Extensive DNA Barcoding of Lepidoptera of Crete (Greece) Reveals Significant Taxonomic and Faunistic Gaps and Supports the First Comprehensive Checklist of the Island’s Fauna
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
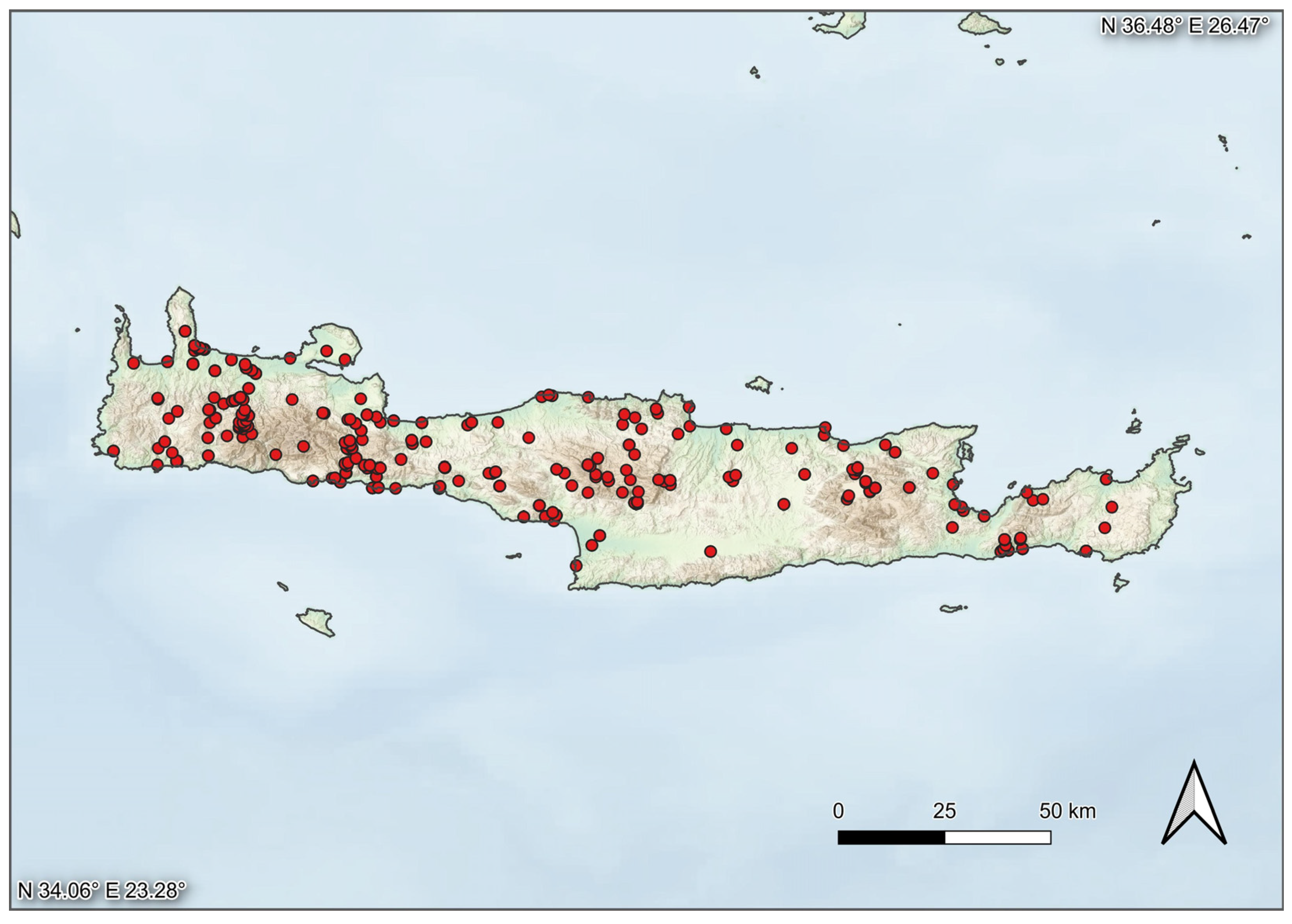
2.1. DNA Barcoding
2.2. Checklist
- Genetically validated additional species (some morphologically examined);
- Unpublished, morphologically verified specimens from collections.
3. Results
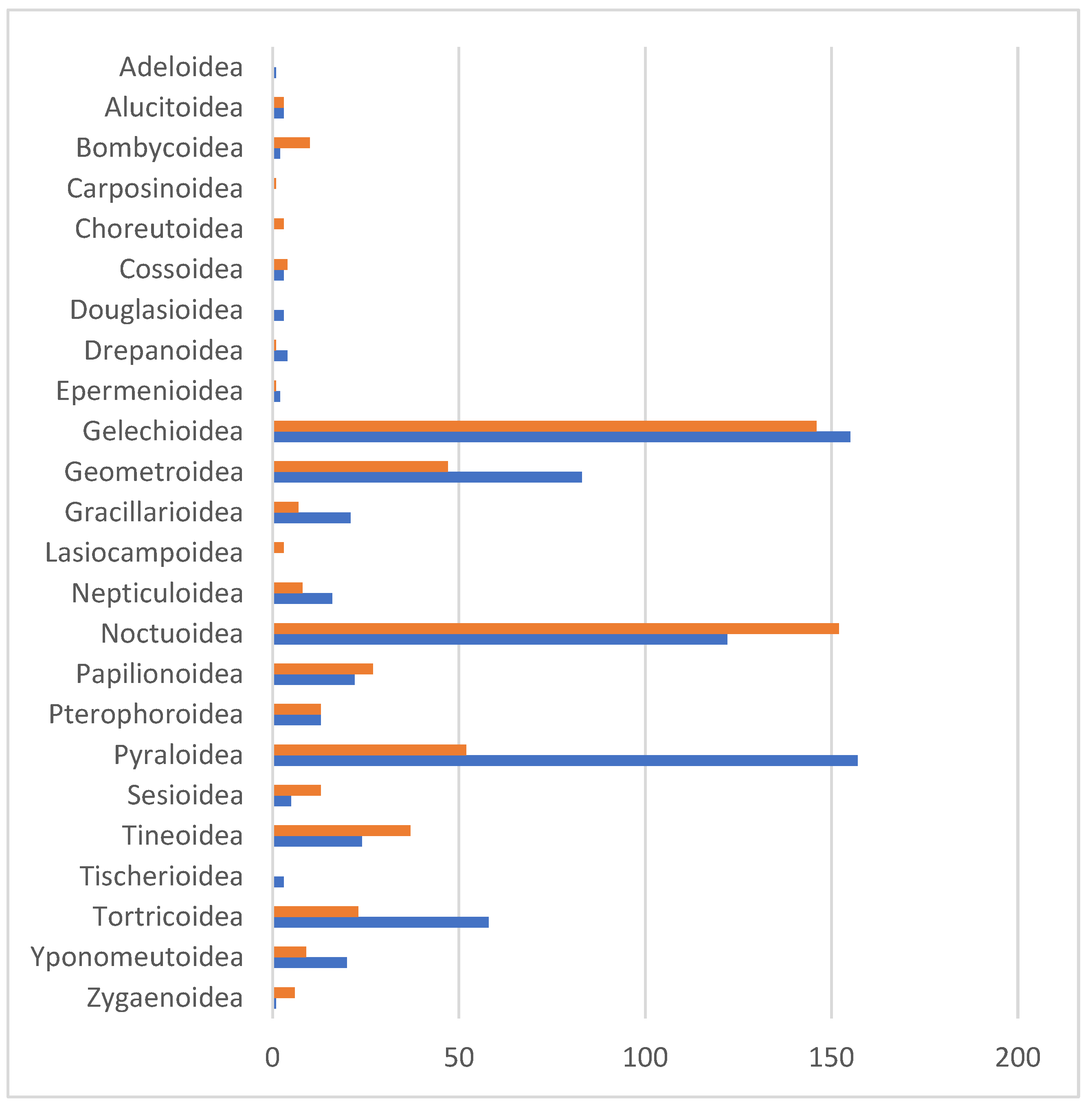
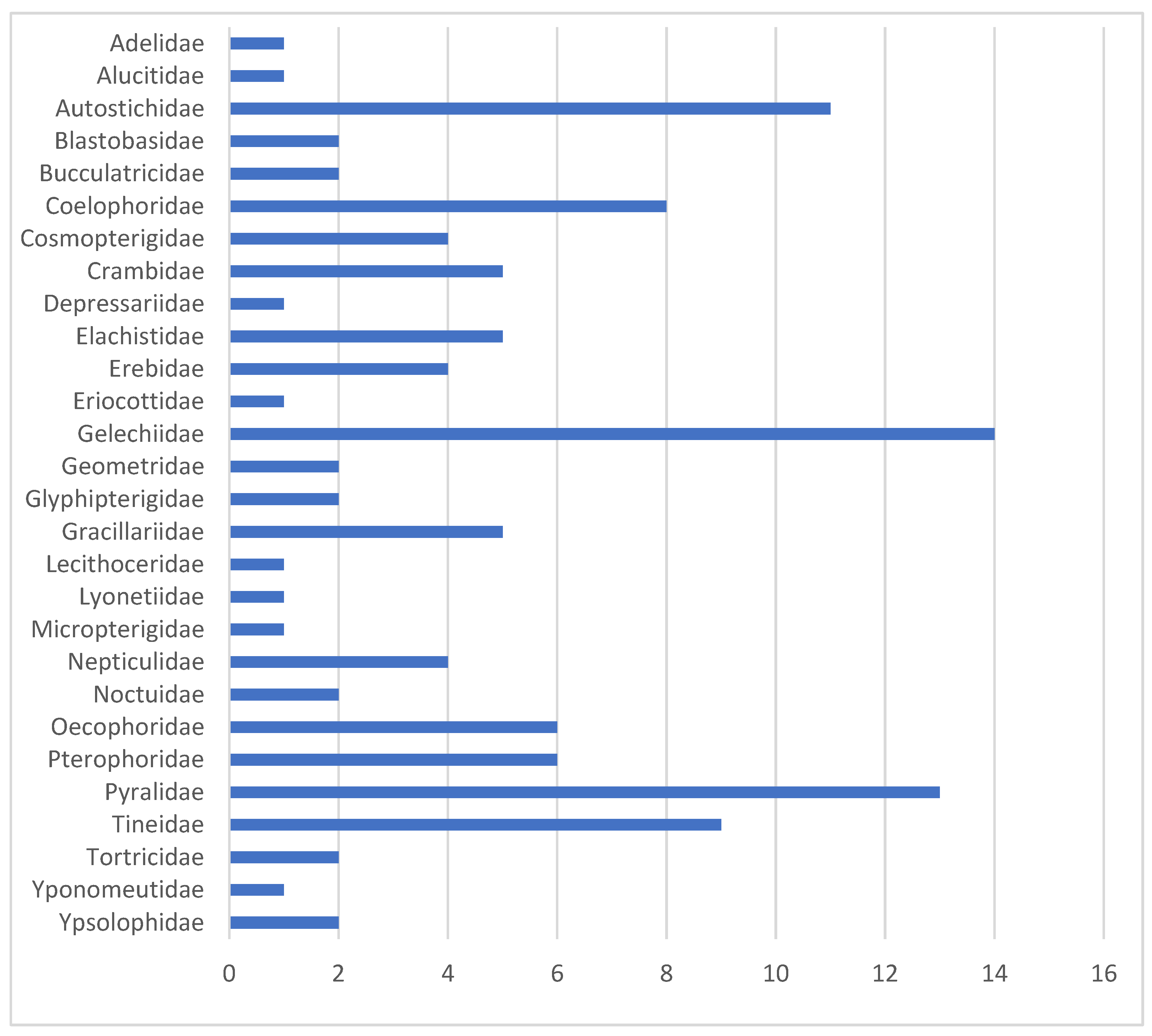
3.1. DNA Barcodes—General Overview
3.2. BINs Attributed to Linnaean Names
3.3. Unassigned BINs—Potential Cryptic Diversity
3.4. New Faunistic Records
3.4.1. New Records with Accompanying DNA Barcodes
3.4.2. New Records Based on Morphology
3.4.3. Reliable New Records Based on Photographs
3.5. Revised and Updated Checklist
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Grove, A.T.; Rackham, O. Threatened landscapes in the Mediterranean: Examples from Crete. Landsc. Urban Plan. 1993, 24, 279–292. [Google Scholar] [CrossRef]
- Rackham, O.; Moody, J.A. The Making of the Cretan Landscape; Manchester University Press: Manchester, UK, 1996; pp. xvii+237. [Google Scholar]
- di Castri, F. Mediterranean–type shrublands of the world. In Mediterranean-Type Shrublands of the World Ecosystems of the World II: Mediterranean-Type Shrublands; di Castri, F., Goodall, D.W., Specht, R.L., Eds.; Elsevier: Amsterdam, The Netherlands, 1981; pp. 1–52. [Google Scholar]
- Vogiatzakis, I.; Rackham, O. Crete. In Mediterranean Island Landscapes: Natural and Cultural Approaches; Vogiatzakis, I., Pungetti, G., Mannion, A.M., Eds.; Springer: Dordrecht, The Netherlands, 2008; pp. 245–270. [Google Scholar] [CrossRef]
- Beerli, P.; Hotz, H.; Uzzell, T. Geologically dated sea barriers calibrate a protein clock for Aegean water frogs. Evolution 1996, 50, 1676–1687. [Google Scholar] [CrossRef] [PubMed]
- Schule, W. Mammals, vegetation and the initial human settlement of the Mediterranean Islands—A palaeoecological approach. J. Biogeogr. 1993, 20, 399–412. [Google Scholar] [CrossRef]
- Muer, T.; Sauerbier, H.; Jahn, R. Flora Cretica. A Complete Handbook of All Flowering Plants, Lycopods and Ferns Occurring on the Island of Crete and Surrounding Islets; Kleinsteuber Books: Karlsruhe, Germany, 2024; pp. 1–1254. [Google Scholar]
- Menteli, V.; Krigas, N.; Avramakis, M.; Turland, N.; Vokou, D. Endemic plants of Crete in electronic trade and wildlife tourism: Current patterns and implications for conservation. J. Biol. Res. Thessalon. 2019, 26, 10. [Google Scholar] [CrossRef]
- Leestmans, R. Histoire de l’éxploration lépidoptérique de l’ile de Crète (Insecta, Lepidoptera). Linneana Belg. 1988, 11, 389–413. [Google Scholar]
- Gozmány, L. The Lepidoptera of Greece and Cyprus. Fauna Graecia IX. Volume 1; Hellenic Zoological Society: Athens, Greece, 2012; pp. 1–409. [Google Scholar]
- Rebel, H. Die Lepidopterenfauna Kretas. Ann. K. K. Naturhist. Hofmus. 1916, 30, 66–172. [Google Scholar]
