1. Introduction
Bagrada bug,
Bagrada hilaris (Burmeister) (Hemiptera: Pentatomidae), is an invasive stink bug species that has been reported as a pest of Brassica crops in Africa, India and the Middle East since the early 1900s [
1,
2,
3]. In the Old World, it is currently widely distributed throughout its native range but also in Southeast Asia and in southern Europe, where it is recorded in the Mediterranean islands of Malta and Pantelleria (Italy) [
2,
4]. In 2008, it was first reported in southern California and it is now rapidly expanding its range throughout several southwestern US states, Hawaii and Mexico. Recently, it has been recorded in Quilicura, Chile [
2,
5,
6].
Bagrada hilaris is a polyphagous pest, infesting a large number of plant species, including crops, weeds and ornamentals belonging to 23 families [
2]. Though most of the economic damage occurs in the family Brassicaceae, it was reported also on wheat, corn, legumes [
7,
8,
9] and on capers in the island of Pantelleria (Italy) [
10,
11]. On brassica crops and capers, the bagrada bug feeds mainly on tender tissues, such as cotyledons and young leaves or flower buds, causing chlorotic lesions that may become necrotic and reduce plant growth or lead to rapid plant death [
2,
11,
12,
13].
Currently, few effective alternatives to protect crops from
B. hilaris are available. Cultural control practices, like the removal of any host plants in crops and adjacent fields, together with field sanitation (removal of fallen vegetables after harvesting), can be useful to prevent
B. hilaris spreading [
2,
11] but are expensive, laborious and time consuming. Chemicals, such as pyrethroids and neo-nicotinoids, are commonly used to prevent
B. hilaris outbreaks in invaded areas but broad-spectrum insecticides are a short-term solution, and their frequent use can induce insecticide resistance. For these reasons more sustainable methods for the economy and the environment are needed [
12,
14].
Classical biological control, based on the introduction of exotic biocontrol agents or enhancement of indigenous ones have shown promising medium- and long-term outcomes controlling bagrada bugs [
15]. Some egg parasitoid wasps have been identified and tested as potential biocontrol agents of bagrada bugs [
2,
16,
17]. Among them, the egg parasitoid
Gryon aetherium Talamas (formerly
G. gonikopalense (Sharma)) (Hymenoptera: Scelionidae) has been selected as the most promising candidate because of its relative host-specificity and its capability to detect and oviposit on buried bagrada bug eggs [
18,
19].
Integrated pest management (IPM) can include classical biological control (CBC) in combination with sterile insect technique (SIT), as components for a synergistic and effective program. SIT is a species-specific method, and it relies on the mass production, sterilization and subsequent inundative release of predominantly sterile male insects [
20]. The additive effect of the combination between the SIT and the classical biological control [
21,
22] further strengthens the opportunity to implement IPM or area-wide eradication programs for managing
B. hilaris within its invasive range [
22,
23,
24].
A pest management program that involves the use of biological control agents (BCAs) requires an effective field monitoring system for their detection, which, for stink bug pests, is based on sentinel eggs for egg parasitoids [
25]. To minimize the risk of inadvertently introducing the pest into the environment by using fertile sentinel eggs, several alternatives were evaluated, such as unfertilized and refrigerated eggs. Recently, Cristofaro et al. [
26] and Roselli et al. [
27] proposed a new concept of using sterile sentinel eggs produced by the SIT for the management of pentatomid pests since wild fertile females mating with irradiated males laid sterile eggs, which are a suitable (and long-life) substrate for the oviposition and the full larval development of egg parasitoids.
Both pentatomid pest species,
B. hilaris and
Halyomorpha halys (Stål) display a gregarious behavior before and during the winter diapause, which could be an opportunity to obtain “wild-type” competitive stink bug adults to irradiate and release in small pilot SIT program contexts [
24,
26,
28].
