with brown shading on posterior margin that extends and forms triangular posterior lateral patches; tergite 10 yellowish with brown submedian streaks. Abdominal sternite 1 blackish; sternites 2–8 creamy yellowish with brown submedian spots and faint ganglionic spots; 9 yellowish. Styliger plate creamy yellowish; forceps gray-brown. Caudal filaments light brown with a reddish-brown ring near the base of each segment. Long joint of forceps with uniform thickness through its length (
Figure 10B). Penes (
Figure 10C and
Figure 15B) with ventral plates not produced into a spine posteriorly, with a row of 3–7 teeth along the ventral margin (
Figure 10D)
Larva: Body length 8–10 mm (holotype 9.3 mm). Dorsal color pattern subtle and variable. Sternal coloration also variable (
Figure 10F–G), but usually pale creamy with conspicuous brown maculae on median and on lateral margin (
Figure 10F). Mandible with distinct setal gap, 20–40% length of setal row (
Figure 10E). Legs not distinctly patterned except for apical black band on tarsi and sometimes another band at tarsal base. Small spines always present on posterior margins of terga 4–10; most individuals also have smaller spines on tergum 2 and 3, which may be scattered. Gill 4 of mature larvae with anal rib narrowly inset, with spines present on both anterior and posterior margins (as in
Figure 5E). Caudal filaments pale at base with a dark band in middle 1/3 (with all articulations similarly colored) and with distal tips darkened.
Type Material. Male imago holotype. Reared from larva, tributary of Storms Creek, 1.6 km E of Ellisonville, Lawrence County, Ohio, USA (38.606389° N, 82.629167° W, elev. 181 m), 7 May 1987, D.H.Funk. Two male imago paratypes, reared from larvae, same data. Types will be deposited at PERC.
Etymology. named for its known distribution, in small tributaries along the Ohio River in Ohio and West Virginia.
Diagnosis. Ameletus ohioensis is a diploid bisexual species. Adult males can be distinguished from all other eastern Nearctic
Ameletus by the following combination of characters: long lateral lobes of the penes extending beyond the base of the forceps; long joint of forceps with uniform thickness (i.e., without distinct dilation in apical 1/3); ventral plates of the penes neither produced into a spine distally, nor with a prominent rounded lobe projecting anteriorly, but extending ventrally into a narrow plate with 3–7 teeth along its ventral margin (
Figure 10D and
Figure 15B). Larvae can be distinguished from most other eastern Nearctic species by the following combination: distinct but narrow setal gap on the mandible; gill 4 with spines on both the costal and anal margins, with anal rib narrowly inset (as in
Figure 5E); dark band in middle 1/3 of caudal filaments with segments uniformly colored; venter of abdomen usually with distinctive maculation (
Figure 10F) but this may be less developed in some specimens (
Figure 10G). Larvae with paler venter and older, faded specimens are not distinguishable from
A. immaculatus other than by the presence of males, or from
A. tarteri or
A. ohioensis other than known geographic range or COI haplotype.
Distribution. Southeastern Ohio and adjacent West Virginia.
Phenology. Ameletus ohioensis is probably univoltine with an obligate summer egg diapause, but this has not been determined experimentally. Emergence is largely in the month of May.
Remarks. I have collected
A. ohioensis from several localities in Lawrence and Washington Counties, OH as well as Cabell County, WV. I have examined specimens (originally reported as male
