Future Climate Predicts Range Shifts and Increased Global Habitat Suitability for 29 Aedes Mosquito Species
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Retrieving Records of the Target Species
2.2. Variables Included in the Models
2.3. Reducing Collinearity
2.4. Predicting Habitat Suitability and the Ranges
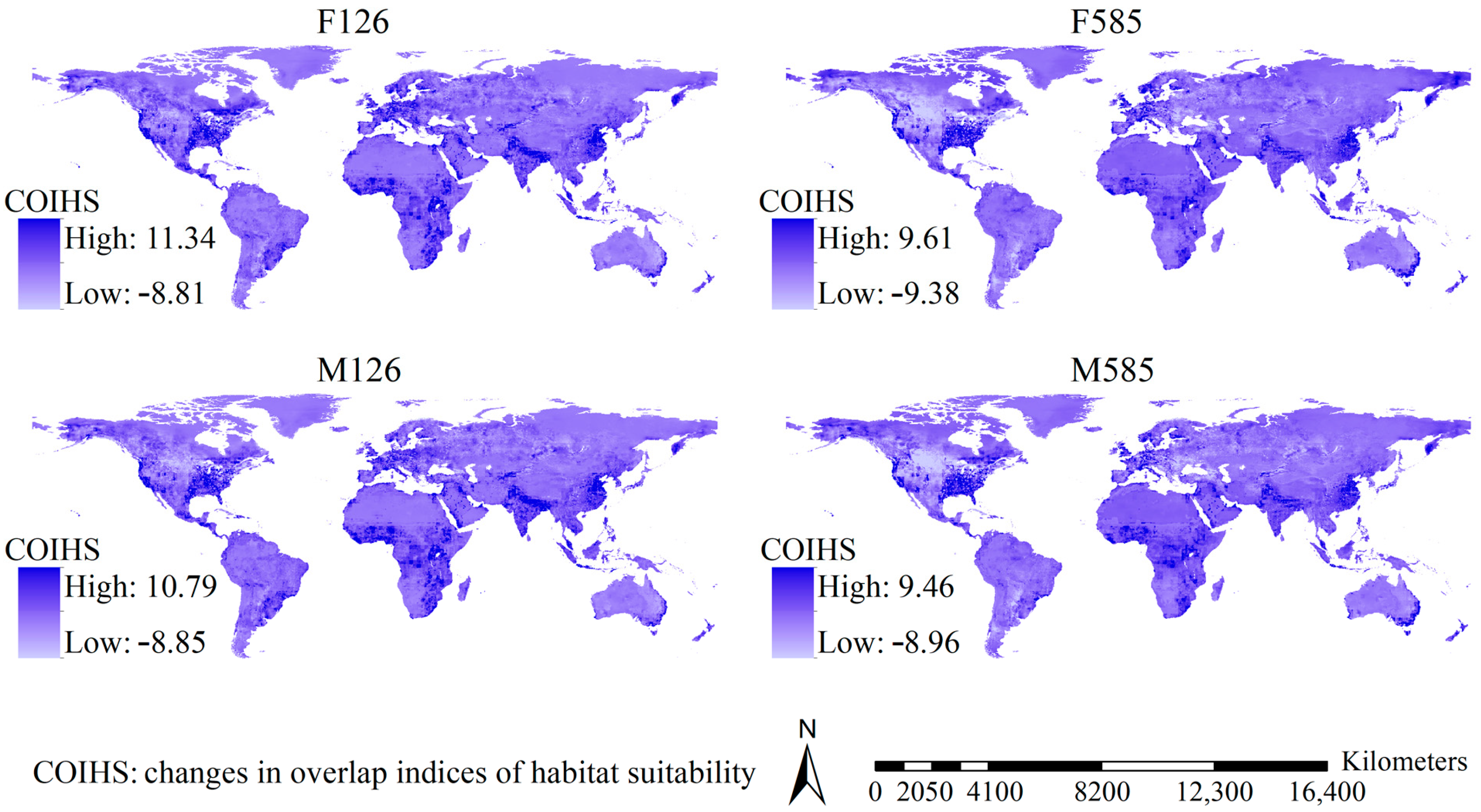
2.5. Investigating the Habitat Suitability Dynamics
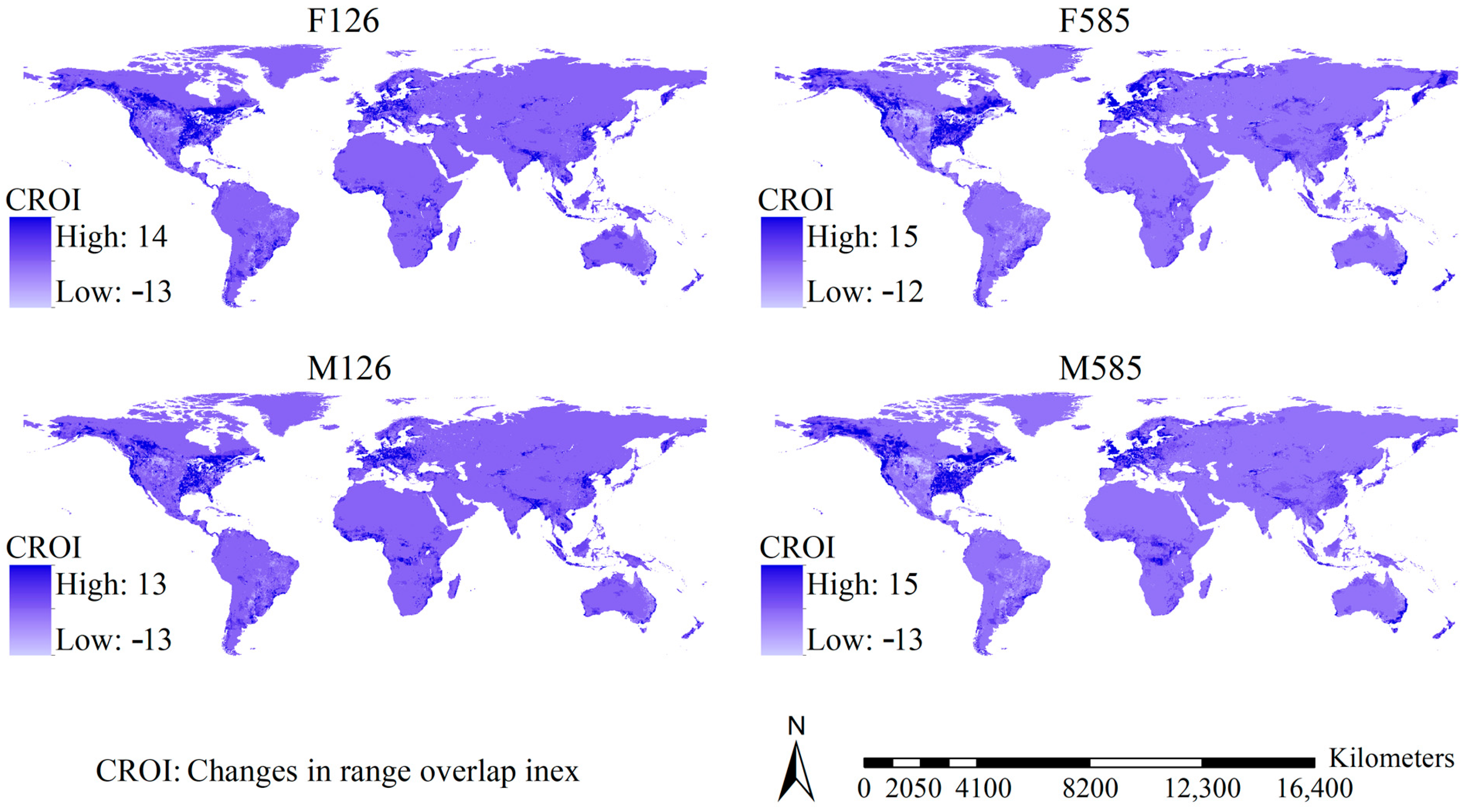
2.6. Investigating Range Shifts and Range Overlaps
3. Results
3.1. Model Performance
3.2. Major Variables Included in the Models
3.3. Habitat Suitability and the Shift Patterns
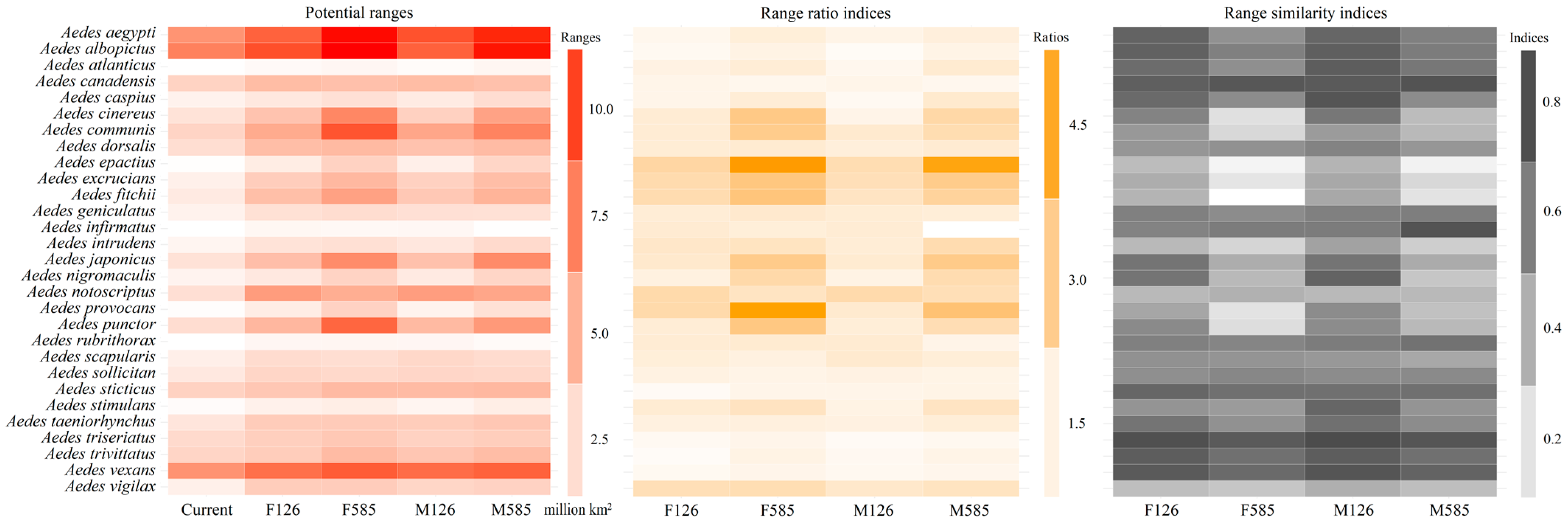
3.4. Potential Ranges, Range Overlaps, and Their Shifts
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. A Global Brief on Vector-Borne Diseases; WHO: Geneva, Switzerland, 2014. [Google Scholar]
- Manguin, S.; Dev, V. (Eds.) Towards Malaria Elimination—A Leap Forward; InTech: London, UK, 2018. [Google Scholar]
- Thacker, S.B. The President’s Malaria Initiative; United States Agency for International Development, Centers for Disease Control and Prevention, Department of Health and Human Services: Atlanta, GA, USA, 2018. [Google Scholar]
- Valenzuela, J.G.; Aksoy, S. Impact of Vector Biology Research on Old and Emerging Neglected Tropical Diseases. PLoS Negl. Trop. Dis. 2018, 12, e0006436. [Google Scholar] [CrossRef]
- Kraemer, M.U.; Sinka, M.E.; Duda, K.A.; Mylne, A.Q.; Shearer, F.M.; Barker, C.M.; Moore, C.G.; Carvalho, R.G.; Coelho, G.E.; Van Bortel, W.; et al. The Global Distribution of the Arbovirus Vectors Aedes aegypti and Ae. albopictus. eLife 2015, 4, e08347. [Google Scholar] [CrossRef]
- Samy, A.M.; Thomas, S.M.; Wahed, A.A.E.; Cohoon, K.P.; Peterson, A.T. Mapping the Global Geographic Potential of Zika Virus Spread. Mem. Inst. Oswaldo Cruz 2016, 111, 559–560. [Google Scholar] [CrossRef]
- Azizi, K.; Dorzaban, H.; Soltani, A.; Alipour, H.; Jaberhashemi, S.A.; Salehi-Vaziri, M.; Mohammadi, T.; Fereydouni, Z.; Paksa, A. Monitoring of Dengue Virus in Field-Caught Aedes Species (Diptera: Culicidae) by Molecular Method, from 2016 to 2017 in Southern Iran. J. Health Sci. Surveill. Syst. 2023, 11, 77–83. [Google Scholar] [CrossRef]
- Roslan, M.A.; Ngui, R.; Abd Karim, M.A.A.; Rosmini, U.S.; Ong, P.S.; Ahmad, M.A.; Lim, Y.A.L.; Wan Sulaiman, W.Y. A Study on Wolbachia-Dengue-Carrying Aedes Mosquitoes (Diptera: Culicidae) Focuses on the Sustainability and Frequency of Wolbachia in High-Rise Buildings in Selangor, Malaysia. Appl. Entomol. Zool. 2024, 59, 225–236. [Google Scholar] [CrossRef]
