Harmful to Parents, Harmless to Offspring: Lethal and Transgenerational Effects of Botanical and Synthetic Insecticides on the Egg Parasitoid Trichogramma atopovirilia
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Maintenance of Trichogramma atopovirilia Colony
2.2. Treatments and Concentrations
2.3. Bioassays
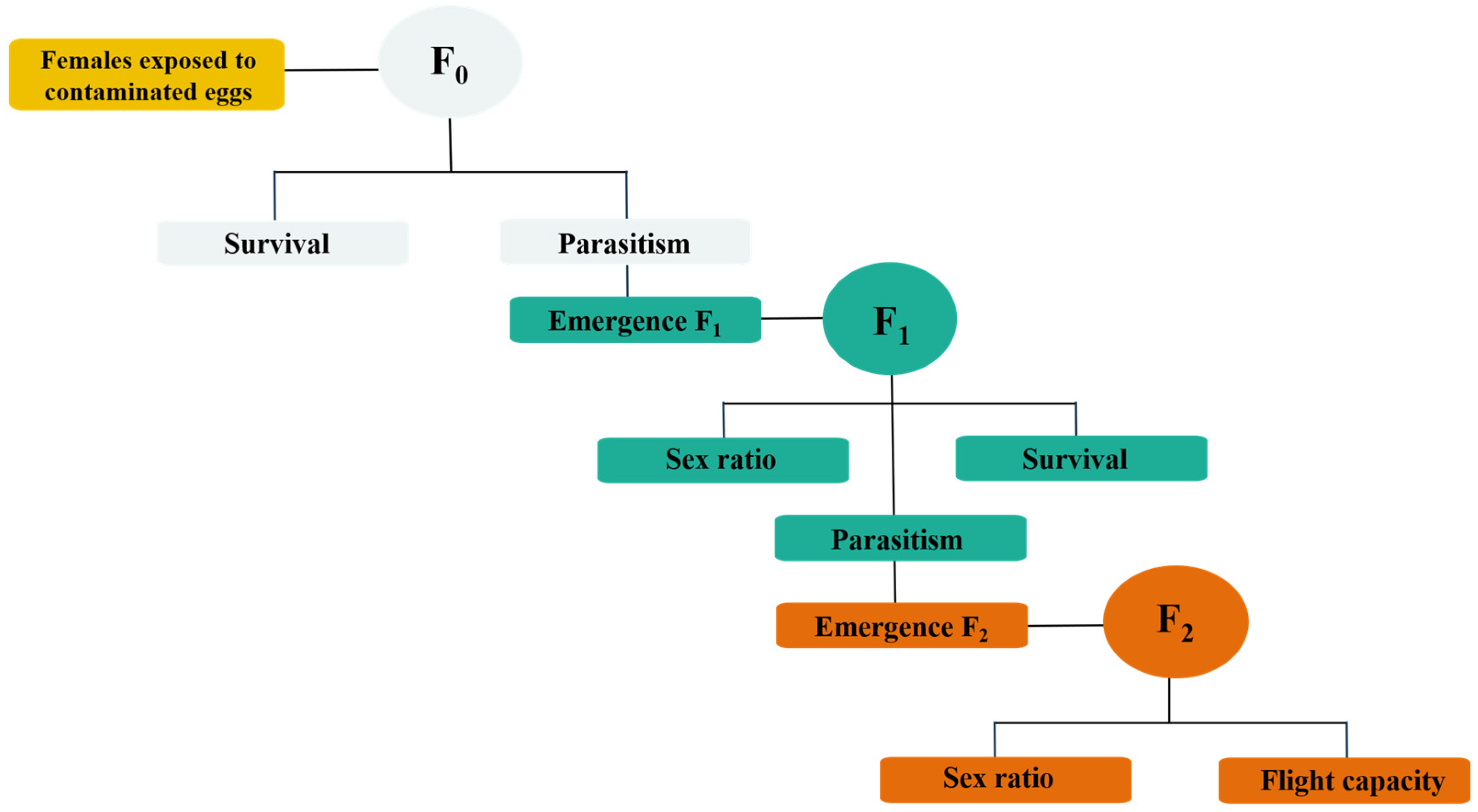
2.3.1. Survival and Parasitism of the F0 Generation (Parental)
2.3.2. Transgenerational Effects on F1 and F2 Generations
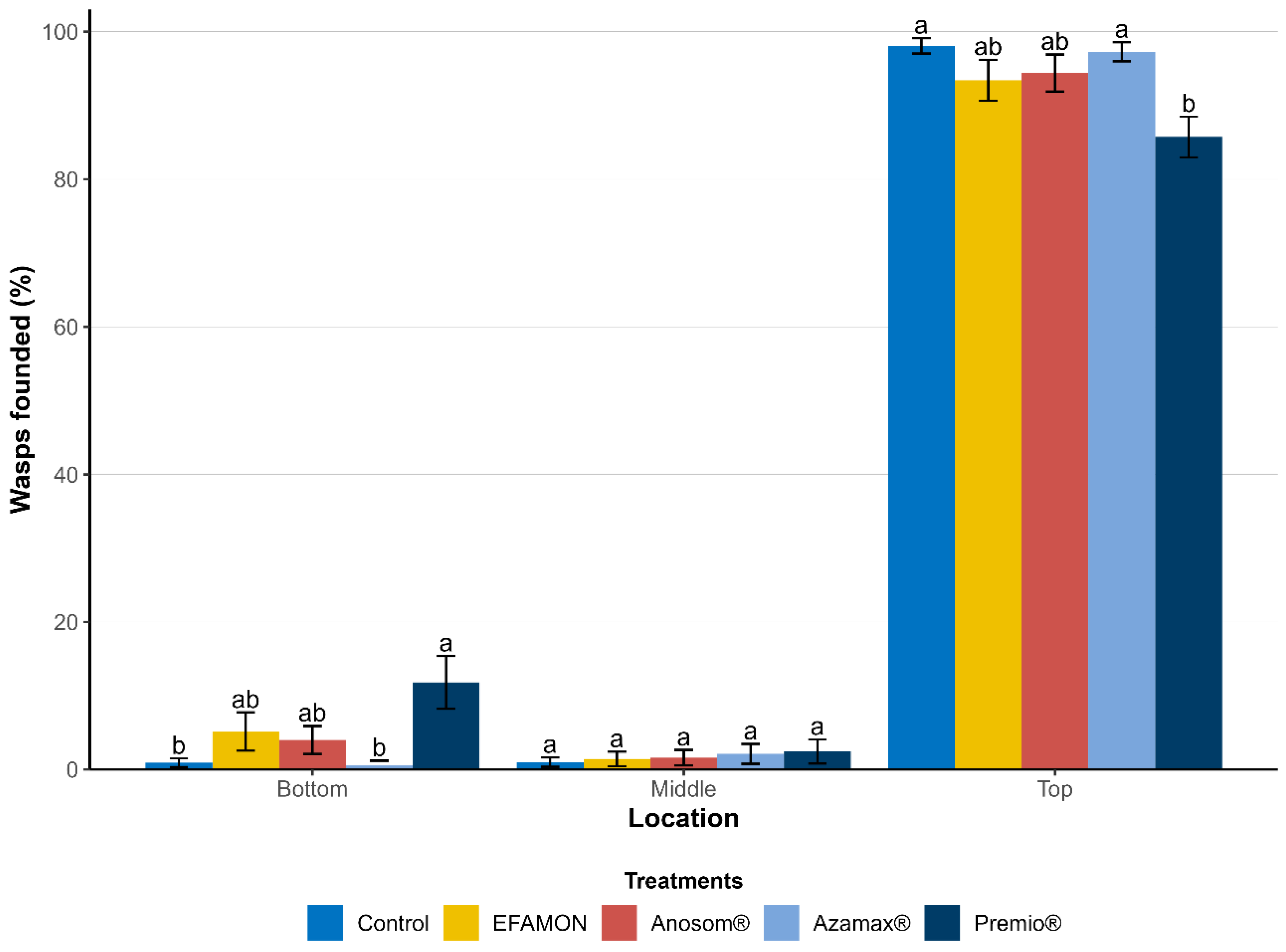
