Suitability of Three Trunk Traps for Capturing Larvae of Lymantria dispar (L.) (Lepidoptera, Erebidae)
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Timeframe and Trap Deployment Areas
2.2. Trap Designs
2.3. Deployment and Monitoring
2.4. Weather Data
2.5. Statistical Analysis of Data
3. Results
3.1. Slovenia
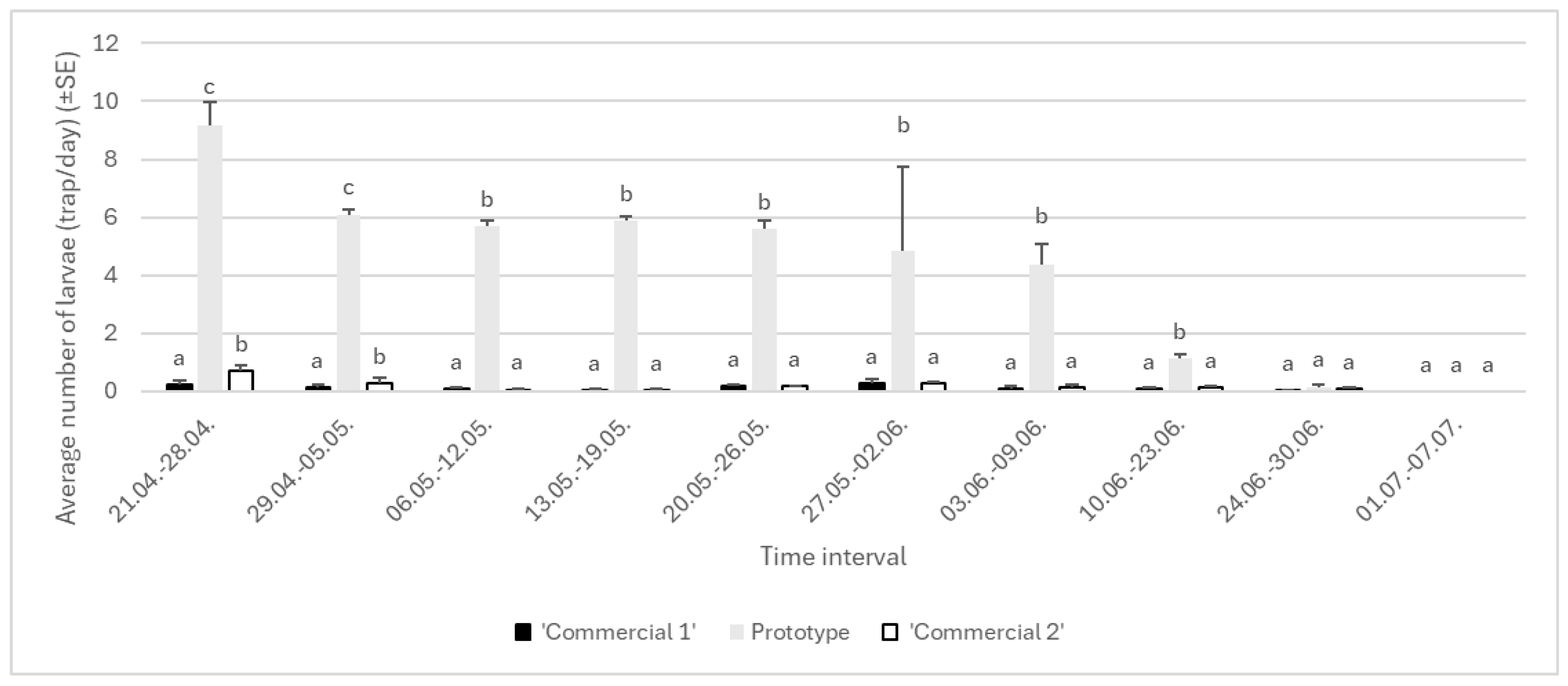
3.1.1. Year 2022
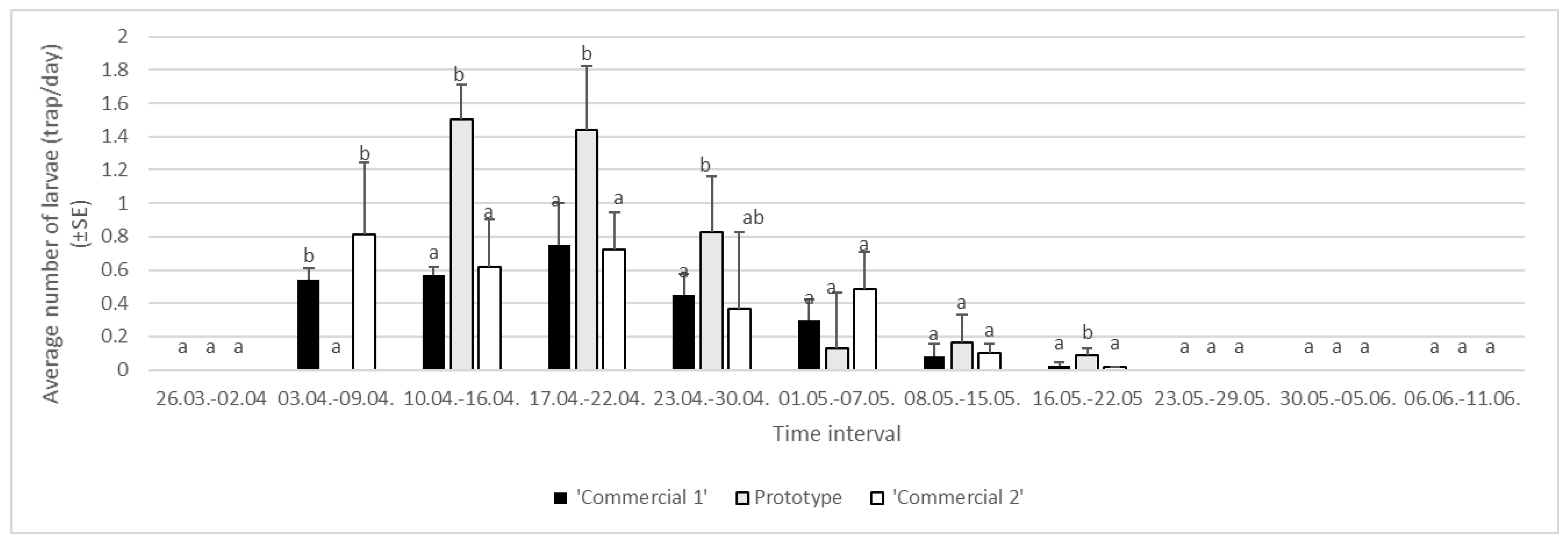
3.1.2. Year 2023
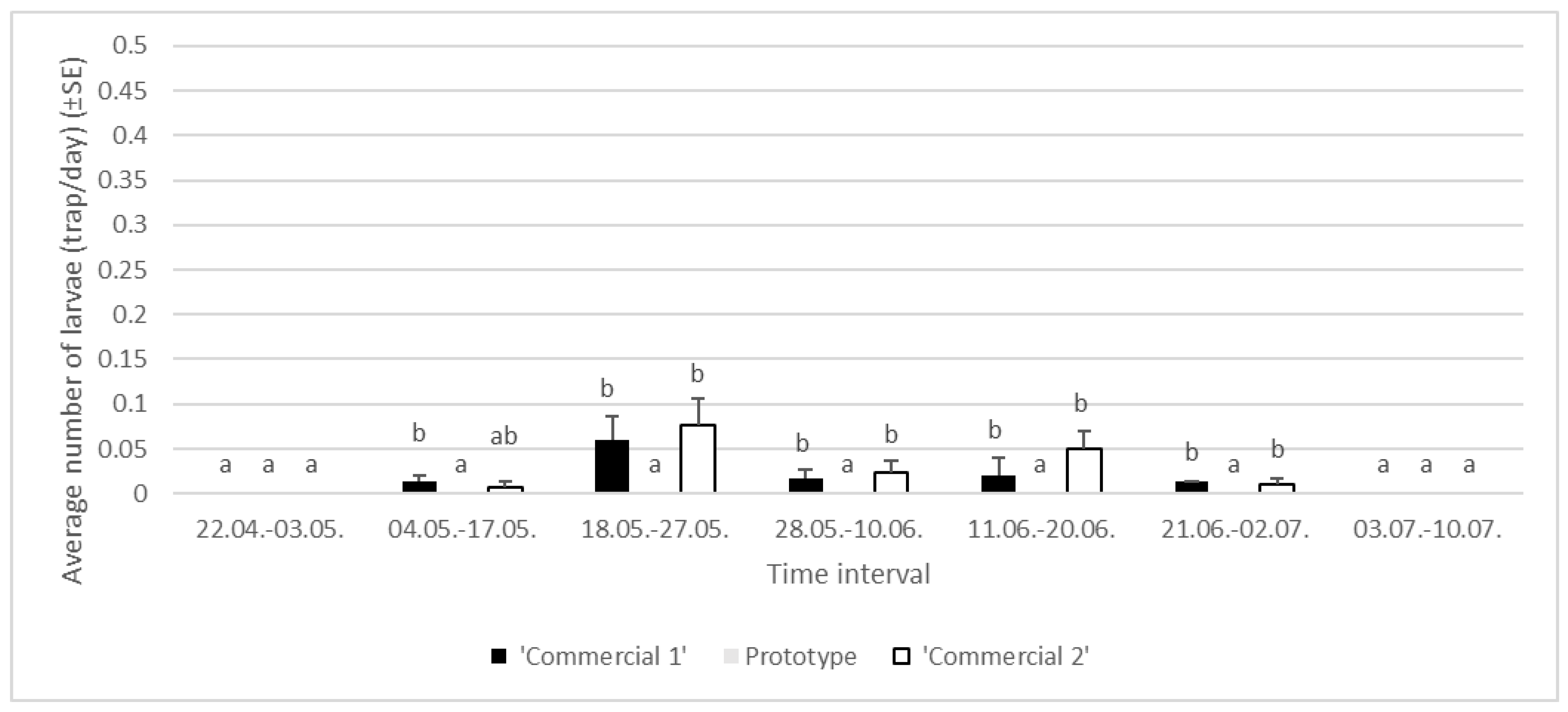
3.1.3. Year 2024
3.2. Greece
3.2.1. Year 2023
3.2.2. Year 2024
3.3. Weather Data from Slovenian Experimental Areas
3.4. Weather Data from Greek Experimental Areas
3.5. DD Computations for Slovenia and Greece
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Boukouvala, M.C.; Kavallieratos, N.G.; Skourti, A.; Pons, X.; Lopez Alonso, C.; Eizaguirre, M.; Benavent Fernandez, E.; Dominguez Soler, E.; Fita, S.; Bohinc, T.; et al. Lymantria dispar (L.) (Lepidoptera: Erebidae): Current status of biology, ecology and management in Europe from notes from North America. Insects 2022, 13, 854. [Google Scholar] [CrossRef] [PubMed]
- Tomé, M.; Almeida, M.H.; Barreiro, S.; Branco, M.R.; Deus, E.; Pinto, G.; Silva, J.S.; Soares, P.; Rodriguez-Soalleiro, R. Opportunities and challenges of Eucalyptus plantations in Europe: The Iberian Peninsula experience. Eur. J. Forest Res. 2021, 140, 489–510. [Google Scholar] [CrossRef]
- Liebhold, A.; Elkinton, J.; Williams, D.; Muzika, R.-M. What causes outbreaks of the gipsy moth in North America? Popul. Ecol. 2000, 42, 257–266. [Google Scholar] [CrossRef]
- Clark, K.L.; Aoki, C.; Ayres, M.; Kabrick, J.; Gallagher, M.R. Insect infestations and the persistence and functioning of oak-pine mixedwood forests in the mid-Atlantic region, USA. PLoS ONE 2022, 17, e0265955. [Google Scholar] [CrossRef]