Previous studied showed the promising effects of the irradiation using gamma rays on the longevity, fecundity and fertility of newly emerged adults of
B. hilaris [
26]; to further the possibility of using field collected stages instead than build bio-factories, here we present a study on the effects of gamma rays on the same physiological parameters when irradiation has been conducted on mature adults and on nymph instars.
4. Discussion
Based on the experience with another pentatomid,
H. halys, critical problem facing SIT implementation on stink bugs is the impracticability of sustainable mass rearing. It has been proposed that this challenge could be mitigated by catching and rearing mature males of previously wild-harvested
B. hilaris when they aggregate before the winter, with the idea of sterilizing and releasing them at the end of the overwintering diapause during the spring [
24,
28].The success of an area-wide pest management program that has the SIT as its strategic core relies on setting up a system to release sterile males able to compete with wild males for mating. Therefore, a recent study was focused on the selection of the optimal irradiation dose, able to induce sperm sterility in irradiated males while at the same time maintaining the mating competitiveness of the sterile bagrada bug males with respect to the fertile insects [
42]. Given the difficulty in mass rearing of this species of stink bugs, the possibility of collecting large numbers of aggregating pre-diapause adults in autumn by extensive mass trapping and use of the sterile irradiated males for SIT small-scale approaches has been considered [
24].
The main objective of the behavioral bioassays carried out here was to demonstrate that gamma irradiation is effective on some physiological parameters of fifth nymphal instars and mature adults of
B. hilaris. Similar work was carried out a few years ago [
26], showing that gamma rays were able to induce sterility in the sperm of newly emerged males, a first important result supporting application of the SIT to control this important alien pest.
In contrast to the results of the previous work [
26], gamma irradiation (from 60 to 120 Gy for the adults and 32 to 100 Gy for fifth-instar nymphs) does not interfere with the longevity of adult females of
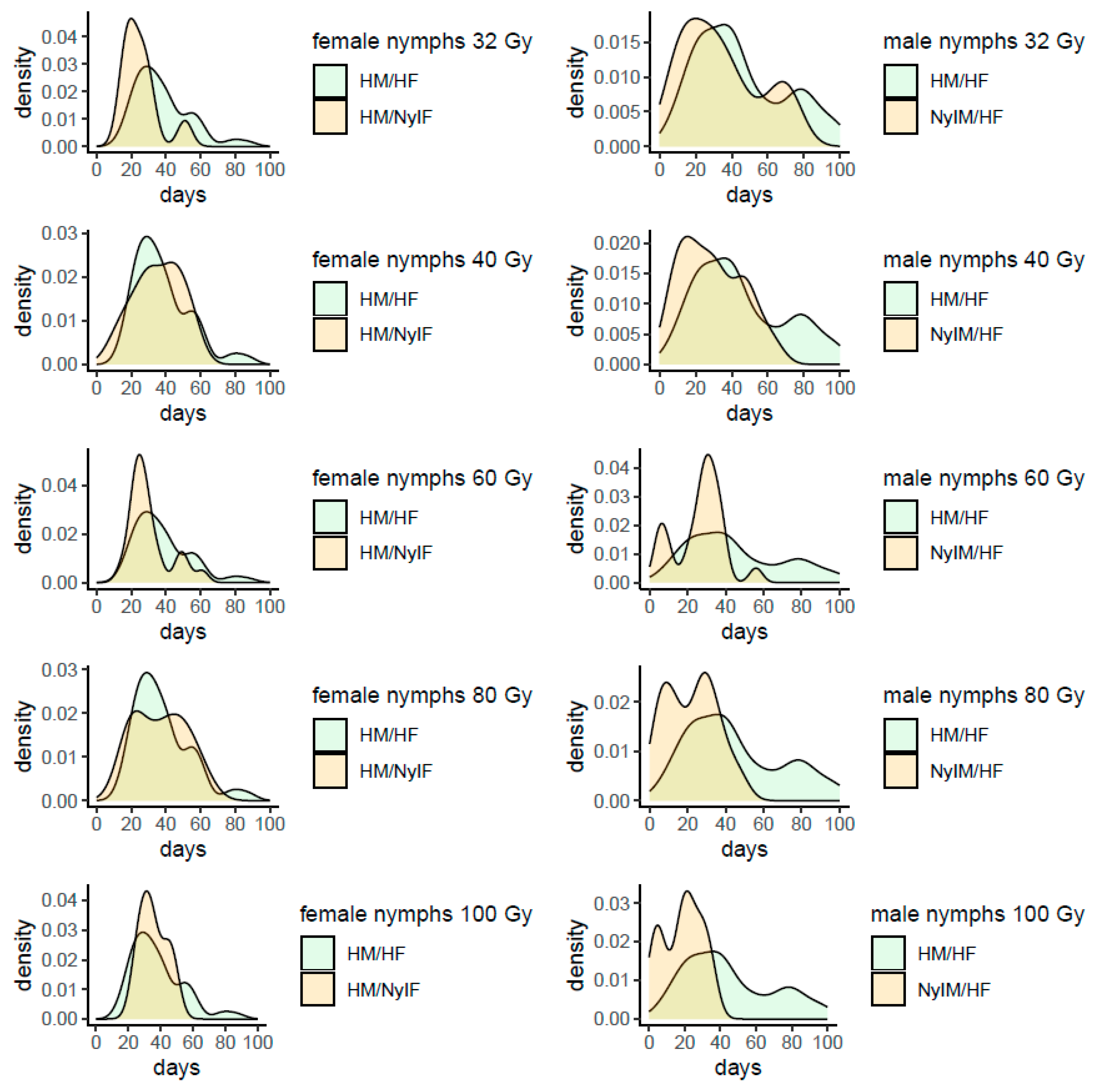