- Richards, S.L.; Ponnusamy, L.; Unnasch, T.R.; Hassan, H.K.; Apperson, C.S. Host-feeding patterns of Aedes albopictus (Diptera: Culicidae) in relation to availability of human and domestic animals in suburban landscapes of central North Carolina. J. Med. Entomol. 2006, 43, 543–551. [Google Scholar] [CrossRef]
- Brès, P. Impact of arboviruses on human and animal health. In The Arboviruses: Epidemiology and Ecology; Monath, T.P., Ed.; CRC Press: Boca Raton, FL, USA, 1988; Volume 1, pp. 1–18. [Google Scholar]
- Socha, W.; Kwasnik, M.; Larska, M.; Rola, J.; Rozek, W. Vector-borne viral diseases as a current threat for human and animal health—One Health perspective. J. Clin. Med. 2022, 11, 3026. [Google Scholar] [CrossRef]
- Liu, Q.; Xie, J.W.; Wang, M.; Du, Y.T.; Yin, Z.G.; Zhou, N.X.; Zhao, T.Y.; Huang, E.J.; Zhang, H.D. Potential Global Distribution of the Invasive Mosquito Aedes koreicus Under a Changing Climate. Trop. Med. Infect. Dis. 2023, 8, 471. [Google Scholar] [CrossRef] [PubMed]
- Aliaga-Samanez, A.; Romero, D.; Murray, K.; Segura, M.; Real, R.; Olivero, J. Potential Climate Change Effects on the Distribution of Urban and Sylvatic Dengue and Yellow Fever Vectors. Pathog. Glob. Health 2024, 118, 397–407. [Google Scholar] [CrossRef]
- Laverdeur, J.; Desmecht, D.; Hayette, M.P.; Darcis, G. Dengue and Chikungunya: Future Threats for Northern Europe? Front. Epidemiol. 2024, 4, 1342723. [Google Scholar] [CrossRef]
- Gan, S.J.; Leong, Y.Q.; bin Barhanuddin, M.F.H.; Wong, S.T.; Wong, S.F.; Mak, J.W.; Ahmad, R.B. Dengue Fever and Insecticide Resistance in Aedes Mosquitoes in Southeast Asia: A Review. Parasites Vectors 2021, 14, 315. [Google Scholar] [CrossRef] [PubMed]
- Cheong, Y.L.; Nazni, W.A.; Lee, H.L.; NoorAfizah, A.; MohdKhairuddin, I.C.; Kamarul, G.M.; Nizam, N.M.; Arif, M.A.; NurZatilAqmar, Z.M.; Irwan, S.M.; et al. Spatial Distribution and Long-Term Persistence of Wolbachia-Infected Aedes aegypti in the Mentari Court, Malaysia. Insects 2023, 14, 373. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Huang, X.; Guo, X.; Cheng, P.; Wang, H.; Liu, L.; Zang, C.; Zhang, C.; Wang, X.; Zhou, G.; et al. Climate Change and Aedes albopictus Risks in China: Current Impact and Future Projection. Infect. Dis. Poverty 2023, 12, 26. [Google Scholar] [CrossRef] [PubMed]
- Velu, R.M.; Kwenda, G.; Bosomprah, S.; Chisola, M.N.; Simunyandi, M.; Chisenga, C.C.; Bumbangi, F.N.; Sande, N.C.; Simubali, L.; Mburu, M.M.; et al. Ecological Niche Modeling of Aedes and Culex Mosquitoes: A Risk Map for Chikungunya and West Nile Viruses in Zambia. Viruses 2023, 15, 1900. [Google Scholar] [CrossRef]