- Payne, N.J. Developments in aerial pesticide application methods for forestry. Crop Prot. 1998, 17, 171–180. [Google Scholar] [CrossRef]
- Kostić, I.; Petrović, O.; Milanović, S.; Popović, Z.; Stankovic, S.; Todorović, G.; Kostić, M. Biological activity of essential oils of Athamanta haynaldii and Myristica fragrans to gypsy moth larvae. Ind. Crops Prod. 2013, 41, 17–20. [Google Scholar] [CrossRef]
- Holuša, J.; Zúbrik, M.; Resnerová, K.; Vanická, H.; Liska, J.; Mertelík, J.; Takov, D.; Trombik, J.; Hajek, A.E.; Pilarska, D. Further spread of the gypsy moth fungal pathogen, Entomophaga maimaiga, to the west and north in Central Europe. J. Plant Dis. Prot. 2020, 128, 323–331. [Google Scholar] [CrossRef]
- Wolz, M.; Höcherl, A.; Hübner, J.; Tschorsnig, H.P.; Whitmore, D.; Leroy, B.M.L.; Weisser, W.W.; Mitesser, O.; Zakharov, E.V.; Hebert, P.D.N.; et al. Response of parasitoid communities to insecticide applicationduring a Lymantria dispar outbreak in mixed oak forests. J. Appl. Ecol. 2024, 61, 2774–2785. [Google Scholar] [CrossRef]
- Webb, R.E.; Ridgway, R.L.; Thorpe, K.W.; Tatman, K.M.; Wieber, A.M.; Venables, L. Development of a specialized gypsy-moth (Lepidoptera, Lymantriidae) management program for suburban parks. J. Econ. Entomol. 1991, 84, 1320–1328. [Google Scholar] [CrossRef]
- Millward, A.A.; Sabir, S. Benefits of a forested urban park: What is the value of Allan Gardens to the city of Toronto, Canada? Landsc. Urban. Plan. 2011, 100, 177–188. [Google Scholar] [CrossRef]
- Hajek, A.E. Larval behavior in Lymantria dispar increases risk of fungal infection. Oecologia 2001, 126, 285–291. [Google Scholar] [CrossRef] [PubMed]
- Allen, V.T.; Miller, O.F.; Tyler, W.B. Gypsy-moth caterpillar dermatitis—Revisited. J. Am. Acad. Dermatol. 1991, 24, 979–981. [Google Scholar] [CrossRef]
- Haq, M.; O’Toole, A.; Beecker, J.; Gooderham, M.J. Return of Lymantria dispar dispar (gypsy moth): A case report. Sage Open Med. Case Rep. 2021, 9, 2050313X211057926. [Google Scholar] [CrossRef] [PubMed]