Ameletus ludens) from Vinton and Guernsey Counties, OH, by Hall [
22]. I have collected both
A. lineatus and
A. immaculatus together with
A. ohioensis in Ohio and West Virginia.
Description. Male holotype imago (fresh, in alcohol) (
Figure 11A). Body length 10.5 mm, forewing length 10.5 mm. In life, head blackish with paler patches on face and between compound eyes and antennae; compound eyes greenish yellow dorsally and dark brown ventrally with dark line dividing in living individuals. Prothoracic tergum blackish; mesotergum yellowish and brown, with yellow streaks along anterolateral margin, scutellum light brown changing to black at the posterior lateral areas; metatergum blackish; thoracic pleuron dark brown with paler areas at the base of wings and mesothoracic spiracle. Fore legs brown; middle and hind legs light brown. Wings nearly transparent with a slight brownish stain. Longitudinal veins and cross-veins dark brown. Abdominal tergites with conspicuous tracheae; tergites 2–9 yellowish with a light brown area on the median and dark brown shading on posterior margin that extends and forms triangular posterior lateral patches; tergite 10 light brown with dark brown submedian streaks. Abdominal sternites with ganglionic markings on sternites 2–7; sternite 1 brown; sternites 2–8 largely pale creamy, with conspicuous tracheae; sternite 9 light brown with dark brown on anterior and lateral margins and styliger plate light brown with darkened distolateral points and brown spots near the base of each forcep. Long joint of the forceps distinctly dilated in distal 2/3 (
Figure 11B). Penes (
Figure 11C) with ventral plates forming a prominent spine with a row of 3–5 teeth on the ventral margin (
Figure 11D). Caudal filaments brown with darker rings at joints. Larva: indistinguishable from
A. ludens except for ploidy level, presence of males and COI haplotype.
Type Material. Reared male holotype: Specimen AL_Nev_57, West Branch Neversink River at Slide Mtn W Trailhead, Sullivan County, New York, USA (42.00866° N, 74.427118° W, elev. 613 m) 21 April 2022, D.H.Funk. Allotype female (AL_Nev_101) and paratype male (AL_Nev_71), same data. Types will be deposited at PERC.
Etymology. patri = father (Latin), paternal source of the hybrid triploid parthenogenetic A. ludens.
Diagnosis. Ameletus patriludens is a diploid bisexual species. Male imagos can be distinguished from all other eastern Nearctic species except
A. matrilineatus by the following combination of characters: penes with long narrow lateral lobes extending beyond the base of the forceps; ventral plates forming a prominent spine with a row of about six teeth on ventral margin; long joint of forceps with distinct dilation in apical 2/3.
Ameletus patriludwns and
A. matrilineatus males are very similar, but the pointed extension formed by the ventral plate is shorter in
A. patriludens (
Figure 15E) and the dilation of the forceps long joint is slightly greater in
A. patriludens (compare
Figure 11B and
Figure 9B). COI sequences from the two differ by about 6%. Larvae of
A. patriludens are distinguished from other eastern Nearctic species by the same combination of characters used to distinguish
A. ludens (see diagnosis under that species). The ventral stripes in
A. patriludens are quite similar to those of
A. ludens: the median stripe is broader and its margins are less defined compared to those of
A. lineatus and
A. matrilineatus (
Figure 16E), and the same is true for the lateral stripes to a lesser degree. However, in
A. patriludens, the stripes often do not coalesce on sternite 9 to the degree they do in
A. ludens, such that white patches between them are evident over most of that segment (
Figure 11F), and in some cases, the stripes do not coalesce at all (
Figure 11G). However,
A. patriludens larvae can only be distinguished with certainty from
A. ludens by ploidy level, the presence of males or COI haplotype.
Distribution. Presently known only from two localities: the type locality in New York and another in north-central Pennsylvania (see Remarks below).
Phenology. Ameletus patriludens is univoltine, with an obligate summer egg diapause. Young larvae first appear in autumn and grow through winter and early spring. Adults first appear in mid-May, and emergence extends into early June.
Remarks. Ameletus patriludens is the paternal progenitor of the hybrid triploid parthenogen A. ludens, whose maternal source is A. matrilineatus. Like its bisexual relative A. matrilineatus, A. patriludens probably once had a broader geographic distribution but is now rare and local, possibly having been displaced by its parthenogenetic offshoots. At the type locality on the upper West Branch of the Neversink, neither A. lineatus nor A. ludens are present. On 21 April 2022 I collected 106 larvae and found a sex ratio of 58% male and 42% female. Thirty-five larvae were sequenced for COI, with the remaining tissue frozen for flow cytometric determination of ploidy. Sixty-two larvae were reared to adults. Of the 45 individuals sequenced, 43 had the same haplotype, while 2 (female larvae) had a second haplotype that differed by a single base substitution (A for G at position 474). Unfertilized eggs were dissected from seven females with the common haplotype, and the parthenogenetic hatch rates for those ranged from <1 to 22%.
