Nesting Preferences of Osmia orientalis (Hymenoptera: Megachilidae) in the Field and Its Potential as a Strawberry Pollinator in Greenhouses
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
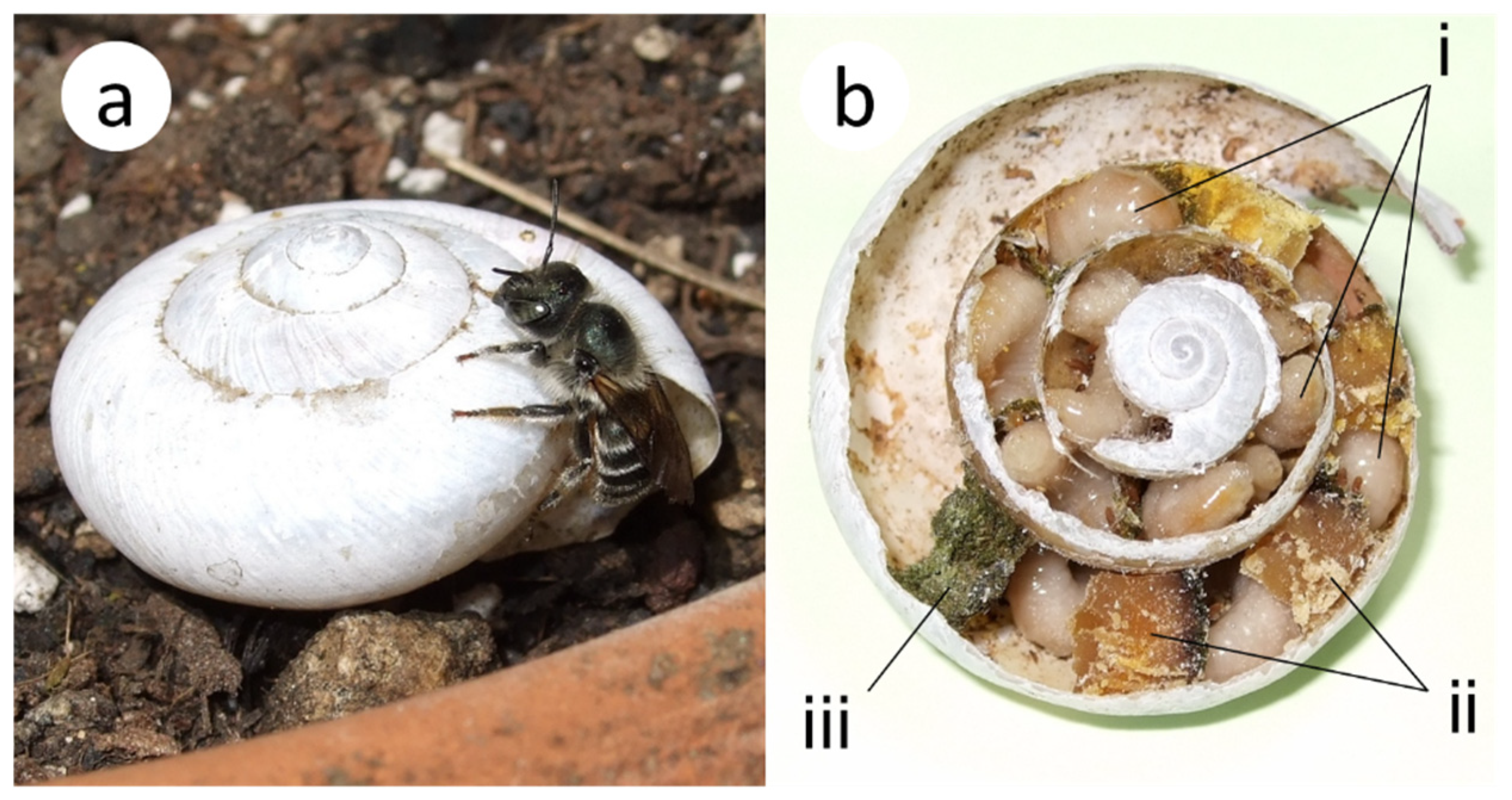
2.1. Study Location and Study Species
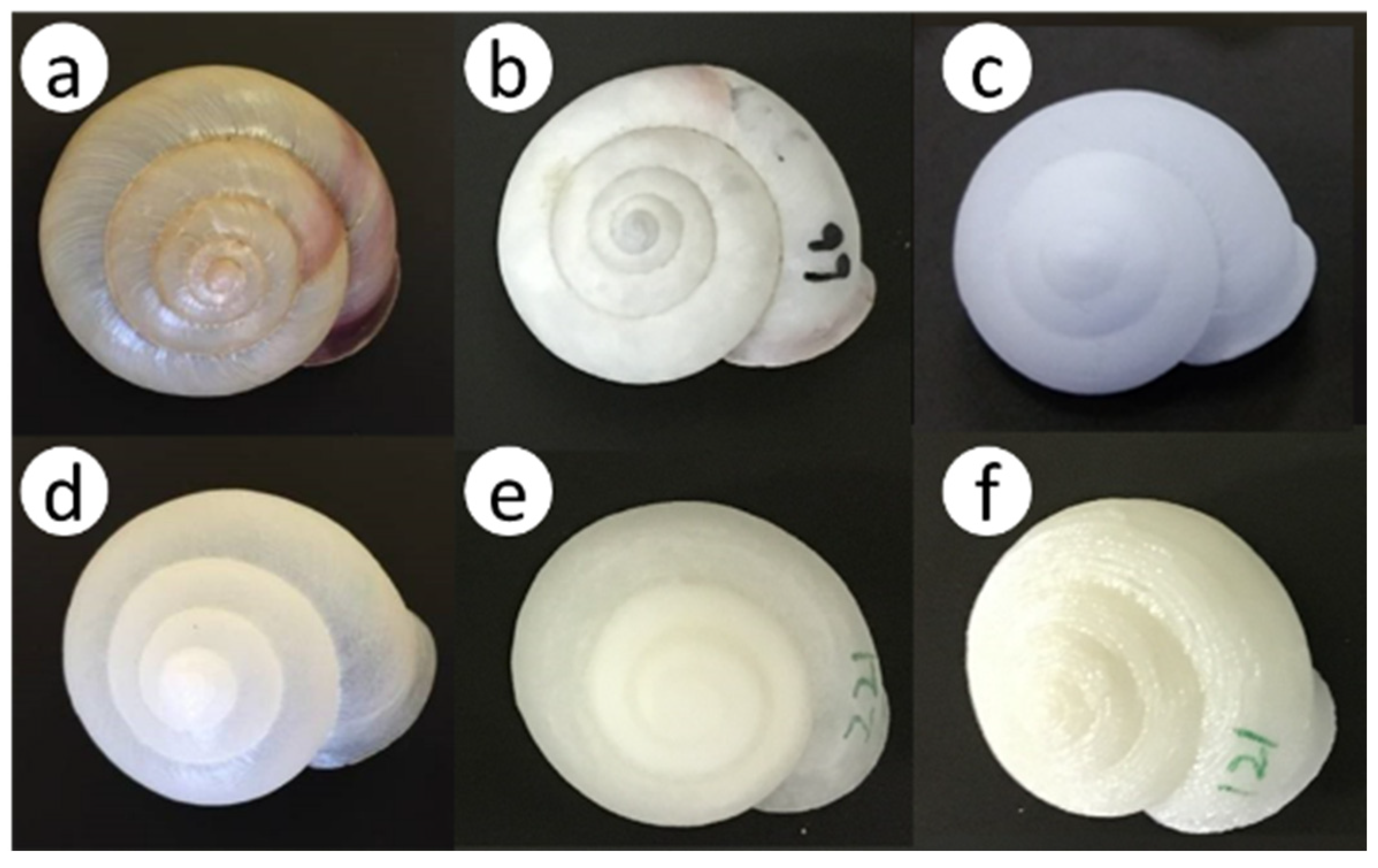
2.2. Experiment 1: Nesting Preferences for Natural Shells and Surrounding Environments in the Field
Data Analysis
2.3. Experiment 2: Per-Visit Strawberry Flower Pollination Efficiency of Apis Mellifera and Osmia orientalis
Data Analysis
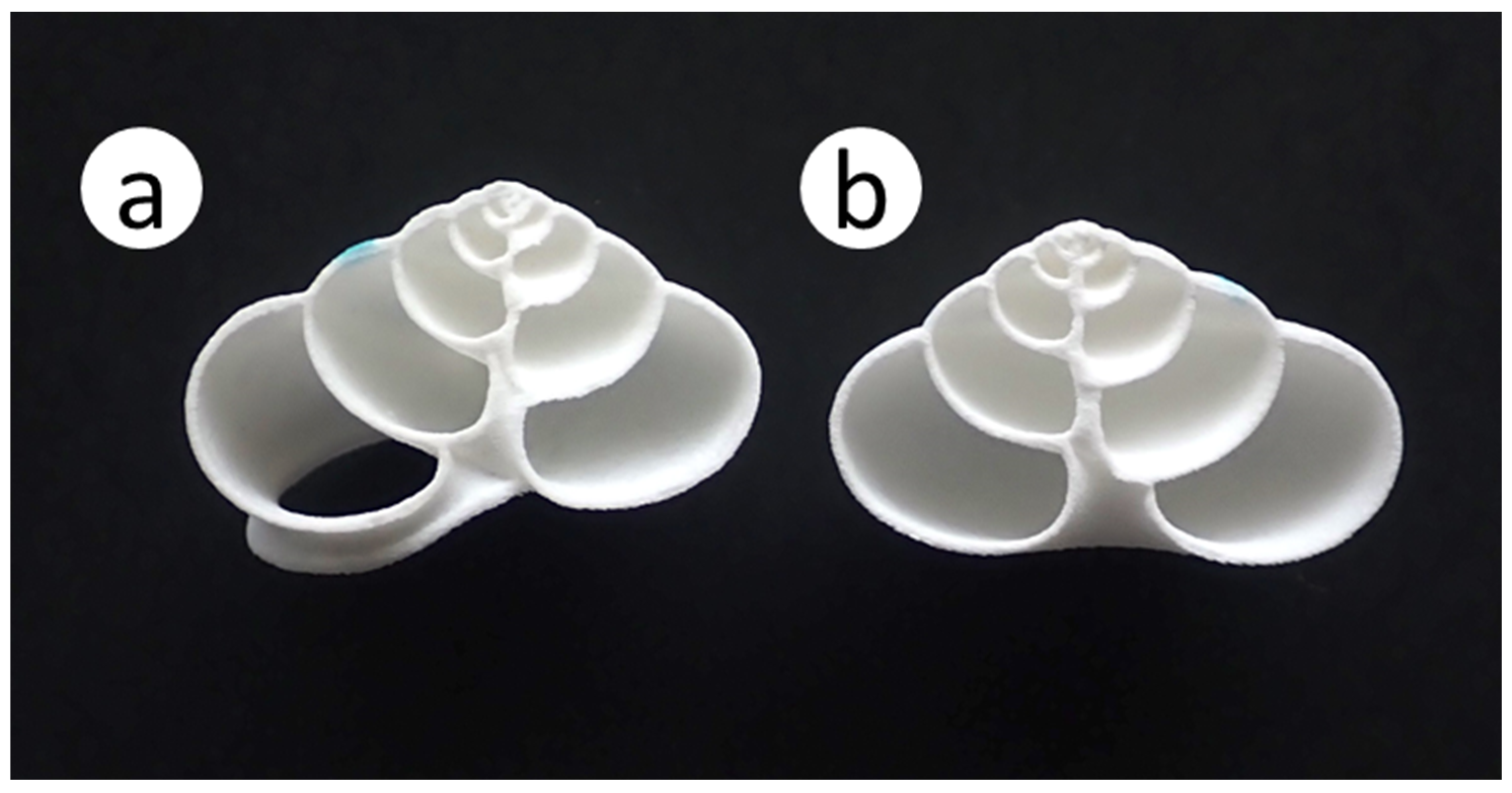
2.4. Experiment 3: Nesting Preference Between Artificial and Natural Shells in Greenhouses
Data Analysis
3. Results
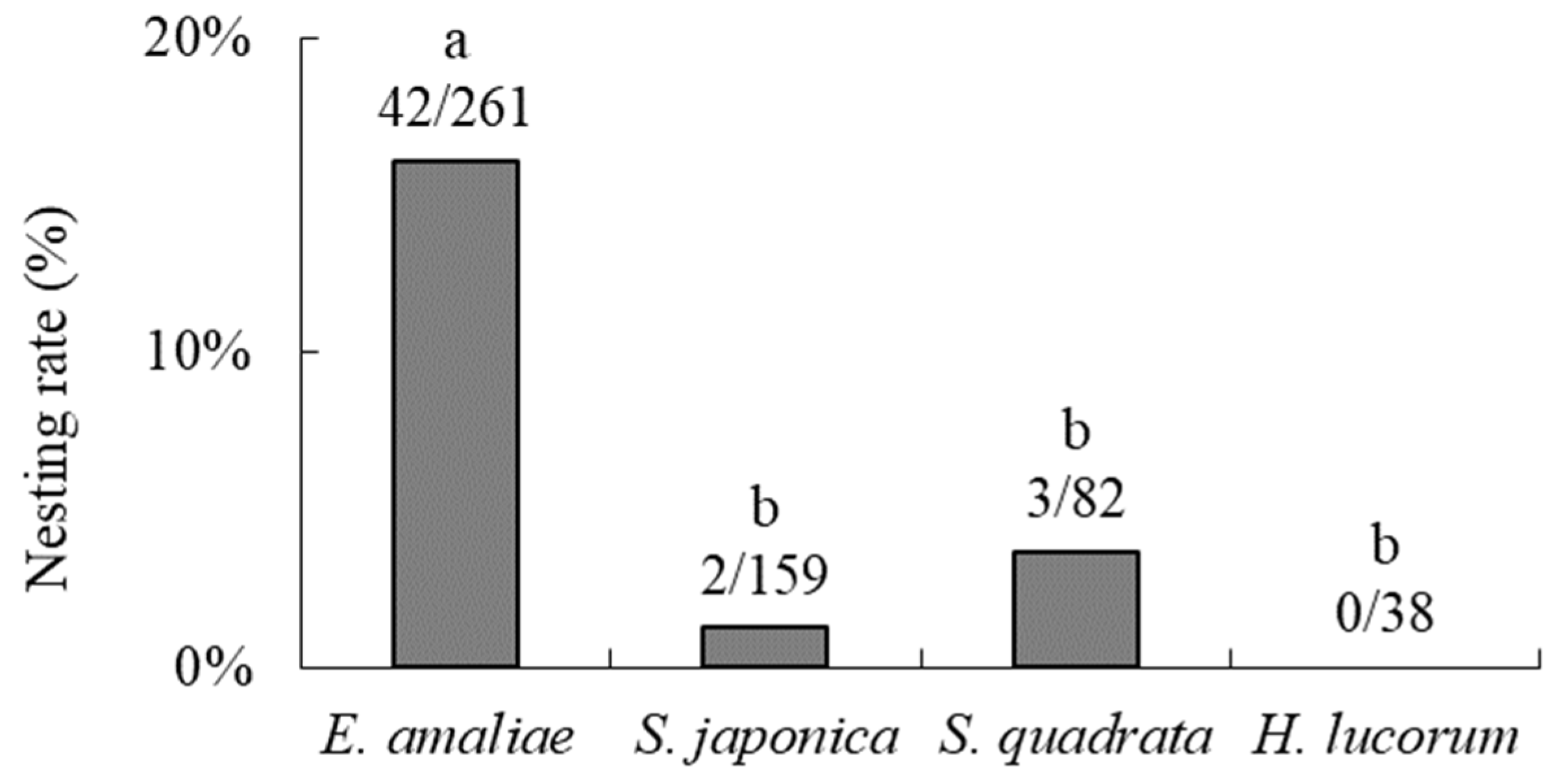
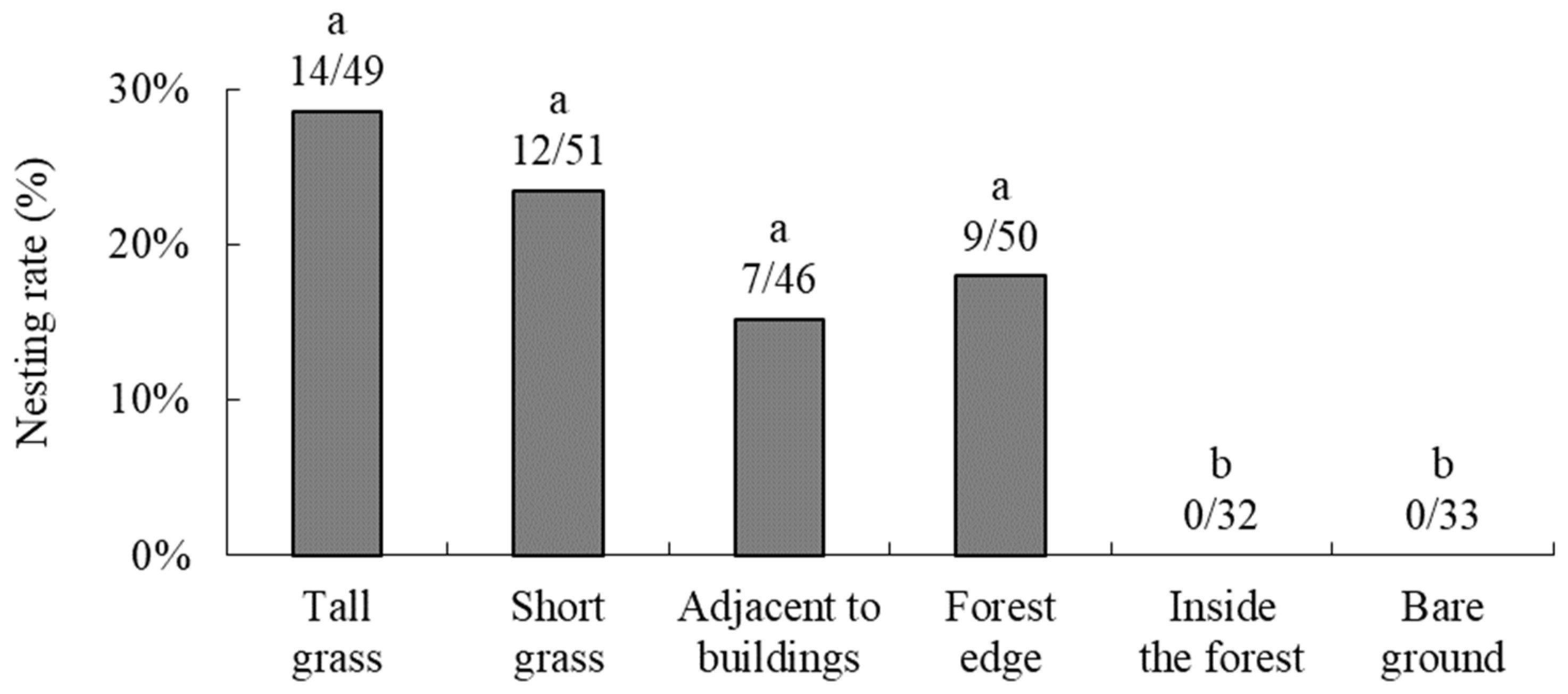
3.1. Nesting Preferences for Natural Shells and Surrounding Environments in the Field
3.2. Per-Visit Strawberry Flower Pollination Efficiency of A. mellifera and O. orientalis
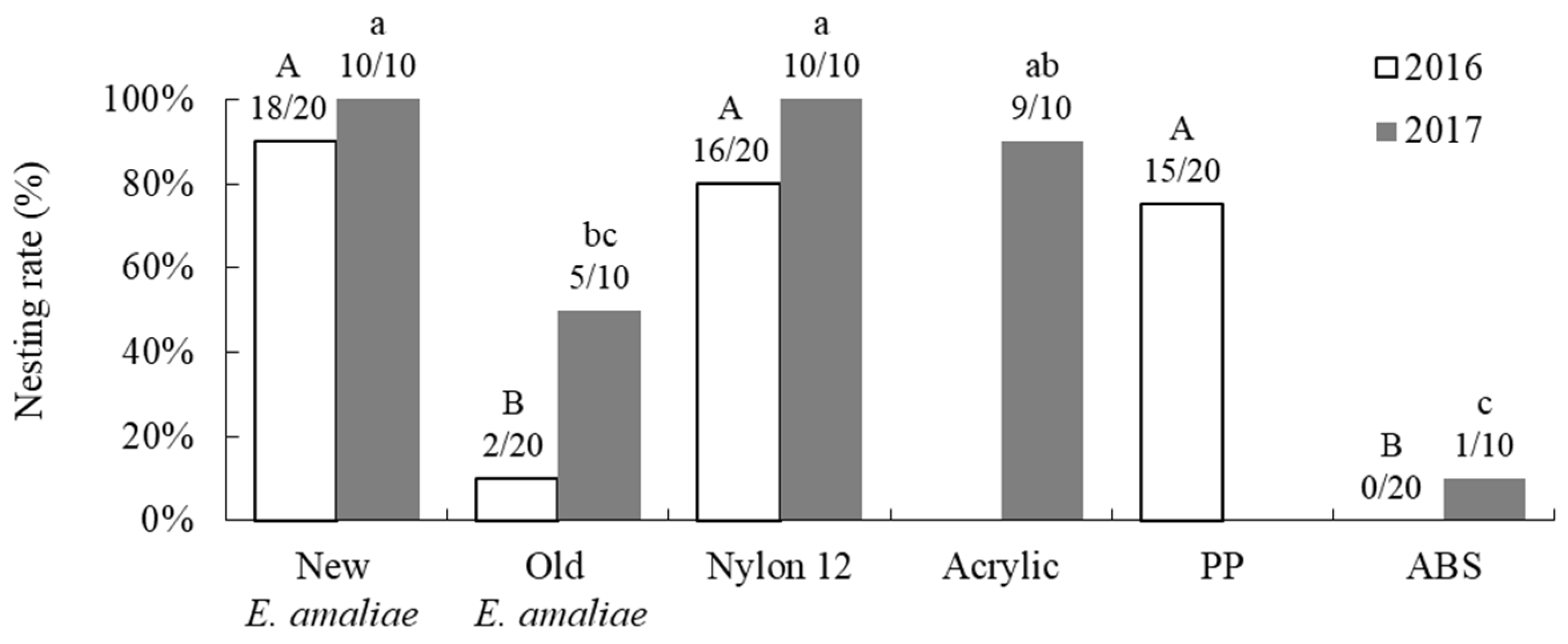
3.3. Nesting Preference Between Artificial and Natural Shells in Greenhouses
4. Discussion
4.1. Nesting Preferences for Natural Shells and Surrounding Environments in the Field
4.1.1. Nesting Preferences for Natural Shells
4.1.2. Nesting Preferences for the Surrounding Environment
4.2. Per-Visit Strawberry Flower Pollination Efficiency of A. Mellifera and O. orientalis
4.3. Nesting Preference Between Artificial and Natural Shells in Greenhouses