- Vulu, F.; Futami, K.; Sunahara, T.; Mampuya, P.; Bobanga, T.L.; Mumba Ngoyi, D.; Minakawa, N. Geographic Expansion of the Introduced Aedes albopictus and Other Native Aedes Species in the Democratic Republic of the Congo. Parasites Vectors 2024, 17, 35. [Google Scholar] [CrossRef]
- Echeverry-Cárdenas, E.; López-Castañeda, C.; Carvajal-Castro, J.D.; Aguirre-Obando, O.A. Potential geographic distribution of the tiger mosquito Aedes albopictus (Skuse, 1894) (Diptera: Culicidae) in current and future conditions for Colombia. PLoS Neglect. Trop. Dis. 2021, 15, e0008212. [Google Scholar] [CrossRef]
- Nie, P.; Feng, J. Niche and Range Shifts of Aedes aegypti and Aedes albopictus Suggest That the Latecomer Shows a Greater Invasiveness. Insects 2023, 14, 810. [Google Scholar] [CrossRef]
- Campbell, L.P.; Sallam, M.F.; Bauer, A.M.; Tavares, Y.; Guralnick, R.P. Climate, Landscape, and Life History Jointly Predict Multidecadal Community Mosquito Phenology. Sci. Rep. 2023, 13, 3866. [Google Scholar] [CrossRef]
- Chura, M.; Healy, K.; Diaz, R.; Kaller, M. Effects of Species, Sex, and Diet on Thermal Tolerance of Aedes aegypti and Culex quinquefasciatus (Diptera: Culicidae). J. Med. Entomol. 2023, 60, 637–643. [Google Scholar] [CrossRef]
- Neoh, K.B.; Bong, L.J.; Silalahi, C.N.; Panthawong, A.; Chareonviriyaphap, T.; Ahmad, I. Life-History Traits of Aedes aegypti (Linnaeus) Distributed Across a Latitudinal Range of 23° N–6° S. J. Med. Entomol. 2024, 61, 611–621. [Google Scholar] [CrossRef]
- Cai, X.; Zhao, J.; Deng, H.; Xiao, J.; Liu, T.; Zeng, W.; Li, X.; Hu, J.; Huang, C.; Zhu, G.; et al. Effects of Temperature, Relative Humidity, and Illumination on the Entomological Parameters of Aedes albopictus: An Experimental Study. Int. J. Biometeorol. 2023, 67, 687–694. [Google Scholar] [CrossRef] [PubMed]
- Lusekelo, E.; Helikumi, M.; Kuznetsov, D.; Mushayabasa, S. Quantifying the Effects of Temperature and Predation on the Growth of Aedes Mosquito Population. Model. Earth Syst. Environ. 2023, 9, 3193–3206. [Google Scholar] [CrossRef]
- Hunt, J.R.; Rehbein, M.M.; Viadero, R.C.; Miller, C.L. Distribution of Invasive Aedes Mosquitoes in West-Central Illinois, 2014–2018: Record Updates for Aedes japonicus and Aedes albopictus. J. Am. Mosq. Control Assoc. 2023, 39, 1–11. [Google Scholar] [CrossRef]
- Janssen, N. Distribution and Population Genetics of the Invasive Asian Bush Mosquito Aedes japonicus (Diptera, Culicidae) in Germany and Eastern Europe. Doctoral Dissertation, University of Munich, Munich, Germany, 2023. [Google Scholar]
