Strategies to Mitigate the Adverse Impacts of Viral Infections on Honey Bee (Apis mellifera L.) Colonies
Simple Summary
Abstract
1. Introduction
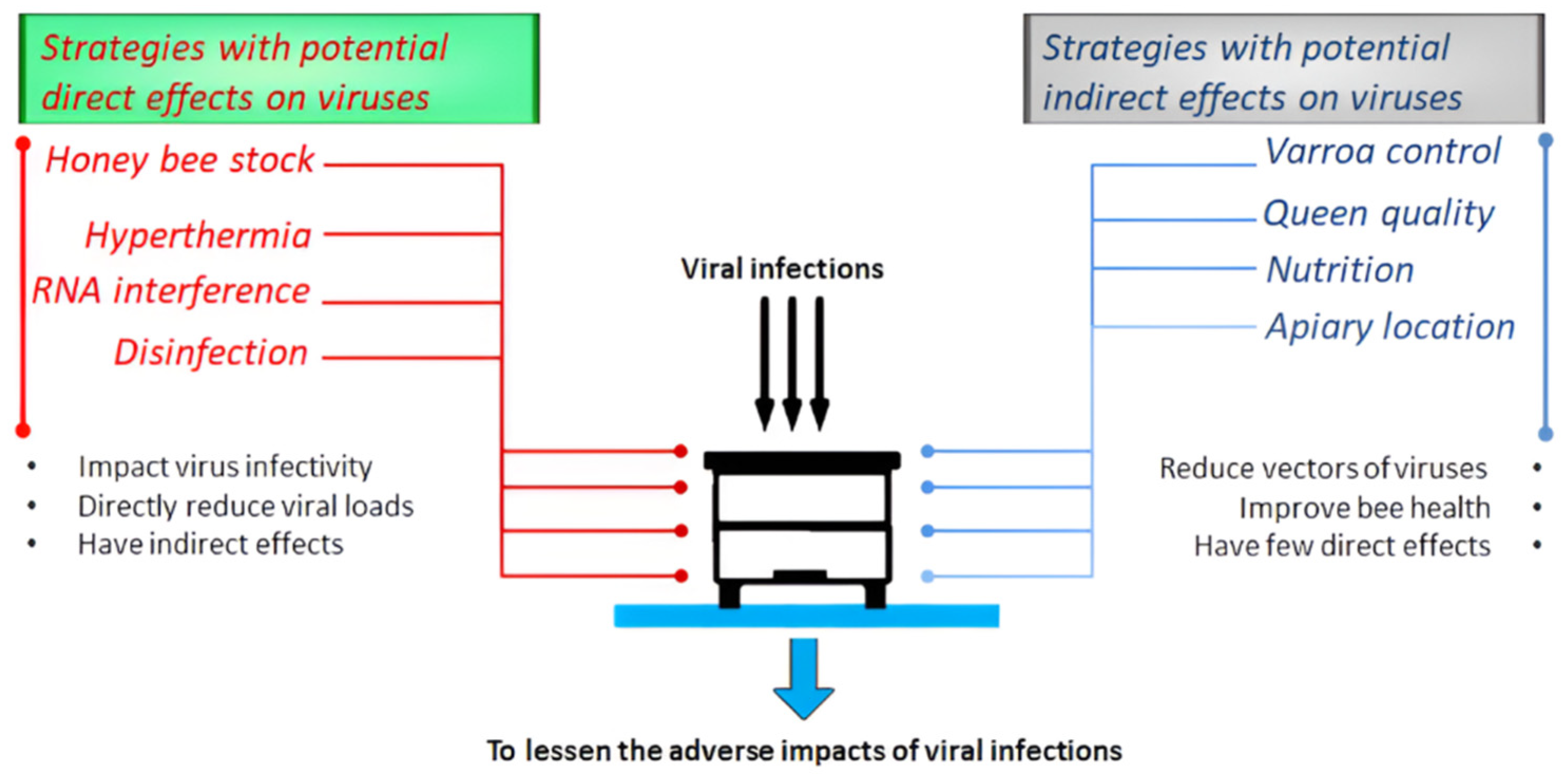
2. Strategies with Potential Indirect Effects on Viruses
2.1. Varroa Control
2.2. Queen Quality
2.3. Nutrition
2.4. Apiary Location
3. Strategies with Potential Direct Effects on Viruses
3.1. Honey Bee Stock
3.2. Thermal Treatment
3.3. RNA Interference
3.4. Disinfection
4. Outlooks
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Paudel, Y.P.; Mackereth, R.; Hanley, R.; Qin, W. Honey bees (Apis mellifera L.) and pollination issues: Current status, impacts, and potential drivers of decline. J. Agric. Sci. 2015, 7, 93–109. [Google Scholar] [CrossRef]
- Patel, V.; Pauli, N.; Biggs, E.; Barbour, L.; Boruff, B. Why bees are critical for achieving sustainable development. Ambio 2021, 50, 49–59. [Google Scholar] [CrossRef] [PubMed]
- Sillman, J.; Uusitalo, V.; Tapanen, T.; Salonen, A.; Soukka, R.; Kahiluoto, H. Contribution of honeybees towards the net environmental benefits of food. ScTEn 2021, 756, 143880. [Google Scholar] [CrossRef]
- Etxegarai-Legarreta, O.; Sanchez-Famoso, V. The role of beekeeping in the generation of goods and services: The interrelation between environmental, socioeconomic, and sociocultural utilities. Agriculture 2022, 12, 551. [Google Scholar] [CrossRef]
- Phiri, B.J.; Fèvre, D.; Hidano, A. Uptrend in global managed honey bee colonies and production based on a six-decade viewpoint, 1961–2017. Sci. Rep. 2022, 12, 21298. [Google Scholar] [CrossRef]
- Panziera, D.; Requier, F.; Chantawannakul, P.; Pirk, C.W.; Blacquière, T. The diversity decline in wild and managed honey bee populations urges for an integrated conservation approach. Front. Ecol. Evol. 2022, 10, 767950. [Google Scholar] [CrossRef]
- Hristov, P.; Shumkova, R.; Palova, N.; Neov, B. Honey bee colony losses: Why are honey bees disappearing? Sociobiology 2021, 68, e5851. [Google Scholar] [CrossRef]
- Williams, G.R.; Tarpy, D.R.; Vanengelsdorp, D.; Chauzat, M.; Cox-Foster, D.L.; Delaplane, K.S.; Neumann, P.; Pettis, J.S.; Rogers, R.E.L.; Shutler, D. Colony collapse disorder in context. Bioessays 2010, 32, 845–846. [Google Scholar] [CrossRef]
- Evans, J.D.; Chen, Y. Colony collapse disorder and honey bee health. In Honey Bee Medicine for the Veterinary Practitioner; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2021; pp. 229–234. [Google Scholar]
- Stankus, T. A review and bibliography of the literature of honey bee Colony Collapse Disorder: A poorly understood epidemic that clearly threatens the successful pollination of billions of dollars of crops in America. J. Agric. Food Inf. 2008, 9, 115–143. [Google Scholar] [CrossRef]
- Piot, N.; Schweiger, O.; Meeus, I.; Yanez, O.; Straub, L.; Villamar-Bouza, L.; De La Rua, P.; Jara, L.; Ruiz, C.; Malmstrom, M.; et al. Honey bees and climate explain viral prevalence in wild bee communities on the continental scale. Sci. Rep. 2022, 12, 1904. [Google Scholar] [CrossRef]
- Piot, N.; Schweiger, O.; Meeus, I.; Yañez, O.; Straub, L.; Villamar-Bouza, L.; De la Rúa, P.; Jara, L.; Ruiz, C.; Malmstrøm, M.; et al. Viral impacts on honey bee populations: A review. Saudi J. Biol. Sci. 2021, 28, 523–530. [Google Scholar]
- McMenamin, A.J.; Genersch, E. Honey bee colony losses and associated viruses. Curr. Opin. Insect Sci. 2015, 8, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Bailey, L.; Ball, B.V. Honey Bee Pathology, 2nd ed.; Academic Press: London, UK, 1991. [Google Scholar]
- Chen, Y.P.; Siede, R. Honey bee viruses. Adv. Virus Res. 2007, 70, 33–80. [Google Scholar]
- Abou-Shaara, H.F.; Bayoumi, S.R. Genetic variations and relationships between deformed wing virus strains infesting honey bees based on structural proteins. Biologia 2021, 76, 3865–3873. [Google Scholar] [CrossRef]
- de Miranda, J.R.; Genersch, E. Deformed wing virus. J. Invertebr. Pathol. 2010, 103, 48–61. [Google Scholar] [CrossRef]
