Simple Summary
It is critical that we identify new eco-friendly methods of controlling spotted wing drosophila (SWD), a destructive fruit and berry crop pest. This study evaluated RNAi insecticides that specifically turned off an essential SWD gene. The RNAi insecticides were produced and delivered to flies in baker’s yeast that was mixed with soda. The yeast effectively killed SWD, but did not impact other insects. These insecticides may represent a new tool for controlling SWD.
Abstract
Spotted wing drosophila (SWD), Drosophila suzukii (Diptera: Drosophilidae), are invasive vinegar flies of East Asian origin that are an increasingly global threat to the small fruit industry. It is essential that new classes of eco-friendly insecticides and cost-effective strategies for SWD control are developed. Here, we describe the preparation of a strain of RNA interference (RNAi) Saccharomyces cerevisiae expressing shRNA that specifically targets the SWD RNA-binding Fox protein 1 (Rbfox1) gene. The yeast effectively silences the SWD Rbfox1 gene, resulting in significant loss of fly neural activity. Laboratory trials demonstrated that the RNAi yeast can be mixed with soda, which functions as SWD attractive targeted sugar bait (ATSB) that can be delivered in a soda bottle feeder. The ATSB, mixed with yeast that was heat-killed prior to suspension in the ATSB, resulted in 92 ± 1% mortality of SWD flies that consumed it, yet had no impact on non-target dipterans. Rbfox.687 yeast delivered in ATSB feeders may one day be a useful component of integrated SWD control programs.
1. Introduction
Drosophila suzukii are vinegar flies of East Asian origin that have wreaked havoc on the small fruit industry worldwide [1,2]. Modeling predicts that D. suzukii will likely be broadly present in the United States, southeast Asia, Australia, and Europe, with considerable expansion of its range in the northern hemisphere [3]. SWD impact most berry crops, cherries, grapes, and other tree fruits [4]. The flies oviposit within ripe fruits, where the larvae hatch and compromise fruit integrity [1]. SWD complete multiple generations in a single year [1]. In locations where SWD are well established, weekly insecticide applications are necessary. These weekly applications result in increased costs and unwanted harm to non-targeted organisms [5]. Calendar spray programs that use broad-spectrum insecticides, which employ organophosphates and pyrethroids, are common among berry farmers [6,7]. With increased insecticide treatments, it was recently demonstrated that there is potential for the emergence of resistance to organophosphates and pyrethroids in this species [8,9,10]. It is therefore critical to identify new classes of pesticides and technologies for controlling SWD.
RNAi is often used to characterize gene function in the laboratory. Although it has generated interest in the insect control realm, few have successfully translated it from the bench to the field [11]. RNAi pesticides targeting neural genes in multiple species of vector mosquitoes were recently generated [12,13]. These mosquito-specific RNAi insecticides were designed to target nucleotide stretches conserved in mosquitoes but not in any other organisms, including humans. For example, an RNAi yeast insecticide was designed to target a conserved site in the mosquito Rbfox1 gene [13]. Rbfox1 genes, which are also referred to as Ataxin 2-binding protein genes, encode an evolutionarily conserved RNA-binding protein that functions in many different biological processes. Rbfox1 protein binds to UGCAUG motifs located in pre-mRNA introns, 3′ UTRS, and pre-miRNA hairpins; this enables the regulation of splicing, mRNA stability, translation, and miRNA processing by Rbfox1 proteins [14,15]. Rbfox1 also promotes ribonucleoprotein granule formation and the survival of cells [16]. In the mosquito Aedes aegypti, silencing of the Rbfox1 gene resulted in high levels of mosquito mortality. The mortality correlated with severe defects in neural activity within the mosquito brain, in which Rbfox was shown to be a positive regulator of Notch signaling. The insecticidal impacts of Rbfox1 silencing were subsequently confirmed in trials conducted on additional human disease vector species, including Aedes albopictus, Anopheles gambiae, and Culex quinquefasciatus [13].
The RNAi insecticides characterized in mosquitoes consisted of short hairpin RNA (shRNA) molecules that were expressed in baker’s yeast, S. cerevisiae, which is consumed by mosquitoes, resulting in silencing of the target neural genes [12,13]. The use of S. cerevisiae enables inexpensive and scalable preparation of the insecticides, and the insecticidal activity of the yeast is maintained when it is heat-killed and dried [17]. Moreover, the yeast can be delivered to mosquitoes as the active ingredient in ATSBs [18], which are presently being assessed for the control of vector insect pests [19,20,21,22]. These insecticidal baits capitalize on the natural sugar-feeding behavior of insects that are attracted to feed on a sugar source laced with a poison [23]. In recent studies, ATSBs targeting mosquitoes were mixed with RNAi yeast [24]. Characterization of the RNAi yeast ATSBs against mosquitoes in both laboratory and semi-field settings has demonstrated the efficacy and biorationality of these pesticides [24]. Several of the targeted genes, which function in the nervous system [24], have orthologues in D. suzukii. Recent studies have shown that baker’s yeast, S. cerevisiae, is an effective attractant of major agricultural pests [25,26]. Moreover, sucrose is known to improve insecticide activity against D. suzukii [27]. Combined, these findings support the hypothesis that species-specific RNAi yeast ATSBs targeting SWD can be generated. Here, we describe the development and characterization of an RNAi yeast ATSB targeting the SWD Rbfox1 gene.
2. Materials and Methods
2.1. Insect Rearing
A D. suzukii strain established from a local Michigan collection was obtained from Juliana Wilson (Michigan State University, East Lansing, MI, USA). The flies were reared at 26 °C and maintained in bottles containing Nutri-Fly® BF media (Genesee Scientific, El Cajon, CA, USA).