- Karsholt, O.; Razowksi, J. The Lepidoptera of Europe. A Distributional Checklist; Apollo Books: Stenstrup, Denmark, 1996; pp. 1–380. [Google Scholar]
- Bartsch, D.; Pühringer, F. Die Glasflügler Kretas (Lepidoptera: Sesiidae). Entomol. Z. 2005, 115, 131–139. [Google Scholar]
- Ruckdeschel, W. Die Geometriden Kretas (Lepidoptera, Geometridae). Nachrichtenblatt Bayer. Entomol. 2007, 56, 2–12. [Google Scholar]
- Huemer, P. Beitrag zur Wicklerfauna Kretas aus Aufsammlungen von Dr. Walter Ruckdeschel (Lepidoptera, Tortricidae). Nachrichtenblatt Bayer. Entomol. 2016, 65, 2–12. [Google Scholar]
- Karsholt, O.; Huemer, P. Review of Gelechiidae (Lepidoptera) from Crete. Linz. Biol. Beiträge 2017, 49, 159–190. [Google Scholar]
- Zlatkov, B.; Huemer, P. Remarkable confusion in some Western Palearctic Clepsis leads to a revised taxonomic concept (Lepidoptera, Tortricidae). ZooKeys 2019, 885, 51–87. [Google Scholar] [CrossRef] [PubMed]
- Zlatkov, B.; Huemer, P. Eucosma subvittana (Staudinger 1892) stat. rev., a Mediterranean species resurrected by DNA barcodes and morphology (Lepidoptera, Tortricidae). Zootaxa 2023, 5361, 451–462. [Google Scholar] [CrossRef] [PubMed]
- Basso, A.; Avtzis, D.; Burban, C.; Kerdelhué, C.; İpekdal, K.; Magnoux, E.; Rousselet, J.; Negrisolo, E.; Battisti, A. The pine processionary moth Thaumetopoea pityocampa (Notodontidae) species complex: A phylogeny-based revision. Arthropod Syst. Phylogeny 2023, 81, 1031–1050. [Google Scholar] [CrossRef]
- Bidzilya, O.; Karsholt, O.; Šumpich, J. A revision of the genus Ivanauskiella (Lepidoptera: Gelechiidae). Entomol. Musei Natl. Pragae 2023, 63, 135–164. [Google Scholar] [CrossRef]
- Bidzilya, O.V.; Huemer, P.; Karsholt, O. Thiotricha sumpichi sp. nov.—A new species of Thiotrichinae (Lepidoptera, Gelechiidae) from south-eastern Europe. ZooKeys 2023, 1173, 85–96. [Google Scholar] [CrossRef]
- Berggren, K.; Aarvik, L.; Karsholt, O.; Slagsvold, P.K.; Marthinsen, G. A new species of Brachmia Hübner (Lepidoptera, Gelechiidae) from South Europe. Nor. J. Entomol. 2023, 70, 34–41. [Google Scholar]
- Aarvik, L.; Berggren, K.; Marthinsen, G. Schiffermuelleria sempronas sp. n., a brilliant moth living in old olive trees in Crete (Lepidoptera, Oecophoridae). Nor. J. Entomol. 2023, 70, 47–54. [Google Scholar]
- Huemer, P.; Aarvik, L.; Berggren, K. A new species of Neurothaumasia Le Marchand (Lepidoptera, Tineidae) from Crete, Greece. Zootaxa 2023, 5318, 401–410. [Google Scholar] [CrossRef]
- Berggren, K.; Aarvik, L. Callima icterinella (Mann, 1867) comb. nov. (Lepidoptera, Oecophoridae), a complex consisting of seven species. Nor. J. Entomol. 2024, 71, 95–108. [Google Scholar]
- Lepiforum: Website zur Bestimmung von Schmetterlingen (Lepidoptera) und ihren Präimaginalstadien. Available online: https://lepiforum.org/ (accessed on 26 December 2024).
- Butterflies of Crete. Available online: https://butterfliesofcrete.com/ (accessed on 1 March 2025).