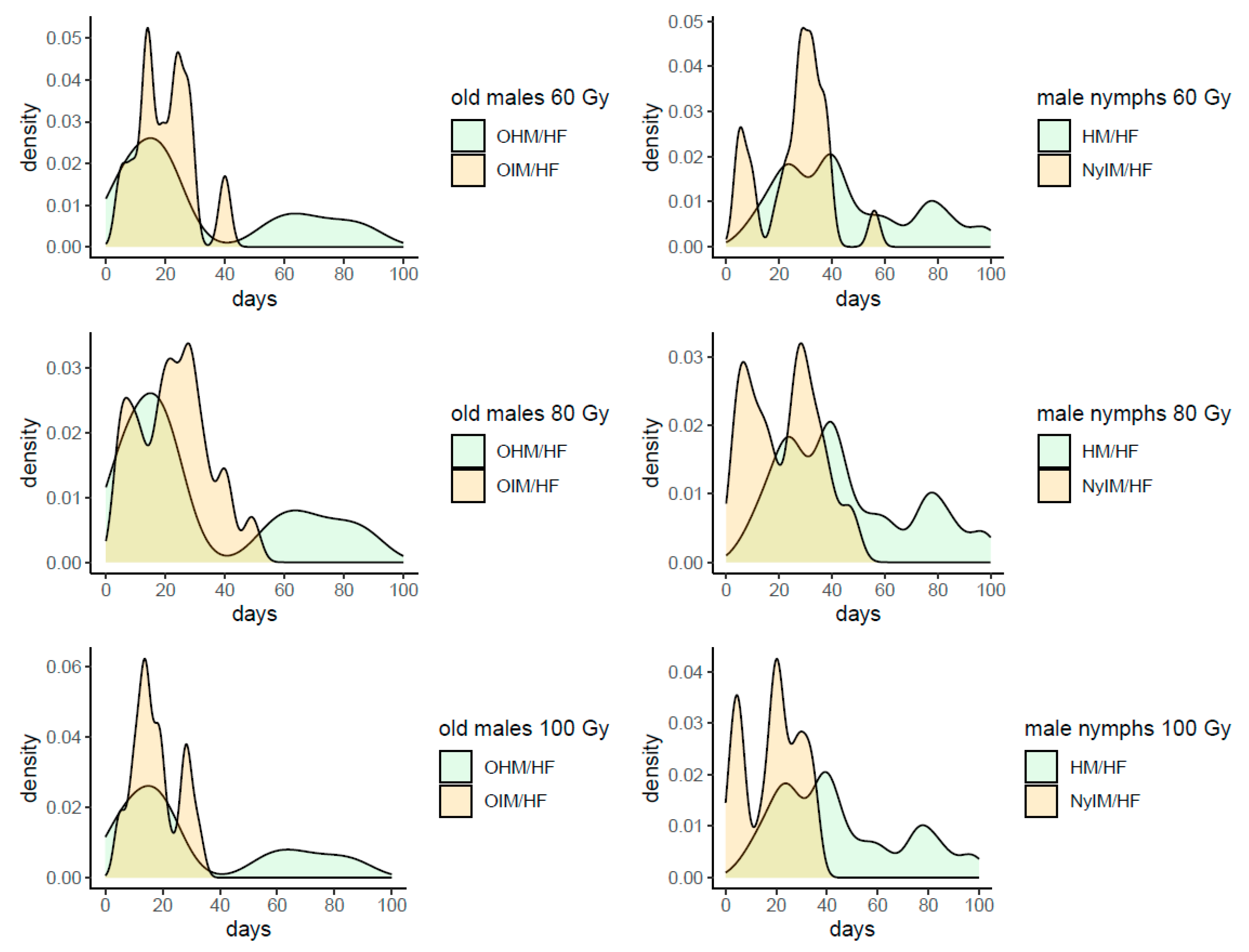
B. hilaris, while having a moderate negative impact on the survival of the males (see
Table 5). In any case, the longevity of irradiated mature males is still long enough to allow their release in the field for a SIT program: in fact, 50% of males survived at least 3 weeks or more when irradiated from 60 Gy upward. Particularly, a peak of frequency in life span of about 30 days was observed at 60 Gy, while at 80 and 100 Gy a portion higher than 50% of males survived 3 weeks at least, although in the latter case more males died within 10 days after emergence (
Figure A2). Even if the test of longevity started 2 weeks after adult emergence, the remaining life span of the males irradiated at mature age was comparable with the longevity of the males irradiated at the nymphal stage, confirming that adult stage has a better capability to contrast the negative effects of gamma irradiation on this physiological aspect (
Table 5,
Figure A2).
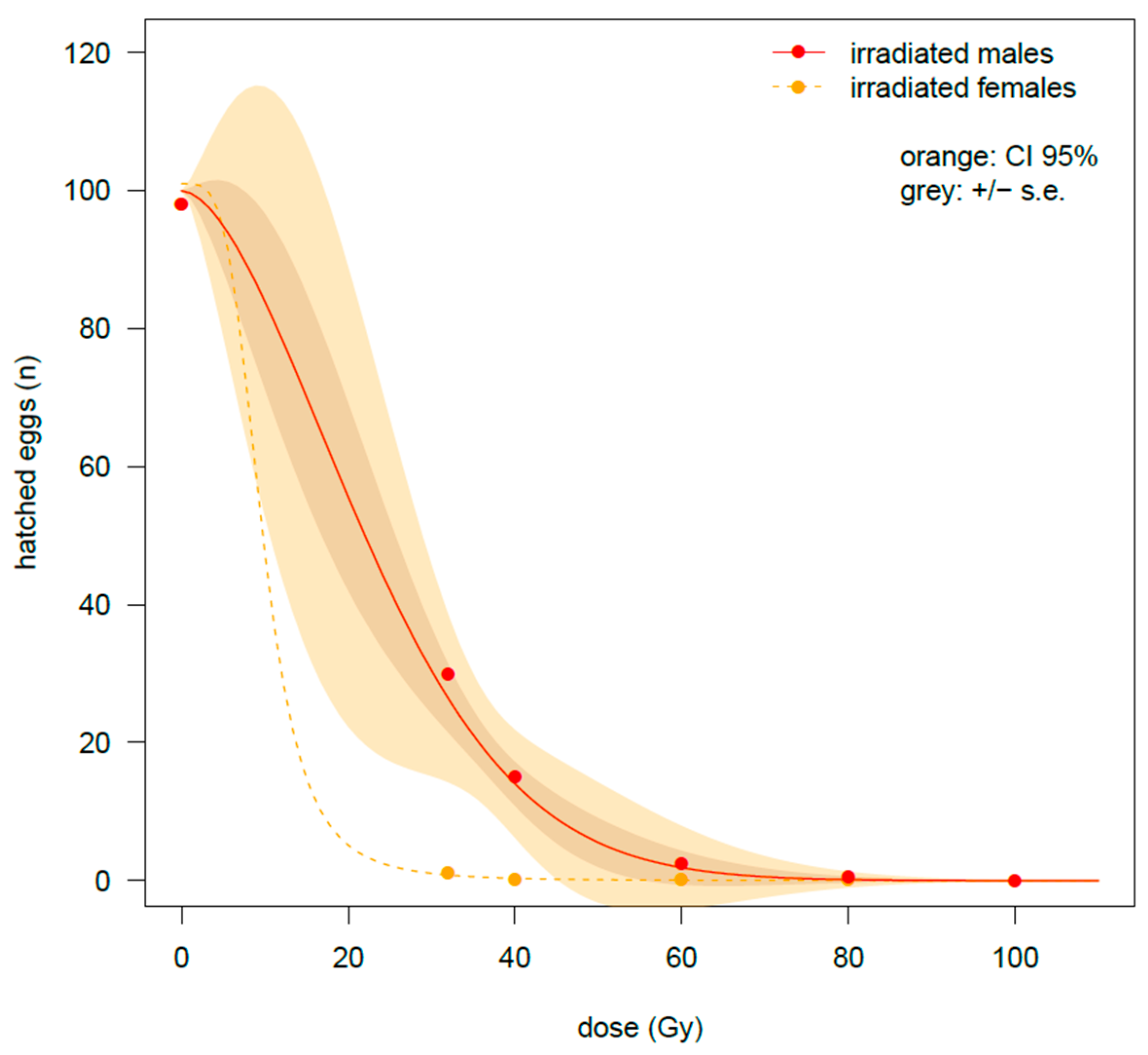
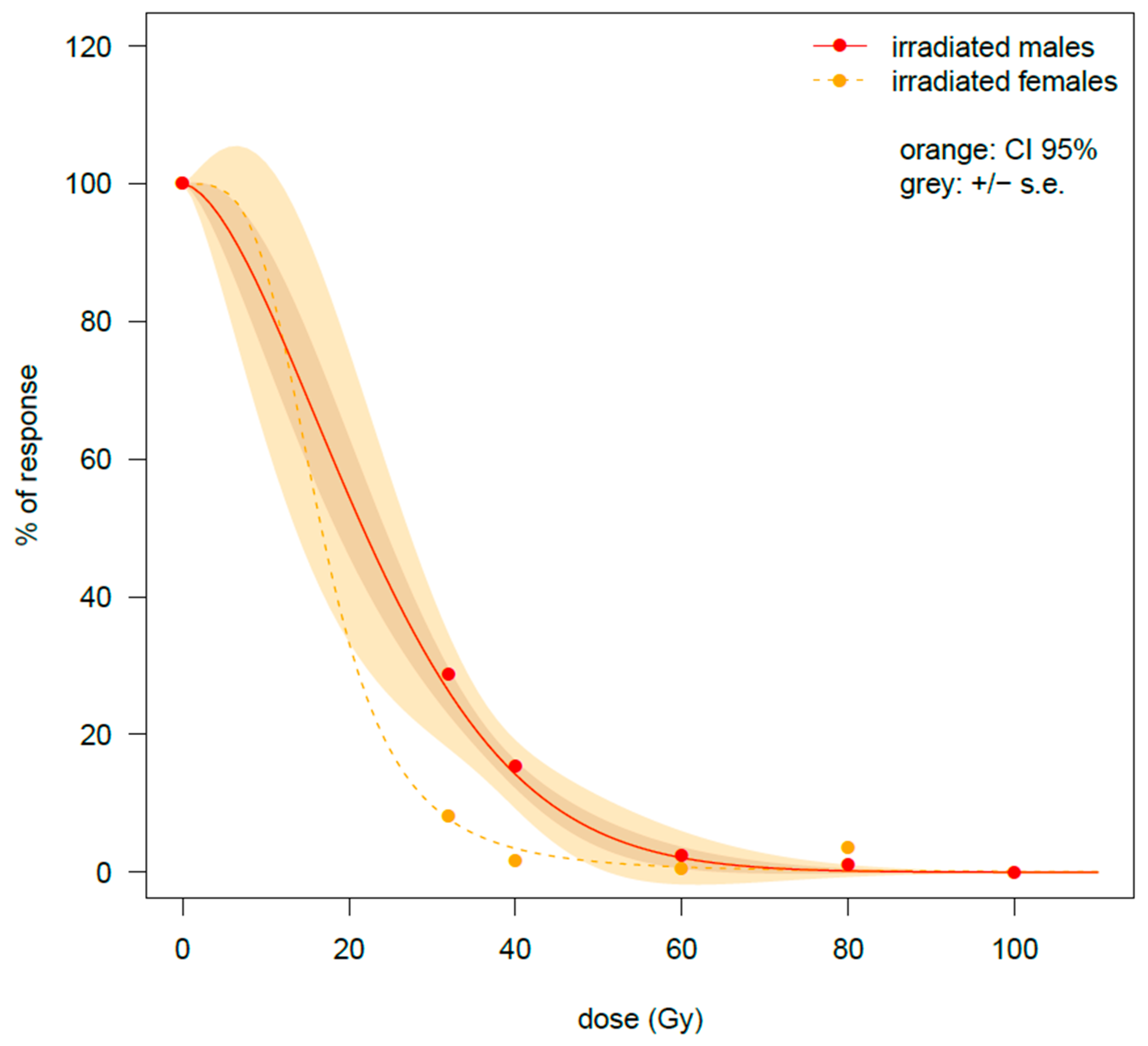
There was a strong negative impact of irradiation dose on hatching rate (number of eggs that hatched), regardless of the physiological stage when bagrada bug males were irradiated (