2.2. Yeast Engineering and Culturing
An RNAi yeast strain expressing shRNA targeting the D. suzukii Rbfox1 gene (NCBI reference sequence: XR_010654136) target site 5′-CCATTGGCGATACTATCCAATCCGG-3′ was prepared as previously described [17]. The S. cerevisiae CEN.PK yeast strain (genotype MATa/α ura3-52/ura3-52 trp1-289/trp1-289 leu2-3_112/leu2-3_112 his3 Δ1/his3 Δ1 MAL2-8C/MAL2-8C SUC2/SUC2) [28] was transformed with a pRS426GPD plasmid [29] containing the Rbfox1 shRNA expression cassette that was prepared as described [30] and selected through growth on a media-lacking uracil. This strain will hereafter be referred to as Rbfox.687. A control yeast strain expressing shRNA with no known target in SWD [17] was also prepared for control studies. Yeast was cultured and heat-killed upon harvesting as described [17] and then lyophilized with 0.025% benzoic acid, which was added as a preservative.
2.3. RNAi Sugar Feeding Assays
Petri dish assays: 3–4 day-old flies were transferred into an empty bottle and starved for 4–5 h. Following the starvation period, the bottle of flies was placed on ice for 15–20 min. A total of 100 µL of a sugar bait–yeast mixture was prepared by mixing 10% sucrose solution (ASB) with 4.5% red food coloring and 40 mg of control RNAi or treatment yeast using a sterile toothpick. Four ~25 µL drops of the ASB + yeast mixture were placed on a Petri dish. Following anesthetization on ice, 25 flies (a mixture of males and females) were placed in the Petri dish, which was subsequently covered with a lid. Flies were permitted to feed overnight in the RNAi yeast–sugar feeding assay, which was conducted at room temperature (~21 ± 1 °C). Feeding was verified in flies by confirming the presence of red food dye in the fly abdomens. Following the overnight feeding, flies were again placed on ice during transfer to a fresh food bottle, in which behavioral phenotypes and survival were evaluated for the next six days.
Dose–response curves: Dose–response assays were performed as previously described [17] by assessing different concentrations of Rbfox.687, which were prepared with varying amounts of control and Rbfox.687 yeast. Each concentration of Rbfox.687 was tested on 25 individual flies per treatment in a Petri dish, which had been prepared as described above. Microsoft Excel 365 software was used to graph the data, which were evaluated through probit analyses conducted using SPSS 25 software (IBM, Armonk, NY, USA).
Yeast endless soda (YES) feeder assays: A feeder system was prepared as described [31] using MUDUODUO automatic bird drinker cups (Amazon, Seattle, WA, USA) that were modified to create the ATSB feeders. A small piece of dehumidifier filter (Honeywell Home, Charlotte, NC, USA) lacking the metal layer was placed in the channel between the reservoir and feeding area. The channel as well as the reservoir were wrapped with parafilm to prevent leakage. The parafilm also prevented the escape of flies into the reservoir and subsequent drowning. To prepare the feeder side, pieces of filter were cut into a 3.3 cm × 7 cm rectangle and folded into a circle. 5 µm (bottom) and 90 µm (top) nylon membranes were placed on top. 200 mg of either control or treatment yeast was mixed with 350 µL of degassed/flat Coca-ColaR, creating a paste that was placed between the membrane layers. At the other end of the feeder, a 12 fl oz soda bottle containing 110 mL of degassed temperature-equilibrated Coca-ColaR and 10 mL/L Tegosept anti-mold agent (Thermofisher Scientific, Waltham, MA, USA) was inverted to serve as a continuous supply of soda sugar bait. For feeding assays, the feeder and 50 3–4 day-old sugar-starved flies were placed in insect cages that were placed in the insectary, where they were monitored for six days, with mortality recorded daily. These data were tabulated and graphed using Microsoft Excel 365 software and evaluated using one-way ANOVA statistics and a Tukey’s post hoc test performed with SPSS 25 (IBM, Armonk, NY, USA).
2.4. Confirmation of Rbfox1 Silencing
Rbfox1 target gene silencing was confirmed through qRT-PCR assays that were performed as described [32]. Fly heads were collected 72 h after ATSB feedings (control or treatment). Total head RNA was extracted from 20 females using the Trizol reagent (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s instructions. The RNA was subsequently treated with DNase I (using the DNA-free kit, Invitrogen, Thermo Fisher Scientific, Waltham, MA, USA) per the manufacturer’s instructions. cDNA was prepared from RNA using the HighCapacity RNA to cDNA Kit (Applied Biosystems, Foster City, CA, USA) according to the manufacturer’s instructions. Real-time PCR quantification assays were performed using a CFX Opus 96 Real-time PCR System (Bio-Rad) using the Power SYBR Green PCR Master Mix as described by the manufacturer (Bio-Rad, Hercules, CA, USA). To amplify the Rbfox1 gene, the following primer sets were used in these reactions: forward 5′-CCCACCACCGGATTGGATAG-3′ and reverse: 5′-GCGGAACGGTATGTTGGAGA-3′. Amplification of alpha tubulin, an internal standard for data normalization, was performed as described [32] with the following primers: forward 5′-AGGATGCGGCGAATAACT-3′ and reverse 5′ CGGTGGATAGTCGCTCAA 3′. PCR amplifications were performed in six replicate wells in each of two separate biological replicate trials, and results were quantified by standardizing reactions to alpha tubulin transcript levels using the ΔΔCt method as described [32]. Data were statistically evaluated using Student’s t-test.
2.5. Evaluation of Yeast Toxicity to Non-Target Insects
The effects of Rbfox.687 yeast feedings on non-target insects were analyzed in Aedes aegypti (Liverpool-IB12), Anopheles stephensi STE2 (obtained through BEI Resources, Manassas, VA, USA, NIAID, NIH: A. stephensi strain STE2, MRA-128, contributed by William E. Collins), Culex quinquefasciatus JHB (provided by the Centers for Disease Control and Prevention for distribution by BEI Resources, Manassas, VA, USA, NIAID, NIH: Eggs, NR-43025), Drosophila melanogaster, and Pogonomyrmex barbatus (Carolina Biologicals, Burlington, NC, USA). These insects were selected because cultures were concurrently being maintained in the lab during the course of the SWD study. In these trials, yeast–sugar feeding assays were performed as previously described [13]. The same preparation of yeast was used for all trials. For a positive control, the same yeast was shown to kill SWD in concurrent assays.
