MPF Regulates Oocyte and Embryo Development During Parthenogenesis Induction in Silkworm, Bombyx mori
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Silkworm and Cell Lines
2.2. Artificially Induced Parthenogenesis
2.3. Protein Structure and Phylogenetics Analysis
2.4. RNA Isolation, cDNA Synthesis, and qPCR Analysis
2.5. Enzyme Inhibition and Activity Detection
2.6. Ovariole and Embryo Observation
2.7. Statistical Analysis
3. Results
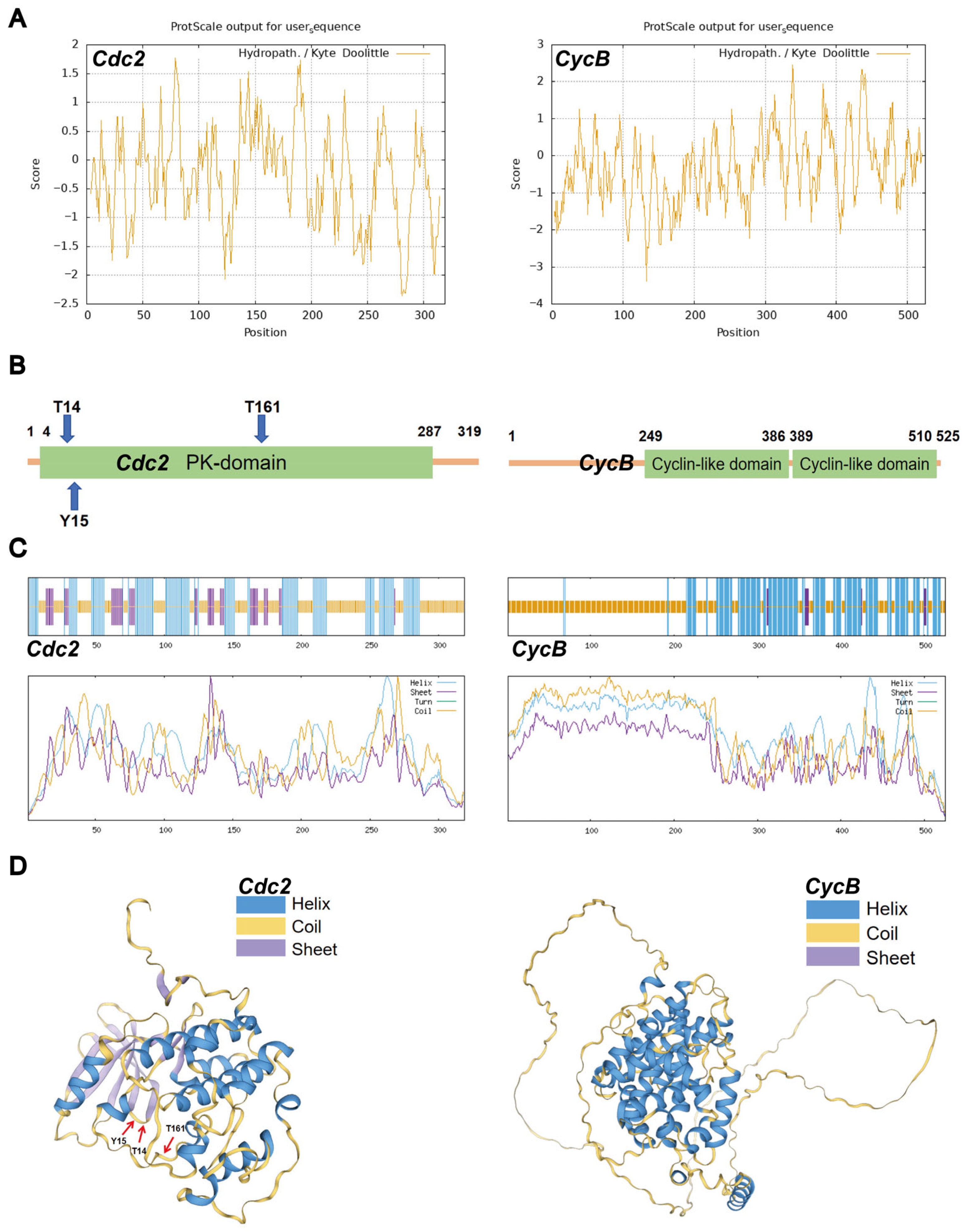
3.1. Protein Structures of Cdc2 and CycB
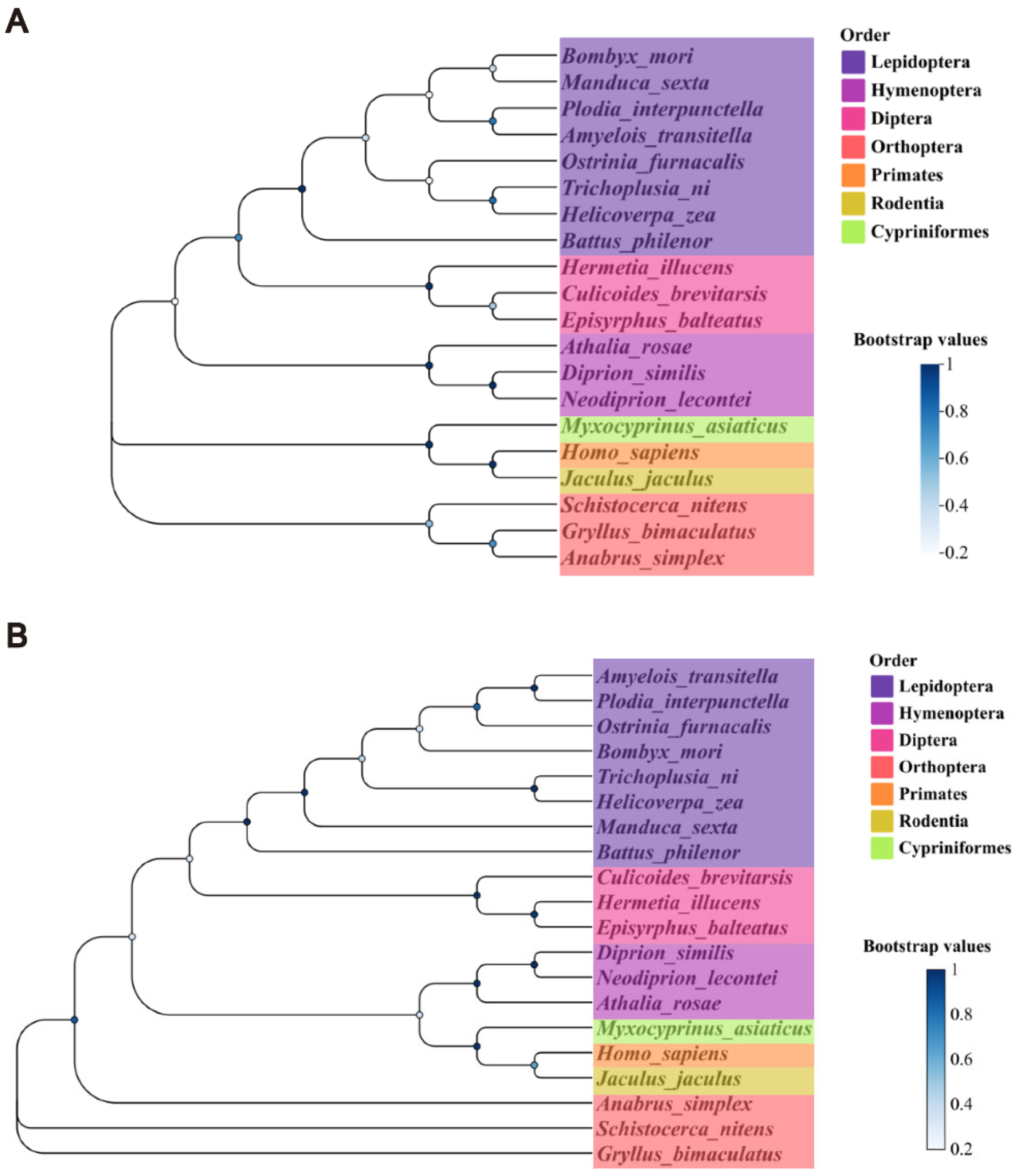
3.2. Phylogenetic Identification of Cdc2 and CycB
3.3. Differential mRNA Expression in Parthenogenetic and Amphigenetic Lines
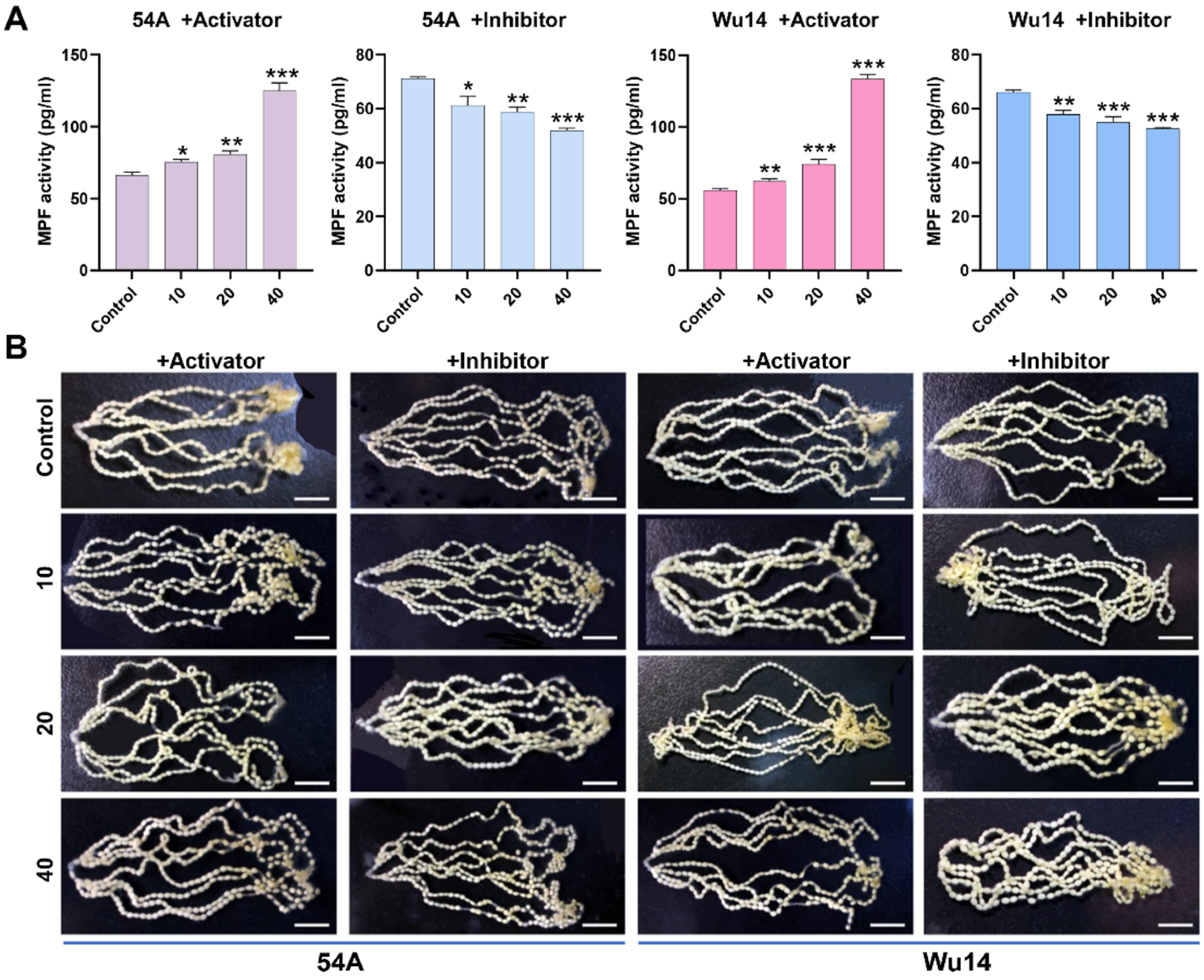
3.4. Activator and Inhibitor Effectively Regulated MPF Activity and Were Not Toxic
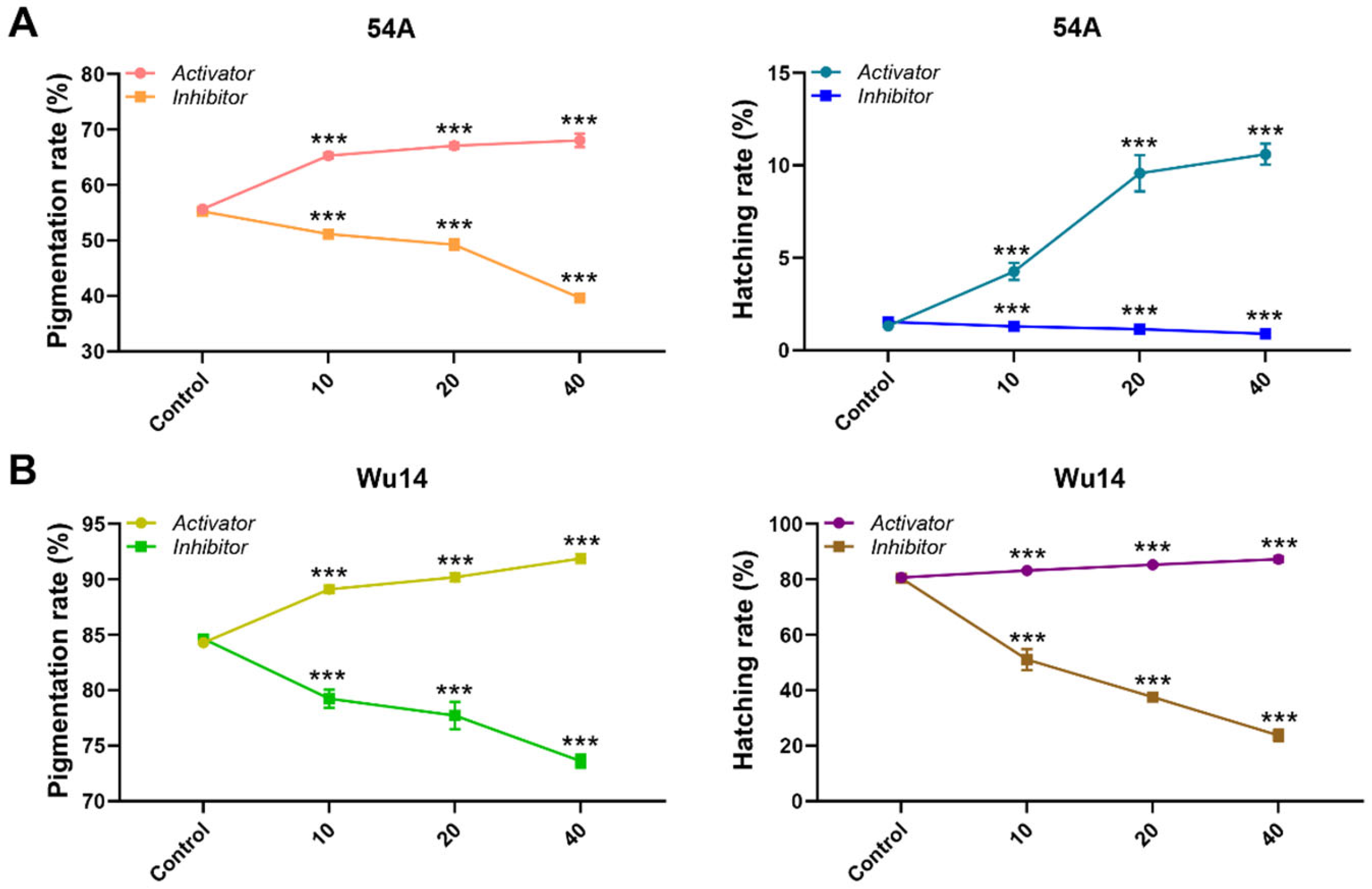
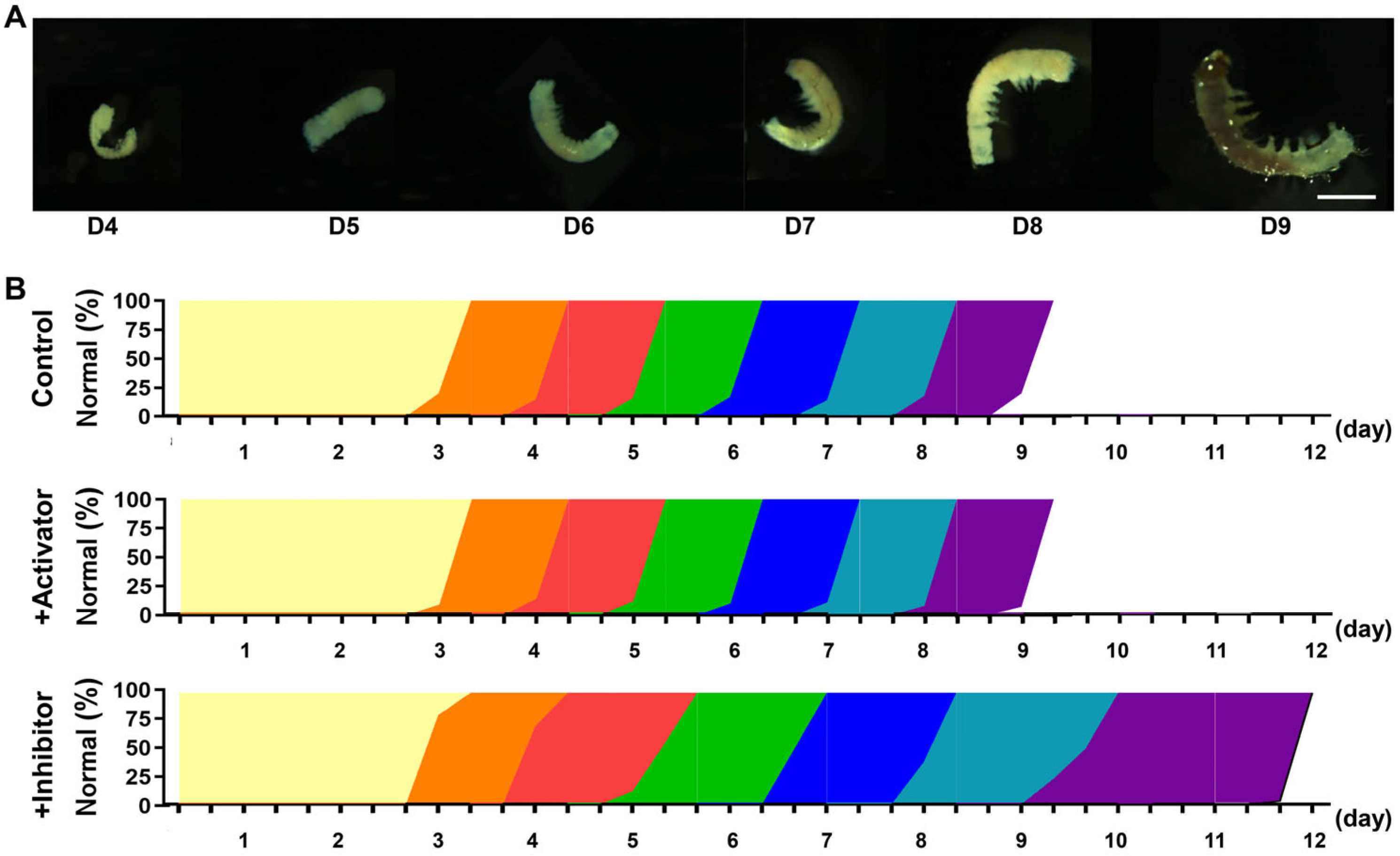
3.5. MPF Activity Affected Parthenogenesis Induction
3.6. Inhibition of MPF Activity Leads to Abnormal Embryonic Development
4. Discussion
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Mita, K.; Kasahara, M.; Sasaki, S.; Nagayasu, Y.; Yamada, T.; Kanamori, H.; Namiki, N.; Kitagawa, M.; Yamashita, H.; Yasukochi, Y.; et al. The Genome Sequence of Silkworm, Bombyx mori. DNA Res. 2004, 11, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Goldsmith, M.R.; Shimada, T.; Abe, H. The genetics and genomics of the silkworm, Bombyx mori. Annu. Rev. Entomol. 2005, 50, 71. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.-C.; Yokoi, K.; Yamamoto, K.; Jouraku, A. An update of KAIKObase, the silkworm genome database. Database 2021, 2021, baaa099. [Google Scholar] [CrossRef] [PubMed]