- Mordecai, G.J.; Wilfert, L.; Martin, S.J.; Jones, I.M.; Schroeder, D.C. Diversity in a honey bee pathogen: First report of a third master variant of the Deformed Wing Virus quasispecies. ISME J. 2016, 10, 1264–1273. [Google Scholar] [CrossRef]
- Kevill, J.L.; Highfield, A.; Mordecai, G.J.; Martin, S.J.; Schroeder, D.C. ABC assay: Method development and application to quantify the role of three DWV master variants in overwinter colony losses of European honey bees. Viruses 2017, 9, 314. [Google Scholar] [CrossRef]
- Tlak Gajger, I.; Kolodziejek, J.; Bakonyi, T.; Nowotny, N. Prevalence and distribution patterns of seven different honeybee viruses in diseased colonies: A case study from Croatia. Apidologie 2014, 45, 701–706. [Google Scholar] [CrossRef]
- Ravoet, J.; Maharramov, J.; Meeus, I.; De Smet, L.; Wenseleers, T.; Smagghe, G.; De Graaf, D.C. Comprehensive bee pathogen screening in Belgium reveals Crithidia mellificae as a new contributory factor to winter mortality. PLoS ONE 2013, 8, e72443. [Google Scholar] [CrossRef]
- Tlak Gajger, I.; Bakarić, K.; Toplak, I.; Šimenc, L.; Zajc, U.; Pislak Ocepek, M. Winter hive debris analysis is significant for assessing the health status of honeybee colonies (Apis mellifera). Insects 2024, 15, 350. [Google Scholar] [CrossRef]
- Martin, S.J. The role of Varroa and viral pathogens in the collapse of honeybee colonies: A modelling approach. J. App. Ecol. 2021, 38, 1082–1093. [Google Scholar] [CrossRef]
- Grozinger, C.M.; Flenniken, M.L. Bee viruses: Ecology, pathogenicity, and impacts. Ann. Rev. Entomol. 2019, 64, 205–226. [Google Scholar] [CrossRef] [PubMed]
- Yañez, O.; Jaffé, R.; Jarosch, A.; Fries, I.; Moritz, R.F.; Paxton, R.J.; De Miranda, J.R. Deformed wing virus and drone mating flights in the honey bee (Apis mellifera): Implications for sexual transmission of a major honey bee virus. Apidologie 2012, 43, 17–30. [Google Scholar] [CrossRef]
- Amiri, E.; Meixner, M.D.; Kryger, P. Deformed wing virus can be transmitted during natural mating in honey bees and infect the queens. Sci. Rep. 2016, 6, 33065. [Google Scholar] [CrossRef]
- Ravoet, J.; De Smet, L.; Wenseleers, T.; de Graaf, D.C. Vertical transmission of honey bee viruses in a Belgian queen breeding program. BMC Vet. Res. 2015, 11, 61. [Google Scholar] [CrossRef]
- Chen, Y.; Evans, J.; Feldlaufer, M. Horizontal and vertical transmission of viruses in the honey bee, Apis mellifera. J. Invertebr. Pathol. 2006, 92, 152–159. [Google Scholar] [CrossRef]
- Schittny, D.; Yañez, O.; Neumann, P. Honey bee virus transmission via hive products. Vet. Sci. 2020, 7, 96. [Google Scholar] [CrossRef]
- Bailes, E.J.; Deutsch, K.R.; Bagi, J.; Rondissone, L.; Brown, M.J.; Lewis, O.T. First detection of bee viruses in hoverfly (syrphid) pollinators. Biol. Lett. 2018, 14, 20180001. [Google Scholar] [CrossRef]
- Tapia-González, J.M.; Morfin, N.; Macías-Macías, J.O.; De la Mora, A.; Tapia-Rivera, J.C.; Ayala, R.; Contreras-Escareño, F.; Gashout, H.A.; Guzman-Novoa, E. Evidence of presence and replication of honey bee viruses among wild bee pollinators in subtropical environments. J. Invertebr. Pathol. 2019, 168, 107256. [Google Scholar] [CrossRef]
- Tlak Gajger, I.; Šimenc, L.; Toplak, I. The First Detection and Genetic Characterization of Four Different Honeybee Viruses in Wild Bumblebees from Croatia. Pathogens 2021, 10, 808. [Google Scholar] [CrossRef]
- Wilfert, L.; Long, G.; Leggett, H.C.; Schmid-Hempel, P.; Butlin, R.; Martin, S.J.M.; Boots, M. Deformed wing virus is a recent global epidemic in honeybees driven by Varroa mites. Science 2016, 351, 594–597. [Google Scholar] [CrossRef] [PubMed]
- Eyer, M.; Chen, Y.P.; Schäfer, M.O.; Pettis, J.; Neumann, P. Small hive beetle, Aethina tumida, as a potential biological vector of honeybee viruses. Apidologie 2009, 40, 419–428. [Google Scholar] [CrossRef]