2.6. Immunohistochemistry Studies
The following reagents were used for immunohistochemical staining of adult D. suzukii brains, which were performed as previously described [33,34]: anti-HRP (Jackson ImmunoResearch Labs, West Grove, PA, USA), mAb nc82 anti-Bruchpilot [35] (DSHB, Iowa City, IA, USA, Iowa Hybridoma Product nc82 deposited by E. Buchner), and TO-PRO-3 iodide (Molecular Probes, Eugene, OR, USA). Immunohistochemical assays were performed in triplicate, and processed tissues were mounted and imaged with a Zeiss 710 confocal microscope. Confocal images of the fly brains were analyzed with FIJI ImageJ version 2.16.0/1.54p, [36] and Adobe Photoshop 2025 software. For quantification of signal intensities, mean gray values (average signal intensity over the selected area) were calculated as described [12] and statistically analyzed using a Student’s t-test.
3. Results and Discussion
3.1. SWD-Specific Insecticidal Activity of Rbfox.687 Yeast
A recent study demonstrated that consumption of yeast targeting a site conserved in mosquito Rbfox1 genes resulted in loss of Rbfox1 transcripts and mosquito mortality [13]. It was therefore hypothesized that RNAi yeast Rbfox.687, which was designed to specifically target a 25 bp sequence present in the D. suzukii Rbfox1 gene, would silence the Rbfox1 gene and lead to fly death. To test the hypothesis, heat-inactivated dried Rbfox.687 yeast was mixed with sucrose and fed to SWD in sugar feeding assays conducted in Petri dishes. As predicted, 72% silencing of Rbfox1 transcripts was observed in the brains of flies that had consumed Rbfox.687 yeast (Figure 1A, p < 0.001 vs. control yeast-treated flies). Significant SWD mortality was observed in flies that had consumed Rbfox.687 (Figure 1B, p < 0.001 vs. sugar bait only or control yeast-treated flies, which survived), with death observed over a six day period (Figure 1C). Dose–response assays revealed an LD50 of 166 μg/μL for Rbfox.687 yeast (Figure 1D). The high levels of SWD mortality observed suggest that the yeast-mediated delivery of insecticidal shRNA can overcome the activity of dsRNAses that are present in the D. suzukii gut, which are known to reduce RNAi efficacy in SWD [37]. These results suggest that RNAi yeast insecticides may prove to have higher field efficacy than other RNAi insecticides.
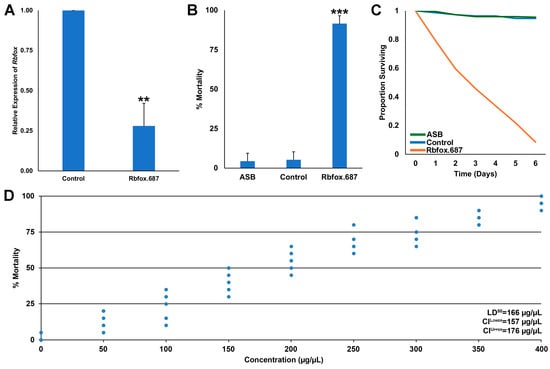
Figure 1.
Rbfox.687 yeast functions as the active ingredient in an ATSB that silences the Rbfox1 gene and kills SWD. (A) qRT-PCR confirmed that the SWD Rbfox1 gene was silenced in the brains of adult flies that consumed Rbfox.687 yeast (** = p < 0.01 vs. control, Student’s t-test). (B) Laboratory trials demonstrated that consumption of Rbfox.687 yeast resulted in significant mortality of SWD flies (*** = p < 0.001 vs. control, Student’s t-test). (C) The corresponding survival curve for these data are shown. Panels (B,C) were compiled from nine replicate trials for each treatment, each of which contained 25 adults. The error bars represent the standard deviation (SD) in (A) and the standard error of the mean (SEM) in (B). (D) Dose-dependent mortality was observed in D. suzukii, with an LD50 of 166 µg/µL; the data shown were compiled from 10 replicate trials (each with 25 flies) for each of the nine different concentrations of yeast.
RNAi insecticides, including Rbfox.687, that use shRNA as the interfering RNA species facilitate precision pest control. For example, the Rbfox insecticide trialed in mosquitoes was designed to match a 25 bp target site conserved in a variety of vector mosquitoes but not found in other genomes; the insecticide killed A. aegypti, A. gambiae, and C. quinquefasciatus, but it did not kill non-targeted insect species [13]. Similarly, the Rbfox.687 yeast was designed to target a short 25 bp site found in D. suzukii but which is not present in mosquitoes and other organisms. NCBI Blast searches failed to detect identical 25 bp target sites in other sequenced genomes. Consequently, although Rbfox.687 kills D. suzukii, sugar feeding trials demonstrated that the yeast did not kill A. aegypti, A. stephensi, C. quinquefasciatus, D. melanogaster, or P. barbatus that consumed Rbfox.687 yeast (p > 0.05 vs. control-yeast treated SWD, Table 1). This lack of toxicity to non-target species, which is enhanced by the use of a very short 25 bp target sequence, would support future regulatory applications. Furthermore, the use of heat-killed dried yeast distinguishes this yeast technology from other systems previously developed for D. suzukii, which used live yeast and targeted long stretches of RNA [38]. This distinction can enable classification of the Rbfox.687 insecticide as a dead microbe. The species-specificity of the dead microbial insecticide is likely to facilitate registry of RNAi yeast insecticides with regulatory authorities.

Table 1.
Rbfox.687 yeast is not toxic to non-target arthropods. Consumption of Rbfox.687 yeast by the indicated non-target insects had no significant impact on insect survival (p > 0.05, Student’s t-test).