Table 2,
Figure 4). When irradiation was applied on fifth-instar nymphs, the emerged females are showing an important decrease in fecundity (number of eggs laid;
Figure 1). This result is similar to what was found previously when irradiation was applied to newly emerged adults [
26]. Combining the results of the effects of irradiation on the longevity and on the fertility (hatching rate of the eggs of fertile females mated with irradiated males), the most suitable dose is confirmed to be 80 Gy, for both bagrada bugs irradiated as old adults or at the fifth nymphal instars. In fact, even if differences were recorded in the amount of irradiation to achieve full sterilization (respectively, 75 Gy for fifth-instar nymphs and 106 Gy for old adults, see
Table 4), the effects of the irradiation on the longevity and the performance are supportive to use the irradiation dose at 80 Gy to reach the most correct trade-off to between sterility, longevity and mating performance in irradiated males [
42].
The use of wild-type adults in biological control in general and SIT programs includes advantages and disadvantages compared to traditional mass rearing. Among the positive aspects, we can consider the following:
Economic. Collecting insects of target species in the field instead of rearing them in huge bio-factory facilities should be less expensive, agronomically supportive as it reduces the pressure of the pest locally, environmentally friendly and, in some cases (i.e., gregarious insects), very easy and fast. At the end of autumn and at the beginning of winter (generally very mild in Pantelleria), mature bagrada nymphs and adults aggregate in huge clusters (from several hundreds to few thousands individuals each): the gregarious behavior in this period is more to create suitable physiological diapause conditions than to maximize the feeding (all the caper plants are without leaves). This would be the perfect period to perform the mass trapping. The mutual effect of removing large numbers of females from the environment and re-introduce sterile (irradiated at 80 Gy) wild-type males for small-scale SIT geo-localized applications, should play an important role in the management of this species, when infestations are very restricted like in the case of Pantelleria Island.