- Marzano, M.; Ambrose-Oji, B.; Hall, C.; Moseley, D. Pests in the City: Managing Public Health Risks and Social Values in Response to Oak Processionary Moth (Thaumetopoea processionea) in the United Kingdom. Forests 2020, 11, 199. [Google Scholar] [CrossRef]
- Thorpe, K.W.; Ridgway, R.L. Effects of Trunk Barriers on Larval Gypsy Moth (Lepidoptera: Lymantriidae) Density in Isolated- and Contiguous-Canopy Oak Trees Get access Arrow. Environ. Entomol. 1994, 23, 832–836. [Google Scholar] [CrossRef]
- Thorpe, K.W.; Tatman, K.M.; Sellers, P.; Webb, R.E.; Ridgway, R.L. Management of gipsy moths using sticky trunk barriers and larval removal. J. Arboric. 1995, 21, 69–76. [Google Scholar]
- Bracchetti, L.; Cocci, P.; Palermo, F.A. Multiple Aspects of the Fight against the Red Palm Weevil in an Urban Area: Study Case, San Benedetto del Tronto (Central Italy). Insects 2023, 14, 502. [Google Scholar] [CrossRef]
- Colacci, M.; Kavallieratos, N.G.; Athanassiou, C.G.; Boukouvala, M.C.; Rumbos, C.I.; Kontodimas, D.C.; Pardo, D.; Sancho, J.; Benavent-Fernandez, E.; Galvez-Settier, S.; et al. Management of the pine processionary moth, Thaumetopoea pityocampa (Lepidoptera: Thaumetopoeidea), in urban and suburban areas: Trials with trunk barrier and adhesive barrier trap devices. J. Econ. Entomol. 2018, 111, 227–238. [Google Scholar] [CrossRef]
- Blumenthal, E.M.; Hoover, C.R. Gypsy-moth (Lepidoptera, Lymantridae) population control using mechanical barriers and contact insecticides applied to tree stems. J. Econom. Entomol. 1986, 79, 1394–1396. [Google Scholar] [CrossRef]
- Žežlina, I.; Seljak, G.; Rebec, E. High densities of gipsy moth (Lymantria dispar L.) on Primorska region and its ascendancy on forest vegetation. In Proceedings of the Lectures and Paper Presented at the 7th Slovenian Conference on Plant Protection, Zreče, Slovenia, 8–10 March 2005; Plant Protection Society of Slovenia: Ljubljana, Slovenia; pp. 392–394. [Google Scholar]