In March of 2024, David I. Rebuck and I discovered a population of A. patriludens in an unnamed tributary of Elk Creek at Gramley Gap, Center Co. Pennsylvania (40.94156° N, 77.40467° W, elev 418 m). These have the same haplotype as predominates at the Neversink. There may be other extant populations of A. patriludens yet to be discovered.
Ameletus tarteri Burrows [
23]: 284; Zloty [
1]: 315.
Type Material. Male imago holotype, reared with exuviae. Hamrick Run at West Virginia Route 39/55 near confluence with North Fork of Cherry River, Greenbrier County, West Virginia, USA (38.22778° N, 80.40111° W, elev. 900 m), 15 June 1983, W. L. Burrows. Deposited at USNM (not examined). Male and female paratypes from the type locality and adjacent Carpenter Run, deposited at USNM, CNC, MU, PERC, FAMU, and ANSP.
Diagnosis. Ameletus tarteri is a diploid bisexual species. Male imagos (
Figure 12A) can be distinguished from other eastern Nearctic
Ameletus species by the following combination of characters: long lateral lobes of the penes extending beyond the base of the forceps; long joint of forceps with uniform thickness (i.e., without dilation in apical 1/3;
Figure 12B); ventral plates of the penes produced into a prominent spine distally (
Figure 12C and
Figure 15G), with 1–4 teeth along its ventral margin (
Figure 12D).
Larvae can be distinguished from most other eastern Nearctic species by the following combination: distinct but narrow setal gap on the mandible (
Figure 12F); gill 4 with spines on both the costal and anal margins, with anal rib narrowly inset (as in
Figure 5E); dark band in middle 1/3 of caudal filaments with segments uniformly colored; venter of abdomen without stripes or distinctive maculation. Not distinguishable from
A. immaculatus other than by the presence of males or COI haplotype, or from
A. burkei other than known geographic range or COI haplotype.
Distribution. Eastern West Virginia and western Maryland.
Phenology. In contrast to its closest relatives, larvae of
A. tarteri are present throughout the year. Matthews and Tarter [
24] reported a univoltine life cycle with a bimodal pattern of emergence, one period occurring in late May to early July and a second period in September. I have found a lack of summer egg diapause and an extended period of hatching in
A. tarteri, suggesting larval recruitment is more or less continuous throughout the year.
Remarks. Burrows [
23] described
A. tarteri from a small area in SE West Virginia. Both the type locality, Hamrick Run, and the adjacent Carpenter Run, where Matthews and Tarter [
24] studied its life history, had very low pH in the 1980s (~4.5). In 1990, I surveyed both streams and found
A. tarteri to be the only
Ameletus species present. However, at several nearby sites with a more circumneutral pH (in the 6.5–7.75 range), I found
A. tarteri together with
A. immaculatus and
A. rebucki. In 2022, I encountered a similar situation in east-central West Virginia in tributaries of the Blackwater and Cheat Rivers:
A. tarteri was the only
Ameletus present in Shay’s Run and Red Run, where pH was 4.4, but at several nearby circumneutral sites (pH 6.5–8.0),
A. tarteri was accompanied by
A. immaculatus,
A. rebucki,
A. ludens, and
A. lineatus. Thus, it appears
A. tarteri is able to tolerate a lower pH than other eastern Nearctic
Ameletus species. From 1987 to 1990, there was an active coal mine in the upper Hamrick Run (type locality) watershed, followed by a reclamation effort, which has resulted in a sharp increase in water pH; in September of 2022, I measured 8.2 at the type locality. It will be interesting to see if and when other
Ameletus species colonize that creek.