- Dalmon, A.; Gayral, P.; Decante, D.; Klopp, C.; Bigot, D.; Thomasson, M.; Herniou, E.A.; Alaux, C.; Le Conte, Y. Viruses in the invasive hornet Vespa velutina. Viruses 2019, 11, 1041. [Google Scholar] [CrossRef] [PubMed]
- Payne, A.N.; Shepherd, T.F.; Rangel, J. The detection of honey bee (Apis mellifera) associated viruses in ants. Sci. Rep. 2020, 10, 2923. [Google Scholar] [CrossRef]
- Beaurepaire, A.; Piot, N.; Doublet, V.; Antunez, K.; Campbell, E.; Chantawannakul, P.; Chejanovsky, N.; Gajda, A.; Heerman, M.; Panziera, D.; et al. Diversity and Global Distribution of Viruses of the Western Honey Bee, Apis mellifera. Insects 2020, 11, 239. [Google Scholar] [CrossRef]
- Bacandritsos, N.; Granato, A.; Budge, G.; Papanastasiou, I.; Roinioti, E.; Caldon, M.; Falcaro, C.; Gallina, A.; Mutinelli, F. Sudden deaths and colony population decline in Greek honey bee colonies. J. Invertebr. Pathol. 2010, 105, 335–340. [Google Scholar] [CrossRef]
- D'Alvise, P.; Seeburger, V.; Gihring, K.; Kieboom, M.; Hasselmann, M. Seasonal dynamics and co-occurrence patterns of honey bee pathogens revealed by high-throughput RT-qPCR analysis. Ecol. Evol. 2019, 9, 10241–10252. [Google Scholar] [CrossRef]
- Peck, D.T.; Smith, M.L.; Seeley, T.D. Varroa destructor mites can nimbly climb from flowers onto foraging honey bees. PLoS ONE 2016, 11, e0167798. [Google Scholar] [CrossRef]
- Han, B.; Wu, J.; Wei, Q.; Liu, F.; Cui, L.; Rueppell, O.; Xu, S. Life-history stage determines the diet of ectoparasitic mites on their honey bee hosts. Nat. Commun. 2024, 15, 725. [Google Scholar] [CrossRef]
- Ramsey, S.D.; Ochoa, R.; Bauchan, G.; Gulbronson, C.; Mowery, J.D.; Cohen, A.; Lim, D.; Joklik, J.; Cicero, J.M.; Ellis, J.D.; et al. Varroa destructor feeds primarily on honey bee fat body tissue and not hemolymph. Proc. Natl. Acad. Sci. USA 2019, 116, 1792–1801. [Google Scholar] [CrossRef]
- Chen, Y.; Pettis, J.S.; Evans, J.D.; Kramer, M.; Feldlaufer, M.F. Transmission of Kashmir bee virus by the ectoparasitic mite Varroa destructor. Apidologie 2004, 35, 441–448. [Google Scholar] [CrossRef]
- Emsen, B.; Guzman-Novoa, E.; Kelly, P.G. Honey production of honey bee (Hymenoptera: Apidae) colonies with high and low Varroa destructor (Acari: Varroidae) infestation rates in eastern Canada. Can. Entomol. 2014, 146, 236–240. [Google Scholar] [CrossRef]
- Aronstein, K.A.; Saldivar, E.; Vega, R.; Westmiller, S.; Douglas, A.E. How Varroa parasitism affects the immunological and nutritional status of the honey bee, Apis mellifera. Insects 2012, 3, 601–615. [Google Scholar] [CrossRef]
- van Dooremalen, C.; Stam, E.; Gerritsen, L.; Cornelissen, B.; van der Steen, J.; van Langevelde, F.; Blacquière, T. Interactive effect of reduced pollen availability and Varroa destructor infestation limits growth and protein content of young honey bees. J. Insect Physiol. 2013, 59, 487–493. [Google Scholar] [CrossRef]
- Jack, C.J.; Ellis, J.D. Integrated pest management control of Varroa destructor (Acari: Varroidae), the most damaging pest of (Apis mellifera L. (Hymenoptera: Apidae)) colonies. J. Insect Sci. 2021, 21, 6. [Google Scholar] [CrossRef]
- Abou-Shaara, H.; Staron, M.; Cermakova, T. Impacts of oxalic acid, thymol, and potassium citrate as Varroa control materials on some parameters of honey bees. Turk. J. Vet. Anim. Sci. 2017, 41, 238–247. [Google Scholar] [CrossRef]
- Smodiš Škerl, M.I.; Rivera-Gomis, J.; Tlak Gajger, I.; Bubnič, J.; Talakić, G.; Formato, G.; Baggio, A.; Mutinelli, F.; Tollenaers, W.; Laget, D.; et al. Efficacy and toxicity of VarroMed® used for controlling Varroa destructor infestation in different seasons and geographical areas. App. Sci. 2021, 11, 8564. [Google Scholar] [CrossRef]
- Bava, R.; Castagna, F.; Palma, E.; Ceniti, C.; Millea, M.; Lupia, C.; Britti, D.; Musella, V. Prevalence of Varroa destructor in Honeybee (Apis mellifera) Farms and Varroosis Control Practices in Southern Italy. Microorganisms 2023, 11, 1228. [Google Scholar] [CrossRef]