- Klymenko, V.V. Parthenogenesis and cloning in the silkworm Bombyx mori L.: Problems and prospects. J. Insect Biotechnol. Sericol. 2001, 70, 155–165. [Google Scholar]
- Wang, Y.; Xu, M.; He, X.; Xia, J. Studies on parthenogenesis of commercial silkworm races. Acta Sericol. Sin. 2001, 27, 20–23. [Google Scholar]
- Liu, P.; Wang, Y.; Du, X.; Yao, L.; Li, F.; Meng, Z. Transcriptome analysis of thermal parthenogenesis of the domesticated silkworm. PLoS ONE 2015, 10, e0135215. [Google Scholar] [CrossRef]
- Strunnikov, V.A. Research on the artificial regulation of sex in animals in the USSR. Ontogenez 1978, 9, 3–19. [Google Scholar]
- Priyadarshini, A.; Basu, D.; Navneet, A.K.; Bhattacharya, A.; Bhattacharya, S.; Maitra, S.; Bhattacharya, S. Activation of both Mos and Cdc25 is required for G2-M transition in perch oocyte. Mol. Reprod. Dev. Inc. Gamete Res. 2009, 76, 289–300. [Google Scholar] [CrossRef]
- Ayeni, J.O.; Varadarajan, R.; Mukherjee, O.; Stuart, D.T.; Sprenger, F.; Srayko, M.; Campbell, S.D. Dual phosphorylation of cdk1 coordinates cell proliferation with key developmental processes in Drosophila. Genetics 2014, 196, 197–210. [Google Scholar] [CrossRef]
- Hanna, C.B.; Yao, S.; Patta, M.C.; Jensen, J.T.; Wu, X. WEE2 is an oocyte-specific meiosis inhibitor in rhesus macaque monkeys. Biol. Reprod. 2010, 82, 1190–1197. [Google Scholar] [CrossRef]
- Nishimura, T.; Shimaoka, T.; Kano, K.; Naito, K. Insufficient amount of Cdc2 and continuous activation of Wee1 B are the cause of meiotic failure in porcine growing oocytes. J. Reprod. Dev. 2009, 55, 553–557. [Google Scholar] [PubMed]
- Qiu, G.F.; Liu, P. On the role of Cdc2 kinase during meiotic maturation of oocyte in the Chinese mitten crab, Eriocheir sinensis. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2009, 152, 243–248. [Google Scholar]