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Klein, A.M.; Vaissiere, B.E.; Cane, J.H.; Steffan-Dewenter, I.; Cunningham, S.A.; Kremen, C.; Tscharntke, T. Importance of pollinators in changing landscapes for world crops. Proc. R. Soc. B-Biol. Sci. 2007, 274, 303–313. [Google Scholar] [CrossRef] [PubMed]
- Bartomeus, I.; Potts, S.G.; Steffan-Dewenter, I.; Vaissière, B.E.; Woyciechowski, M.; Krewenka, K.M.; Tscheulin, T.; Roberts, S.P.M.; Szentgyörgyi, H.; Westphal, C.; et al. Contribution of insect pollinators to crop yield and quality varies with agricultural intensification. Peerj 2014, 2, e328. [Google Scholar] [CrossRef] [PubMed]
- Khalifa, S.A.M.; Elshafiey, E.H.; Shetaia, A.A.; Abd El-Wahed, A.A.; Algethami, A.F.; Musharraf, S.G.; AlAjmi, M.F.; Zhao, C.; Masry, S.H.D.; Abdel-Daim, M.M.; et al. Overview of Bee Pollination and Its Economic Value for Crop Production. Insects 2021, 12, 688. [Google Scholar] [CrossRef] [PubMed]
- Maccagnani, B.; Burgio, G.; Stanisavljevic, L.Z.; Main, S. Osmia cornuta management in pear orchards. Bull. Insectol. 2007, 60, 77–82. [Google Scholar]
- Zhang, H.; Shan, S.; Gu, S.H.; Huang, X.Z.; Li, Z.B.; Khashaveh, A.; Zhang, Y.J. Prior Experience with Food Reward Influences the Behavioral Responses of the Honeybee Apis mellifera and the Bumblebee Bombus lantschouensis to Tomato Floral Scent. Insects 2020, 11, 884. [Google Scholar] [CrossRef] [PubMed]
- Cooley, H.; Vallejo-Marín, M. Buzz-Pollinated Crops: A Global Review and Meta-analysis of the Effects of Supplemental Bee Pollination in Tomato. J. Econ. Entomol. 2021, 114, 505–519. [Google Scholar] [CrossRef]