3.2. Silencing of Rbfox1 Results in Loss of Neural Activity in the Brain and Behavioral Phenotypes in Adult Flies
In adult mosquitoes, consumption of yeast corresponding to the Rbfox1 gene resulted in loss of neural activity in the adult brain [13]. Based on these results, it was hypothesized that silencing of Rbfox1 in D. suzukii would result in comparable neural defects. Treatments with Rbfox.687 yeast resulted in a reduction in nc82 levels in the adult SWD brain (Figure 2A1 vs. Figure 2B1; green), which correlates well with the loss of the Rbfox1 transcript observed in the fly head (Figure 1A). Although levels of Bruchpilot, a marker of active synapses [35] that are labeled with the nc82 antibody, as well as HRP levels (Figure 2A2 vs. Figure 2B2) were significantly reduced in flies that consumed the insecticidal yeast, no significant difference in TO-PRO nuclear staining levels (Figure 2A3 vs. Figure 2B3) was observed in the fly brain (Figure 2C; p > 0.05). These results, which were similar to those observed in A. aegypti [13], suggested that loss of nc82 signal likely results from a reduction in neural activity rather than loss of neural density.
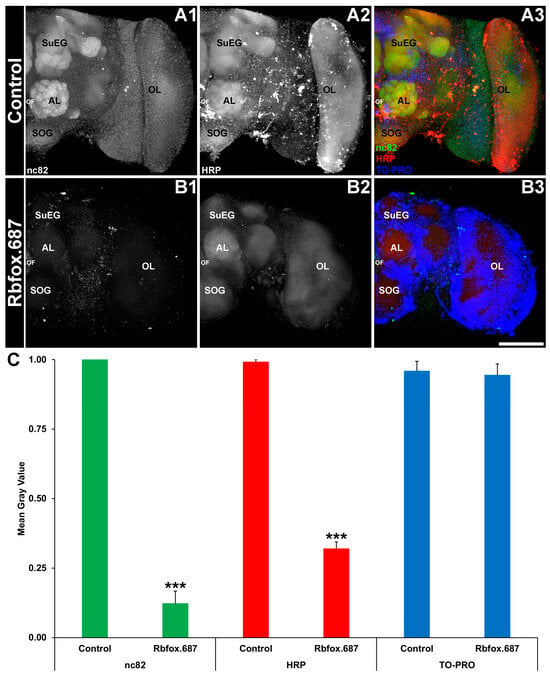
Figure 2.
Neural defects are detected in SWD that consumed Rbfox.687 yeast ATSB. Adult brains prepared from flies that consumed control (A1–A3) or Rbfox.687 (B1–B3) yeast were labeled with mAbnc82 (marker for active synapses; white in (A1,B1), green in (A3,B3)), anti-HRP (neural marker; white in (A2,B2), red in (A3,B3)) and TO-PRO (nuclear stain; blue in (A3,B3)). Although nc82 and HRP levels were significantly reduced in Rbfox.687-treated brains (C); *** p < 0.001 vs. control), no significant levels of nuclear staining were detected (C, p > 0.05). The data in panel (C) are shown as average mean gray values with error bars denoting the SEM and were analyzed with Student’s t-test. Representative larval brains are oriented dorsal upward and labeled as follows: AL, larval antennal lobe; OF, esophageal foramen; OL, optic lobe; SOG, sub-esophageal ganglion; and SuEG, supraesophageal ganglion. Scale Bar = 100 μm. N = 75 brains/treatment from three replicate trials.
The silencing of SWD Rbfox 1 (Figure 1A) and neural deficits (Figure 2) correlated with a locomotor defect and the inability to fly. Although flies can survive for up to six days following yeast treatment in the laboratory, it is likely that they would die sooner in field assays in which they were susceptible to predation or exposed to outdoor elements. This was the case for mosquitoes that had locomotor defects resulting from Shaker gene silencing, which died within one day following yeast treatment in semi-field experiments conducted outdoors in Trinidad and in Thailand [24].
3.3. An ATSB Station for Delivery of Insecticidal RNAi Yeast
The use of RNAi yeast insecticides for SWD control would require the development of means for delivering the insecticides in the field. Coca-colaR is known to be an excellent sugar bait attractant for Aedes japonicus [39], and we have recently begun to explore the use of Coca-ColaR as a sugar bait in a variety of insects, including SWD [31]. Recent laboratory trials demonstrated that yeast could be delivered to insects in a feeder composed of a soda bottle attached to a reservoir containing yeast, which had been treated with a mold inhibitor [31]. The bottle feeder, which is shown in Figure 3A, was constructed so that the yeast, which was covered by a membrane, was constantly rewetted, thereby preventing yeast drying. Rbfox.687 yeast was mixed with Coca-ColaR and supplied to SWD in the soda bottle feeders that continuously rewetted the yeast, which was consumed by the flies through a membrane (Figure 3B). Rbfox.687 yeast delivered to SWD in this manner resulted in significant SWD mortality (92 ± 1%, Figure 3C,D, p < 0.001 vs. soda only or soda + control yeast-treated flies).
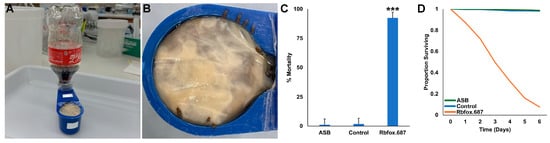
Figure 3.
Rbfox.687 yeast and soda delivered in a soda bottle feeder induced significant SWD mortality. (A) The soda bottle feeder system is shown. (B) SWD feeding on the yeast-soda mixture were observed. (C) Significant mortality was observed in D. suzukii that drank from a soda bottle ATSB feeder prepared with Coca-ColaR and Rbfox.687 yeast (*** p < 0.001 vs. flies that fed on soda alone or soda prepared with control yeast, ANOVA). (D) The flies died over a six day period. Data compiled from nine replicate trials for each treatment, each with 50 adults are shown in (C,D).