- Li, Y.; An, Q.; Sun, Z.; Gao, X.; Wang, H. Distribution Areas and Monthly Dynamic Distribution Changes of Three Aedes Species in China: Aedes aegypti, Aedes albopictus and Aedes vexans. Parasites Vectors 2023, 16, 297. [Google Scholar] [CrossRef] [PubMed]
- Talbot, B.; Kulkarni, M.A.; Rioux-Rousseau, M.; Siebels, K.; Kotchi, S.O.; Ogden, N.H.; Ludwig, A. Ecological Niche and Positive Clusters of Two West Nile Virus Vectors in Ontario, Canada. EcoHealth 2023, 20, 249–262. [Google Scholar] [CrossRef]
- Georgiades, P.; Proestos, Y.; Lelieveld, J.; Erguler, K. Machine Learning Modeling of Aedes albopictus Habitat Suitability in the 21st Century. Insects 2023, 14, 447. [Google Scholar] [CrossRef]
- Leisnham, P.T.; Juliano, S.A. Impacts of Climate, Land Use, and Biological Invasion on the Ecology of Immature Aedes Mosquitoes: Implications for La Crosse Emergence. EcoHealth 2012, 9, 217–228. [Google Scholar] [CrossRef]
- Zahouli, J.B.; Koudou, B.G.; Müller, P.; Malone, D.; Tano, Y.; Utzinger, J. Urbanization Is a Main Driver for the Larval Ecology of Aedes Mosquitoes in Arbovirus-Endemic Settings in South-Eastern Côte d’Ivoire. PLoS Negl. Trop. Dis. 2017, 11, e0005751. [Google Scholar] [CrossRef]
- Dickens, B.L.; Sun, H.; Jit, M.; Cook, A.R.; Carrasco, L.R. Determining Environmental and Anthropogenic Factors Which Explain the Global Distribution of Aedes aegypti and Aedes albopictus. BMJ Glob. Health 2018, 3, e000801. [Google Scholar] [CrossRef]
- Liu, B.; Gao, X.; Ma, J.; Jiao, Z.; Xiao, J.; Hayat, M.A.; Wang, H. Modeling the Present and Future Distribution of Arbovirus Vectors Aedes aegypti and Aedes albopictus Under Climate Change Scenarios in Mainland China. Sci. Total Environ. 2019, 664, 203–214. [Google Scholar] [CrossRef]
- Dudov, S.V. Modeling of Species Distribution with the Use of Topography and Remote Sensing Data on the Example of Vascular Plants of the Tukuringra Ridge Low Mountain Belt (Zeya State Nature Reserve, Amur Oblast). Biol. Bull. Rev. 2017, 7, 246–257. [Google Scholar] [CrossRef]
- Tang, C.Q.; Matsui, T.; Ohashi, H.; Dong, Y.F.; Momohara, A.; Herrando-Moraira, S.; Qian, S.; Yang, Y.; Ohsawa, M.; Luu, H.T.; et al. Identifying Long-Term Stable Refugia for Relict Plant Species in East Asia. Nat. Commun. 2018, 9, 4488. [Google Scholar] [CrossRef] [PubMed]
- Virkkala, R.; Marmion, M.; Heikkinen, R.K.; Thuiller, W.; Luoto, M. Predicting Range Shifts of Northern Bird Species: Influence of Modelling Technique and Topography. Acta Oecol. 2010, 36, 269–281. [Google Scholar] [CrossRef]
- Lippi, C.A.; Stewart-Ibarra, A.M.; Loor, M.F.B.; Zambrano, J.E.D.; Lopez, N.A.E.; Blackburn, J.K.; Ryan, S.J. Geographic Shifts in Aedes aegypti Habitat Suitability in Ecuador Using Larval Surveillance Data and Ecological Niche Modeling: Implications of Climate Change for Public Health Vector Control. PLoS Negl. Trop. Dis. 2019, 13, e0007322. [Google Scholar] [CrossRef]