- Kato, M.; Shibata, A.; Yasui, T.; Nagamasu, H. Impact of introduced honeybees, Apis mellifera, upon native bee communities in the Bonin (Ogasawara) Islands. Res. Popul. Ecol. 1999, 41, 217–228. [Google Scholar] [CrossRef]
- Dohzono, I.; Yokoyama, J. Impacts of alien bees on native plant-pollinator relationships: A review with special emphasis on plant reproduction. Appl. Entomol. Zool. 2010, 45, 37–47. [Google Scholar] [CrossRef]
- Elbgami, T.; Kunin, W.E.; Hughes, W.O.H.; Biesmeijer, J.C. The effect of proximity to a honeybee apiary on bumblebee colony fitness, development, and performance. Apidologie 2014, 45, 504–513. [Google Scholar] [CrossRef]
- Bosch, J.; Kemp, W.P. Developing and establishing bee species as crop pollinators: The example of Osmia spp. (Hymenoptera: Megachilidae) and fruit trees. Bull. Entomol. Res. 2002, 92, 3–16. [Google Scholar] [CrossRef]
- Yamada, M.; Oyama, N.; Sekita, T.; Shirasaki, S.; Tsugawa, C. The ecology of the megachilid bee Osmia cornifrons and its utilization for apple pollination. Bull. Aomori Apple Exp. Stn. 1971, 15, 1–80. (In Japanese) [Google Scholar]
- Maeta, Y. Comparative studies on the biology of bees of the genus Osmia of Japan, with special reference to their management for pollinations of crops (Hymenoptera: Megachilidae). Bull. Tohoku Nat. Agric. Exp. Stn. 1978, 57, 1–221. (In Japanese) [Google Scholar]
- Torchio, P.F. In-nest biologies and development of immature stages of 3 Osmia species (Hymenoptera, Megachilidae). Ann. Entomol. Soc. Am. 1989, 82, 599–615. [Google Scholar] [CrossRef]