The results of these simulated field soda bottle feeder assays are promising. It will be important to next investigate the efficacy of RNAi yeast soda and bottle feeders in the field. The results of such trials will be critical for determining when and where to place the feeders and whether the soda–yeast system can effectively compete against natural SWD sugar sources. During the cold season in temperate zones, the adult population is composed primarily of flies that are active during daytime hours, when temperature permits, and reside within areas containing wild vegetation [40]. Although the source of food during the cold season is presently unknown for SWD, it is likely that sugar source availability is restricted. It may therefore be useful to deploy ATSB feeders toward the end of the cold season, when temperatures are warmer, but before fruit crops are available. Recent studies have demonstrated that winter morphs, which are thought to be the main source for infestations of fruit crops [41], are highly attracted to yeast, which could be beneficial [42]. Insecticide sprays are frequently used for SWD control [40], suggesting that the development of RNAi yeast spray formulations might also be of interest. Such sprays have been developed for bacterial larvicides [43]. SWD primarily come into contact with insecticides sprayed on fruits and foliage where they feed, oviposit, and develop to maturity [40], so it would be important to evaluate the best areas to deploy RNAi yeast spray insecticides if such sprays were to be developed.
If the efficacy of RNAi yeast insecticides is demonstrated, the use of this technology could benefit SWD integrated pest management (IPM) programs, which presently rely heavily on the use of chemical insecticides [6,40]. The introduction of RNAi yeast-dead microbial pesticides to the current repertoire could potentially help reduce chemical inputs while providing an SWD-specific pesticide that would be safer for non-target organisms. Moreover, this dead microbial pesticide could potentially be of interest to organic fruit farmers, who have been particularly hard hit by the invasion of SWD due to the limited ability of effective insecticides that can be used to protect organic crops [40].
The cost of RNAi insecticides will of course also be a critical factor [40]. The use of yeast is expected to significantly reduce the cost of RNAi-based interventions. Recent studies demonstrated that commercial-ready RNAi yeast could be produced at pilot scale with no indication that special media, which increase prices substantially, would be necessary, suggesting that the yeast could be produced at competitive prices [44]. The Rbfox.687 laboratory yeast strain used in the present study is not suitable for scaled fermentation. For scaling, it would be useful to integrate multiple copies of the Rbfox.687 shRNA expression construct into a commercial yeast strain. Cas-CLOVER was recently used to produce such yeast strains, in which shRNA production levels increased ~30 fold in pilot scaled fermentations [44].
Inexpensive scaled production of insecticidal yeast would facilitate further evaluation of RNAi yeast efficacy when deployed in YES feeders. In a recent study [45], ATSB stations deployed on household structures for mosquito control in Western Zambia were often damaged. These findings underline the importance of assessing whether the YES feeders or other potential deployment systems are suitable for use in agricultural settings and how often the feeders might need to be replaced. Potential agricultural stakeholders should also have the opportunity to voice any concerns regarding the use of this novel technology and to make any recommendations for improvement. When community acceptance of the ATSB stations for mosquito control was evaluated in Zambia, stakeholders accepted the novel mosquito control intervention [46]. The demonstration of similar levels of acceptance of the RNAi yeast/YES feeders in conjunction with significantly reduced SWD densities in multiple agricultural settings would be ideal. ATSB stations significantly reduced the incidence of malaria mosquito vectors in Mali [21,22], suggesting that similarly reduced densities of SWD might be achieved. The ATSB used in Mali was shown to effectively compete with natural nectar sources [47], suggesting that the intervention has the capacity to reduce mosquito densities following deployment. In a related mosquito ATSB study conducted in Western Zambia, ATSB feeding rates in Anopheles funestus mosquitoes were consistent with those expected to reduce malaria transmission; however, vector light trapping densities were not significantly reduced [48]. Consistent with these findings, the deployment of two ATSB stations per household in rural Western Zambia did not result in a statistically significant reduction in clinical malaria incidences among children [20]. The authors concluded that further research is needed to optimize the impact of ATSB deployment for vector control in Zambia and other settings [20]. Thus, future research endeavors to assess the efficacy of RNAi yeast/YES feeders for SWD control should be performed in multiple agricultural contexts in various crop and climate settings.
4. Conclusions
The results of these studies indicate that Rbfox.687, a species-specific RNAi yeast insecticide that targets D. suzukii, can serve as a highly toxic component of an ATSB that effectively kills SWD, yet has no impact on non-target insects. Rbfox.687 yeast ATSB silences the D. suzukii Rbfox1 gene, resulting in neural defects in the adult SWD brain. The Rbfox.687 yeast was delivered in a soda bottle feeder that effectively killed D. suzukii under simulated field conditions. RNAi yeast insecticides represent a new class of effective, species-specific biorational insecticides that could one day become an important component of integrated pest management programs for SWD control following field studies designed to optimize the impacts of RNAi yeast-ATSB deployment in a variety of agricultural contexts.
Author Contributions
Individual author contributions were as follows: conceptualization, A.T.M.S., K.M., L.S., M.D.-S. and M.H.-A.; methodology, A.T.M.S., K.M., L.S., M.D.-S. and M.H.-A.; validation, J.G., K.M., L.S., M.H.-A., S.N. and T.M.N.; formal analysis, K.M., M.H-A, M.D.-S., S.N. and T.M.N.; investigation, J.G., K.M., M.H-A., S.N. and T.M.N.; writing—original draft preparation, K.M. and M.D.-S.; writing—review and editing, K.M., T.M.N., M.H-A. and A.T.M.S.; visualization, K.M.; supervision, A.T.M.S., K.M., T.M.N., M.H-A. and M.D.-S.; project administration, M.D.-S.; funding acquisition, M.D.-S. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by the Regents of the University of Minnesota as a subaward (H011114529 to M.D.S.) from their United States Department of Agriculture North Central Sustainable Agriculture Research and Education (NCR-SARE) award 2023-38640-39573.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Acknowledgments
Juliana Wilson provided the SWD. We thank Britton Sofhauser for technical assistance.
