CRISPR/Cas9-Mediated Knockout of BmGDAP2 in the Silkworm, Bombyx mori: Extended Lifespan and Altered Gene Expression Impacting Developmental Pathways
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Bioinformatics Analysis
2.2. Silkworm
2.3. RNA Extraction and RT-qPCR
2.4. Plasmid Construction
2.5. Microinjection and Screening
2.6. Phenotypic Observation and Statistics
2.7. Analyses of RNA-Seq Data
2.8. Statistical Analysis
3. Results
3.1. GDAP2 Exhibits High Homology and Conserved Phylogenetic Relationships and Tissue Expression Analysis
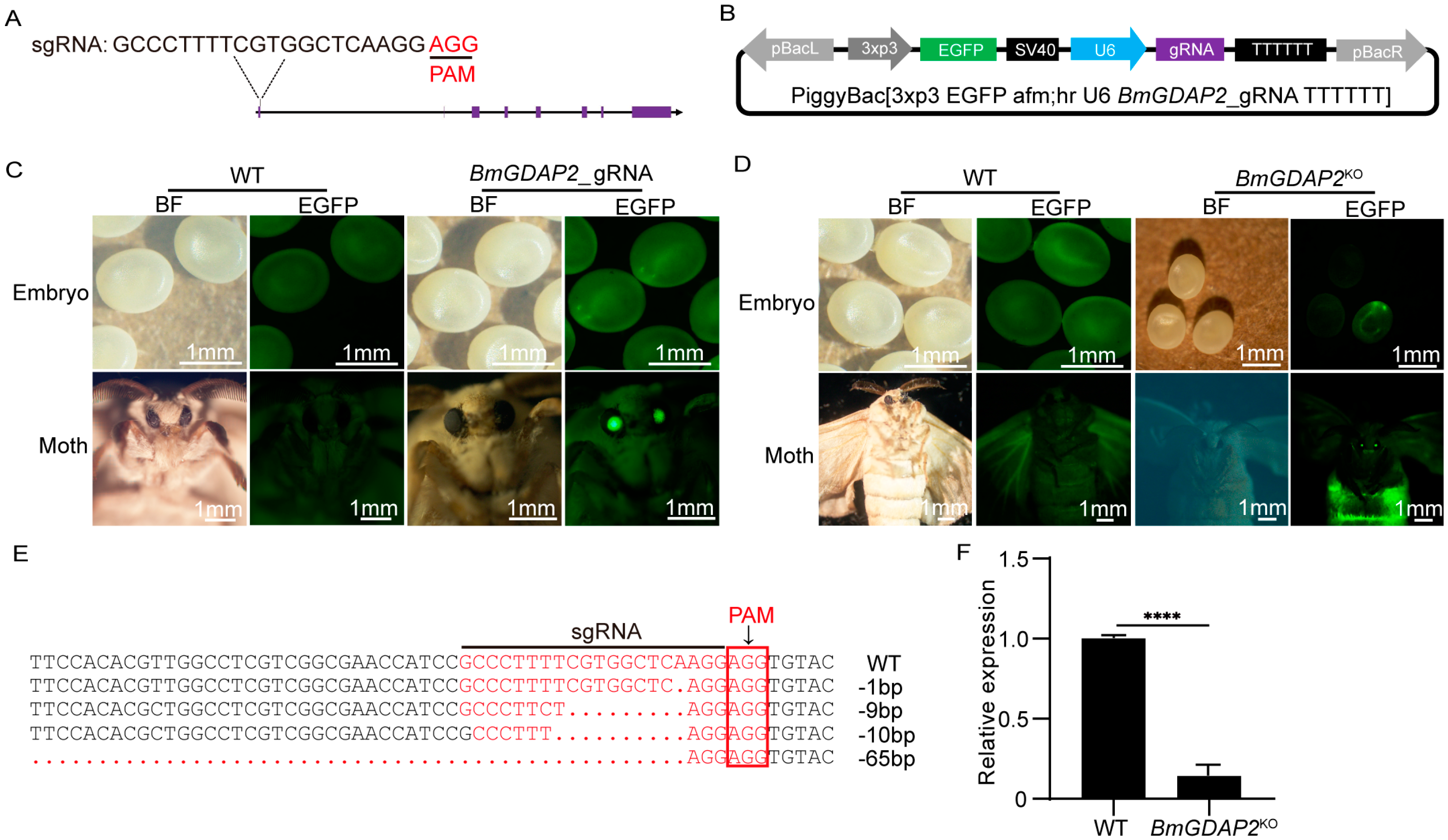
3.2. CRISPR/Cas9-Mediated Mutagenesis of BmGDAP2
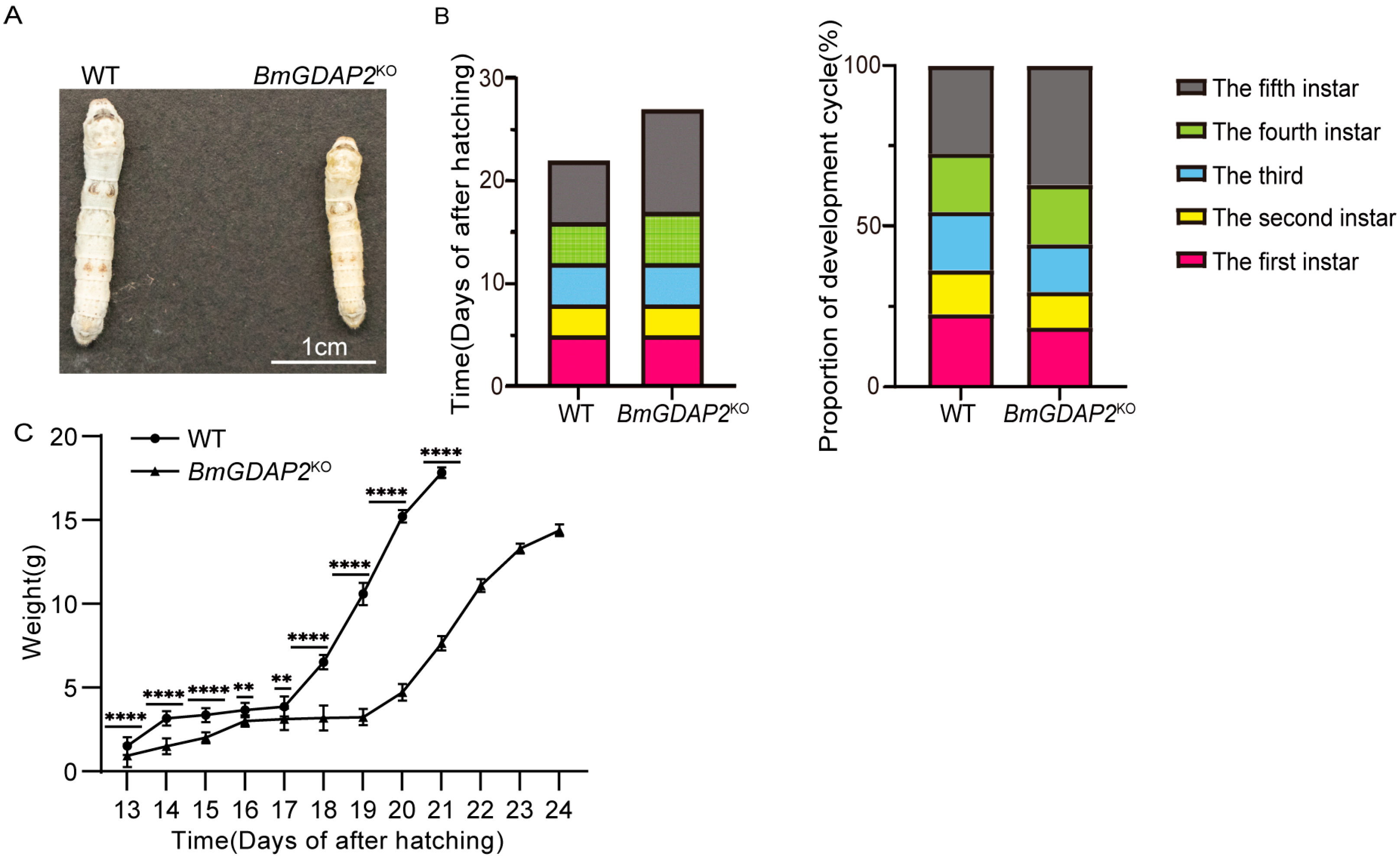