- Bosch, J. The nesting behavior of the mason bee Osmia cornuta (Latreille) with special reference to its pollinating potential (Hymenoptera, Megachilidae). Apidologie 1994, 25, 84–93. [Google Scholar] [CrossRef]
- Bosch, J.; Kemp, W.P. Exceptional cherry production in an orchard pollinated with blue orchard bees. Bee World 1999, 80, 163–173. [Google Scholar] [CrossRef]
- Müller, A.; Praz, C.; Dorchin, A. Biology of Palaearctic Wainia bees of the subgenus Caposmia including a short review on snail shell nesting in osmiine bees (Hymenoptera, Megachilidae). J. Hymenopt. Res. 2018, 65, 61–89. [Google Scholar] [CrossRef]
- Shibuya, T. Habits of Osmia orientalis Benoist nesting in snail shells and its natural enemy, Chrysis (Holochrysis) sp. Mushi 1939, 12, 41–55. (In Japanese) [Google Scholar]
- Maeta, Y. Appendix of the nest structure of Osmia (Chalcosmia) orientalis Benoist. Bull. Iwate Insect Club 1980, 5, 1–4. (In Japanese) [Google Scholar]
- Kandori, I.; Hirao, T.; Matsunaga, S.; Kurosaki, T. An invasive dandelion unilaterally reduces the reproduction of a native congener through competition for pollination. Oecologia 2009, 159, 559–569. [Google Scholar] [CrossRef]
- Yokoi, T.; Kandori, I. Comparison of foraging activity between mason bee Osmia orientalis (Hymenoptera: Megachilidae) and honybees for wild raspberry Rubus hirsutus (Rosales: Rosaceae). Entomol. News 2016, 125, 363–367. [Google Scholar] [CrossRef]
- Azuma, M. Colored Illustrations of the Land Snails of Japan; Hoikusha: Osaka, Japan, 1982. [Google Scholar]