Conflicts of Interest
The funders of this investigation had no role in the design of the study, in the collection, analyses, or interpretation of data, in the writing of the manuscript; or in the decision to publish the results. M.D.S. and K.M. are inventors on a pending U.S. patent application related to this work, which was filed by Indiana University; however, this did not impact their analysis of the data described herein or the decision to publish it. The other authors have no competing interests to declare.
Abbreviations
The following abbreviations are used in this manuscript:
| ATSB | Attractive targeted sugar bait |
| IPM | Integrated pest management |
| RNAi | RNA interference |
| Rbfox1 | RNA Binding Fox-1 Homolog |
| SD | Standard deviation |
| SEM | Standard error of the mean |
| shRNA | Short hairpin RNA |
| SWD | Spotted-wing drosophila |
| YES | Yeast endless soda |
References
- Reyes, J.A.; Lira-Noriega, A. Current and future global potential distribution of the fruit fly Drosophila suzukii (Diptera: Drosophilidae). Can. Entomol. 2020, 152, 587–599. [Google Scholar] [CrossRef]
- Walsh, D.B.; Bolda, M.P.; Goodhue, R.E.; Dreves, A.J.; Lee, J.; Bruck, D.J.; Walton, V.M.; O’Neal, S.D.; Zalom, F.G. Drosophila suzukii (Diptera: Drosophilidae): Invasive pest of ripening soft fruit expanding its geographic range and damage potential. J. Integr. Pest Manag. 2011, 2, G1–G7. [Google Scholar] [CrossRef]
- Asplen, M.K.; Anfora, G.; Biondi, A.; Choi, D.-S.; Chu, D.; Daane, K.M.; Gibert, P.; Gutierrez, A.P.; Hoelmer, K.A.; Hutchison, W.D.; et al. Invasion biology of spotted wing Drosophila (Drosophila suzukii): A global perspective and future priorities. J. Pest Sci. 2015, 88, 469–494. [Google Scholar] [CrossRef]
- Cai, P.; Song, Y.; Yi, C.; Zhang, Q.; Xia, H.; Lin, J.; Zhang, H.; Yang, J.; Ji, Q.; Chen, J. Potential host fruits for Drosophila suzukii: Olfactory and oviposition preferences and suitability for development. Entomol. Exp. Appl. 2019, 167, 880–890. [Google Scholar] [CrossRef]
- Sarkar, N.; Rhodes, E.M.; Spies, J.; Roubos, C.R.; Little, B.A.; Sial, A.A.; Fanning, P.D.; Isaacs, R.; Liburd, O.E. Evaluation of non-target effects of OMRI-listed insecticides for management of Drosophila suzukii Matsumura in berry crops. J. Appl. Entomol. 2020, 144, 12–25. [Google Scholar] [CrossRef]
- Diepenbrock, L.M.; Rosensteel, D.O.; Hardin, J.A.; Sial, A.A.; Burrack, H.J. Season-long programs for control of Drosophila suzukii in southeastern US blueberries. Crop Prot. 2016, 81, 76–84. [Google Scholar] [CrossRef]
- Fanning, P.; Grieshop, M.; Isaacs, R. Efficacy of biopesticides on spotted wing drosophila, Drosophila suzukii Matsumura in fall red raspberries. J. Appl. Entomol. 2017, 142, 26–32. [Google Scholar] [CrossRef]
- Ganjisaffar, F.; Demkovich, M.R.; Chiu, J.C.; Zalom, F.G. Characterization of field-derived Drosophila suzukii (Diptera: Drosophilidae) Resistance to Pyrethroids in California Berry Production. J. Econ. Entomol. 2022, 115, 1676–1684. [Google Scholar] [CrossRef]
- Rijal, J. Tackling Pesticide resistance of spotted wing drosophila in cherries. Progress. Crop Consult. 2023, 25, 2023. [Google Scholar]
- Disi, J.O.; Sial, A.A. Laboratory selection and assessment of resistance risk in Drosophila suzukii (Diptera: Drosophilidae) to Spinosad and Malathion. Insects 2021, 12, 794. [Google Scholar] [CrossRef]
- Airs, P.M.; Bartholomay, L.C. RNA Interference for mosquito and mosquito-borne disease control. Insects 2017, 8, 4. [Google Scholar] [CrossRef]
- Mysore, K.; Hapairai, L.K.; Sun, L.; Li, P.; Wang, C.W.; Scheel, N.D.; Lesnik, A.; Igiede, J.; Scheel, M.P.; Wei, N.; et al. Characterization of a dual-action adulticidal and larvicidal interfering RNA pesticide targeting the Shaker gene of multiple disease vector mosquitoes. PLoS Negl. Trop. Dis. 2020, 14, e0008479. [Google Scholar] [CrossRef] [PubMed]
- Mysore, K.; Sun, L.; Hapairai, L.K.; Wang, C.W.; Roethele, J.B.; Igiede, J.; Scheel, M.P.; Scheel, N.D.; Li, P.; Wei, N.; et al. A broad-based mosquito yeast interfering RNA pesticide targeting Rbfox1 represses Notch signaling and kills both larvae and adult mosquitoes. Pathogens 2021, 10, 1251. [Google Scholar] [CrossRef]
- Conboy, J.G. Developmental regulation of RNA processing by Rbfox proteins. Wiley Interdiscip. Rev. RNA 2017, 8, e1398. [Google Scholar] [CrossRef]
- Gazzara, M.R.; Mallory, M.J.; Roytenberg, R.; Lindberg, J.P.; Jha, A.; Lynch, K.W.; Barash, Y. Ancient antagonism between CELF and RBFOX families tunes mRNA splicing outcomes. Genome Res. 2017, 27, 1360–1370. [Google Scholar] [CrossRef] [PubMed]