- Elkinton, J.S.; Liebhold, A.M. Population dynamics of gipsy moth in North Amerika. Annu. Rev. Entomol. 1990, 35, 571–596. [Google Scholar] [CrossRef]
- Zúbrik, M.; Kunca, A.; Kulfan, J.; Rell, S.; Nikolov, C.; Galko, J.; Vakula, J.; Gubka, A.; Leontovyč, R.; KonÔpka, B.; et al. Occurrence of gypsy moth (Lymantria dispar L.) in the Slovak Republic and its outbreaks during 1945–2020. Cent. Eur. For. J. 2021, 67, 55–71. [Google Scholar] [CrossRef]
- Agrafioti, P.; Lampiri, E.; Bohinc, T.; Roig, A.; Levi-Mourao, A.; Boukouvala, M.; Skourti, A.; Lopez, C.; Eizaguirre, M.; Pons, X.; et al. Influence of trap type on the captures of Lymantria dispar L. (Lepidoptera: Erebidae): Trials from different European countries. J. Econ. Entomol. 2024, 117, 2545–2556. [Google Scholar] [CrossRef] [PubMed]
- Mešić, A.; Barčić, J.; Barčić, J.I.; Miličević, T.; Duralija, B.; Culjak, T.G. A low environmental impact method to control horse chestnut leaf miner Cameraria ohridella (Deschka & Dimic). J. Food Agric. Environ. 2008, 6, 421–427. [Google Scholar]
- Trematerra, P.; Colacci, M.; Athanassiou, C.G.; Kavallieratos, N.G.; Rumbos, C.I.; Boukouvala, M.C.; Nikolaidou, A.J.; Kontodimas, D.C.; Benavent-Fernández, E.; Gálvez-Settier, S. Evaluation of Mating Disruption For the Control of Thaumetopoea pityocampa (Lepidoptera: Thaumetopoeidae) in Suburban Recreational Areas in Italy and Greece. J. Econ. Entomol. 2019, 112, 2229–2235. [Google Scholar] [CrossRef]
- Slovenian Environmental Agency. Available online: https://meteo.arso.gov.si/met/sl/app/webmet/#webmet==8Sdwx2bhR2cv0WZ0V2bvEGcw9ydlJWblR3LwVnaz9SYtVmYh9iclFGbt9SaulGdugXbsx3cs9mdl5WahxXYyNGapZXZ8tHZv1WYp5mOnMHbvZXZulWYnwCchJXYtVGdlJnOn0UQQdSf (accessed on 1 December 2024).