- Kandori, I.; Tamaru, M.; Yokoi, T. Nesting Habits of a Pollinator: Osmia orientalis (Hymenoptera: Megachilidae). Jpn. J. Appl. Entomol. Zool. 2010, 54, 77–84, (In Japanese with English Summary). [Google Scholar] [CrossRef]
- Nitsch, J.P. Growth and morphogenesis of the strawberry as related to auxin. Am. J. Bot. 1950, 37, 211–215. [Google Scholar] [CrossRef]
- Kakutani, T.; Inoue, T.; Tezuka, T.; Maeta, Y. Pollination of strawberry by the stingless bee, Trigona minangkabau, and the honey bee, Apis mellifera: An experimental study of fertilization efficiency. Res. Popul. Ecol. 1993, 35, 95–111. [Google Scholar] [CrossRef]
- Kandori, I.; Shimaoka, R.; Tsukamoto, T.; Kamiya, K.; Yokoi, T. Multiyear study of pollinator efficiency and importance of a wide array of pollinators in a field-cultivated strawberry plot. PLoS ONE 2024, 19, e0297130. [Google Scholar] [CrossRef]
- IBM SPSS. SPSS Base 28.0 User’s Guide; SPSS Inc.: Chicago, IL, USA, 2021. [Google Scholar]
- Hopfenmüller, S.; Holzschuh, A.; Steffan-Dewenter, I. Effects of grazing intensity, habitat area and connectivity on snail-shell nesting bees. Biol. Conserv. 2020, 242, 108406. [Google Scholar] [CrossRef]
- Maeta, Y.; Kitamura, T. Pollination efficiency by Osmia cornifrons in relation to required number of nesting bees for economic fruit production. Honeybee Sci. 1981, 2, 65–72. (In Japanese) [Google Scholar]
- Bosch, J.; Blas, M. Foraging behavior and pollinating efficiency of Osmia cornuta and Apis mellifera on almond (Hymenoptera, Megachilidae and Apidae). Appl. Entomol. Zool. 1994, 29, 1–9. [Google Scholar] [CrossRef]