2.3.3. Flight Capacity of the F2 Generation
2.4. Statistical Analysis
3. Results
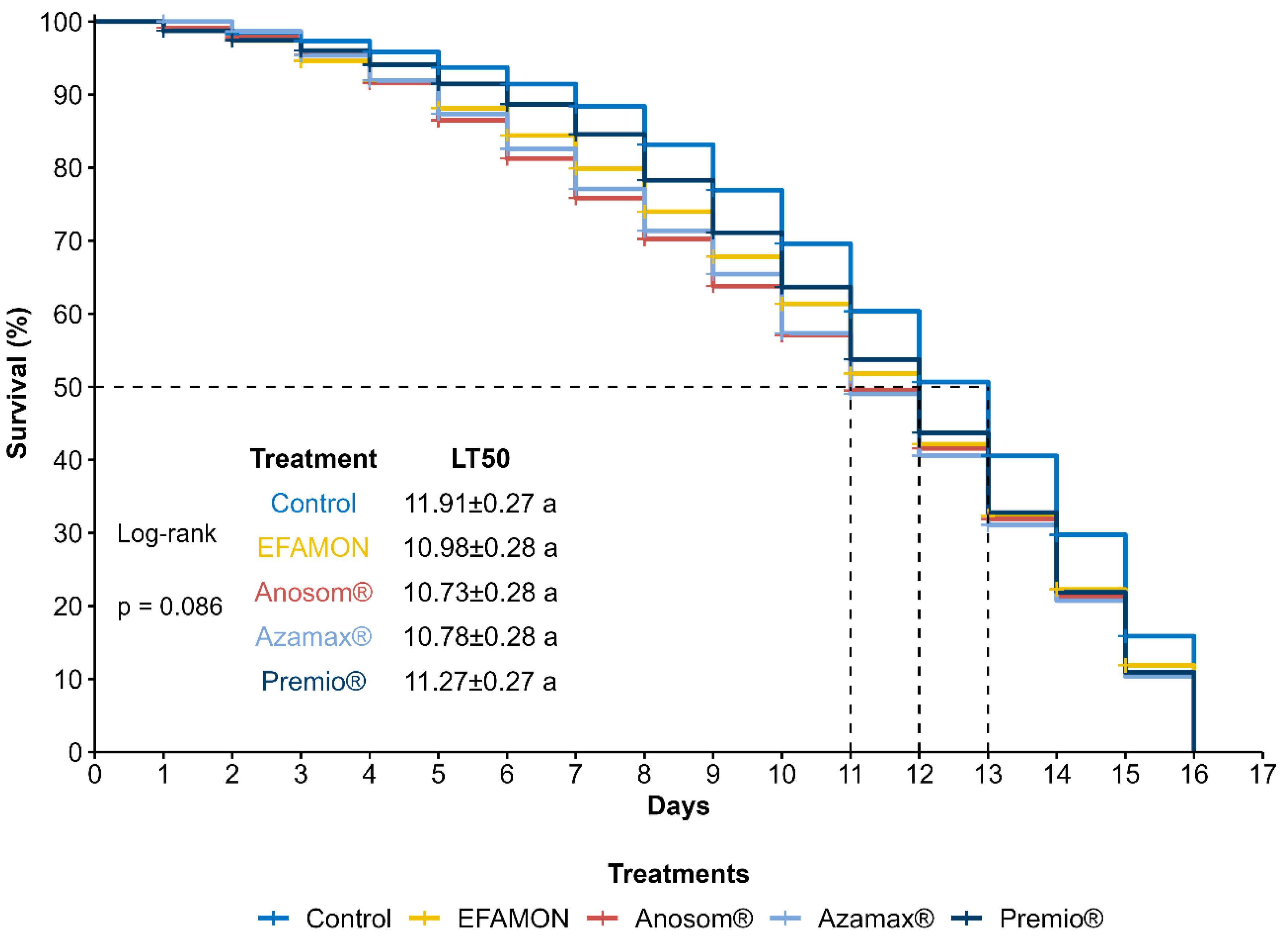
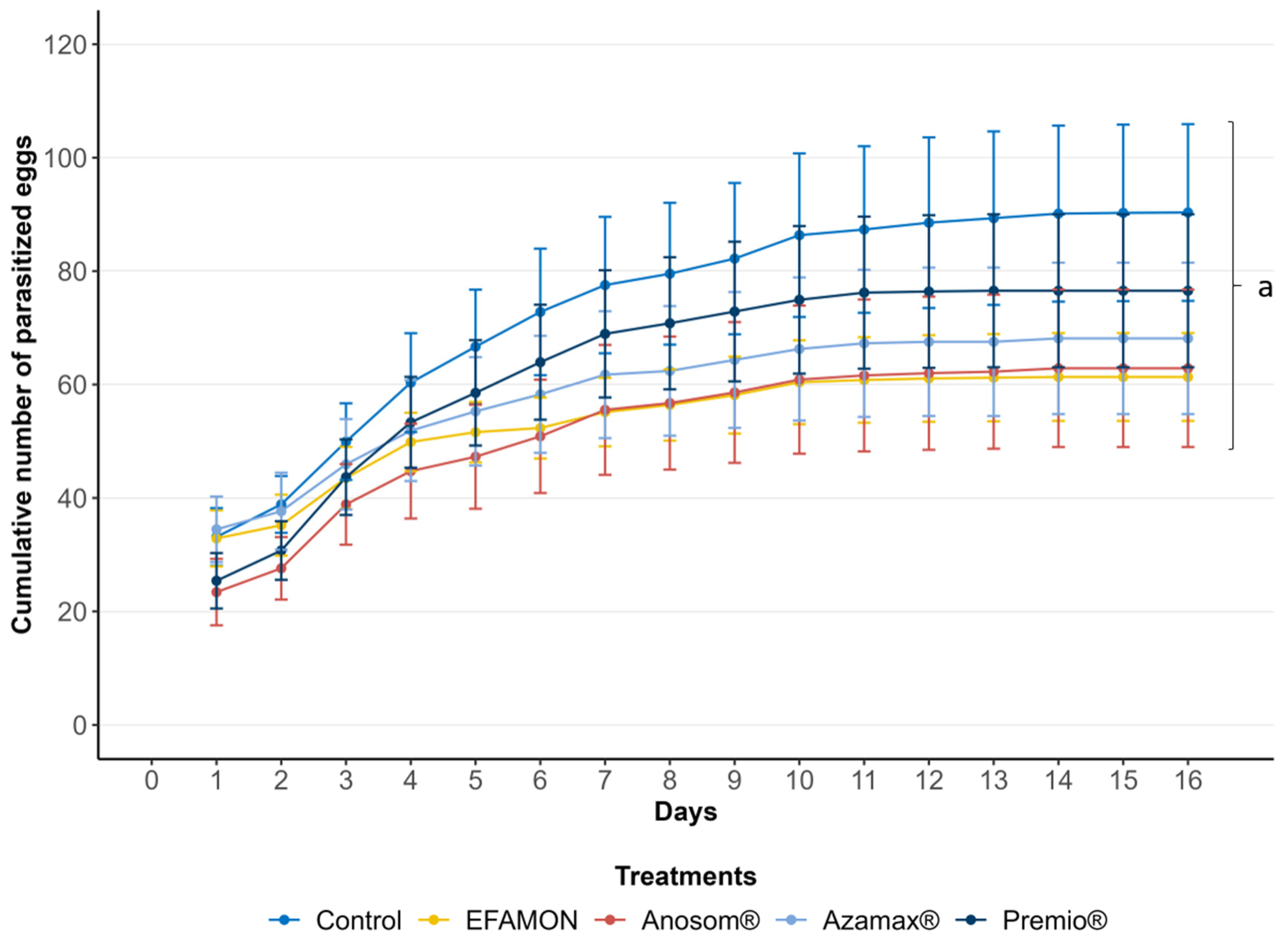
3.1. Survival and Parasitism of the F0 Generation (Parental)
3.2. Transgenerational Effects on the F1 and F2 Generations
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Montezano, D.G.; Specht, D.; Sosa-Gómez, V.F.; Roque-Specht, J.; Sousa-Silva, J.C.; Paula-Moraes, S.V.; Peterson, J.A.; Hunt, T.E. Host plants of Spodoptera frugiperda (Lepidoptera: Noctuidae) in the Americas. Afr. Entomol. 2018, 26, 286–300. [Google Scholar] [CrossRef]
- Midega, C.; Hadi, B.; McGuire, S.; Zaidi, Z.; Rajagopalan, P. Fall armyworm: Measuring damage and loss caused by a novel invasive pest as a guide to sustainable management. In Statistics Working Paper Series, No. 23–38; FAO: Rome, Italy, 2023. [Google Scholar] [CrossRef]
- Mota-Sanchez, D.; Wise, J.C. The Arthropod Pesticide Resistance Database; Michigan State University: East Lansing, MI, USA. Available online: http://www.pesticideresistance.org (accessed on 26 December 2024).