- Ponomarev, V.I.; Klobukov, G.I.; Napalkova, V.V.; Akhanaev, Y.B.; Pavlushin, S.V.; Yakimova, M.E.; Subbotina, A.O.; Picq, S.; Cusson, M.; Martemyanov, V.V. Phenological features of the spongy moth, Lymantria dispar (L.) (Lepidoptera: Erebidae), in the northernmost portions of its Eurasian range. Insects 2023, 14, 276. [Google Scholar] [CrossRef]
- McMaster, G.S.; Wilhelm, W.W. Growing degree-days: One equation, two interpretations. Agric. Forest. Meteor. 1997, 87, 291–300. [Google Scholar] [CrossRef]
- Devetak, M.; Bohinc, T.; Kač, M.; Trdan, S. Seasonal dynamics of the cabbage armyworm (Mamestra brassicae [L.]) and the bright-line brown-eyes moth (Mamestra oleracea [L.]) in Slovenia. Hortic. Sci. 2014, 41, 80–88. [Google Scholar] [CrossRef]
- Zar, J.H. Biostatistical Analysis; Pearson Education Limited: Essex, UK, 2014. [Google Scholar]
- Statgraphics Centurion X.I.X.; Statpoint Technologies Inc.: Warrenton, VA, USA, 2020.
- Jurc, M. Hrasti–žuželke na poganjkih, listih in iglicah. Gozdarski Vestn. 2006, 64, 253–268. (In Slovenian) [Google Scholar]
- Trdan, S.; Vidrih, M. Quantifying the damage of red deer (Cervus elaphus) grazing on grassland production in southeastern Slovenia. Eur. J. Wildwlife Res. 2008, 54, 138–141. [Google Scholar] [CrossRef]
- Acebes-Doria, A.L.; Leskey, T.C.; Bergh, J.C. Development and comparison of trunk traps to monitor movement of Halyomorpha halys nymphs on host trees. Entomol. Exp. App. 2015, 158, 44–53. [Google Scholar] [CrossRef]
- Shi, L.; He, H.; Yang, G.; Huang, H.; Vasseur, L.; You, M. Are Yellow Sticky Cards and Light Traps Effective on Tea Green Leafhoppers and Their Predators in Chinese Tea Plantations? Insects 2021, 12, 14. [Google Scholar] [CrossRef] [PubMed]
- Leonard, D.E. Feeding rhythm in larvae of the gypsy moth. J. Econ. Entomol. 1970, 63, 1454–1457. [Google Scholar] [CrossRef]
- Weseloh, R.M. Behavioural responses of gypsy moth (Lepidoptera: Lymantridae) larvae to abiotic environmental factors. Environ. Entomol. 1989, 18, 361–367. [Google Scholar] [CrossRef]
- Caputo, B.; Ienco, A.; Manica, M.; Petrarca, V.; Rosà, R.; della Torre, A. New adhesive traps to monitor urban mosquitoes with a case study to assess the efficacy of insecticide control strategies in temperate areas. Parasites Vectors 2015, 8, 134. [Google Scholar] [CrossRef]
- Anderson, J.R.; Poorbaugh, J.H. Refinements for collecting and processing sticky fly tapes used for sampling populations of domestic flies. J. Econ. Entomol. 1965, 58, 497–500. [Google Scholar] [CrossRef]
- Limbu, S.; Keena, M.; Chen, F.; Cook, G.; Nadel, H.; Hoover, K. Effects of temperature on development of Lymantria dispar asiatica and Lymantria dispar japonica (Lepidoptera: Erebidae). Environ. Entomol. 2017, 46, 1012–1024. [Google Scholar] [CrossRef]
- Haavik, L.J.; Stephen, F.M. Insect ecology. In Forest Entomology and Pathology; Springer Nature: Cham, Switzerland, 2023. [Google Scholar]
- Engel, J.; Hertzog, L.; Tiede, J.; Wagg, J.; Ebeling, A.; Briesen, H.; Weisser, W.W. Pittfall trap sampling bias depends on body mass, temperature and trap number: Insights from an individual-based model. Ecosphere 2017, 8, e01790. [Google Scholar] [CrossRef]
- Filgueiras, C.C.; Willett, D.S. Phenology and monitoring of the lesser Chestnut weevil (Curculio sayi). Insects 2022, 13, 713. [Google Scholar] [CrossRef]