- Sampson, B.J.; Cane, J.H. Pollination efficiencies of three bee (Hymenoptera: Apoidea) species visiting rabbiteye blueberry. J. Econ. Entomol. 2000, 93, 1726–1731. [Google Scholar] [CrossRef] [PubMed]
- Vicens, N.; Bosch, J. Pollinating efficacy of Osmia cornuta and Apis mellifera (Hymenoptera: Megachilidae, Apidae) on ‘red Delicious’ apple. Environ. Entomol. 2000, 29, 235–240. [Google Scholar] [CrossRef]
- Cane, J.H. Pollination potential of the bee Osmia aglaia for cultivated red raspberries and blackberries (Rubus: Rosaceae). Hortscience 2005, 40, 1705–1708. [Google Scholar] [CrossRef]
- Rader, R.; Howlett, B.G.; Cunningham, S.A.; Westcott, D.A.; Edwards, W. Spatial and temporal variation in pollinator effectiveness: Do unmanaged insects provide consistent pollination services to mass flowering crops? J. Appl. Ecol. 2012, 49, 126–134. [Google Scholar] [CrossRef]
- Layek, U.; Kundu, A.; Bisui, S.; Karmakar, P. Impact of managed stingless bee and western honey bee colonies on native pollinators and yield of watermelon: A comparative study. Ann. Agric. Sci. 2021, 66, 38–45. [Google Scholar] [CrossRef]
- Kamo, T.; Nikkeshi, A.; Inoue, H.; Yamamoto, S.; Sawamura, N.; Nakamura, S.; Kishi, S. Pollinators of Oriental persimmon in Japan. Appl. Entomol. Zool. 2022, 57, 237–248. [Google Scholar] [CrossRef]
- Kamo, T.; Nikkeshi, A.; Tawaratsumida, T.; Tanaka, Y.; Nakamura, S.; Kishi, S. Pollination efficiency of bumblebee, honeybee, and hawkmoth in kabocha squash, Cucurbita maxima, production in Kagoshima, Japan. Appl. Entomol. Zool. 2022, 57, 119–129. [Google Scholar] [CrossRef]