- Paredes-Sánchez, F.A.; Rivera, G.; Bocanegra-García, V.; Martínez-Padrón, H.Y.; Berrones-Morales, M.; Niño-García, N.; Herrera-Mayorga, V. Advances in control strategies against Spodoptera frugiperda. A review. Molecules 2021, 26, 5587. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.M.; Gadratagi, B.G.; Paramesh, V.; Kumar, P.; Madivalar, Y.; Narayanappa, N.; Ullah, F. Sustainable management of invasive fall armyworm, Spodoptera frugiperda. Agronomy 2022, 12, 2150. [Google Scholar] [CrossRef]
- Navik, O.; Yele, Y.; Kedar, S.C.; Sushil, S.N. Biological Control of Fall Armyworm Spodoptera frugiperda (JE Smith) using egg parasitoids, Trichogramma species (Hymenoptera: Trichogrammatidae): A review. Egypt. J. Biol. Pest Control 2023, 33, 118. [Google Scholar] [CrossRef]
- Zucchi, R.A.; Querino, R.B. Historical note on the genus Trichogramma (Hymenoptera, Trichogrammatidae) in Brazil, focusing on taxonomy and diversity. Neotrop. Entomol. 2024, 53, 773–785. [Google Scholar] [CrossRef]
- Jaraleño-Teniente, J.; Lomeli-Flores, J.R.; Rodríguez-Leyva, E.; Bujanos-Muñiz, R.; Rodríguez-Rodríguez, S.E. Egg parasitoids survey of Spodoptera frugiperda (Smith) (Lepidoptera: Noctuidae) in maize and sorghum in Central Mexico. Insects 2020, 11, 157. [Google Scholar] [CrossRef]
- Jaraleño-Teniente, J.; Refugio Lomeli-Flores, J.; Rodríguez-Leyva, E.; Bujanos-Muñiz, R.; Rodríguez-Rodríguez, S.E. Efficiency of three egg parasitoid species on fall armyworm (Lepidoptera: Noctuidae) in laboratory and field cages. J. Entomol. Sci. 2021, 56, 519–526. [Google Scholar] [CrossRef]
- Gonzalez-Cabrera, J.; García-García, R.E.; Vega-Chavez, J.L.; Contreras-Bermudez, Y.; Mejía-García, N.; Ángeles-Chavez, E.; Sanchez-Gonzalez, J.A. Biological and population parameters of Telenomus remus and Trichogramma atopovirilia as biological control agents for Spodoptera frugiperda. Crop Prot. 2025, 188, 106995. [Google Scholar] [CrossRef]
- Dequech, S.T.B.; Camera, C.; Soares Sturza, V.; Ribeiro, L.d.P.; Querino, R.B.; Poncio, S. Flutuação populacional de ovos de Spodoptera frugiperda e parasitismo natural por Trichogramma em milho. Acta Sci. Agron. 2013, 35, 295–300. [Google Scholar] [CrossRef]
- Beserra, E.B.; Parra, J.R.P. Comportamento de parasitismo de Trichogramma atopovirilia Oatman & Platner e Trichogramma pretiosum Riley (Hymenoptera, Trichogrammatidae) em posturas de Spodoptera frugiperda (J. E. Smith) (Lepidoptera, Noctuidae). Rev. Bras. Entomol. 2004, 47, 205–209. [Google Scholar] [CrossRef]
- Tay, W.T.; Meagher, R.L.; Czepak, C.; Groot, A.T. Spodoptera frugiperda: Ecology, evolution, and management options of an invasive species. Annu. Rev. Entomol. 2023, 68, 299–317. [Google Scholar] [CrossRef] [PubMed]
- Hikal, W.M.; Baeshen, R.S.; Said-Al Ahl, H.A.H. Botanical insecticide as simple extractives for pest control. Cogent Biol. 2017, 3, 1404274. [Google Scholar] [CrossRef]
- Ansante, T.F.; do Prado Ribeiro, L.; Bicalho, K.U.; Fernandes, J.B.; das Graças Fernandes da Silva, M.F.; Vieira, P.C.; Vendramim, J.D. Secondary metabolites from neotropical Annonaceae: Screening, bioguided fractionation, and toxicity to Spodoptera frugiperda (J. E. Smith) (Lepidoptera: Noctuidae). Ind. Crops Prod. 2015, 74, 969–976. [Google Scholar] [CrossRef]
- Ansante, T.F.; do Prado Ribeiro, L.; Vendramim, J.D. Acute and chronic toxicities of an Annonin-based commercial bioinsecticide and a joint mixture with a Limonoid-based formulation to the fall armyworm. Neotrop. Entomol. 2017, 46, 216–222. [Google Scholar] [CrossRef]
- Hidalgoa, J.R.; Gilaberta, M.; Cabedoc, N.; Cortesb, D.; Neske, A. Phytochemistry letters Montanacin-L and Montanacin-K two previously non-described acetogenins from Annona montana twigs and leaves. Phytochem. Lett. 2020, 38, 78–83. [Google Scholar] [CrossRef]
- Di Toto Blessing, L.; Ramos, J.; Diaz, S.; Ben Altabef, A.; Bardón, A.; Brovetto, M.; Seoane, G.; Neske, A. Insecticidal properties of annonaceous acetogenins and their analogues. interaction with lipid membranes. Nat. Prod. Commun. 2012, 7, 1215–1218. [Google Scholar] [CrossRef]