- Oqani, R.K.; Lin, T.; Lee, J.E.; Kim, S.Y.; Kang, J.W.; Jin, D.I. Effects of CDK inhibitors on the maturation, transcription, and MPF activity of porcine oocytes. Reprod. Biol. 2017, 17, 320–326. [Google Scholar] [PubMed]
- Lim, S.; Kaldis, P. Cdks, cyclins and CKIs: Roles beyond cell cycle regulation. Development 2013, 140, 3079–3093. [Google Scholar]
- Yamashita, M. Toward Modeling of a General Mechanism of MPF Formation during Oocyte Maturation in Vertebrates. Zool. Sci. 2000, 17, 841–851. [Google Scholar] [CrossRef]
- Fattaey, A.; Booher, R.N. Myt1: A Wee1-type kinase that phosphorylates Cdc2 on residue Thr14. Prog. Cell Cycle Res. 1997, 3, 233–240. [Google Scholar]
- Guadagno, T.M.; Newport, J.W. Cdk2 kinase is required for entry into mitosis as a positive regulator of Cdc2–cyclin B kinase activity. Cell 1996, 84, 73–82. [Google Scholar]
- Kuroda, T.; Naito, K.; Sugiura, K.; Yamashita, M.; Takakura, I.; Tojo, H. Analysis of the roles of cyclin B1 and cyclin B2 in porcine oocyte maturation by inhibiting synthesis with antisense RNA injection. Biol. Reprod. 2004, 70, 154–159. [Google Scholar]
- Liu, J.; Lu, X.; Wang, W.; Zhu, J.; Li, Y.; Luo, L.; Zhang, W. Activity of MPF and expression of its related genes in mouse MI oocytes exposed to cadmium. Food Chem. Toxicol. 2018, 112, 332–341. [Google Scholar]
- Li, J.; Qian, W.P.; Sun, Q.Y. Cyclins regulating oocyte meiotic cell cycle progression. Biol. Reprod. 2019, 101, 878–881. [Google Scholar]
- Jessus, C.; Ozon, R. Regulation of cell divisions during oogenesis of vertebrates: The Xenopus oocyte paradigm. Comp. Biochem. Physiol. Part A Physiol. 1993, 106, 431–448. [Google Scholar]