- Rahman, M.S.; Pientong, C.; Zafar, S.; Ekalaksananan, T.; Paul, R.E.; Haque, U.; Rocklöv, J.; Overgaard, H.J. Mapping the Spatial Distribution of the Dengue Vector Aedes aegypti and Predicting Its Abundance in Northeastern Thailand Using Machine-Learning Approach. One Health 2021, 13, 100358. [Google Scholar] [CrossRef]
- Arnoldi, I.; Negri, A.; Soresinetti, L.; Brambilla, M.; Carraretto, D.; Montarsi, F.; Roberto, P.; Mosca, A.; Rubolini, D.; Bandi, C.; et al. Assessing the Distribution of Invasive Asian Mosquitoes in Northern Italy and Modelling the Potential Spread of Aedes koreicus in Europe. Acta Trop. 2022, 232, 106536. [Google Scholar] [CrossRef]
- Rohlf, F.J. Classification of Aedes by numerical taxonomic methods (Diptera: Culicidae). Ann. Entomol. Soc. Am. 1963, 56, 798–804. [Google Scholar]
- Mattingly, P.F. Taxonomy of Aedes aegypti and related species. Bull. World Health Organ. 1967, 36, 552. [Google Scholar]
- Zavortink, T.J. Classical taxonomy of mosquitoes—A memorial to John N. Belkin. J. Am. Mosq. Control Assoc. 1990, 6, 593–599. [Google Scholar]
- Becker, N.; Petrić, D.; Zgomba, M.; Boase, C.; Madon, M.B.; Dahl, C.; Kaiser, A. Mosquitoes: Identification, Ecology and Control; Springer Nature: Berlin/Heidelberg, Germany, 2020. [Google Scholar]
- Wilkerson, R.C.; Linton, Y.M.; Strickman, D. Mosquitoes of the World; Johns Hopkins University Press: Baltimore, MD, USA, 2021. [Google Scholar]
- Lambrechts, L.; Scott, T.W.; Gubler, D. Consequences of the expanding global distribution of Aedes albopictus for dengue virus transmission. PLoS Negl. Trop. Dis. 2010, 4, e646. [Google Scholar] [CrossRef]
- Powell, J.R. Mosquitoes and their role in forest ecosystems, with special reference to carbon cycling. Tree Physiol. 2011, 31, 1–10. [Google Scholar]
- Powell, J.R.; Tabachnick, W.J. History of domestication and spread of Aedes aegypti—A review. Mem. Inst. Oswaldo Cruz 2013, 108 (Suppl. S1), 11–17. [Google Scholar] [PubMed]
- Brown, J.E.; Evans, B.; Zheng, W.; Obas, V.; Barrera-Martinez, L.; Egizi, A.; Zhao, H.; Caccone, A.; Powell, J.R. Human impacts have shaped historical and recent evolution in Aedes aegypti, the dengue and yellow fever mosquito. Evolution 2014, 68, 514–525. [Google Scholar] [PubMed]
- Khormi, H.M.; Kumar, L. Climate change and the potential global distribution of Aedes aegypti: Spatial modelling using geographical information system and CLIMEX. Geospat. Health 2014, 8, 405–415. [Google Scholar]