- Kucherenko, M.M.; Shcherbata, H.R. Stress-dependent miR-980 regulation of Rbfox1/A2bp1 promotes ribonucleoprotein granule formation and cell survival. Nat. Commun. 2018, 9, 312. [Google Scholar] [CrossRef] [PubMed]
- Hapairai, L.K.; Mysore, K.; Chen, Y.; Harper, E.I.; Scheel, M.P.; Lesnik, A.M.; Sun, L.; Severson, D.W.; Wei, N.; Duman-Scheel, M. Lure-and-kill yeast interfering RNA larvicides targeting neural genes in the human disease vector mosquito Aedes aegypti. Sci. Rep. 2017, 7, 13223. [Google Scholar] [CrossRef]
- Njoroge, T.M.; Hamid-Adiamoh, M.; Duman-Scheel, M. Maximizing the potential of attractive targeted sugar baits (ATSBs) for integrated vector management. Insects 2023, 14, 585. [Google Scholar] [CrossRef]
- Eisele, T.P.; Kleinschmidt, I.; Sarrassat, S.; TerKuile, F.; Miller, J.; Chanda, J.; Silumbe, K.; Samuels, A.; Janssen, J.; Ogwang, C.; et al. Attractive targeted sugar bait phase III trials in Kenya, Mali, and Zambia. Trials 2022, 23, 640. [Google Scholar] [CrossRef]
- Ashton, R.A.; Saili, K.; Chishya, C.; Banda Yikona, H.; Arnzen, A.; Orange, E.; Chitoshi, C.; Chulu, J.; Tobolo, T.; Ndalama, F.; et al. Efficacy of attractive targeted sugar bait stations against malaria in Western Province Zambia: Epidemiological findings from a two-arm cluster randomized phase III trial. Malar. J. 2024, 23, 343. [Google Scholar] [CrossRef]
- Traore, M.M.; Junnila, A.; Traore, S.F.; Doumbia, S.; Revay, E.E.; Kravchenko, V.D.; Schlein, Y.; Arheart, K.L.; Gergely, P.; Xue, R.D.; et al. Large-scale field trial of attractive toxic sugar baits (ATSB) for the control of malaria vector mosquitoes in Mali, West Africa. Malar. J. 2020, 19, 72. [Google Scholar] [CrossRef] [PubMed]
- Traore, M.M.; Junnila, A.; Traore, S.F.; Doumbia, S.; Revay, E.E.; Schlein, Y.; Yakovlev, R.V.; Saldaitis, A.; Cui, L.; Petrányi, G.; et al. The efficacy of attractive targeted sugar baits in reducing malaria vector abundance in low-endemicity settings of northwest Mali. Malar. J. 2024, 23, 319. [Google Scholar] [CrossRef]
- Fiorenzano, J.M.; Koehler, P.G.; Xue, R.D. Attractive toxic sugar bait (ATSB) for control of mosquitoes and its impact on non-target organisms: A review. Int. J. Environ. Res. Public Health 2017, 14, 398. [Google Scholar] [CrossRef] [PubMed]
- Stewart, A.T.M.; Mysore, K.; Njoroge, T.M.; Winter, N.; Feng, R.S.; Singh, S.; James, L.D.; Singkhaimuk, P.; Sun, L.; Mohammed, A.; et al. Demonstration of RNAi yeast insecticide activity in semi-field larvicide and attractive targeted sugar bait trials conducted on Aedes and Culex mosquitoes. Insects 2023, 14, 950. [Google Scholar] [CrossRef]
- Hamby, K.A.; Becher, P.G. Current knowledge of interactions between Drosophila suzukii and microbes, and their potential utility for pest management. J. Pest Sci. 2016, 89, 621–630. [Google Scholar] [CrossRef]
- Becher, P.G.; Hagman, A.; Verschut, V.; Chakraborty, A.; Rozpędowska, E.; Lebreton, S.; Bengtsson, M.; Flick, G.; Witzgall, P.; Piškur, J. Chemical signaling and insect attraction is a conserved trait in yeasts. Ecol. Evol. 2018, 8, 2962–2974. [Google Scholar] [CrossRef]
- Cowles, R.S.; Rodriguez-Saona, C.; Holdcraft, R.; Loeb, G.M.; Elsensohn, J.E.; Hesler, S.P. Sucrose improves insecticide activity against Drosophila suzukii (Diptera: Drosophilidae). J. Econ. Entomol. 2015, 108, 640–653. [Google Scholar] [CrossRef]
- van Dijken, J.P.; Bauer, J.; Brambilla, L.; Duboc, P.; Francois, J.M.; Gancedo, C.; Giuseppin, M.L.; Heijnen, J.J.; Hoare, M.; Lange, H.C.; et al. An interlaboratory comparison of physiological and genetic properties of four Saccharomyces cerevisiae strains. Enzym. Microb. Technol. 2000, 26, 706–714. [Google Scholar] [CrossRef]
- Mumberg, D.; Müller, R.; Funk, M. Yeast vectors for the controlled expression of heterologous proteins in different genetic backgrounds. Gene 1995, 156, 119–122. [Google Scholar] [CrossRef]
- Mysore, K.; Hapairai, L.K.; Wei, N.; Realey, J.S.; Scheel, N.D.; Severson, D.W.; Duman-Scheel, M. Preparation and use of a yeast shRNA delivery system for gene silencing in mosquito larvae. Methods Mol. Biol. 2019, 1858, 213–231. [Google Scholar] [CrossRef]
- Mysore, K.; Graham, J.; Njoroge, T.M.; Stewart, A.T.M.; Nelaturi, S.; Duman-Scheel. Development of an eco-friendly RNAi yeast attractive targeted sugar bait that silences the Shaker gene in spotted-wing drosophila, Drosophila suzukii. bioRxiv 2025. [Google Scholar] [CrossRef]
- Zhai, Y.; Lin, Q.; Zhou, X.; Zhang, X.; Liu, T.; Yu, Y. Identification and validation of reference genes for quantitative real-time PCR in Drosophila suzukii (Diptera: Drosophilidae). PLoS ONE 2014, 9, e106800. [Google Scholar] [CrossRef]