- Moths of Crete. Available online: https://butterfliesofcrete.com/moths-of-crete/a-z-moth-families/ (accessed on 1 March 2025).
- Dinca, V.; Dapporto, L.; Somervuo, P.; Vodă, R.; Cuvelier, S.; Gascoigne-Pees, M.; Huemer, P.; Mutanen, M.; Hebert, P.D.N.; Vila, R. High resolution DNA barcode library for European butterflies reveals continental patterns of mitochondrial genetic diversity. Commun. Biol. 2021, 4, 315. [Google Scholar] [CrossRef]
- Huemer, P.; Karsholt, O.; Aarvik, L.; Berggren, K.; Bidzilya, O.; Junnilainen, J.; Landry, J.-F.; Mutanen, M.; Nupponen, K.; Segerer, A.; et al. DNA barcode library for European Gelechiidae (Lepidoptera) suggests greatly underestimated species diversity. ZooKeys 2020, 921, 141–157. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Vaamonde, C.; Kirichenko, N.; Cama, A.; Doorenweerd, C.; Godfray, H.C.J.; Guiguet, A.; Gomboc, S.; Huemer, P.; Landry, J.-F.; Laštůvka, A.; et al. Evaluating DNA barcoding for species identification and discovery in European gracillariid moths. Front. Ecol. Evol. 2021, 9, 626752. [Google Scholar] [CrossRef]
- Huemer, P.; Mutanen, M. An incomplete European barcode library has a strong impact on the identification success of Lepidoptera from Greece. Diversity 2022, 14, 118. [Google Scholar] [CrossRef]
- Tabell, J.; Siloaho, R.; Sippola, L. The Casebearer Moths (Coleophoridae) of Northern Europe—Genitalia; Tibiale Insect Equipment Ltd.: Helsinki, Finland, 2024; pp. 1–248. [Google Scholar]
- deWaard, J.R.; Ivanova, N.V.; Hajibabaei, M.; Hebert, P.D.N. Assembling DNA Barcodes: Analytical Protocols. Methods in Molecular Biology: Environmental Genomics; Martin, C.C., Ed.; Humana Press Inc.: Totowa, NJ, USA, 2008; pp. 275–293. [Google Scholar]
- Folmer, O.; Black, M.; Hoeh, W.; Lutz, R.; Vrijenhoek, R. DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Mol. Mar. Biol. Biotechnol. 1994, 3, 294–299. [Google Scholar]
- Hebert, P.D.N.; Penton, E.H.; Burns, J.M.; Janzen, D.H.; Hallwachs, W. Ten species in one: DNA barcoding reveals cryptic species in the neotropical skipper butterfly Astraptes fulgerator. Proc. Natl. Acad. Sci. USA 2004, 101, 14812–14817. [Google Scholar] [CrossRef]
- Camacho, C.; Coulouris, G.; Avagyan, V.; Ma, N.; Papadopoulos, J.; Bealer, K.; Madden, T.L. BLAST+: Architecture and applications. BMC Bioinform. 2009, 10, 421. [Google Scholar] [CrossRef]
- Ratnasingham, S.; Hebert, P.D.N. BOLD: The Barcode of Life Data System (www.barcodinglife.org). Mol. Ecol. Notes 2007, 7, 355–364. [Google Scholar] [CrossRef]
- Ratnasingham, S.; Hebert, P.D.N. A DNA-based registry for all animal species: The Barcode Index Number (BIN) System. PLoS ONE 2013, 8, e66213. [Google Scholar] [CrossRef]
- iNaturalist. Available online: https://www.inaturalist.org (accessed on 1 February 2025).
- Observation. Available online: https://www.observation.org (accessed on 1 February 2025).
- Karsholt, O.; van Nieukerken, E.J. Lepidoptera, Moths. Fauna Europaea 2013, Version 2021.12. Available online: https://fauna-eu.org (accessed on 1 December 2021). [currently offline].