Competitive. Wild-type collected insects should perform better than reared ones. In the case of mass rearing, one of the challenging requirement to successfully apply the SIT, is producing huge numbers of insects maintaining an adequate quality for the subsequent release in the field [
43]. In the past, poor performance of sterile males in terms of mating competitiveness has been always attributed to side effects of irradiation [
44,
45] and was addressed by “overflooding” ratios; on the contrary, it has been demonstrated that the mass-rearing process can promote genetic drifts, selecting genotypic characteristics in laboratory populations more suitable for captivity [
26].
Conservative. The suggested approach (catch–irradiate–release) will not introduce new organisms in the area where it is applied, rather it will utilize wild males already in the field. This type of small-scale SIT could therefore be applied in areas like national parks, where the voluntary release of non-indigenous organisms (even if beneficial) is not allowed.
Impactful. Field traps trigged with the aggregation pheromone or just massive collections during the autumn in the areas where B. hilaris is aggregating can provide large numbers of alive adults of both sexes: keeping (or eliminating) caught females and releasing wild males after irradiation in the environment can give more chances to the sterile males to find fertile females with whom to mate, removing at the same time the additional feeding impact induced by the massive release (according to the suitable overflooding ratio) of new adults reared in bio-factory facilities.
On the other hand, there are also some negative aspects around the use of wild-collected insects for the SIT:
Pathogenic. Arthropods collected in nature could be affected by some pathogenic diseases, such as bacteria, fungi and protozoa. Even in cases where the infection rate at the beginning is very low, performing mass trapping and consequently confining large numbers of the collected insects in laboratory cages can cause the spreading of the infection to the full colony. An important mitigation would be to periodically preserve in ETOH 95% small samples of individuals from each site to detect the eventual presence of pathogens, keeping them separated in small cages to limit and manage the contamination effect.
Marking. Unfortunately, B. hilaris is very sensitive to marking methods tested so far. For this reason, it is still not possible to mark the irradiated individuals to release in the open field, making it difficult to conduct early monitoring detection of the validity of the SIT program. Among the possible solutions to solve this issue, in addition to finding a suitable (easy and fast) marking system, is to verify the suitability of the system in semi-field conditions, starting from a known number of fertile individuals, detecting the eventual decrease in the population in the presence of sterile irradiated males.
Logistically. The idea to build a structure similar to the Calliope Irradiation Unit at the ENEA Casaccia is unrealistic and not sustainable. SIT applications on a small scale need the use of irradiation sources alternative to the
60Co. One possible alternative is given by linear accelerators, producing high-energy photons and used in hospitals for medical purposes: they have been successfully tested with another pentatomid pest species, achieving a sterility level of 95% exposing
H. halys males at a 32 Gy X-ray irradiation dose [
28].
Considering the differences in terms of fertility and fecundity, we can hypothesize that the “holistic” irradiation of wild-type
B. hilaris, followed by the release of sterile bagrada bugs at different physiological stages and of both sexes, has a good chance to be successful as an SIT program suppressing the target populations of bagrada bugs. This is before considering the additive effect with biological control by the use of egg parasitoids, which should increase the effectiveness of control. An integrated approach, combining the SIT and biological control, could be particularly helpful because fertile females mating with irradiated males will lay the same number of eggs (no interference with the fecundity, see
Table A2) but the eggs will be sterile. Similarly to what has been proved on the brown marmorated stink bug (
H. halys) [
27], those “sterile” sentinel eggs could be a suitable substrate for the oviposition and full larval development of the egg parasitoid
G. aetherium [
46].