Burrows [
23] reported larval
A. tarteri (of unspecified gender) from Virginia and New York. However,
A. tarteri larvae cannot be distinguished with certainty from those of
A.burkei,
A. ohioensis, or
A. immaculatus, if they are female. Given what we now know of the distribution of these four species, Burrows’ Virginia and New York specimens were almost certainly
A. immaculatus.
| |
| Ameletus subnotatus species group |
| |
Three species from the Nearctic, the transcontinental
A. subnotatus, the Western
A. oregonensis and the Eastern
A. walleyi, form a distinct group diagnosed by the unique shape of the male genitalia [
1], with short, broad lateral lobes not reaching to the base of the forceps (Figure 2 in Zloty [
10];
Figure 13D). Larvae of
A. walleyi are unknown, but together
A. subnotatus and
A. oregonensis can be distinguished from other western Nearctic
Ameletus by the combination of numerous spines on the posterior margins of sternites 6–8 and caudal filaments with alternating dark and light segments (i.e., speckled) as in
Figure 13H. These two also have the anal rib of gill 4 inset far from the anal margin (
Figure 13F). It is likely that the larvae of
A. walleyi, once discovered, will share these characters.
Ameletus subnotatus Eaton [
25]:211; Spieth [
26]:92; Zloty [
1]: 314.
Type Material. Male lectotype as designated by Spieth [
26]: Colorado, USA; NHM (not examined).
Diagnosis. Ameletus subnotatus is a bisexual species. Male imagos have wings that are mostly transparent with distinctive black patches and margining around some cross-veins, giving them a speckled appearance (
Figure 13A). Body length ranges from 7–10 mm, forewing length 8–10 mm. Upper portion of the compound eye dark brown to black in life. Males can be distinguished from most other eastern Nearctic species by the distinctively shaped penes (
Figure 13C), the lateral lobes of which do not extend as far as the base of the forceps (
Figure 13D vs.
Figure 13E). The only other
Ameletus in the eastern Nearctic with this type of penes is
A. walleyi, which does not have speckled wings and differs in the fine details of the penes.
Larvae can be distinguished from all other known eastern Nearctic species by the following combination: wide setal gap on the mandible that is about as long as the setal row (
Figure 13G); gill 4 with anal rib inset far from anal margin and with spines on both the costal and anal margins (
Figure 13F); caudal filaments dark from base to about ¾ of their length, with every fourth segment pale, giving them a speckled appearance (
Figure 13H); spines present on posterior margins of tergites 2– or 3–10.
Distribution. Transcontinental species with known eastern populations restricted to southern Canada (Ontario, Quebec, New Brunswick, and Newfoundland; Zloty 1996) and adjacent areas in the U.S.
Phenology. I followed this species in rivers near Sept-Iles, Quebec, where emergence occurred in mid- to late-June and young larvae reached collectable size by early September. There, A. subnotatus appears to be univoltine, without a summer egg diapause, but this has not been thoroughly documented.
Remarks. Ameletus subnotatus,
A. walleyi, and the western North American
A. oregonensis form a distinct group based on male genitalia. Larval specimens from Quebec have caudal filament coloration as described above and shown in
Figure 13H. However, Zloty’s [
10] description based on material from Alberta differs: “Caudal filaments with a few brown basal segments followed by a light brown band (about one-third of each filament’s length), followed by a band consisting of alternating brown and pale rings (covering about one-third of each filament’s length), followed by a pale band, and a few brown apical segments.” The taxonomic significance, if any, of this difference is unclear.
Ameletus walleyi Harper [
27]: 603; Zloty [
1]: 319.
Type Material. Male holotype and female allotype: Headwaters of the Eramosa River, Wellington County, Ontario, Canada, 3 and 1 May 1969, respectively, P.P. Harper; in CNC (one male paratype examined)
Diagnosis. Ameletus walleyi is a bisexual species. Male imagos have wings that are mostly transparent with amber shading at the base and with dark brown longitudinal veins and brown cross-veins (Figure 44A in Zloty [
1]). Body length ranges from 11 to 12 mm, forewing length 10 mm. Upper portion of the compound eye dark brown to black in life. Males may be distinguished from most other eastern Nearctic species by the distinctively shaped penes (similar to
Figure 13C), the lateral lobes of which do not extend as far as the base of the forceps (
Figure 13D vs.