- Bassi, G.; Graf, F.; Slamka, F. New or interesting Pyraloidea for the European and Italian faunas (Insecta: Lepidoptera). SHILAP Rev. Lepidopterol. 2025, 53, 189–201. [Google Scholar] [CrossRef]
- Sattin, L.; Fiumi, G.; Floriani, A.; Govi, G. Leucochlaena labrys, a new species from Crete, Greece (Lepidoptera: Noctuidae, Xyleninae, Episemini). Ecol. Montenegrina 2025, 81, 43–53. [Google Scholar] [CrossRef]
- Timossi, G.; Huemer, P. Adela paludicolella Zeller, and Adela orientella Staudinger, Lepidoptera, Adelidae: Two distinct species revealed by morphological analysis and DNA barcoding, 1850, 1870 sp. rev. Zootaxa 2025, 5621, 249–261. [Google Scholar] [CrossRef]
- Bidzilya, O.V.; Budashkin, Y.I.; Gaedike, R. A revision of the Eudarcia glaseri-species group (Lepidoptera, Meesiidae) with description of two new species from Greece and Crimea. Zootaxa 2016, 4179, 547–560. [Google Scholar] [CrossRef] [PubMed]
- Gaedike, R. New West Palaearctic Meessiidae and Tineidae (Lepidoptera: Tineoidea). SHILAP Rev. Lepidopterol. 2019, 47, 75–86. [Google Scholar] [CrossRef]
- Gaedike, R.; Henderickx, H. A new species of Eudarcia subgenus Abchagleris and description of the hitherto unknown female of E. (A.) sutteri (Tineidae). Nota Lepidopterol. 1999, 22, 2–9. [Google Scholar]
- Gaedike, R. New species and new records of Palaearctic Meessiidae and Tineidae (Lepidoptera: Tineoidea). SHILAP Rev. Lepidopterol. 2021, 49, 627–639. [Google Scholar] [CrossRef]
- Arnscheid, W.R. Taleporia henderickxi sp. n., a new psychid species of the subfamily Taleporiinae from Crete (Lepidoptera, Psychidae). Nota Lepidopterol. 2016, 39, 93–100. [Google Scholar] [CrossRef]
- Sobczyk, T. Eochorica vardarica und Penestoglossa sutteri—Zwei neue Arten der Typhoniinae aus Südosteuropa (Lepidoptera: Psychidae). Entomol. Z. 2013, 123, 19–24. [Google Scholar]
- Hauser, E. Eine neue Schmetterlingsart aus Kreta: Peloponnesia haettenschwileri n. sp. (Lepidoptera: Psychidae). Entomol. Z. 1996, 106, 58–67. [Google Scholar]
- Hauser, E. Ein neues Subgenus und eine neue Art aus Kreta: Reisseronia (Tsikalasia) malickyi (Lepidoptera: Psychidae). Entomol. Z. 1996, 106, 433–439. [Google Scholar]
- Nel, J.; Nel, A. Contribution à la connaissance des Lépidoptères de l’île de Crète (Grèce) (Lepidoptera). Bull. Société Entomol. Fr. 2003, 108, 277–282. [Google Scholar] [CrossRef]
- Gaedike, R. Microlepidoptera of Europe. In Tineidae I (Dryadaulinae, Hapsiferinae, Euplocaminae, Scardiinae, Nemapogoninae and Meessiinae); Brill: Leiden, The Netherlands; Boston, MA, USA, 2015; Volume 7, pp. 1–308. [Google Scholar]
- Gaedike, R. Microlepidoptera of Europe. In Tineidae II (Myrmecozelinae, Perissomasticinae, Tineinae, Hieroxestinae, Teichobiinae and Stathmopolitinae); Brill: Leiden, The Netherlands; Boston, MA, USA, 2019; Volume 9, pp. 1–248. [Google Scholar]
- Petersen, G. Beitrag zur Kenntnis der ostmediterranen Tineiden (Lepidoptera: Tineidae, exclus. Nemapogoninae). Acta Entomol. Bohemoslov. 1968, 65, 52–66. [Google Scholar]
- Gaedike, R.; Sammut, P.; Seguna, A. Proterospastis orientalis Petersen, 1959, a species new to the lepidopterofauna of Malta, Greece and the whole of Europe (Lepidoptera: Tineidae, Tineinae). SHILAP Rev. Lepidopterol. 2011, 39, 37–38. [Google Scholar]
- Huemer, P.; van Nieukerken, E. Identity of some recently described Lepidoptera from France—Re-assessed with DNA barcodes and morphology. Zootaxa 2021, 4941, 301–337. [Google Scholar] [CrossRef] [PubMed]
- Mey, W. Phyllobrostis minoica sp. n. from Crete (Greece)—An expected discovery (Lepidoptera, Yponomeutoidea, Lyonetiidae). Nota Lepidopterol. 2014, 37, 161–165. [Google Scholar] [CrossRef][Green Version]
- Tokár, Z. Bucculatrix kaptarae sp. nov. (Lepidoptera: Bucculatricidae) from the mountains of western Crete. Entomol. Gaz. 2017, 68, 99–102. [Google Scholar]
- Deschka, G. Bucculatrix cretica sp. n. (Lepidoptera, Bucculatricidae). Linzer Biol. Beitr. 1991, 23, 527–531. [Google Scholar]
- Laštůvka, A.; Laštůvka, Z. Southern European Phyllonorycter species mining Quercus, with two new species (Lepidoptera: Gracillariidae). Acta Univ. Agric. Silvic. Mendel. Brun. 2007, 55, 95–110. [Google Scholar] [CrossRef]
- Nel, J.; Peslier, S. Trois microlépidoptères nouveaux pour la Crète (Grèce) (Lepidoptera, Carposinidae et Pyralidae Phycitinae). Rev. Assoc. Roussillon Entomol. 2023, 32, 148–149. [Google Scholar]
- Scholz, A.; Jäckh, E. Taxonomie und Verbreitung der Westpalaearktischen Alucita-Arten (Lepidoptera: Alucitidae [Orneodidae]). Alexanor 1994, 18, 3–64. [Google Scholar]
- Fazekas, I.; Edmunds, H. New records of Alucitidae and Pterophoridae species from Crete (Lepidoptera). Lepidopterol. Hung. 2023, 19, 25–36. [Google Scholar]
- Arenberger, E. Microlepidoptera Palaearctica. Zwölfter Band. Pterophoridae. 3. Teilband. Platyptiliinae: Platyptiliini: Stenoptilia; Goecke & Evers: Keltern, Germany, 2005; pp. 1–191. [Google Scholar]
- Gaedike, R. Two new species of the genus Epermenia Hübner, [1825] and some new distributional and taxonomic records (Lepidoptera: Epermeniidae). SHILAP Rev. Lepidopterol. 2022, 50, 755–765. [Google Scholar] [CrossRef]
- Gaedike, R. Die Genitalien der europäischen Epermeniidae (Lepidoptera:Epermeniidae). Beitr. Entomol. 1966, 16, 633–692. [Google Scholar]
- Razowski, J. Neue und wenig bekannte palaearktische Wickler-Arten (Lepidoptera, Tortricidae). Z. Wien. Entomol. Ges. 1959, 44, 81–87, pls. 2–3. [Google Scholar]
- Razowski, J. Descriptions of new Cnephasia Curtis (Tortricidae). Nota Lepidopterol. 1983, 6, 235–238. [Google Scholar]
- Osthelder, L. Beitrag zur Kleinschmetterlingsfauna Kretas. Mitt. Münchn. Entomol. Ges. 1941, 31, 365–370, pl. XV. [Google Scholar]
- Pröse, H.; Sutter, R. Cydia marathonana sp. nov., eine neue Art der succedana-Gruppe aus Griechenland und Bemerkungen über Cydia trogodana Pröse, 1988 (Lepidoptera: Tortricidae). Entomol. Z. 2003, 113, 168–169. [Google Scholar]