- Bubnič, J.; Prešern, J.; Pietropaoli, M.; Cersini, A.; Moškrič, A.; Formato, G.; Manara, V.; Smodiš Škerl, M.I. Integrated pest management strategies to control Varroa mites and their effect on viral loads in honey bee colonies. Insects 2024, 5, 115. [Google Scholar] [CrossRef]
- Garrido, P.M.; Porrini, M.P.; Alberoni, D.; Baffoni, L.; Scott, D.; Mifsud, D.; Eguaras, M.J.; Di Gioia, D. Beneficial bacteria and plant extracts promote honey bee health and reduce Nosema ceranae infection. Probiotics Antimicrob. Proteins 2024, 16, 259–274. [Google Scholar] [CrossRef]
- Dietemann, V.; Nazzi, F.; Martin, S.J.; Anderson, D.L.; Locke, B.; Delaplane, K.S.; Wauquiez, Q.; Tannahill, C.; Frey, E.; Ziegelmann, B.; et al. Standard methods for varroa research. J. Apic. Res. 2013, 52, 1–54. [Google Scholar] [CrossRef]
- Morfin, N.; Foster, L.J.; Guzman-Novoa, E.; Van Westendorp, P.; Currie, R.W.; Higo, H. Varroa destructor economic injury levels and pathogens associated with colony losses in Western Canada. Front. Bee Sci. 2024, 2, 1355401. [Google Scholar] [CrossRef]
- Abou-Shaara, H.F.; Al-Ghamdi, A.A.; Khan, K.A.; Al-Kahtani, S.N. Genetic network analysis between Apis mellifera subspecies based on mtDNA argues the purity of specimens from North Africa, the Levant and Saudi Arabia. Saudi J. Biol. Sci. 2021, 28, 2718–2725. [Google Scholar] [CrossRef]
- Güneşdoğdu, M.; Şekeroğlu, A. Factors affecting queen bee quality. Turk. J. Agric.-Food Sci. Technol. 2020, 8, 197–202. [Google Scholar]
- Amiri, E.; Abou-Shaara, H.; McAfee, A. The effect of major abiotic stressors on honey bee (Apis mellifera L.) queens and potential impact on their progeny. Apidologie 2025, 56, 2. [Google Scholar] [CrossRef]
- Cobey, S.W. Comparison studies of instrumentally inseminated and naturally mated honey bee queens and factors affecting their performance. Apidologie 2007, 38, 390–410. [Google Scholar] [CrossRef]
- Brutscher, L.M.; Baer, B.; Niño, E.L. Putative drone copulation factors regulating honey bee (Apis mellifera) queen reproduction and health: A review. Insects 2019, 10, 8. [Google Scholar] [CrossRef]
- Amiri, E.; Kryger, P.; Meixner, M.D.; Strand, M.K.; Tarpy, D.R.; Rueppell, O. Quantitative patterns of vertical transmission of deformed wing virus in honey bees. PLoS ONE 2018, 13, e0195283. [Google Scholar] [CrossRef]
- Akyol, E.; Yeninar, H.; Korkmaz, A.; Çakmak, I. An observation study on the effects of queen age on some characteristics of honey bee colonies. Ital. J. Anim. Sci. 2008, 7, 19–25. [Google Scholar] [CrossRef]
- Brodschneider, R.; Crailsheim, K. Nutrition and health in honey bees. Apidologie 2010, 41, 278–294. [Google Scholar] [CrossRef]
- Vaudo, A.D.; Tooker, J.F.; Grozinger, C.M.; Patch, H.M. Bee nutrition and floral resource restoration. Curr. Opin. Insect Sci. 2015, 10, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Ponton, F.; Tan, Y.X.; Forster, C.C.; Austin, A.J.; English, S.; Cotter, S.C.; Wilson, K. The complex interactions between nutrition, immunity and infection in insects. J. Exp. Biol. 2023, 226, 245714. [Google Scholar] [CrossRef] [PubMed]
- Grimble, R.F. Nutritional modulation of immune function. Proc. Nutr. Soc. 2001, 60, 389–397. [Google Scholar] [CrossRef] [PubMed]
- Schmid-Hempel, P. Evolutionary ecology of insect immune defenses. Annu. Rev. Entomol. 2005, 50, 529–551. [Google Scholar] [CrossRef]
- Berenbaum, M.R.; Calla, B. Honey as a functional food for Apis mellifera. Ann. Rev. Entomol. 2021, 66, 185–208. [Google Scholar] [CrossRef]
- Di Pasquale, G.; Salignon, M.; Le Conte, Y.; Belzunces, L.P.; Decourtye, A.; Kretzschmar, A.; Suchail, S.; Brunet, J.-L.; Alaux, C. Influence of pollen nutrition on honey bee health: Do pollen quality and diversity matter? PLoS ONE 2013, 8, e72016. [Google Scholar] [CrossRef]