- Clemons, A.; Flannery, E.; Kast, K.; Severson, D.; Duman-Scheel, M. Immunohistochemical analysis of protein expression during Aedes aegypti development. Cold Spring Harb. Protoc. 2010, 2010, pdb.prot5510. [Google Scholar] [CrossRef] [PubMed]
- Mysore, K.; Flister, S.; Muller, P.; Rodrigues, V.; Reichert, H. Brain development in the yellow fever mosquito Aedes aegypti: A comparative immunocytochemical analysis using cross-reacting antibodies from Drosophila melanogaster. Dev. Genes. Evol. 2011, 221, 281–296. [Google Scholar] [CrossRef]
- Wagh, D.A.; Rasse, T.M.; Asan, E.; Hofbauer, A.; Schwenkert, I.; Durrbeck, H.; Buchner, S.; Dabauvalle, M.C.; Schmidt, M.; Qin, G.; et al. Bruchpilot, a protein with homology to ELKS/CAST, is required for structural integrity and function of synaptic active zones in Drosophila. Neuron 2006, 49, 833–844. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2019, 9, 676–682. [Google Scholar] [CrossRef] [PubMed]
- Yoon, J.S.; Ahn, S.J.; Flinn, C.M.; Choi, M.Y. Identification and functional analysis of dsRNases in spotted-wing drosophila, Drosophila suzukii. Arch. Insect Biochem. Physiol. 2021, 107, e21822. [Google Scholar] [CrossRef]
- Murphy, K.A.; Tabuloc, C.A.; Cervantes, K.R.; Chiu, J.C. Ingestion of genetically modified yeast symbiont reduces fitness of an insect pest via RNA interference. Sci. Rep. 2016, 6, 22587. [Google Scholar] [CrossRef]
- Fryzlewicz, L.; VanWinkle, A.; Lahondere, C. Development of an attractive toxic sugar bait for the control of Aedes j. japonicus (Diptera: Culicidae). J. Med. Entomol. 2022, 59, 308–313. [Google Scholar] [CrossRef]
- Tait, G.; Mermer, S.; Stockton, D.; Lee, J.; Avosani, S.; Abrieux, A.; Anfora, G.; Beers, E.; Biondi, A.; Burrack, H.; et al. Drosophila suzukii (Diptera: Drosophilidae): A decade of research towards a sustainable integrated pest management program. J. Econ. Entomol. 2021, 114, 1950–1974. [Google Scholar] [CrossRef]
- Panel, A.D.C.; Zeeman, L.; van der Sluis, B.J.; van Elk, P.; Pannebakker, B.A.; Wertheim, B.; Helsen, H.H.M. Overwintered Drosophila suzukii are the main source for infestations of the first fruit crops of the season. Insects 2018, 9, 145. [Google Scholar] [CrossRef] [PubMed]
- Escobedo-Quevedo, K.; Lankheet, M.J.; Pen, I.; Trienens, M.; Helsen, H.H.M.; Wertheim, B. Studying foraging behavior to improve bait sprays application to control Drosophila suzukii. BMC Ecol. Evol. 2024, 24, 60. [Google Scholar] [CrossRef] [PubMed]
- Bohari, R.; Jin Hin, C.; Matusop, A.; Abdullah, M.R.; Ney, T.G.; Benjamin, S.; Lim, L.H. Wide area spray of bacterial larvicide, Bacillus thuringiensis israelensis strain AM65-52, integrated in the national vector control program impacts dengue transmission in an urban township in Sibu district, Sarawak, Malaysia. PLoS ONE 2020, 15, e0230910. [Google Scholar] [CrossRef]
- Brizzee, C.; Mysore, K.; Njoroge, T.M.; McConnell, S.; Hamid-Adiamoh, M.; Stewart, A.T.M.; Kinder, J.T.; Crawford, J.; Duman-Scheel, M. Targeting mosquitoes through generation of an insecticidal RNAi yeast strain using Cas-CLOVER and super piggyBac engineering in Saccharomyces cerevisiae. J. Fungi 2023, 9, 1056. [Google Scholar] [CrossRef]
- Kyomuhangi, I.; Yukich, J.; Saili, K.; Orange, E.; Masuzyo, M.H.; Mwenya, M.; Mambo, P.; Hamainza, B.; Wagman, J.; Miller, J.; et al. Evaluating trends in damage to attractive targeted sugar baits (ATSBs) deployed during the second year of a two-year Phase III trial in Western Zambia. Malar. J. 2024, 23, 263. [Google Scholar] [CrossRef] [PubMed]
- Orange, E.; Arnzen, A.; Muluma, C.; Akalalambili, S.; Tobolo, T.; Ndalama, F.; Chishya, C.; Saili, K.; Ashton, R.A.; Eisele, T.P.; et al. Community acceptance of a novel malaria intervention, Attractive Targeted Sugar Baits, in the Zambia phase III trial. Malar. J. 2024, 23, 240. [Google Scholar] [CrossRef]
- Yalla, N.; Polo, B.; McDermott, D.P.; Kosgei, J.; Omondi, S.; Agumba, S. A comparison of the attractiveness of flowering plant blossoms versus attractive targeted sugar baits (ATSBs) in western Kenya. PLoS ONE 2023, 18, e0286679. [Google Scholar] [CrossRef]
- Chanda, J.; Wagman, J.; Chanda, C.; Kaniki, T.; Ng’andu, M.; Muyabe, R.; Mwenya, M.; Sakala, J.; Miller, J.; Mwaanga, G.; et al. Feeding rates of malaria vectors from a prototype attractive sugar bait station in Western Province, Zambia: Results of an entomological validation study. Malar. J. 2024, 22, 70. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).