- Sussarellu, R.; Suquet, M.; Thomas, Y.; Lambert, C.; Fabioux, C.; Pernet, M.E.J.; Le Goïc, N.; Quillien, V.; Mingant, C.; Epelboin, Y.; et al. Oyster reproduction is affected by exposure to polystyrene microplastics. Proc. Natl. Acad. Sci. USA 2016, 113, 2430–2435. [Google Scholar] [CrossRef]
- Karlsson, T.M.; Vethaak, A.D.; Almroth, B.C.; Ariese, F.; van Velzen, M.; Hassellöv, M.; Leslie, H.A. Screening for microplastics in sediment, water, marine invertebrates and fish: Method development and microplastic accumulation. Mar. Pollut. Bull. 2017, 122, 403–408. [Google Scholar] [CrossRef]
- Clukey, K.E.; Lepczyk, C.A.; Balazs, G.H.; Work, T.M.; Lynch, J.M. Investigation of plastic debris ingestion by four species of sea turtles collected as bycatch in pelagic Pacific longline fisheries. Mar. Pollut. Bull. 2017, 120, 117–125. [Google Scholar] [CrossRef]



| Environment (Number of Locations) | Environmental Characteristics |
|---|---|
| Tall grass (n = 6) | Herbaceous plants over 1 m in height (e.g., ferns, goldenrod Solidago altissima, and silver grass Miscanthus sinensis) grew densely. Direct sunlight was rare. |
| Short grass (n = 6) | Herbaceous plants, approximately 30 cm in height, grew somewhat densely. Frequent direct sunlight. |
| Adjacent to buildings (n = 5) | Shade or semi-shade under the eaves or rim of buildings, such as work sheds and school buildings. There was almost no rain and no viable grass. |