- Kobayashi, H.; Minshull, J.; Ford, C.; Golsteyn, R.; Poon, R.; Hunt, T. On the synthesis and destruction of A- and B-type cyclins during oogenesis and meiotic maturation in Xenopus laevis. J. Cell Biol. 1991, 114, 755–765. [Google Scholar] [PubMed]
- Kubelka, M.; Anger, M.; Kalous, J.; Schultz, R.M.; Motlík, J. Chromosome condensation in pig oocytes: Lack of a requirement for either cdc2 kinase or MAP kinase activity. Mol. Reprod. Dev. Inc. Gamete Res. 2002, 63, 110–118. [Google Scholar] [CrossRef] [PubMed]
- Kanayama, N.; Miyano, T.; Lee, J. Acquisition of meiotic competence in growing pig oocytes correlates with their ability to activate Cdc2 kinase and MAP kinase. Zygote 2002, 10, 261–270. [Google Scholar] [PubMed]
- Begum, N.; Shen, W.; Manganiello, V. Role of PDE3A in regulation of cell cycle progression in mouse vascular smooth muscle cells and oocytes: Implications in cardiovascular diseases and infertility. Curr. Opin. Pharmacol. 2011, 11, 725–729. [Google Scholar]
- Adhikari, D.; Liu, K. The regulation of maturation promoting factor during prophase I arrest and meiotic entry in mammalian oocytes. Mol. Cell. Endocrinol. 2014, 382, 480–487. [Google Scholar]
- Pei, Z.; Deng, K.; Xu, C.; Zhang, S. The molecular regulatory mechanisms of meiotic arrest and resumption in Oocyte development and maturation. Reprod. Biol. Endocrinol. 2023, 21, 90. [Google Scholar] [CrossRef]
- Phillips, K.P.; Petrunewich, M.A.F.; Collins, J.L.; Booth, R.A.; Liu, X.J.; Baltz, J.M. Inhibition of MEK or cdc2 Kinase Parthenogenetically Activates Mouse Eggs and Yields the Same Phenotypes as Mos−/− Parthenogenotes. Dev. Biol. 2002, 247, 210–223. [Google Scholar] [CrossRef]