- Gloria-Soria, A.; Ayala, D.; Bheecarry, A.; Calderon-Arguedas, O.; Chadee, D.D.; Chiappero, M.; Coetzee, M.; Elahee, K.B.; Fernandez-Salas, I.; Kamal, H.A.; et al. Global genetic diversity of Aedes aegypti. Mol. Ecol. 2016, 25, 5377–5395. [Google Scholar]
- Santos, J.; Meneses, B.M. An integrated approach for the assessment of the Aedes aegypti and Aedes albopictus global spatial distribution, and determination of the zones susceptible to the development of Zika virus. Acta Trop. 2017, 168, 80–90. [Google Scholar]
- Ding, F.; Fu, J.; Jiang, D.; Hao, M.; Lin, G. Mapping the spatial distribution of Aedes aegypti and Aedes albopictus. Acta Trop. 2018, 178, 155–162. [Google Scholar]
- Kamal, M.; Kenawy, M.A.; Rady, M.H.; Khaled, A.S.; Samy, A.M. Mapping the global potential distributions of two arboviral vectors Aedes aegypti and Ae. albopictus under changing climate. PLoS ONE 2018, 13, e0210122. [Google Scholar]
- Liu-Helmersson, J.; Brännström, Å.; Sewe, M.O.; Semenza, J.C.; Rocklöv, J. Estimating past, present, and future trends in the global distribution and abundance of the arbovirus vector Aedes aegypti under climate change scenarios. Front. Public Health 2019, 7, 148. [Google Scholar]
- Laporta, G.Z.; Potter, A.M.; Oliveira, J.F.; Bourke, B.P.; Pecor, D.B.; Linton, Y.M. Global distribution of Aedes aegypti and Aedes albopictus in a climate change scenario of regional rivalry. Insects 2023, 14, 49. [Google Scholar] [CrossRef]
- Mejía-Jurado, E.; Echeverry-Cárdenas, E.; Aguirre-Obando, O.A. Potential current and future distribution for Aedes aegypti and Aedes albopictus in Colombia: Important disease vectors. Biol. Invasions 2024, 26, 2119–2137. [Google Scholar]
- Zhou, Y.; Wu, C.; Nie, P.; Feng, J.; Hu, X. Invasive Pest and Invasive Host: Where Might Spotted-Wing Drosophila (Drosophila suzukii) and American Black Cherry (Prunus serotina) Cross Paths in Europe? Forests 2024, 15, 206. [Google Scholar] [CrossRef]
- Fick, S.E.; Hijmans, R.J. WorldClim2: New 1km Spatial Resolution Climate Surfaces for Global Land Areas. Int. J. Climatol. 2017, 37, 4302–4315. [Google Scholar] [CrossRef]
- Zhang, M.Z.; Xu, Z.F.; Han, Y.; Guo, W.D. Evaluation of CMIP6 Models Toward Dynamical Downscaling over 14 CORDEX Domains. Clim. Dyn. 2022, 62, 4475–4489. [Google Scholar] [CrossRef]
- Dormann, C.F.; Elith, J.; Bacher, S.; Buchmann, C.; Carl, G.; Carré, G.; Carré, G.; Marquéz, J.R.G.; Gruber, B.; Lafourcade, B.; et al. Collinearity: A Review of Methods to Deal with It and a Simulation Study Evaluating Their Performance. Ecography 2013, 36, 27–46. [Google Scholar] [CrossRef]