- Zhang, H.; Wu, H.; Liao, A.; He, B.; Liu, J.; Wang, N.; Xia, Y.; Cao, Y.; Zhu, Z.; Fu, C. Effects of trunk distance and rainfall on throughfall and associated chemical alterations with a subtropical deciduous forests. Forests 2022, 13, 1707. [Google Scholar] [CrossRef]
- Keena, M.A.; Shi, J. Effects of temperature on first instar Lymantria (Lepidoptera: Erebidae) survival and development with and without food. Environ. Entomolol. 2019, 48, 655–666. [Google Scholar] [CrossRef]
- Pfrieme, A.K.; Stahl, A.; Pillen, K.; Will, T. Comparison of two different experimental environments for screening for the leafhoppeer-transmitter wheat dwarf virus. J. Plant Dis. Prot. 2024, 131, 1525–1535. [Google Scholar] [CrossRef]
- Lance, D.; Barbosa, P. Host tree influences on the dispersal of late instar gypsy moths, Lymantria dispar. Oikos 1982, 38, 1–7. [Google Scholar] [CrossRef]
- Ghaderi, S.; Fathipour, Y.; Mirhosseini, M.A.; Asgari, S. Field-based thermal requirements study to improve Tuta absoluta (Lepidoptera: Gelechiidae) management. J. Crop. Prot. 2020, 9, 591–599. [Google Scholar]
- Graffigna, S.; González-Vaquero, R.A.; Torretta, J.P.; Marrero, H.J. Importance of urban green areas’ connectivity for the conservation of pollinators. Urban. Ecosyst. 2024, 27, 417–426. [Google Scholar] [CrossRef]
- Kolimenakis, A.; Solomou, A.D.; Proutsos, N.; Avramidou, E.V.; Korakaki, E.; Karetsos, G.; Kontogianni, A.B.; Kontos, K.; Georgiadis, C.; Maroulis, G. Public Perceptions of the Socioeconomic Importance of Urban Green Areas in the Era of COVID-19: A Case Study of a Nationwide Survey in Greece. Land 2023, 11, 2290. [Google Scholar] [CrossRef]
- Buenrostro, J.H.; Hufbauer, R.A. Urban environments have species-specific associations with invasive insect herbivores. J. Urban. Ecol. 2022, 8, juac011. [Google Scholar] [CrossRef]
- Bigsby, K.M.; Ambrose, M.J.; Tobin, P.C.; Sills, E.O. The cost of gipsy moth sex in the city. Urban. For. Urban. Green. 2014, 13, 459–468. [Google Scholar] [CrossRef]
- Li, H.P.; Wickham, J.D.; Bushley, K.; Wang, Z.G.; Zhang, B.; Sun, J.H. New approaches in urban forestry to mininize invasive species impact: The case of Xiongan new area in China. Insects 2020, 11, 300. [Google Scholar] [CrossRef]
- Lankinen, Å.; Witzel, J.; Aleklett, K.; Furenhed, S.; Karsson Green, K.; Latz, M.; Liljeroth, E.; Larsson, R.; Lőfkvist, K.; Meijer, J.; et al. Challanges and opportunities for increasing the use of low-risk plant protection products in sustainable production. A review. Agron. Sustain. Develop. 2024, 44, 21. [Google Scholar] [CrossRef]
- Southwood, T.R.E.; Henderson, P.A. Ecological Methods, 3rd ed.; Wiley-Blackwell: Hoboken, NJ, USA, 2000; 594p. [Google Scholar]
- Life eGymer. Life Project. Available online: https://egymer.eu/contact/ (accessed on 9 May 2024).







| AREA 1 and AREA 2 | AREA 3 | |||||||
|---|---|---|---|---|---|---|---|---|
| Time Interval | Average Daily Temperature (°C) | Average Daily Precipitation (mm) | Sum of Precipitation (mm) | Number of Rainy Days | Average Daily Temperature (°C) | Average Daily Precipitation (mm) | Sum of Precipitation (mm) | Number of Rainy Days |
| 5 May–11 May | 16.04 | 2.76 | 19.30 | 4 | 18.16 | 2.33 | 16.30 | 2 |
| 12 May–18 May | 20.01 | 0.41 | 2.90 | 2 | 20.18 | 0.46 | 141.30 | 1 |
| 19 May–27 May | 19.47 | 3.69 | 33.20 | 2 | 19.96 | 2.98 | 26.80 | 2 |
| 28 May–02 June | 15.53 | 3.28 | 19.70 | 3 | 16.20 | 3.42 | 25.20 | 1 |