- Liao, C.; Xu, Y.; Sun, Y.; Lehnert, M.S.; Xiang, W.; Wu, J.; Wu, Z. Feeding behavior of honey bees on dry sugar. J. Insect Physiol. 2020, 124, 104059. [Google Scholar] [CrossRef]
- Papežíková, I.; Palíková, M.; Syrová, E.; Zachová, A.; Somerlíková, K.; Kováčová, V.; Pecková, L. Effect of feeding honey bee (Apis mellifera Hymenoptera: Apidae) colonies with honey, sugar solution, inverted sugar, and wheat starch syrup on Nosematosis prevalence and intensity. J. Econom. Entomol. 2020, 113, 26–33. [Google Scholar] [CrossRef]
- De Jong, D.; da Silva, E.J.; Kevan, P.G.; Atkinson, J.L. Pollen substitutes increase honey bee haemolymph protein levels as much as or more than does pollen. J. Apic. Res. 2009, 48, 34–37. [Google Scholar] [CrossRef]
- Tlak Gajger, I.; Smodiš Škerl, M.I.; Šoštarić, P.; Šuran, J.; Sikirić, P.; Vlainić, J. Physiological and Immunological Status of Adult Honeybees (Apis mellifera) Fed Sugar Syrup Supplemented with Pentadecapeptide BPC 157. Biology 2021, 10, 891. [Google Scholar] [CrossRef]
- Schmehl, D.R.; Teal, P.E.; Frazier, J.L.; Grozinger, C.M. Genomic analysis of the interaction between pesticide exposure and nutrition in honey bees (Apis mellifera). J. Insect Physiol. 2014, 71, 177–190. [Google Scholar] [CrossRef] [PubMed]
- Tlak Gajger, I.; Nejedli, S.; Cvetnić, L. Influence of Probiotic Feed Supplement on Nosema spp. Infection Level and the Gut Microbiota of Adult Honeybees (Apis mellifera L.). Microorganisms 2023, 11, 610. [Google Scholar] [CrossRef] [PubMed]
- Abou-Shaara, H.; Amro, A.; Omar, E. Effects of acetylsalicylic acid, Echinacea purpurea extract, and vitamin C on survival, immunity and performance of honey bees. Egypt. Acad. J. Biol. Sci. A Entomol. 2023, 16, 81–91. [Google Scholar] [CrossRef]
- Abd-El-Samie, E.M.; Seyam, H.; El-Deeb, A.; El-Mohandes, S.; Badr, M.S.; Surano, A.; Abou Kubaa, R. The antiviral activities of Egyptian ethanolic propolis extract and honey bee venom against honey bees infected with multiple viruses in vitro. J. Apic. Res. 2024, 1–14. [Google Scholar] [CrossRef]
- DeGrandi-Hoffman, G.; Chen, Y. Nutrition, immunity and viral infections in honey bees. Curr. Opin. Insect Sci. 2015, 10, 170–176. [Google Scholar] [CrossRef]
- Tlak Gajger, I.; Ribarić, J.; Smodiš Škerl, M.; Vlainić, J.; Sikirić, P. Stable gastric pentadecapeptide BPC 157 in honeybee (Apis mellifera) therapy, to control Nosema ceranae invasions in apiary conditions. J. Vet. Pharmacol. Therap. 2018, 41, 614–621. [Google Scholar] [CrossRef]
- Seeley, T.D.; Smith, M.L. Crowding honeybee colonies in apiaries can increase their vulnerability to the deadly ectoparasite Varroa destructor. Apidologie 2015, 46, 716–727. [Google Scholar] [CrossRef]
- Dynes, T.L.; Berry, J.A.; Delaplane, K.S.; Brosi, B.J.; de Roode, J.C. Reduced density and visually complex apiaries reduce parasite load and promote honey production and overwintering survival in honey bees. PLoS ONE 2019, 14, e0216286. [Google Scholar] [CrossRef]
- Dalmon, A.; Diévart, V.; Thomasson, M.; Fouque, R.; Vaissière, B.E.; Guilbaud, L.; Le Conte, Y.; Henry, M. Possible spillover of pathogens between bee communities foraging on the same floral resource. Insects 2021, 12, 122. [Google Scholar] [CrossRef]
- Nanetti, A.; Bortolotti, L.; Cilia, G. Pathogens Spillover from Honey Bees to Other Arthropods. Pathogens 2021, 10, 1044. [Google Scholar] [CrossRef]
- Piché-Mongeon, V.; Guzman-Novoa, E. Pathogen spillover from honey bees (Apis mellifera L.) to wild bees in North America. Discov. Anim. 2024, 1, 33. [Google Scholar] [CrossRef]
- Giacobino, A.; Molineri, A.I.; Pacini, A.; Fondevila, N.; Pietronave, H.; Rodríguez, G.; Palacio, A.; Cagnolo, N.B.; Orellano, E.; Salto, C.E.; et al. Varroa destructor and viruses association in honey bee colonies under different climatic conditions. Environ. Microbiol. Rep. 2016, 8, 407–412. [Google Scholar] [CrossRef] [PubMed]