- Karisch, T.; Pinzari, M. Cydia molybdana (Constant, 1884): A valid species (Lepidoptera: Tortricidae: Olethreutinae). Beitr. Entomol. 2010, 60, 463–470. [Google Scholar] [CrossRef]
- Gozmány, L. Holcopogonidae. Microlepidoptera Palaearctica. Volume 10. Holcopogonidae; Goecke & Evers: Keltern, Germany, 2000; pp. 1–176. [Google Scholar]
- Aarvik, L. Symmoca sparsella Joannis, 1891 (Gelechioidea, Autostichidae) new to Europe. Nota Lepidopterol. 2012, 35, 181–183. [Google Scholar]
- Gozmány, L.A. A new species of the genus Aprominta Gozm. from Crete (Lep. Symmocidae). Dtsch. Entomol. Z. 1964, 11, 11–12. [Google Scholar] [CrossRef]
- Gozmány, L.A. Some new considerations on the generic group Symmoca Hbn. (Lep., Gelechiidae). Acta Zool. Acad. Sci. Hung. 1959, 5, 41–48. [Google Scholar]
- Gozmány, L.A. The description of some new Symmocoid taxa (Lepidoptera: Gelechiidae). Acta Zool. Acad. Sci. Hung. 1961, 7, 97–110. [Google Scholar]
- Gozmány, L.A. New Symmocid species (Lepidoptera) from the Mediterranean region. Acta Zool. Acad. Sci. Hung. 1977, 23, 87–97. [Google Scholar]
- Karsholt, O.; Lvovsky, A. A new species of the genus Herrichia Staudinger (Lepidoptera: Oecophoridae) from the Island of Crete. Entomol. Rec. J. Var. 2018, 130, 307–312. [Google Scholar]
- Rebel, H. Versammlung am 9. Februar 1917. Verh. K. K. Zool.-Bot. Ges. Wien 1917, 67, 45–55. [Google Scholar]
- Corley, M.F.V. Taxonomic notes on Portuguese Microlepidoptera I. Cacochroa rosetella Corley, sp. n. (Lepidoptera: Depressariidae, Cryptolechiinae). SHILAP Rev. Lepidopterol. 2018, 46, 75–79. [Google Scholar]
- Buchner, P.; Corley, M.F.V. Agonopterix olusatri, a new species of Depressariidae (Lepidoptera) from the West Palaearctic region. Misc. Pap. (Cent. Entomol. Stud. Ank.) 2019, 196, 1–13. [Google Scholar]
- Buchner, P.; Corley, M.F.V. Microlepidoptera of Europe. Volume 10. Depressariidae; Brill: Leiden, The Netherlands; Boston, MA, USA, 2024; pp. 1–624. [Google Scholar]
- Buchner, P. Redescription of Agonopterix selini (Heinemann, 1870) with description of Agonopterix lessini sp.n. and Agonopterix paraselini sp.n. (Lepidoptera, Gelechoidea). Gortania 2017, 38, 71–101. [Google Scholar]
- Corley, M.F.V. Five new species of microlepidoptera from Portugal. Entomol. Rec. J. Var. 2014, 126, 229–243. [Google Scholar]
- Huemer, P. DNA-based faunistics—Exemplified by surveys of Lepidoptera in Greece. Entomol. Rec. J. Var. 2024, 136, 27–35. [Google Scholar]
- Karsholt, O.; Rutten, T. The genus Bryotropha Heinemann in the Western Palaearctic (Lepidoptera: Gelechiidae). Tijdschr. Voor Entomol. 2005, 148, 77–207. [Google Scholar] [CrossRef]
- Huemer, P.; Karsholt, O. Microlepidoptera of Europe. Volume 3. Gelechiidae I; Apollo Books: Stenstrup, Denmark, 1999; pp. 1–356. [Google Scholar]
- Huemer, P.; Karsholt, O. Additions to the fauna of Gelechiidae (Gelechiinae: Teleiodini and Gelechiini) of Europe. Nota Lepidopterol. 2001, 24, 41–55. [Google Scholar]
- Koster, S.; Sinev, S.Y. Microlepidoptera of Europe. Volume 5. Momphidae s. l.; Apollo Books: Stenstrup, Denmark, 2003; pp. 1–387. [Google Scholar]
- Stübner, A. Taxonomische Revision der Coleophora frischella-Artengruppe (Coleophoridae). Nota Lepidopterol. 2007, 30, 121–172. [Google Scholar]
- Baldizzone, G. Records of the Lepidoptera of Greece based on the collections of G. Christensen and L. Gozmány: III, Coleophoridae. Contribuzioni alla conoscenza dei Coleophoridae, XXXII. Annls. Mus. Goulandris 1983, 6, 207–248. [Google Scholar]
- Baldizzone, G.; van der Wolf, H.W. Corrections of and additions to the Checklist of European Coleophoridae (Lepidoptera: Coleophoridae). SHILAP Rev. Lepidopterol. 2000, 28, 395–428. [Google Scholar]
- Baldizzone, G. Fauna d’Italia. Volume 53. Lepidoptera, Coleophoridae; Calderini: Bologna, Italy, 2019; pp. 1–907. [Google Scholar]
- Kaila, L. Notes on the genus Perittia of the West Palaearctic region with descriptions of three new species (Lepidoptera: Elachistidae). Zootaxa 2009, 2230, 16–28. [Google Scholar] [CrossRef]
- Kaila, L. A taxonomic revision of the Elachista bedellella (Sircom) complex (Lepidoptera: Elachistidae: Elachistinae). Zootaxa 2007, 1629, 1–25. [Google Scholar] [CrossRef]
- Traugott-Olsen, E. Three new Elachista–species & supplement to the description of the five n.sp. from Sierra Nevada. SHILAP Rev. Lepidopterol. 1985, 13, 169–174, pls. 1–4. [Google Scholar]
- Kaila, L. New Palearctic species of the Elachista bifasciella group (Lepidoptera: Gelechioidea, Elachistidae). SHILAP Rev. Lepidopterol. 2015, 43, 385–423. [Google Scholar]
- Parenti, U.; Varalda, P.G. Revision of European Elachistidae (Lepidoptera, Elachistidae). The genus Cosmiotes Clemens, 1860. Boll. Mus. Regionale. Sci. Nat. Torino 2003, 20, 347–370. [Google Scholar]
- Bengtsson, B.A. Microlepidoptera of Europe. Volume 2. Scythrididae; Apollo Books: Stenstrup, Denmark, 1997; pp. 1–301. [Google Scholar]
- Reisser, H. Weitere neue Heteroceren aus Kreta. Z. Wien. Entomol. Ges. 1962, 47, 193–216. [Google Scholar]
- Špatenka, K. Neue paläarktische Glasflügler-Arten (Lepidoptera: Sesiidae). Entomol. Z. 2001, 111, 75–80. [Google Scholar]
- Rebel, H. Bericht der Sektion für Lepidopterologie. Versammlung am 4. Dezember 1903. Verh. K. K. Zool.-Bot. Ges. Wien 1904, 54, 1–4. [Google Scholar]
- Pamperis, L. The butterflies of Greece—An Update of Distribution Maps, Plates and Diagrams 3.3, in Map 3.4, in Chart 4.15 and in Chart 4.16; Editions Pamperis: Athens, Greece, 2022; pp. 1–245. [Google Scholar]
- Freyer, C.F. Neuere Beiträge zur Schmetterlingskunde mit Abbildungen nach der Natur. 5. Band; C.F. Freyer: Augsburg, Germany, 1842–1845; pp. 1–166, pls. 385–480. [Google Scholar]
- Slamka, F. Pyraloidea of Europe (Lepidoptera). Volume 1. Pyralinae, Galleriinae, Epipaschiinae, Cathariinae & Odontiinae; F. Slamka: Bratislava, Slovakia, 2006; pp. 1–138. [Google Scholar]
- Slamka, F. Pyraloidea of Europe (Lepidoptera). Volume 4. Phycitinae—Part 1; F. Slamka: Bratislava, Slovakia, 2019; pp. 1–432. [Google Scholar]