- Costa, S.; De Paula, S.O.; Martins, G.F. Multiple Modes of action of the Squamocin in the midgut cells of Aedes aegypti larvae. PLoS ONE 2016, 11, e0160928. [Google Scholar] [CrossRef]
- Pompanon, F.; Fouillet, P.; Bouletreau, M. Physiological and genetic factors as sources of varietion in locomotion and activity rhythm in a parasitoid wasp (Trichogramma brassicae). Physiol. Entomol. 1999, 24, 346–357. [Google Scholar] [CrossRef]
- Woelke, J.B.; Bukovinszky, T.; Huigens, M.E. Nocturnal parasitism of moth eggs by Trichogramma wasps. Biocontrol Sci. Technol. 2017, 27, 769–780. [Google Scholar] [CrossRef]
- Cantori, L.V.; Iost Filho, F.H.; Pazini, J.d.B.; Diniz, A.J.F.; Yamamoto, P.T.; Parra, J.R.P. Is integrated management of Gymnandrosoma aurantianum possible with Trichogramma atopovirilia and novel products used in citrus orchards in Brazil? Insects 2023, 14, 419. [Google Scholar] [CrossRef]
- Braga-Maia, J.; Andrade-Carvalho, G.; Santos-Leite, M.I.; Lopes De Oliveira, R.; Makyama, L. Selectivity of insecticides used in corn crops to adult Trichogramma atopovirilia (Hymenoptera: Trichogrammatidae). Rev. Colomb. Entomol. 2010, 36, 202–206. [Google Scholar] [CrossRef]
- Braga Maia, J.; Andrade Carvalho, G.; Lopes de Oliveira, R.; Lasmar, O.; Santos Leite, M.I. Effects of insecticides used in corn on immature stages of Trichogramma atopovirilia (Hymenoptera: Trichogrammatidae). Rev. Colomb. Entomol. 2013, 39, 205–210. [Google Scholar] [CrossRef]
- Manzoni, C.; Grützmacher, A.; Giolo, F.; Härter, W.; Castilhos, R.; Paschoal, M. Seletividade de agroquímicos utilizados na produção integrada de maçã aos parasitóides Trichogramma pretiosum Riley e Trichogramma atopovirilia Oatman & Platner (Hymenoptera: Trichogrammatidae). BioAssay 2007, 2, 50. [Google Scholar] [CrossRef]
- Giolo, F.P.; Grützmacher, A.D.; Manzoni, C.G.; Nörnberg, S.D.; Härter, W.D.R.; Castilhos, R.V. Seletividade de produtos fitossanitários utilizados na cultura do pessegueiro nos estágios imaturos de Trichogramma atopovirilia Oatman & Platner, 1983 (Hymenoptera: Trichogrammatidae). Cienc. Rural 2008, 38, 1220–1226. [Google Scholar] [CrossRef]
- Pratissoli, D.; Milanez, A.M.; Celestino, F.N.; Barbosa, W.F. Trichogramma atopovirilia Oatman & Platner (Hymenoptera: Trichogrammatidae) em condições de laboratório. Rev. Ceres 2011, 58, 661–664. [Google Scholar]
- Castle, S.; Naranjo, S.E. Sampling plans, selective insecticides and sustainability: The case for IPM as “Informed Pest Management”. Pest Manag. Sci. 2009, 65, 1321–1328. [Google Scholar] [CrossRef]
- Torres, J.B.; Bueno, A.D.F. Conservation biological control using selective insecticides—A valuable tool for IPM. Biol. Control 2018, 126, 53–64. [Google Scholar] [CrossRef]
- Serrão, J.E.; Plata-Rueda, A.; Martínez, L.C.; Zanuncio, J.C. Side-effects of pesticides on non-target insects in agriculture: A mini-review. Sci. Nat. 2022, 109, 17. [Google Scholar] [CrossRef]
- de Bueno, A.F.; Carvalho, G.A.; dos Santos, A.C.; Sosa-Gómez, D.R.; Silva, D.M.D. Pesticide selectivity to natural enemies: Challenges and constraints for research and field recommendation. Ciência Rural 2017, 47, e20160829. [Google Scholar] [CrossRef]
- Sterk, G.; Hassan, S.A.; Baillod, M.; Bakker, F.; Bigler, F.; Blümel, S.; Bogenschütz, H.; Boller, E.; Bromand, B.; Brun, J.; et al. Results of the seventh joint pesticide testing programme carried out by the IOBC/WPRS-working group ‘Pesticides and Beneficial Organisms’. BioControl 1999, 44, 99–117. [Google Scholar] [CrossRef]
- Costa, M.A.; Farias, E.S.; Andrade, E.D.; Carvalho, V.C.; Carvalho, G.A. Lethal, sublethal and transgenerational effects of insecticides labeled for cotton on immature Trichogramma pretiosum. J. Pest Sci. (2004) 2023, 96, 119–127. [Google Scholar] [CrossRef]
- Costa, M.A.; Moscardini, V.F.; da Costa Gontijo, P.; Carvalho, G.A.; de Oliveira, R.L.; de Oliveira, H.N. Sublethal and transgenerational effects of insecticides in developing Trichogramma galloi (Hymenoptera: Trichogrammatidae): Toxicity of insecticides to Trichogramma galloi. Ecotoxicology 2014, 23, 1399–1408. [Google Scholar] [CrossRef] [PubMed]
- MAPA. Agrofit. Available online: http://agrofit.agricultura.gov.br/agrofit_cons/principal_agrofit_cons (accessed on 10 January 2024).