| 3 June–09 June | 20.77 | 8.96 | 62.70 | 5 | 21.33 | 5.87 | 41.10 | 4 |
| 10 June–16 June | 20.07 | 0.49 | 3.40 | 2 | 20.32 | 0.20 | 1.40 | 1 |
| 17 June–23 June | 21.40 | 0.89 | 6.20 | 2 | 22.11 | 2.54 | 17.80 | 3 |
| 24 June–30 June | 24.91 | 4.70 | 32.90 | 1 | 25.84 | 1.57 | 11.00 | 1 |
| 1 July–06 July | 23.17 | 4.37 | 26.20 | 1 | 23.45 | 3.42 | 3.42 | 3 |
| 7 July–15 July | 19.22 | 0.79 | 7.10 | 1 | 19.52 | 0.54 | 4.90 | 2 |
| Σ */SUM ** | 20.06 * | 3.03 * | 213.60 ** | 23 ** | 20.71 * | 2.33 * | 289.22 ** | 20 ** |
| AREA 1 and AREA 2 | AREA 3 | |||||||
|---|---|---|---|---|---|---|---|---|
| Time Interval | Average Daily Temperature (°C) | Average Daily Precipitation (mm) | Sum of Precipitation (mm) | Number of Rainy Days | Average Daily Temperature (°C) | Average Daily Precipitation (mm) | Sum of Precipitation (mm) | Number of Rainy Days |
| 20 April–26 April | 12.30 | 1.16 | 8.10 | 2 | 13.16 | 2.26 | 15.80 | 1 |
| 27 April–4 May | 12.60 | 1.85 | 14.80 | 2 | 13.28 | 3.19 | 22.50 | 4 |
| 5 May–10 May | 14.18 | 0.70 | 4.20 | 1 | 15.23 | 0.98 | 5.90 | 2 |
| 11 May–17 May | 11.81 | 13.94 | 97.60 | 6 | 12.40 | 9.84 | 68.90 | 6 |
| 18 May–26 May | 17.28 | 1.13 | 10.20 | 1 | 17.89 | 1.08 | 9.70 | 2 |
| 27 May–1 June | 18.55 | 0.23 | 1.40 | 1 | 19.02 | 0.23 | 1.10 | 1 |
| 2 June–8 June | 17.94 | 1.30 | 9.10 | 2 | 18.44 | 4.96 | 34.70 | 4 |
| 9 June–13 June | 17.56 | 5.96 | 29.80 | 3 | 19.02 | 3.12 | 15.60 | 1 |
| 14 June–21 June | 21.38 | 0.00 | 0.00 | 0 | 21.59 | 0.00 | 0.00 | 0 |
| 22 June–28 June | 21.14 | 5.91 | 41.40 | 3 | 21.47 | 8.13 | 56.90 | 3 |
| 29 June–5 July | 20.96 | 2.33 | 16.30 | 3 | 21.51 | 4.03 | 28.20 | 2 |
| 6 July–11 July | 23.10 | 0.30 | 1.80 | 1 | 24.00 | 3.20 | 19.40 | 1 |
| 12 July–19 July | 23.55 | 6.35 | 50.80 | 4 | 22.08 | 6.94 | 55.50 | 4 |
| Σ */SUM ** | 17.87 * | 3.17 * | 285.50 ** | 29 ** | 18.39 * | 3.69 * | 334.20 ** | 31 ** |
| AREA 1 and AREA 2 | AREA 3 | |||||||
|---|---|---|---|---|---|---|---|---|
| Time Interval | Average Daily Temperature (°C) | Average Daily Precipitation (mm) | Sum of Precipitation (mm) | Number of Rainy Days | Average Daily Temperature (°C) | Average Daily Precipitation (mm) | Sum of Precipitation (mm) | Number of Rainy Days |
| 22 April–3 May | 12.33 | 2.15 | 25.80 | 5 | 12.49 | 2.28 | 31.50 | 1 |
| 4 May–17 May | 15.48 | 4.63 | 64.80 | 5 | 16.57 | 2.78 | 38.90 | 4 |
| 18 May–27 May | 17.86 | 4.78 | 47.80 | 3 | 18.35 | 4.94 | 49.40 | 4 |
| 28 May–10 June | 19.34 | 3.17 | 44.44 | 6 | 19.88 | 3.82 | 53.60 | 6 |
| 11 June–20 June | 20.00 | 2.36 | 23.60 | 1 | 19.95 | 2.89 | 28.90 | 3 |
| 21 June–3 July | 22.48 | 1.93 | 25.10 | 3 | 22.62 | 1.40 | 18.20 | 1 |
| 4 July–10 July | 23.33 | 0.00 | 0.00 | 0 | 24.01 | 0.00 | 0.00 | 0 |
| Σ */SUM ** | 18.69 * | 2.72 * | 231.54 ** | 23 ** | 19.12 * | 2.59 * | 220.50 ** | 19 ** |
| Time Interval | Average Daily Temperature (°C) | Average Daily Precipitation (mm) | Sum of Precipitation (mm) | Number of Rainy Days |
|---|---|---|---|---|
| 21 April–28 April | 15.72 | 0.12 | 1 | 0 |
| 29 April–5 May | 16.63 | 3.66 | 25.60 | 4 |
| 6 May–12 May | 18 | 0.06 | 0.40 | 0 |
| 13 May–19 May | 20.04 | 0.26 | 1.80 | 1 |
| 20 May–26 May | 19.74 | 3.06 | 21.40 | 3 |
| 27 May–2 June | 21.04 | 3.57 | 25.00 | 2 |
| 3 June–9 June | 22.48 | 0 | 0 | 0 |
| 10 June–23 June | 23.35 | 1.21 | 17 | 1 |
| 24 June–30 June | 25.55 | 0 | 0 | 0 |