- Ilyasov, R.A.; Lee, M.L.; Takahashi, J.I.; Kwon, H.W.; Nikolenko, A.G. A revision of subspecies structure of western honey bee Apis mellifera. Saudi J. Biol. Sci. 2020, 27, 3615–3621. [Google Scholar] [CrossRef] [PubMed]
- Meixner, M.D.; Pinto, M.A.; Bouga, M.; Kryger, P.; Ivanova, E.; Fuchs, S. Standard methods for characterizing subspecies and ecotypes of Apis mellifera. J. Apic. Res. 2013, 52, 1–28. [Google Scholar] [CrossRef]
- Ropars, L.; Affre, L.; Geslin, B. Morphometric identification of honey bee subspecies reveals a high proportion of hybrids within a Mediterranean protected area. J. Apicul. Res. 2021, 60, 871–874. [Google Scholar] [CrossRef]
- Calfee, E.; Agra, M.N.; Palacio, M.A.; Ramírez, S.R.; Coop, G. Selection and hybridization shaped the rapid spread of African honey bee ancestry in the Americas. PLoS Gen. 2020, 16, e1009038. [Google Scholar] [CrossRef]
- Nganso, B.T.; Fombong, A.T.; Yusuf, A.A.; Pirk, C.W.; Stuhl, C.; Torto, B. Hygienic and grooming behaviors in African and European honeybees—New damage categories in Varroa destructor. PLoS ONE 2017, 12, e0179329. [Google Scholar] [CrossRef]
- Le Conte, Y.; Meixner, M.D.; Brandt, A.; Carreck, N.L.; Costa, C.; Mondet, F.; Büchler, R. Geographical distribution and selection of European honey bees resistant to Varroa destructor. Insects 2020, 11, 873. [Google Scholar] [CrossRef]
- Mondet, F.; Alaux, C.; Severac, D.; Rohmer, M.; Mercer, A.R.; Le Conte, Y. Antennae hold a key to Varroa-sensitive hygiene behaviour in honey bees. Sci. Rep. 2015, 5, 10454. [Google Scholar] [CrossRef]
- Bąk, B.; Wilde, J. Grooming behavior by worker bees of various subspecies of honey bees to remove Varroa destructor mites. J. Apicul. Res. 2015, 54, 207–215. [Google Scholar] [CrossRef]
- O’Shea-Wheller, T.A.; Rinkevich, F.D.; Danka, R.G.; Simone-Finstrom, M.; Tokarz, P.G.; Healy, K.B. A derived honey bee stock confers resistance to Varroa destructor and associated viral transmission. Sci. Rep. 2022, 12, 4852. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, S.; Baral, S.S.; Vega Melendez, C.; Amiri, E.; Rueppell, O. Comparing survival of Israeli acute paralysis virus infection among stocks of US honey bees. Insects 2021, 12, 60. [Google Scholar] [CrossRef] [PubMed]
- Cambron-Kopco, L.; Underwood, R.M.; Given, J.K.; Harpur, B.A.; López-Uribe, M.M. Honey bee stocks exhibit high levels of intra-colony variation in viral loads. J. Apic. Res. 2024, 63, 256–259. [Google Scholar] [CrossRef]
- Dalmon, A.; Peruzzi, M.; Le Conte, Y.; Alaux, C.; Pioz, M. Temperature-driven changes in viral loads in the honey bee Apis mellifera. J. Invertebr. Pathol. 2019, 160, 87–94. [Google Scholar] [CrossRef]
- McMenamin, A.J.; Daughenbaugh, K.F.; Flenniken, M.L. The heat shock response in the western honey bee (Apis mellifera) is antiviral. Viruses 2020, 12, 245. [Google Scholar] [CrossRef]
- Xu, X.; Zhou, S.; Huang, J.; Geng, F.; Zhu, X.; Abou-Shaara, H.F. Influence of hyperthermia treatment on varroa infestation, viral infections, and honey bee health in beehives. Insects 2025, 16, 168. [Google Scholar] [CrossRef]
- Kablau, A.; Berg, S.; Härtel, S.; Scheiner, R. Hyperthermia treatment can kill immature and adult Varroa destructor mites without reducing drone fertility. Apidologie 2020, 51, 307–315. [Google Scholar] [CrossRef]
- Porporato, M.; Manino, A.; Cuttini, D.; Lorenzon, S.; Ciaudano, S.; Parodi, V. Varroa control by means of a hyperthermic device. Appl. Sci. 2022, 12, 8138. [Google Scholar] [CrossRef]
- Sandrock, C.; Wohlfahrt, J.; Brunner, W.; Brunner, P. Efficacy and trade-offs of an innovative hyperthermia device to control Varroa destructor in honeybee colonies. J. Pest Sci. 2023, 97, 1433–1450. [Google Scholar] [CrossRef]
- Huang, Z. Mite zapper-a new and effective method for Varroa mite control. Am. Bee J. 2001, 141, 730–732. [Google Scholar]
- Goras, G.; Tananaki, C.H.; Gounari, S.; Dimou, M.; Lazaridou, E.; Karazafiris, E.; Kanelis, D.; Liolios, V.; El Taj, H.F.; Thrasyvoulou, A. Hyperthermia–a non-chemical control strategy against Varroa. J. Hell. Vet. Med. Soc. 2015, 66, 249–256. [Google Scholar] [CrossRef]