- Roesler, U. Phycitinen-Studien VI (Lepidoptera, Pyralidae). Entomol. Z. 1969, 79, 149–154. [Google Scholar]
- Goater, B.; Nuss, M.; Speidel, W. Microlepidoptera of Europe. In Pyraloidea I (Crambidae: Acentropinae, Evergestinae, Heliothelinae, Schoernobiinae, Scopariinae); Apollo Books: Stenstrup, Denmark, 2005; Volume 4, pp. 1–304. [Google Scholar]
- Leraut, P. Contribution à l’étude des Scopariinae 2. Onze nouveaux taxa (dont deux nouveaux genres) de la zone ouest-paléarctique (Lep. Crambidae). Alexanor 1982, 12 (Suppl. S6), 1–18. [Google Scholar]
- Slamka, F. Pyraloidea of Europe (Lepidoptera). Volume 2. Crambinae & Schoenobiinae; F. Slamka: Bratislava, Slovakia, 2008; pp. 1–223. [Google Scholar]
- Slamka, F. Pyraloidea of Europe (Lepidoptera). Volume 3. Pyraustinae & Spilomelinae; F. Slamka: Bratislava, Slovakia, 2013; pp. 1–357. [Google Scholar]
- Mally, R.; Segerer, A.H.; Nuss, M. Udea ruckdescheli sp. n. from Crete and its phylogenetic relationships (Pyraloidea, Crambidae, Spilomelinae). Nota Lepidopterol. 2016, 39, 123–135. [Google Scholar] [CrossRef][Green Version]
- Hausmann, A. The Geometrid Moths of Europe. Volume 1. Introduction. Archiearinae, Orthostixinae, Desmobathrinae, Alsophilinae, Geometrinae; Apollo Books: Stenstrup, Denmark, 2001; pp. 1–282. [Google Scholar]
- Skou, P.; Sihvonen, P. The Geometrid Moths of Europe. Volume 5. Subfamily Ennominae I; Brill: Leiden, The Netherlands; Boston, MA, USA, 2015; pp. 1–657. [Google Scholar]
- Müller, B.; Erlacher, S.; Hausmann, A.; Rajaei, H.; Sihvonen, P.; Skou, P. The Geometrid Moths of Europe. Volume 6. Subfamily Ennominae II (Boarmiini, Gnophini, Additions to Previous Volumes); Brill: Leiden, The Netherlands; Boston, MA, USA, 2019; Part 1: 1–562, part 2: 563–906. [Google Scholar]
- Reisser, H. Neue Heteroceren aus Kreta. Z. Wien. Entomol. Ges. 1958, 43, 105–128. [Google Scholar]
- Hausmann, A. The Geometrid Moths of Europe. Volume 2. Sterrhinae; Apollo Books: Stenstrup, Denmark, 2004; pp. 1–600. [Google Scholar]
- Hausmann, A. Zweiter Beitrag zur Taxonomie und Systematik der Gattung Glossotrophia Prout. 1913. Mitt. Münchn. Entomol. Ges. 1993, 83, 77–107. [Google Scholar]
- Reisser, H. Beiträge zur Kenntnis der Sterrhinae (Lep. Geom.) II. Codonia (Cosymbia) ariadne spec. nov. Z. Wien. EntVer. 1939, 23, 169–170. [Google Scholar]
- Hausmann, A.; Viidalepp, J. The Geometrid Moths of Europe. Volume 3. Larentiinae I; Apollo Books: Vester Skerninge, Denmark, 2012; pp. 1–743. [Google Scholar]
- Reisser, H. Anaitis cretica n. sp. (Lepidoptera: Geometridae). Z. ArbGem. Österr. Entomol. 1974, 24, 133–139. [Google Scholar]
- Mironov, V. The Geometrid Moths of Europe. Volume 4. Larentiinae II (Perizomini and Eupitheciini); Apollo Books: Stenstrup, Denmark, 2003; pp. 1–464. [Google Scholar]
- Hacker, H. Die Noctuidae Griechenlands. Mit einer Übersicht über die Fauna des Balkanraumes. Herbipoliana 1989, 2, 1–589. [Google Scholar]
- Witt, T.J.; Ronkay, L. (Eds.) Noctuidae Europaeae. Volume 13. Lymantriinae and Arctiinae Including Phylogeny and Check List of the Quadrifid Noctuoidea of Europe; Entomological Press: Sorø, Denmark, 2011; pp. 1–448. [Google Scholar]
- Reisser, H. Ocneria eos sp. nov., eine neue Lymantriide aus Kreta (Vorläufige Beschreibung). Nachrichtenblatt Bayer. Entomol. 1962, 11, 9–10. [Google Scholar]
- de Freina, J.J. Paidia minoica sp. n. von Kreta, eine neue Paidia für Europa. Beschreibung und Angaben zur Phänologie der Art (Lepidoptera: Arctiidae, Lithosiinae). Mitt. Münchn. Entomol. Ges. 2006, 96, 21–27. [Google Scholar]
- Fibiger, M.; Ronkay, L.; Yela, J.L.; Zilli, A. Noctuidae Europaeae. Volume 12. Rivulinae, Boletobiinae, Hypenodinae, Araeopteroninae, Eublemminae, Herminiinae, Hypeninae, Phytometrinae, Euteliinae, and Micronoctuidae. Including Supplement to Volumes 1–11; Entomological Press: Sorø, Denmark, 2010; pp. 1–451. [Google Scholar]
- Goater, B.; Ronkay, L.; Fibiger, M. Noctuidae Europaeae. Volume 10. Catocalinae & Plusiinae; Entomological Press: Sorø, Denmark, 2003; pp. 1–452. [Google Scholar]
- Fibiger, M.; Ronkay, L.; Steiner, A.; Zilli, A. Noctuidae Europaeae. Volume 11. Pantheinae—Bryophilinae; Entomological Press: Sorø, Denmark, 2009; pp. 1–504. [Google Scholar]
- Pinker, R.; Reisser, H. Eine neue Allophyes aus Creta (Lep., Noctuidae). Z. ArbGem. Österr. Entomol. 1978, 29, 92. [Google Scholar]
- Svendsen, P.; Fibiger, M. New endemic taxa from Crete: Cryphia omalosi, sp. n. and Chersotis larixia idai, subsp. n. (Noctuidae, Bryophilinae, Noctuinae). Esperiana 1998, 6, 469–471. [Google Scholar]
- Fibiger, M.; Hacker, H. Noctuidae Europaeae. Volume 9. Amphipyrinae, Condicinae, Eriopinae, Xyleninae (Part); Entomological Press: Sorø, Denmark, 2007; pp. 1–410, 12 pls. [Google Scholar]
- Hacker, H. Revision of the genus Caradrina Ochsenheimer, 1816, with notes on other genera of the tribus Caradrini (Lepidoptera, Noctuidae). Esperiana 2004, 10, 7–690, pls. 1–27. [Google Scholar]
- Ronkay, L.; Varga, Z. Neue Arten und Unterarten aus der Gattung Ammoconia Lederer, 1857 (Lepidoptera: Noctuidae). Acta Zool. Acad. Sci. Hung. 1984, 30, 481–491. [Google Scholar]
- Fibiger, M. Contribution to the knowledge of the Lepidoptera fauna of Greece. Noctuidae in Crete during November 1991—With a description of one new species and three new subspecies (Lepidoptera, Noctuidae). Esperiana 1993, 3, 379–390. [Google Scholar]
- Herrich-Schäffer, G.A.W. Systematische Bearbeitung der Schmetterlinge von Europa, zugleich als Text, Revision und Supplement zu Jakob Hübner’s Sammlung Europäischer Schmetterlinge. Zweiter Band. Die Schwärmer, Spinner und Eulen; G.J. Manz: Regensburg, Germany, 1843–1855; pp. 1–450, Index pp. 1–64, Hepialides pl. 1, Cossides pl. 1–2, Zygaenides pl. 1–16, Sesiides pl. 1–10, Sphingides pl. 1–4, Bombycides pl. 1–32, Noctuides pl. 1–124, Nycteolidae pl. 1. [Google Scholar]