- Biasini, M.; Bienert, S.; Waterhouse, A.; Arnold, K.; Studer, G.; Schmidt, T.; Kiefer, F.; Gallo Cassarino, T.; Bertoni, M.; Bordoli, L.; et al. SWISS-MODEL: Modelling protein tertiary and quaternary structure using evolutionary information. Nucleic Acids Res. 2014, 42, 252–258. [Google Scholar]
- Wirawan, I.G.P.; Dewi, N.K.E.S.; Sasadara, M.M.V.; Sunyamurthi, I.G.N.A.; Jawi, I.M.; Wijaya, I.N.; Darmawati, I.A.P.; Suada, I.K.; Krisnandika, A.A.K. Phytochemical Analysis and Molecular Identification of Green Macroalgae Caulerpa spp. from Bali, Indonesia. Molecules 2022, 27, 4879. [Google Scholar] [CrossRef]
- Mitsumasu, K.; Azuma, M.; Niimi, T.; Yamashita, O.; Yaginuma, T. Changes in the Expression of Soluble and Integral-Membrane Trehalases in the Midgut During Metamorphosis in Bombyx mori. Zool. Sci. 2008, 25, 693–698. [Google Scholar]
- Jang, W.I.; Lin, Z.L.; Lee, S.H.; Namgoong, S.; Kim, N.H. A specific inhibitor of CDK1, RO-3306, reversibly arrests meiosis during in vitro maturation of porcine oocytes. Anim. Reprod. Sci. 2014, 144, 102–108. [Google Scholar] [PubMed]
- Ichikawa, D.; Nakamura, M.; Murota, W.; Osawa, S.; Matsushita, M.; Yanagawa, H.; Hattori, Y. A phenylphthalimide derivative, TC11, induces apoptosis by degrading MCL1 in multiple myeloma cells. Biochem. Biophys. Res. Commun. 2020, 521, 252–258. [Google Scholar] [PubMed]
- Anguita, B.; Jimenez-Macedo, A.R.; Izquierdo, D.; Mogas, T.; Paramio, M.T. Effect of oocyte diameter on meiotic competence, embryo development, p34 (cdc2) expression and MPF activity in prepubertal goat oocytes. Theriogenology 2007, 67, 526–536. [Google Scholar]
- Nagahama, Y.; Yamashita, M. Regulation of oocyte maturation in fish. Dev. Growth Differ. 2008, 50, 195–219. [Google Scholar]