- Tihelka, E. History of Varroa heat treatment in Central Europe (1981–2013). Bee World 2016, 93, 4–6. [Google Scholar] [CrossRef]
- Yang, D.; Xu, X.; Zhao, H.; Yang, S.; Wang, X.; Zhao, D.; Diao, Q.; Hou, C. Diverse factors affecting efficiency of RNAi in honey bee viruses. Front. Genet. 2018, 9, 384. [Google Scholar] [CrossRef]
- Liu, J.; Swevers, L.; Iatrou, K.; Huvenne, H.; Smagghe, G. Bombyx mori DNA/RNA non-specific nuclease: Expression of isoforms in insect culture cells, subcellular localization and functional assays. J. Insect Physiol. 2012, 58, 1166–1176. [Google Scholar] [CrossRef]
- Brutscher, L.M.; Daughenbaugh, K.F.; Flenniken, M.L. Antiviral defense mechanisms in honey bees. Curr. Opin. Insect Sci. 2015, 10, 71–82. [Google Scholar] [CrossRef]
- Jarosch, A.; Moritz, R.A. Systemic RNA-interference in the honeybee Apis mellifera: Tissue-dependent uptake of fluorescent siRNA after intra-abdominal application observed by laser-scanning microscopy. J. Insect Physiol. 2011, 57, 851–857. [Google Scholar] [CrossRef]
- Jarosch, A.; Stolle, E.; Crewe, R.M.; Moritz, R.A. Alternative splicing of a single transcription factor drives selfish reproductive behavior in honeybee workers (Apis mellifera). Proc. Natl. Acad. Sci. USA 2011, 108, 15282–15287. [Google Scholar] [CrossRef]
- Amdam, G.V.; Guidugli, K.R.; Norberg, K.; Omholt, S.W. Disruption of vitellogenin gene function in adult honeybees by intra-abdominal injection of double-stranded RNA. BMC Biotechnol. 2003, 3, 1. [Google Scholar] [CrossRef]
- Aronstein, K.; Saldivar, E. Characterization of a honey bee Toll related receptor gene Am18w and its potential involvement in antimicrobial immune defense. Apidologie 2005, 36, 3–14. [Google Scholar] [CrossRef]
- Chapman, N.C.; Oldroyd, B.P.; Hughes, W.O. Differential responses of honeybee (Apis mellifera) patrilines to changes in stimuli for the generalist tasks of nursing and foraging. Behav. Ecol. Sociobiol. 2007, 61, 1185–1194. [Google Scholar] [CrossRef]
- Chen, Y.; Zhao, Y.; Hammond, J.; Hsu, H.; Evans, J.; Feldlaufer, M. Multiple virus infections in the honey bee and genome divergence of honey bee viruses. J. Invertebr. Pathol. 2004, 87, 84–93. [Google Scholar] [CrossRef] [PubMed]
- Todd, J.H.; Miranda, J.R.D.; Ball, B.V. Incidence and molecular characterization of viruses found in dying New Zealand honey bee (Apis mellifera) colonies infested with Varroa destructor. Apidologie 2007, 4, 354–367. [Google Scholar] [CrossRef]
- de Miranda, J.R.; Cordoni, G.; Budge, G. The acute bee paralysis virus-Kashmir bee virus-Israeli acute paralysis virus complex. J. Invertebr. Pathol. 2010, 103, 30–47. [Google Scholar] [CrossRef] [PubMed]
- Ricigliano, V.A.; McMenamin, A.; Martin Ewert, A.; Adjaye, D.; Simone-Finstrom, M.; Rainey, V.P. Green biomanufacturing of edible antiviral therapeutics for managed pollinators. Sustain. Agricul. 2024, 2, 4. [Google Scholar] [CrossRef]
- Bojanić Rašović, M. The most important methods of disinfection in beekeeping. Agric. For. 2021, 67, 167–176. [Google Scholar]
- Borum, A.E. Biosecurity and good beekeeping practices in beekeeping. Uludağ Arıcılık Dergisi 2022, 22, 246–276. [Google Scholar] [CrossRef]
- Rašović, M.B. The importance of applying good beekeeping practice in the production of beekeeping products in Montenegro. J. Hyg. Eng. Des. 2022, 38, 52–57. [Google Scholar]
- Tlak Gajger, I.; Tomljanović, Z.; Mutinelli, F.; Granato, A.; Vlainić, J. Effects of Disinfectants on Bacterium Paenibacillus larvae in Laboratory Conditions. Insects 2024, 15, 268. [Google Scholar] [CrossRef]
- Colwell, M.J.; Pernal, S.F.; Currie, R.W. Treatment of waxborne honey bee (Hymenoptera: Apidae) viruses using time, temperature, and electron-beam irradiation. J. Econom. Entomol. 2024, 117, 34–42. [Google Scholar] [CrossRef]