- Ronkay, L.; Hacker, H. Episema gozmanyi sp. n. from Crete (Lepidoptera: Noctuidae). Nota Lepidopterol. 1985, 8, 69–76. [Google Scholar]
- Zilli, A.; Ronkay, L.; Fibiger, M. Noctuidae Europaeae. Volume 8. Apamaeini; Entomological Press: Sorø, Denmark, 2005; pp. 1–323. [Google Scholar]
- Hacker, H.; Ronkay, L.; Hreblay, M. Noctuidae Europaeae. Volume 4. Hadeninae I; Entomological Press: Sorø, Denmark, 2002; pp. 1–419. [Google Scholar]
- Fibiger, M. (Ed.) Noctuidae Europaeae. Volume 1. Noctuinae I; Entomological Press: Sorø, Denmark, 1990; pp. 1–208, pls. 1–16. [Google Scholar]
- Fibiger, M. (Ed.) Noctuidae Europaeae. Volume 3. Noctuinae III; Entomological Press: Sorø, Denmark, 1997; pp. 1–418. [Google Scholar]
- Rebel, H. Versammlung am 6. April 1906. Verh. K. K. Zool.-Bot. Ges. Wien 1917, 56, 232–243. [Google Scholar]
- Prozorov, A.M.; Prozorova, T.A.; Volkova, J.S.; Yakovlev, R.V.; Sitar, C.; Saldaitis, A.; Petrányi, G.; Revay, E.; Müller, G.C. Notes on Pachypasa otus and the description of a new Iranian Pachypasa species (Lepidoptera, Lasiocampidae, Lasiocampinae, Lasiocampini). Ecol. Montenegrina 2022, 56, 14–27. [Google Scholar] [CrossRef]
- Danner, F.; Eitschberger, U.; Surholt, B. Die Schwärmer der Westlichen Palaearktis. Bausteine zu Einer Revision (Lepidoptera, Sphingidae); Herbipoliana; ConchBooks: Harxheim, Germany, 1998; Volume 4, pp. 1–719. [Google Scholar]
- Arnscheid, W.R.; Weidlich, M. Microlepidoptera of Europe. Volume 8. Psychidae; Brill: Leiden, The Netherlands; Boston, MA, USA, 2017; pp. 1–423. [Google Scholar]
- Hofmann, A.F.; Tremewan, W.G. The Natural History of Burnet Moths (Zygaena Fabricius, 1775) (Lepidoptera: Zygaenidae); Proceedings of the Museum Witt; Museum Witt: Munich, Germany, 2020; Volume 6, i–xxvi; pp. 1–1097. [Google Scholar]
- Petsopoulos, D.; Leblois, R.; Sauné, L.; İpekdal, K.; Aravanopoulos, F.A.; Kerdelhué, C.; Avtzis, D.N. Crossing the Mid-Aegean Trench: Vicariant evolution of the Eastern pine processionary moth, Thaumetopoea wilkinsoni (Lepidoptera: Notodontidae), in Crete. Biol. J. Linn. Soc. 2018, 124, 228–236. [Google Scholar] [CrossRef]
- Evans, W.H. A revision of the genus Tarucus (Lepidoptera: Lycaenidae) of Europe, North Africa and Asia. Entomologist 1955, 88, 179–187. [Google Scholar]





| Alucitidae | Nepticulidae |
|---|---|
| Alucita grammodactyla (Zeller, 1841) * | Stigmella basiguttella (Heinemann, 1862) |
| Bedelliidae | Stigmella fasciata (van Nieukerken and Johansson, 2003) |
| Bedellia ehikella (Szőcs, 1967) | Trifurcula eurema (Tutt, 1899) |
| Blastobasidae | Noctuidae |
| Blastobasis glandulella (Riley, 1871) * | Bryophila felina (Eversmann, 1852) |
| Tecmerium perplexus (Gozmány, 1957) | Caradrina kadenii Freyer, [1836] |
| Bucculatricidae | Nolidae |
| Bucculatrix bechsteinella (Bechstein and Scharfenberg, 1805) * | Meganola kolbi (Daniel, 1935) |
| Bucculatrix phagnalella (Walsingham, 1908) * | Nola subchlamydula (Staudinger, 1870) |
| Coleophoridae | Oecophoridae |
| Coleophora berbera (Baldizzone, 1988) * | Batia lunaris (Haworth, 1828) |
| Coleophora curictae (Baldizzone, 2016) * | Batia samosella (Sutter, 2003) |
| Coleophora deauratella (Lienig and Zeller, 1846) | Plutellidae |
| Coleophora nutantella (Mühlig and Frey, 1857) | Rhigognostis annulatella (Curtis, 1832) |
| Coleophora oriolella (Zeller, 1849) | Pyralidae |
| Coleophora variicornis (Toll, 1952) | Acrobasis advenella (Zincken, 1818) |
| Cosmopterigidae | Acrobasis centunculella (Mann, 1859) |
| Anatrachyntis badia (Hodges, 1962) | Acrobasis consociella (Hübner, [1813]) |
| Crambidae | Acrobasis getuliella (Zerny, 1914) * |
| Agriphila bleszynskiella (Amsel, 1961) ** | Acrobasis glaucella (Staudinger, 1859) |
| Cataclysta lemnata (Linnaeus, 1758) | Acrobasis marmorea (Haworth, 1811) |
| Euchromius ramburiellus (Duponchel, 1836) | Acrobasis obtusella (Hübner, 1796) |
| Euchromius vinculellus (Zeller, 1847) | Amphithrix sublineatella (Staudinger, 1859) * |
| Metasia carnealis (Treitschke, 1829) | Coenochroa ablutella (Zeller, 1839) |
| Pediasia contaminella (Hübner, 1796) | Dioryctria mendacella (Staudinger, 1859) |
| Ostrinia furnacalis (Guenée, 1854) ** | Dioryctria pineae (Staudinger, 1859) |
| Depressariidae | Elegia atrifasciella (Ragonot, 1887) |
| Agonopterix nodiflorella (Millière, 1866) | Ephestia cypriusella (Roesler, 1965) |
| Agonopterix straminella (Staudinger, 1859) | Epischnia asteris (Staudinger, 1870) * |
| Depressaria bantiella (Rocci, 1934) * | Epischnia cretaciella (Mann, 1869) |
| Depressaria discipunctella Herrich-Schäffer, [1854] | Etiella zinckenella (Treitschke, 1832) |
| Rosetea corfuella (Lvovsky, 2000) | Euzophera bigella (Zeller, 1848) |
| Douglasiidae | Euzophera lunulella (Costa, [1836]) |
| Tinagma ocnerostomella (Stainton, 1850) | Euzophera osseatella (Treitschke, 1832) |
| Drepanidae | Euzopherodes vapidella (Mann, 1857) |
| Cilix glaucata (Scopoli, 1763) | Keradere tengstroemiella (Erschoff, 1874) |
| Elachistidae | Melathrix coenulentella (Zeller, 1846) * |
| Blastodacna atra (Haworth, 1828) | Merulempista brucella (Staudinger, 1879) |
| Elachista differens (Parenti, 1978) | Merulempista turturella (Zeller, 1848) * |
| Elachista gleichenella (Fabricius, 1781) | Morosaphycita cleopatrella (Ragonot, 1887) * |
| Elachista pigerella (Herrich-Schäffer, [1854]) | Myelois ossicolor (Ragonot, 1893) * |
| Elachista scirpi (Stainton, 1887) | Nyctegretis ruminella (De la Harpe, 1860) |
| Perittia echiella (De Joannis, 1902) * | Pempelia brephiella (Staudinger, 1879) * |
| Epermeniidae | Phycita acericola (Kuznetsov, 1960) |
| Epermenia chaerophyllella (Goeze, 1783) | Phycita torrenti (Agenjo, 1962) |
| Erebidae | Phycitodes inquinatella (Ragonot, 1887) |
| Eilema muscula (Staudinger, 1899) | Phycitodes lacteella (Rothschild, 1915) |
| Gelechiidae | Phycitodes saxicola (Vaughan, 1870) |
| Anarsia leberonella (Réal, 1994) | Psorosa mediterranella (Amsel, 1953) * |
| Aproaerema montanata (Gozmány, 1957)* | Pyralis kacheticalis (Christoph, 1893) |