- Zhang, D.X.; Park, W.J.; Sun, S.C.; Xu, Y.N.; Li, Y.H.; Cui, X.S.; Kim, N.H. Regulation of maternal gene expression by MEK/MAPK and MPF signaling in porcine oocytes during in vitro meiotic maturation. J. Reprod. Dev. 2011, 57, 49–56. [Google Scholar] [CrossRef]
- Chesnel, F.; Bazile, F.; Pascal, A.; Kubiak, J.Z. Cyclin B dissociation from CDK1 precedes its degradation upon MPF inactivation in mitotic extracts of Xenopus laevis embryos. Cell Cycle 2006, 5, 1687–1698. [Google Scholar]
- Grallert, A.; Patel, A.; Tallada, V.A.; Chan, K.Y.; Bagley, S.; Krapp, A.; Simanis, V.; Hagan, I.M. Centrosomal MPF triggers the mitotic and morphogenetic switches of fission yeast. Nat. Cell Biol. 2013, 15, 88–95. [Google Scholar]
- Cheng, M.; Chen, X.; Han, M.; Luo, X.; Yu, Y.; Lv, Y.; Han, Y.; Cao, L.; Zhang, J.; Wang, M.; et al. miR-155-5p improves oocyte maturation in porcine cumulus cells through connexin 43-mediated regulation of MPF activity. Theriogenology 2024, 214, 124–133. [Google Scholar]
- Liu, P.; Wang, Y.; Du, X.; Shi, F.; Meng, Z. A comparative proteomic analysis of parthenogenetic lines and amphigenetic lines of silkworm. Biotechnol. Bioprocess Eng. 2014, 19, 641–649. [Google Scholar]
- Dutertre, S.; Cazales, M.; Quaranta, M.; Froment, C.; Trabut, V.; Dozier, C.; Mirey, G.; Bouché, J.P.; Theis-Febvre, N.; Schmitt, E.; et al. Phosphorylation of CDC25B by Aurora-A at the centrosome contributes to the G2–M transition. J. Cell Sci. 2004, 117, 2523–2531. [Google Scholar] [PubMed]
- Yu, B.; Wang, Y.; Liu, Y.; Liu, Y.; Li, X.; Wu, D.; Zong, Z.; Zhang, J.; Yu, D. Protein kinase A regulates cell cycle progression of mouse fertilized eggs by means of MPF. Dev. Dyn. 2005, 232, 98–105. [Google Scholar] [PubMed]