| 1 July–7 July | 25.86 | 0 | 0 | 0 |
| Σ */SUM ** | 20.84 * | 1.19 * | 92.20 ** | 11 ** |
| Time Interval | Average Daily Temperature (°C) | Average Daily Precipitation (mm) | Sum of Precipitation (mm) | Number of Rainy Days |
|---|---|---|---|---|
| 26 March–2 April | 17.05 | 0.18 | 1.40 | 0 |
| 3 April–9 April | 15.51 | 0.17 | 1.20 | 0 |
| 10 April–16 April | 17.80 | 0.03 | 0.20 | 0 |
| 17 April–22 April | 17.03 | 3.33 | 20.00 | 2 |
| 23 April–30 April | 18.01 | 0.63 | 5.00 | 1 |
| 1 May–7 May | 17.50 | 0.71 | 5.00 | 1 |
| 8 May–15 May | 18.30 | 0.78 | 6.20 | 2 |
| 16 May–22 May | 23.44 | 0.00 | 0.00 | 0 |
| 23 May–29 May | 20.21 | 0.14 | 1 | 0 |
| 30 May–5 June | 22.97 | 0.00 | 0 | 0 |
| 6 June–11 June | 24.43 | 0.00 | 0 | 0 |
| Σ */SUM ** | 19.32 * | 0.54 * | 40 ** | 6 ** |
| Year and Area | Time Interval | First/Last Occurrence | DD Value (°C) |
|---|---|---|---|
| 2022 | |||
| Areas 1 and 2 | 19–27 May | First—OL | 433.40 |
| 24–30 June | Last—OL | 898.70 | |
| Area 3 | 19–27 May | First—OL | 479.90 |
| 1–6 July | Last—OL | 1065.10 | |
| 2023 | |||
| Areas 1 and 2 | 20–6 April | First—YL | 133.90 |
| 27 May–1 June | First—OL | 417.30 | |
| 6–11 July | Last—OL | 995.00 | |
| Area 3 | 20–6 April | First—YL | 172.60 |
| 27 May–1 June | First—OL | 480.10 | |
| 6–11 July | Last—OL | 939.90 | |
| 2024 | |||
| Area 1 | 4-17 May | First—OL | 434.00 |
| 21 June–2 July | Last—OL | 1036.10 | |
| Area 2 | 4-17 May | First—OL | 434.00 |
| 3–10 July | Last—OL | 1166.7 | |
| Area 3 | 18–27 May | First—OL | 638.30 |
| Last—OL | 947.40 |
| Year and Area | Time Interval | First/Last Occurrence | DD Value (°C) |
|---|---|---|---|
| 2023 | |||
| Areas 1, 2, and 3 | 21–28 April | first | 648.10 |
| 24–30 June | last | 1538.1 | |
| 2024 | |||
| Areas 1, 2, and 3 | 3–9 April | first | 595.20 |
| 16–22 May | last | 1098.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bohinc, T.; Agrafioti, P.; Vasilopoulos, S.; Lampiri, E.; Boukouvala, M.C.; Skourti, A.; Gidari, D.L.S.; Kavallieratos, N.G.; Pons, X.; Levi-Mourao, A.; et al. Suitability of Three Trunk Traps for Capturing Larvae of Lymantria dispar (L.) (Lepidoptera, Erebidae). Insects 2025, 16, 522. https://doi.org/10.3390/insects16050522
Bohinc T, Agrafioti P, Vasilopoulos S, Lampiri E, Boukouvala MC, Skourti A, Gidari DLS, Kavallieratos NG, Pons X, Levi-Mourao A, et al. Suitability of Three Trunk Traps for Capturing Larvae of Lymantria dispar (L.) (Lepidoptera, Erebidae). Insects. 2025; 16(5):522. https://doi.org/10.3390/insects16050522
Chicago/Turabian StyleBohinc, Tanja, Paraskevi Agrafioti, Stelios Vasilopoulos, Evagelia Lampiri, Maria C. Boukouvala, Anna Skourti, Demeter Lorentha S. Gidari, Nickolas G. Kavallieratos, Xavier Pons, Alexandre Levi-Mourao, and et al. 2025. "Suitability of Three Trunk Traps for Capturing Larvae of Lymantria dispar (L.) (Lepidoptera, Erebidae)" Insects 16, no. 5: 522. https://doi.org/10.3390/insects16050522
APA StyleBohinc, T., Agrafioti, P., Vasilopoulos, S., Lampiri, E., Boukouvala, M. C., Skourti, A., Gidari, D. L. S., Kavallieratos, N. G., Pons, X., Levi-Mourao, A., Domínguez Solera, E., Benavent Fernandez, E., Roig Pinãs, A., Athanassiou, C. G., & Trdan, S. (2025). Suitability of Three Trunk Traps for Capturing Larvae of Lymantria dispar (L.) (Lepidoptera, Erebidae). Insects, 16(5), 522. https://doi.org/10.3390/insects16050522