| Aristotelia subdecurtella (Stainton, 1859) | Rhodophaea formosa (Haworth, 1811) * |
| Bryotropha figulella (Staudinger, 1859) | Scythropiidae |
| Carpatolechia decorella (Haworth, 1812) | Scythropia crataegella (Linnaeus, 1767) |
| Mondeguina mediterranella (Nel and Varenne, 2012) * | Tineidae |
| Monochroa hornigi (Staudinger, 1883) * | Nemapogon hungaricus (Gozmány, 1960) |
| Oxypteryx immaculatella (Douglas, 1850) * | Opogona omoscopa (Meyrick, 1893) * |
| Parastenolechia nigrinotella (Zeller, 1847) * | Tischeriidae |
| Geometridae | Coptotriche angusticolella (Duponchel, [1843]) |
| Chloroclysta siterata (Hufnagel, 1767) | Tortricidae |
| Glyphipterigidae | Bactra lancealana (Hübner, [1799]) * |
| Acrolepiopsis vesperella (Zeller, 1850) | Cnephasia genitalana (Pierce and Metcalfe, 1915) * |
| Orthotelia sparganella (Thunberg, 1788) | Cydia conicolana (Heylaerts, 1874) * |
| Gracillariidae | Cydia johanssoni (Aarvik and Karsholt, 1993) |
| Acrocercops brongniardella (Fabricius, 1798) | Cydia splendana (Hübner, [1799]) |
| Acrocercops tacita (Triberti, 2001) | Epiblema cirsiana (Zeller, 1843) * |
| Aspilapteryx tringipennella (Zeller, 1839) | Epiblema cnicicolana (Zeller, 1847) * |
| Calybites phasianipennella (Hübner, [1813]) | Notocelia cynosbatella (Linnaeus, 1758) |
| Parornix anguliferella (Zeller, 1847) | Pammene blockiana (Herrich-Schäffer, 1851) * |
| Parornix tenella (Rebel, 1919) * | Pammene gallicolana (Lienig and Zeller, 1846) |
| Phyllonorycter cephalariae (Lhomme, 1934) | Pelochrista caecimaculana (Hübner, [1799]) * |
| Phyllonorycter corylifoliella (Hübner, 1796) | Pelochrista modicana (Zeller, 1847) |
| Phyllonorycter distentella (Zeller, 1846) * | Phtheochroa ochralana (Chrétien, 1915) |
| Phyllonorycter triflorella (de Peyerimhoff, 1871) | Zeiraphera isertana (Fabricius, 1794) * |
| Triberta cistifoliella (Groschke, 1944) | Yponomeutidae |
| Momphidae | Paradoxus osyridellus (Millière, 1869) |
| Mompha epilobiella ([Denis and Schiffermüller], 1775) | Ypsolophidae |
| Ypsolopha alpella ([Denis and Schiffermüller], 1775) |
| Taxon | Family |
|---|---|
| Coleophora kroneella (Fuchs, 1899) | Coleophoridae |
| Coleophora nigridorsella (Amsel, 1935) | Coleophoridae |
| Coleophora ptarmicia (Walsingham, 1910) | Coleophoridae |
| Phlyctaenomorpha sinuosalis (Le Cerf, [1910]) | Crambidae |
| Tegostoma comparalis (Hübner, 1796) | Crambidae |
| Depressaria depressana (Fabricius, 1775) | Depressariidae |
| Eublemma purpurina ([Denis and Schiffermüller], 1775) | Erebidae |
| Caryocolum mucronatella (Chrétien, 1900) | Gelechiidae |
| Lanceoptera panochra (Janse, 1960) | Gelechiidae |
| Phyllonorycter platani (Staudinger, 1870) | Gracillariidae |
| Phyllonorycter sublautella (Stainton, 1869) | Gracillariidae |
| Mompha divisella Herrich-Schäffer, [1854] | Momphidae |
| Stigmella muricatella (Klimesch, 1978) | Nepticulidae |
| Mesapamea secalis (Linnaeus, 1758) | Noctuidae |
| Meganola kolbi (Daniel, 1935) | Nolidae |
| Meganola togatulalis ([Denis and Schiffermüller], 1775) | Nolidae |
| Pyla fusca (Haworth, 1811) | Pyralidae |
| Nemapogon variatella (Clemens, 1859) | Tineidae |
| Aethes bilbaensis (Rössler, 1877) | Tortricidae |
| Cnephasia ecullyana Réal, 1951 | Tortricidae |
| Epinotia festivana (Hübner, [1799]) | Tortricidae |
| Notocelia mediterranea (Obraztsov, 1952) | Tortricidae |
| Rhyacionia buoliana ([Denis and Schiffermüller], 1775) | Tortricidae |
| Taxon | Family |
|---|---|
| Heliothela ophideresana (Walker, 1863) | Crambidae |
| Palepicorsia ustrinalis (Christoph, 1877) | Crambidae |
| Pyrausta purpuralis (Linnaeus, 1758) | Crambidae |
| Acantholipes regularis (Hübner, 1813) | Erebidae |
| Euproctis chrysorrhoea (Linnaeus, 1758) | Erebidae |
| Apocheima hispidaria ([Denis and Schiffermüller], 1775) | Geometridae |
| Agrotis herzogi (Rebel, 1911) | Noctuidae |
| Noctua interjecta Hübner, [1803] | Noctuidae |
| Aphomia cephalonica (Stainton, 1866) | Pyralidae |
| Denticera divisella (Duponchel, [1843]) | Pyralidae |
| Galleria mellonella (Linnaeus, 1758) | Pyralidae |
| Myelois circumvoluta (Geoffroy in Fourcroy, 1785) | Pyralidae |
| Hemaris croatica (Esper, 1800) | Sphingidae |
| Mimas tiliae (Linnaeus, 1758) | Sphingidae |
| Cochylimorpha langeana (Kalchberg, 1898) | Tortricidae |
| Zelleria hepariella (Stainton, 1849) | Yponomeutidae |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huemer, P.; Berggren, K.; Aarvik, L.; Rennwald, E.; Hausmann, A.; Segerer, A.; Staffoni, G.; Aspaas, A.M.; Trichas, A.; Hebert, P.D.N. Extensive DNA Barcoding of Lepidoptera of Crete (Greece) Reveals Significant Taxonomic and Faunistic Gaps and Supports the First Comprehensive Checklist of the Island’s Fauna. Insects 2025, 16, 438. https://doi.org/10.3390/insects16050438
Huemer P, Berggren K, Aarvik L, Rennwald E, Hausmann A, Segerer A, Staffoni G, Aspaas AM, Trichas A, Hebert PDN. Extensive DNA Barcoding of Lepidoptera of Crete (Greece) Reveals Significant Taxonomic and Faunistic Gaps and Supports the First Comprehensive Checklist of the Island’s Fauna. Insects. 2025; 16(5):438. https://doi.org/10.3390/insects16050438
Chicago/Turabian StyleHuemer, Peter, Kai Berggren, Leif Aarvik, Erwin Rennwald, Axel Hausmann, Andreas Segerer, Giorgia Staffoni, Aina Mærk Aspaas, Apostolos Trichas, and Paul D. N. Hebert. 2025. "Extensive DNA Barcoding of Lepidoptera of Crete (Greece) Reveals Significant Taxonomic and Faunistic Gaps and Supports the First Comprehensive Checklist of the Island’s Fauna" Insects 16, no. 5: 438. https://doi.org/10.3390/insects16050438
APA StyleHuemer, P., Berggren, K., Aarvik, L., Rennwald, E., Hausmann, A., Segerer, A., Staffoni, G., Aspaas, A. M., Trichas, A., & Hebert, P. D. N. (2025). Extensive DNA Barcoding of Lepidoptera of Crete (Greece) Reveals Significant Taxonomic and Faunistic Gaps and Supports the First Comprehensive Checklist of the Island’s Fauna. Insects, 16(5), 438. https://doi.org/10.3390/insects16050438