- Thuiller, W.; Lafourcade, B.; Engler, R.; Araujo, M.B. BIOMOD—A Platform for Ensemble Forecasting of Species Distributions. Ecography 2009, 32, 369–373. [Google Scholar] [CrossRef]
- Barbet-Massin, M.; Jiguet, F.; Albert, C.H.; Thuiller, W. Selecting Pseudo-Absences for Species Distribution Models: How, Where and How Many? Methods Ecol. Evol. 2012, 3, 327–338. [Google Scholar] [CrossRef]
- Liu, C.; Newell, G.; White, M. On the Selection of Thresholds for Predicting Species Occurrence with Presence-Only Data. Ecol. Evol. 2016, 6, 337–348. [Google Scholar] [CrossRef][Green Version]
- Yang, R.J.; Cao, R.Y.; Gong, X.; Feng, J.M. Large Shifts of Niche and Range in the Golden Apple Snail (Pomacea canaliculata), an Aquatic Invasive Species. Ecosphere 2023, 14, e4391. [Google Scholar] [CrossRef]
- Khan, S.U.; Ogden, N.H.; Fazil, A.A.; Gachon, P.H.; Dueymes, G.U.; Greer, A.L.; Ng, V. Current and Projected Distributions of Aedes aegypti and Aedes albopictus in Canada and the US. Environ. Health Perspect. 2020, 128, 057007. [Google Scholar] [CrossRef]
- Oliveira, S.; Rocha, J.; Sousa, C.A.; Capinha, C. Wide and Increasing Suitability for Aedes albopictus in Europe Is Congruent Across Distribution Models. Sci. Rep. 2021, 11, 9916. [Google Scholar] [CrossRef]
- Outammassine, A.; Zouhair, S.; Loqman, S. Global Potential Distribution of Three Underappreciated Arbovirus Vectors (Aedes japonicus, Aedes vexans and Aedes vittatus) Under Current and Future Climate Conditions. Transbound. Emerg. Dis. 2022, 69, e1160–e1171. [Google Scholar] [CrossRef] [PubMed]
- Sirami, C.; Caplat, P.; Popy, S.; Clamens, A.; Arlettaz, R.; Jiguet, F.; Brotons, L.; Martin, J.L. Impacts of Global Change on Species Distributions: Obstacles and Solutions to Integrate Climate and Land Use. Glob. Ecol. Biogeogr. 2017, 26, 385–394. [Google Scholar] [CrossRef]
- Bennett, K.L.; Gómez Martínez, C.; Almanza, A.; Rovira, J.R.; McMillan, W.O.; Enriquez, V.; Barraza, E.; Diaz, M.; Sanchez-Galan, J.E.; Whiteman, A.; et al. High infestation of invasive Aedes mosquitoes in used tires along the local transport network of Panama. Parasites Vectors 2019, 12, 264. [Google Scholar]
- Malla, R.K.; Mandal, K.K.; Dutta, M.; Chandra, G. An estimation of monthly propagation of dengue vector Aedes aegypti in rain-water filled tires. Int. J. Pest Manag. 2020, 66, 239–242. [Google Scholar]
- Mohammadi, A.; Mostafavi, E.; Zaim, M.; Enayati, A.; Basseri, H.R.; Mirolyaei, A.; Poormozafari, J.; Gouya, M.M. Imported tires; a potential source for the entry of Aedes invasive mosquitoes to Iran. Travel Med. Infect. Dis. 2022, 49, 102389. [Google Scholar]