- Bowen, W.R.; Stern, V.M. Effect of temperature on the production of males and sexual mosaics in a uniparental race of Trichogramma semifumatum (Hymenoptera: Trichogrammatidae). Ann. Entomol. Soc. 1996, 59, 823–834. [Google Scholar] [CrossRef]
- Prezotti, L.; Parra, J.R.R.; Vencovsky, R.; Dias, C.T.D.S.; Cruz, I.; Chagas, M.C.M. Flight test as quality assessment grades of Trichogramma pretiosum Riley (Hymenoptera: Trichogrammatidae): Methodology adaptation. Neotrop. Entomol. 2002, 31, 411–417. [Google Scholar] [CrossRef]
- Dutton, A.; Bigler, F. Flight activity assessment of the egg parasitoid Trichogramma brassicae (Hymenoptera: Trichogrammatidae) in laboratory and field conditions A. Entomophaga 1995, 40, 223–233. [Google Scholar] [CrossRef]
- Therneau, T. A Package for Survival Analysis in R. R Package Version 3.8-3. Available online: https://cran.r-project.org/web/packages/survival/vignettes/survival.pdf (accessed on 12 January 2025).
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021; Available online: https://www.r-project.org/ (accessed on 15 March 2024).
- Terry, M.; Therneau, P.M.G. Modeling Survival Data: Extending the Cox Mode; Springer: New York, NY, USA, 2000; Volume 3, ISBN 0387987843. [Google Scholar]
- Moral, R.A.; Hinde, J.; Demétrio, C.G.B. Half-normal plots and overdispersed models in R: The Hnp package. J. Stat. Softw. 2017, 81, 1–23. [Google Scholar] [CrossRef]
- Bueno, R.C.O.d.F.; Bueno, A.d.F.; Parra, J.R.P.; Vieira, S.S.; de Oliveira, L.J. Biological characteristics and parasitism capacity of Trichogramma pretiosum Riley (Hymenoptera, Trichogrammatidae) in eggs of Spodoptera frugiperda (J. E. Smith) (Lepidoptera, Noctuidae). Rev. Bras. Entomol. 2010, 54, 322–327. [Google Scholar] [CrossRef]
- Bernardi, D.; Ribeiro, L.; Andreazza, F.; Neitzke, C.; Oliveira, E.E.; Botton, M.; Nava, D.E.; Vendramim, J.D. Potential use of Annona by products to control Drosophila suzukii and toxicity to its parasitoid Trichopria anastrephae. Ind. Crops Prod. 2017, 110, 30–35. [Google Scholar] [CrossRef]
- Ribeiro, L.d.P.; Gonçalves, G.L.P.; Bicalho, K.U.; Fernandes, J.B.; Vendramim, J.D. Rolliniastatin-1, a bis-tetrahydrofuran acetogenin: The major compound of Annona mucosa Jacq. (Annonaceae) has potent grain-protective properties. J. Stored Prod. Res. 2020, 89, 101686. [Google Scholar] [CrossRef]
- Alali, F.Q.; Liu, X.; Mclaughlin, J.L. Annonaceous acetogenins: Recent Progress. J. Nat. Prod. 1999, 62, 504–540. [Google Scholar] [CrossRef]
- Tormo, J.R.; Gallardo, T.; Cortes, D.; Estornell, E. Specific interactions of monotetrahydrofuranic Annonaceous acetogenins as inhibitors of mitochondrial complex I. Chem. Biol. Interact. 1999, 122, 171–183. [Google Scholar] [PubMed]
- Machado, A.; Araujo, A.; De Freitas, C.; Emanuel, V.; Martins, P.; Auxiliadora, M.; Ferreira, P.; Ana, C.; Letícia, C.; Cabeça, S.; et al. Larvicidal activity of Annona mucosa jacq. extract and main constituents rolliniastatin 1 and rollinicin against Aedes aegypti and Aedes albopictus. Ind. Crops Prod. 2021, 169, 113678. [Google Scholar] [CrossRef]
- Bombasaro, J.A.; Blessing, L.D.T.; Diaz, S.; Neske, A.; Suvire, F.D.; Enriz, R.D.; Rodríguez, A.M. Theoretical and experimental study of the interactions of annonaceous acetogenins with artificial lipid bilayers. J. Mol. Struct. 2011, 1003, 87–91. [Google Scholar] [CrossRef]
- Ribeiro, L.d.P.; Vendramim, J.D.; Bicalho, K.U.; Andrade, M.d.S.; Fernandes, J.B.; Moral, R.d.A.; Demétrio, C.G.B. Annona Mucosa Jacq. (Annonaceae): A promising source of bioactive compounds against Sitophilus zeamais Mots. (Coleoptera: Curculionidae). J. Stored Prod. Res. 2013, 55, 6–14. [Google Scholar] [CrossRef]
- Gomes, F.L. Phytochemical Study and Insecticidal Activity of the Phytosanitary Composition of Annona squamosa L. and Annona mucosa (Jacq.) Baill. (Annonaceae) for the Control of Plutella xylostella (L., 1758) (Lepidoptera: Plutellidae). Ph.D. Thesis, Federal University of Alagoas, Alagoas, Brazil, 2018. [Google Scholar]
- Bicalho, K.U. Annona mucosa (Annonaceae) for Pest Control: Biomonitored Phytochemical Study, Mimic Synthesis, and Development of Nanoencapsulation Processes. Ph.D. Thesis, Federal University of São Carlos, São Carlos, Brazil, 2016. [Google Scholar]