- Majoroš, A.; Tlak Gajger, I.; Smodiš Škerl, M.I. Nutritional stress of honeybee colonies (Apis mellifera L.): Causes, effects and loss prevention measures. Vet. Stn. 2022, 53, 461–474. [Google Scholar]
- Büchler, R.; Andonov, S.; Bernstein, R.; Bienefeld, K.; Costa, C.; Du, M.; Gabel, M.; Giveng, K.; Hatjinah, F.; Harpur, B.A.; et al. Standard methods for rearing and selection of Apis mellifera queens 2.0. J. Apic. Res. 2024, 64, 555–611. [Google Scholar] [CrossRef]
- Chaimanee, V.; Chantawannakul, P.; Chen, Y.; Evans, J.D.; Pettis, J.S. Effects of host age on susceptibility to infection and immune gene expression in honey bee queens (Apis mellifera) inoculated with Nosema ceranae. Apidologie 2014, 45, 451–463. [Google Scholar] [CrossRef]
- Medina, R.G.; Paxton, R.J.; Hernández-Sotomayor, S.T.; Pech-Jiménez, C.; Medina-Medina, L.A.; Quezada-Euán, J.J.G. Heat stress during development affects immunocompetence in workers, queens and drones of Africanized honey bees (Apis mellifera L.) (Hymenoptera: Apidae). J. Thermal Biol. 2020, 89, 102541. [Google Scholar] [CrossRef] [PubMed]
- McAfee, A.; Chapman, A.; Pettis, J.S.; Foster, L.J.; Tarpy, D.R. Trade-offs between sperm viability and immune protein expression in honey bee queens (Apis mellifera). Commun. Biol. 2021, 4, 48. [Google Scholar] [CrossRef]
- Erez, T.; Osabutey, A.F.; Hamdo, S.; Bonda, E.; Otmy, A.; Chejanovsky, N.; Soroker, V. Ontogeny of immunity and natural viral infection in Apis mellifera drones and workers. J. Invertebr. Pathol. 2024, 205, 108124. [Google Scholar] [CrossRef]
- Zmarlicki, C.; Morse, R.A. Drone congregation areas. J. Apic. Res. 1963, 2, 64–66. [Google Scholar] [CrossRef]
- Koeniger, N.; Koeniger, G.; Pechhacker, H. The nearer the better? Drones (Apis mellifera) prefer nearer drone congregation areas. Insects Sociaux 2005, 52, 31–35. [Google Scholar] [CrossRef]
- Galindo-Cardona, A.; Carolina Monmany, A.; Moreno-Jackson, R.; Rivera-Rivera, C.; Huertas-Dones, C.; Caicedo-Quiroga, L.; Giray, T. Landscape analysis of drone congregation areas of the honey bee, Apis mellifera. J. Insect Sci. 2012, 12, 122. [Google Scholar] [CrossRef]
- Mortensen, A.N.; Ellis, J.D. Scientific note on a single-user method for identifying drone congregation areas. J. Apic. Res. 2014, 53, 424–425. [Google Scholar] [CrossRef]
- Abou-Shaara, H.F.; Kelany, M.M. A methodology to assist in locating drone congregation area using remote sensing technique. J. Apic. Res. 2023, 62, 468–470. [Google Scholar] [CrossRef]
- Forfert, N.; Natsopoulou, M.E.; Paxton, R.J.; Moritz, R.F. Viral prevalence increases with regional colony abundance in honey bee drones (Apis mellifera L). Infect. Genet. Evol. 2016, 44, 549–554. [Google Scholar] [CrossRef]
- Mortensen, A.N.; Jack, C.J.; Ellis, J.D. The discovery of Varroa destructor on drone honey bees, Apis mellifera, at drone congregation areas. Parasitol. Res. 2018, 117, 3337–3339. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tlak Gajger, I.; Abou-Shaara, H.F.; Smodiš Škerl, M.I. Strategies to Mitigate the Adverse Impacts of Viral Infections on Honey Bee (Apis mellifera L.) Colonies. Insects 2025, 16, 509. https://doi.org/10.3390/insects16050509
Tlak Gajger I, Abou-Shaara HF, Smodiš Škerl MI. Strategies to Mitigate the Adverse Impacts of Viral Infections on Honey Bee (Apis mellifera L.) Colonies. Insects. 2025; 16(5):509. https://doi.org/10.3390/insects16050509
Chicago/Turabian StyleTlak Gajger, Ivana, Hossam F. Abou-Shaara, and Maja Ivana Smodiš Škerl. 2025. "Strategies to Mitigate the Adverse Impacts of Viral Infections on Honey Bee (Apis mellifera L.) Colonies" Insects 16, no. 5: 509. https://doi.org/10.3390/insects16050509
APA StyleTlak Gajger, I., Abou-Shaara, H. F., & Smodiš Škerl, M. I. (2025). Strategies to Mitigate the Adverse Impacts of Viral Infections on Honey Bee (Apis mellifera L.) Colonies. Insects, 16(5), 509. https://doi.org/10.3390/insects16050509