| Forest edge (n = 6) | Half-shade at the edge of a forest. Fallen leaves were piled up, and viable grass was sparse. |
| Within a forest (n = 5) | Approximately 10 m inside a broad-leaved forest dominated by Quercus serrata and Q. glauca. The area was shaded all day with almost no viable grass. |
| Bare ground (n = 4) | A 2-m-radius circle with bare soil and no growing plants. We weeded the area, which originally had relatively little grass. The area received direct sunlight all day. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kandori, I.; Ogata, Y.; Yokoi, T. Nesting Preferences of Osmia orientalis (Hymenoptera: Megachilidae) in the Field and Its Potential as a Strawberry Pollinator in Greenhouses. Insects 2025, 16, 473. https://doi.org/10.3390/insects16050473
Kandori I, Ogata Y, Yokoi T. Nesting Preferences of Osmia orientalis (Hymenoptera: Megachilidae) in the Field and Its Potential as a Strawberry Pollinator in Greenhouses. Insects. 2025; 16(5):473. https://doi.org/10.3390/insects16050473
Chicago/Turabian StyleKandori, Ikuo, Yudai Ogata, and Tomoyuki Yokoi. 2025. "Nesting Preferences of Osmia orientalis (Hymenoptera: Megachilidae) in the Field and Its Potential as a Strawberry Pollinator in Greenhouses" Insects 16, no. 5: 473. https://doi.org/10.3390/insects16050473
APA StyleKandori, I., Ogata, Y., & Yokoi, T. (2025). Nesting Preferences of Osmia orientalis (Hymenoptera: Megachilidae) in the Field and Its Potential as a Strawberry Pollinator in Greenhouses. Insects, 16(5), 473. https://doi.org/10.3390/insects16050473