- Lopes Baldin, E.L.; Soares, M.C.E.; da Silva Santana, A.; Hunt, T.E.; McMechan, J.; Vélez Arango, A.M.; Louis, J. Efficacy of ethanolic seed extracts of Annona spp. against Aphis glycines. Crop Prot. 2023, 170, 106268. [Google Scholar] [CrossRef]
- Neske, A.; Ruiz Hidalgo, J.; Cabedo, N.; Cortes, D. Acetogenins from Annonaceae family. Their potential biological applications. Phytochemistry 2020, 174, 112332. [Google Scholar] [CrossRef]
- Durán-Ruiz, C.A.; González-Esquinca, A.R.; de-la-Cruz-Chacón, I. Annonaceous acetogenins: A comparative analysis of insecticidal activity. Rev. Bras. Frutic. 2024, 46, e-508. [Google Scholar] [CrossRef]
- Rodrigues, R.; Jaras, L.I.; Poltronieri, A.S.; Pimentel, I.C.; Zawadneak, M.A.C. Selectivity of insect growth regulators and botanical insecticides on the parasitism of three Trichogramma species in eggs of Duponchelia fovealis Zeller (Lepidoptera: Crambidae). EntomoBrasilis 2017, 10, 26–32. [Google Scholar] [CrossRef]
- Luckmann, D.; de Gouvea, A.; Potrich, M.; da Silva, E.R.L.; Puretz, B.; Dallacort, S.; Gonçalves, T.E. Selectivity of commercial natural products to Trichogramma pretiosum (Riley, 1879) (Hymenoptera: Trichogrammatidae). Rev. Ceres 2014, 61, 924–931. [Google Scholar] [CrossRef]
- Mordue(Luntz), A.J.; Nisbet, A.J. Azadirachtin from the neem tree Azadirachta indica: Its action against insects. An. Soc. Entomológica Bras. 2000, 29, 615–632. [Google Scholar] [CrossRef]
- Chatterjee, S.; Bag, S.; Biswal, D.; Sarkar Paria, D.; Bandyopadhyay, R.; Sarkar, B.; Mandal, A.; Dangar, T.K. Neem-based products as potential eco-friendly mosquito control agents over conventional eco-toxic chemical pesticides-a review. Acta Trop. 2023, 240, 106858. [Google Scholar] [CrossRef] [PubMed]
- Alouani, A.; Rehimi, N.; Soltani, N. Larvicidal activity of a neem tree extract (Azadirachtin) against mosquito larvae in the republic of algeria. Jordan J. Biol. Sci. 2009, 2, 15–22. [Google Scholar]
- Kala, S.; Naik, S.N.; Patanjali, P.K.; Sogan, N. Neem oil water dispersible tablet as effective larvicide, ovicide and oviposition deterrent against Anopheles culicifacies. S. Afr. J. Bot. 2019, 123, 387–392. [Google Scholar] [CrossRef]
- Khaldi, R.; Rehimi, N.; Kharoubi, R.; Soltani, N. Phytochemical composition of almond oil from Melia azedarach l. and its larvicidal, ovicidal, repellent and enzyme activities in Culex pipiens L. Trop. Biomed. 2022, 39, 531–538. [Google Scholar] [CrossRef]
- Nauen, R. Insecticide Mode of Action: Return of the ryanodine receptor. Pest Manag. Sci. 2006, 692, 690–692. [Google Scholar] [CrossRef]
- Lahm, G.P.; Cordova, D.; Barry, J.D. Bioorganic & medicinal chemistry new and selective ryanodine receptor activators for insect control. Bioorg. Med. Chem. 2009, 17, 4127–4133. [Google Scholar] [CrossRef]
- Dos Santos, V.P.; Damascena, A.P.; Pratissoli, D.; De Carvalho, J.R.; Bueno, R.C.O.d.F.; Zago, H.B.; Pereira, J.B.; Oliveira, D.V. Toxicity of recommended insecticides for the tomato culture on Trichogramma pretiosum Riley, 1879 (Hymenoptera: Trichogrammatidae). Contrib. Ciencias Soc. 2023, 16, 721–731. [Google Scholar] [CrossRef]
- Li, H.R.; Li, C.Y.; Dai, P.; Zang, L.S.; Desneux, N.; Xu, W. Selective and persistent toxicity of seven insecticides to five egg parasitoids of Spodoptera frugiperda. CABI Agric. Biosci. 2024, 5, 8. [Google Scholar] [CrossRef]
- Veronica, C.S.; Ivan, G.M.; Francisco, G.G. Evolutionary consequences of pesticide exposure include transgenerational plasticity and potential terminal investment transgenerational effects. Evolution 2022, 76, 2649–2668. [Google Scholar] [CrossRef]
- da Silva, M.R. Insecticides in Citrus Orchards and Their Selectivity on the Parasitoid Trichogramma atopovirilia. (Hymenoptera: Trichogrammatidae). Master’s Thesis, Univerity of São Paulo, Piracicaba, Brazil, 2021. [Google Scholar]
- Soares, M.A.; Passos, L.C.; Campos, M.R.; Collares, L.J.; Desneux, N.; Carvalho, G.A. Side effects of insecticides commonly used against Tuta absoluta on the predator Macrolophus basicornis. J. Pest Sci. (2004) 2019, 92, 1447–1456. [Google Scholar] [CrossRef]