- Hawley, W.A.; Reiter, P.; Copeland, R.S.; Pumpuni, C.B.; Craig, G.B., Jr. Aedes albopictus in North America: Probable introduction in used tires from northern Asia. Science 1987, 236, 1114–1116. [Google Scholar]
- Rolland, C. Spatial and Seasonal Variations of Air Temperature Lapse Rates in Alpine Regions. J. Clim. 2003, 16, 1032–1046. [Google Scholar] [CrossRef]
- Joly, D.; Castel, T.; Pohl, B.; Richard, Y. Influence of Spatial Information Resolution on the Relation Between Elevation and Temperature. Int. J. Climatol. 2018, 38, 5677–5688. [Google Scholar] [CrossRef]
- Invasive Species Specialist Group. Global Invasive Species Database. Available online: http://www.iucngisd.org/gisd/100_worst.php (accessed on 1 January 2024).
- Trájer, A.J. The Potential Habitat and Environmental Fitness Change of Aedes albopictus in Western Eurasia for 2081–2100. J. Vector Dis. 2024, 61, 243–252. [Google Scholar] [CrossRef]
- Kesavaraju, B.; Leisnham, P.T.; Keane, S.; Delisi, N.; Pozatti, R. Interspecific competition between Aedes albopictus and A. sierrensis: Potential for competitive displacement in the Western United States. PLoS ONE 2014, 9, e89698. [Google Scholar] [CrossRef] [PubMed]
- Steinwascher, K. Competition among Aedes aegypti larvae. PLoS ONE 2018, 13, e0202455. [Google Scholar] [CrossRef] [PubMed]
- Lizuain, A.A.; Maffey, L.; Garzón, M.; Leporace, M.; Soto, D.; Diaz, P.; Salomón, O.D.; Santini, M.S.; Schweigmann, N. Larval competition between Aedes albopictus and Aedes aegypti (Diptera: Culicidae) in Argentina: Coexistence and implications in the distribution of the Asian Tiger mosquito. J. Med. Entomol. 2022, 59, 1636–1645. [Google Scholar] [CrossRef] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Mei, H.; Nie, P.; Hu, X.; Feng, J. Future Climate Predicts Range Shifts and Increased Global Habitat Suitability for 29 Aedes Mosquito Species. Insects 2025, 16, 476. https://doi.org/10.3390/insects16050476
Zhang X, Mei H, Nie P, Hu X, Feng J. Future Climate Predicts Range Shifts and Increased Global Habitat Suitability for 29 Aedes Mosquito Species. Insects. 2025; 16(5):476. https://doi.org/10.3390/insects16050476
Chicago/Turabian StyleZhang, Xueyou, Hongyan Mei, Peixiao Nie, Xiaokang Hu, and Jianmeng Feng. 2025. "Future Climate Predicts Range Shifts and Increased Global Habitat Suitability for 29 Aedes Mosquito Species" Insects 16, no. 5: 476. https://doi.org/10.3390/insects16050476
APA StyleZhang, X., Mei, H., Nie, P., Hu, X., & Feng, J. (2025). Future Climate Predicts Range Shifts and Increased Global Habitat Suitability for 29 Aedes Mosquito Species. Insects, 16(5), 476. https://doi.org/10.3390/insects16050476