Figure 13E). The only other
Ameletus in the eastern Nearctic with this type of penes is
A. subnotatus, which has distinctively speckled wings and differs in the fine details of the penes (Figures 35 and 36B in Zloty [
1]).
Larvae are unknown.
Distribution. Known only from two localities in southern Ontario (Wellington County).
Phenology. The only known specimens were collected as adults around the first of May.
Remarks. Ameletus walleyi, A. subnotatus, and the western North American A. oregonensis form a distinct group based on male genitalia. Larvae of A. walleyi are currently unknown, but are likely similar to A. subnotatus.
| |
| Ameletus tertius species group |
| |
Among the eastern Nearctic
Ameletus, male imagos of this group can be distinguished by the basal lobes of the ventral plates of the penes (
Figure 14C) and the relatively long accessory teeth on the ventral margin of those plates. Larvae lack spines on the anal margin of gill 4 and the anal rib is not inset (
Figure 14H). Here, I consider this group monotypic, but two COI haplotype groups from the southern Appalachians, which differ considerably from both the northern group and each other, may eventually prove worthy of specific status (see Remarks below and
Section 4.1.1).
Ameletus tertius McDunnough [
28]:27; Zloty [
1]: 316.
Type Material. Male holotype and female allotype: Baddeck Forks, Cape Breton Island, Nova Scotia, Canada 13 July 1936, J. McDunnough; No. 4287 in CNC (male paratype examined).
Diagnosis. Ameletus tertius is a diploid bisexual species. Male imagos (
Figure 14A) are clear-winged with a distinct amber tinge in the costal region of the forewing, with light brown veins and cross-veins. Upper portion of the eyes light green in life. They can be distinguished from other eastern Nearctic species by the following combination of characters: penes with long lateral lobes extending beyond the base of the forceps; long joint of forceps with uniform thickness through its length (
Figure 14B); ventral plates of the penes narrow, not produced into a spine distally (
Figure 15A), with prominent rounded basal lobe projecting anteriorly (
Figure 14C) and 4–7 relatively long (18–23 µm) spinules along ventral margin (
Figure 14D).
Larvae can be distinguished from all other eastern Nearctic species by the following combination: no setal gap on the mandible (
Figure 14I); gill 4 with spines on the costal but not anal margins, with anal rib on anal margin (i.e., not inset;
Figure 14H); dark band in middle portion of caudal filaments evenly colored (
Figure 14E) and may extend to the base (
Figure 14F); dorsum of abdomen distinctively patterned, with tergites 2–6 gray-brown with large submedian pale spots, tergites 7–8 lighter brown and 9–10 dark (
Figure 14E); venter without dark stripes running the length of the abdomen but sternite 8 with median and sublateral dark maculae and sternite 9 almost entirely dark (
Figure 14G); spinules present on posterior margins of all tergites.
Distribution. Quebec and Labrador, the Canadian Maritimes, and New England south to North Carolina.
Phenology. Univoltine, with emergence in spring to mid-summer (generally later than other eastern Nearctic species). May have a summer egg diapause.
Remarks. Although the penes of
A. tertius resemble those of
A. burkei and
A. ohioensis, larval characters and genetic data (both COI sequences and allozymes) indicate
A. tertius is not closely related to any other eastern Nearctic species. Although populations from Pennsylvania northward are genetically uniform (as expected due to the recency of their post-glacial dispersal into this area from refugia in Pennsylvania), there is considerable differentiation in mitochondrial COI between these and populations from the southern Appalachians (see
Section 4.1.1). Larval coloration in some North Carolina populations is distinctive in that the dark band on the caudal filaments extends from, or nearly from, their base (
Figure 14F). The latter are treated as “
A.sp.3” in the larval key of Morse et al. [
29], but adult males I have reared from these have genitalia identical to those from larvae having the more typical color pattern.