- Newport, J.W.; Kirschner, M.W. Regulation of the cell cycle during early Xenopus development. Cell 1984, 37, 731–742. [Google Scholar] [PubMed]
- Hashimoto, N.; Kishimoto, T. Cell cycle dynamics of maturation-promoting factor during mouse oocyte maturation. Tokai J. Exp. Clin. Med. 1986, 11, 471–477. [Google Scholar]
- Baek, J.I.; Seol, D.W.; Lee, A.R.; Lee, W.S.; Yoon, S.Y.; Lee, D.R. Maintained MPF Level After Oocyte Vitrification Improves Embryonic Development After IVF, but not After Somatic Cell Nuclear Transfer. Mol. Cells 2017, 40, 871–879. [Google Scholar]

| Primer Name | Primer Sequence (5′–3′) |
|---|---|
| BmCdc2-F | ATGGACTCCCTTGGTTCTGG |
| BmCdc2-R | CACTGGAACACCGAATGCTC |
| BmCycB-F | ATGTGCAAACTGGGCTTCTG |
| BmCycB-R | CTGCTAGTTCTGACGGTCGA |
| BmRP49-F | TCAATCGGATCGCTATGACA |
| BmRP49-R | ATGACGGGTCTTCTTGTTGG |
| Species Name | GenBank Accession Numbers | Order | |
|---|---|---|---|
| Cdc2 | CycB | ||
| Bombyx mori | NP_001037512.1 | NP_001037343.1 | Lepidoptera |
| Manduca sexta | XP_030037649.1 | XP_030029390.1 | Lepidoptera |
| Trichoplusia ni | XP_026740648.1 | XP_026731829.1 | Lepidoptera |
| Ostrinia furnacalis | XP_028177540.1 | XP_028156235.1 | Lepidoptera |
| Amyelois transitella | XP_013193284.1 | XP_013191183.2 | Lepidoptera |
| Helicoverpa zea | XP_047033055.1 | XP_047023653.1 | Lepidoptera |
| Battus philenor | XP_068623488.1 | XP_068626558.1 | Lepidoptera |
| Plodia interpunctella | XP_053617113.1 | XP_053610622.1 | Lepidoptera |
| Diprion similis | XP_046738554.1 | XP_046746195.1 | Hymenoptera |
| Neodiprion lecontei | XP_015520372.1 | XP_046598379.1 | Hymenoptera |
| Athalia rosae | XP_012254072.1 | XP_012254238.2 | Hymenoptera |
| Hermetia illucens | XP_037903055.1 | XP_037904258.1 | Diptera |