- Naiara Gomes, I.; Ingred Castelan Vieira, K.; Moreira Gontijo, L.; Canto Resende, H. Honeybee survival and flight capacity are compromised by insecticides used for controlling melon pests in Brazil. Ecotoxicology 2020, 29, 97–107. [Google Scholar] [CrossRef]


| Treatments | N | LC90 (95% CI) 1 | Slope ± SE | X2 (df) | p | Manufacturer |
|---|---|---|---|---|---|---|
| EFAMON | 452 | 10,949.0 mg kg−1 (8115.5–14,771.8) | 1.93 ± 0.20 | 9.39 (5) | 0.094 | Pre-commercial |
| ESAM | 672 | 1882.0 mg kg−1 (13,486.0–3830.0) 2 | 3.44 ± 0.64 | 9.24 (4) | 0.838 | Pre-commercial |
| Anosom® 1 EC, 1 g a.i L−1 (Acetogenin annonin) | 480 | 2959.0 mg kg−1 (2596.0–3504.0) 3 | 3.53 ± 0.41 | 0.78 (2) | 0.160 | Agri Life (Hyderabad, India) |
| Azamax® 1.2 EC, 1.2 g a.i L−1 (Azadirachtin + 3-tigloylazadirachtol) | 442 | 65.42 mg kg−1 (44.28–96.68) | 1.62 ± 0.16 | 8.04 (4) | 0.090 | UPL Brazil (Campinas, Brazil) |
| Premio® SC, 200 g a.i. L−1 (Chlorantraniliprole) | 523 | 0.43 mg kg−1 (0.37–0.50) | 3.28 ± 0.28 | 2.58 (4) | 0.630 | FMC Brazil (Campinas, Brazil) |
| F0 | |||
|---|---|---|---|
| Treatments | 24 h Parasitism (%) 1 | Parasitism Reduction (%) | IOBC/WPRS Classification 2 |
| Control | 19.19 ± 0.72 a | ||
| EFAMON | 7.28 ± 1.25 b | 62.02 | 2 |
| ESAM | 0.04 ± 0.05 d | 99.76 | 4 |
| Anosom® | 1.12 ± 0.36 cd | 94.13 | 3 |
| Azamax® | 1.46 ± 0.52 c | 92.36 | 3 |
| Premio® | 13.77 ± 1.34 a | 28.21 | 1 |
| F1 | ||||
|---|---|---|---|---|
| Treatments | 24 h Emergence (%) 1 | Emergence Reduction (%) | IOBC/WPRS Classification 2 | Sex Ratio |
| Control | 83.23 ± 2.64 ab | 0.64 ± 0.03 | ||
| EFAMON | 87.24 ± 3.45 a | 9.30 | 1 | 0.65 ± 0.07 |
| ESAM | - | - | - | - |
| Anosom® | 61.39 ± 11.08 b | 25.17 | 1 | 0.77 ± 0.12 |
| Azamax® | 70.89 ± 10.93 ab | 13.59 | 1 | 0.69 ± 0.26 |
| Premio® | 85.26 ± 2.33 ab | 0.00 | 1 | 0.54 ± 0.12 |
| F1 | |||
|---|---|---|---|
| Treatments | 24 h Parasitism (%) 1 | Parasitism Reduction (%) | IOBC/WPRS Classification 2 |
| Control | 22.08 ± 3.39 a | - | - |
| EFAMON | 21.91 ± 3.29 a | 0.80 | 1 |
| Anosom® | 22.57 ± 3.34 a | 29.38 | 1 |
| Azamax® | 22.97 ± 2.30 a | 0.00 | 1 |
| Premio® | 16.93 ± 3.26 a | 23.34 | 1 |
| F2 | ||||
|---|---|---|---|---|
| Treatments | 24 h Emergence (%) 1 | Emergence Reduction (%) | IOBC/WPRS Classification 2 | Sex Ratio |
| Control | 83.55 ± 4.06 a | - | - | 0.62 ± 0.06 |
| EFAMON | 73.65 ± 5.33 a | 11.86 | 1 | 0.69 ± 0.04 |
| ESAM | - | - | - | - |
| Anosom® | 69.09 ± 8.16 a | 17.31 | 1 | 0.60 ± 0.16 |
| Azamax® | 81.37 ± 3.82 a | 2.62 | 1 | 0.82 ± 0.13 |
| Premio® | 79.20 ± 4.74 a | 5.22 | 1 | 0.81 ± 0.19 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santana, E.D.R.; Thiesen, L.V.; Ribeiro, L.d.P.; Takahashi, T.A.; Parra, J.R.P.; Yamamoto, P.T. Harmful to Parents, Harmless to Offspring: Lethal and Transgenerational Effects of Botanical and Synthetic Insecticides on the Egg Parasitoid Trichogramma atopovirilia. Insects 2025, 16, 493. https://doi.org/10.3390/insects16050493
Santana EDR, Thiesen LV, Ribeiro LdP, Takahashi TA, Parra JRP, Yamamoto PT. Harmful to Parents, Harmless to Offspring: Lethal and Transgenerational Effects of Botanical and Synthetic Insecticides on the Egg Parasitoid Trichogramma atopovirilia. Insects. 2025; 16(5):493. https://doi.org/10.3390/insects16050493
Chicago/Turabian StyleSantana, Emile Dayara Rabelo, Leonardo Vinicius Thiesen, Leandro do Prado Ribeiro, Tamara Akemi Takahashi, José Roberto Postali Parra, and Pedro Takao Yamamoto. 2025. "Harmful to Parents, Harmless to Offspring: Lethal and Transgenerational Effects of Botanical and Synthetic Insecticides on the Egg Parasitoid Trichogramma atopovirilia" Insects 16, no. 5: 493. https://doi.org/10.3390/insects16050493
APA StyleSantana, E. D. R., Thiesen, L. V., Ribeiro, L. d. P., Takahashi, T. A., Parra, J. R. P., & Yamamoto, P. T. (2025). Harmful to Parents, Harmless to Offspring: Lethal and Transgenerational Effects of Botanical and Synthetic Insecticides on the Egg Parasitoid Trichogramma atopovirilia. Insects, 16(5), 493. https://doi.org/10.3390/insects16050493