| Culicoides brevitarsis | XP_063700522.1 | XP_063700924.1 | Diptera |
| Episyrphus balteatus | XP_055836824.1 | XP_055837226.1 | Diptera |
| Gryllus bimaculatus | GLH05238.1 | GLG94543.1 | Orthoptera |
| Anabrus simplex | XP_067015382.1 | XP_066990889.1 | Orthoptera |
| Schistocerca nitens | XP_049799429.1 | XP_049815951.1 | Orthoptera |
| Homo sapiens | NP_001307847.1 | NP_114172.1 | Primates |
| Jaculus jaculus | XP_004657918.1 | XP_045016300.1 | Rodentia |
| Myxocyprinus asiaticus | XP_051502898.1 | XP_051559981.1 | Cypriniformes |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, C.; Xu, F.; Hu, C.; Cui, C.; Du, X.; Chen, J.; Zhu, L.; Yu, S.; He, X.; Yu, W.; et al. MPF Regulates Oocyte and Embryo Development During Parthenogenesis Induction in Silkworm, Bombyx mori. Insects 2025, 16, 361. https://doi.org/10.3390/insects16040361
Ma C, Xu F, Hu C, Cui C, Du X, Chen J, Zhu L, Yu S, He X, Yu W, et al. MPF Regulates Oocyte and Embryo Development During Parthenogenesis Induction in Silkworm, Bombyx mori. Insects. 2025; 16(4):361. https://doi.org/10.3390/insects16040361
Chicago/Turabian StyleMa, Chenkai, Fang Xu, Chengjie Hu, Chunguang Cui, Xin Du, Jine Chen, Linbao Zhu, Shaofang Yu, Xingjian He, Wei Yu, and et al. 2025. "MPF Regulates Oocyte and Embryo Development During Parthenogenesis Induction in Silkworm, Bombyx mori" Insects 16, no. 4: 361. https://doi.org/10.3390/insects16040361
APA StyleMa, C., Xu, F., Hu, C., Cui, C., Du, X., Chen, J., Zhu, L., Yu, S., He, X., Yu, W., Wang, Y., & Xu, X. (2025). MPF Regulates Oocyte and Embryo Development During Parthenogenesis Induction in Silkworm, Bombyx mori. Insects, 16(4), 361. https://doi.org/10.3390/insects16040361