3.3. Phenotypes Induced by Disruption of BmGDAP2
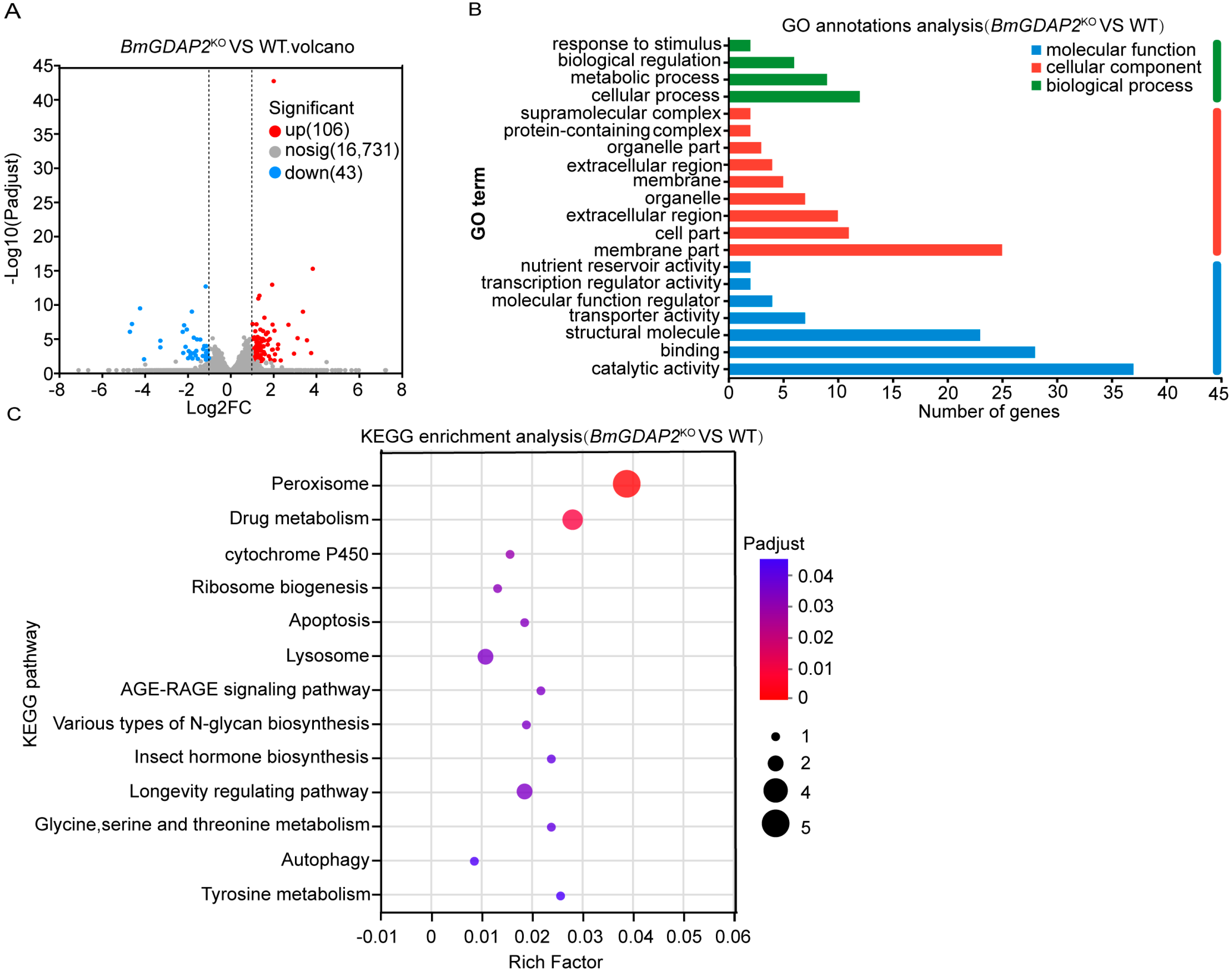
3.4. Differentially Expressed Genes (DEGs) and Functional Enrichment Analysis from BmGDAP2KO-Mutant and Wild Silkworms
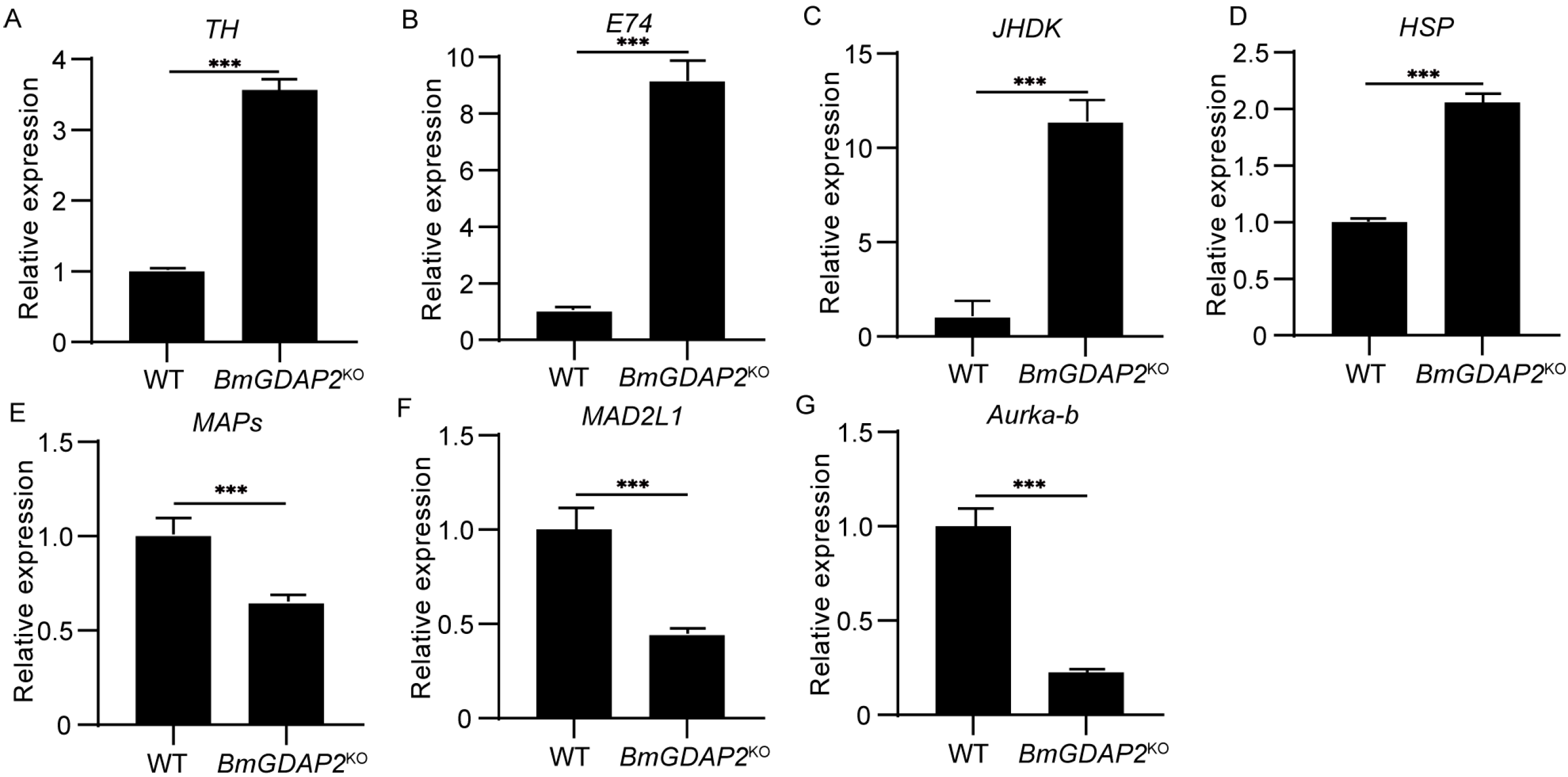
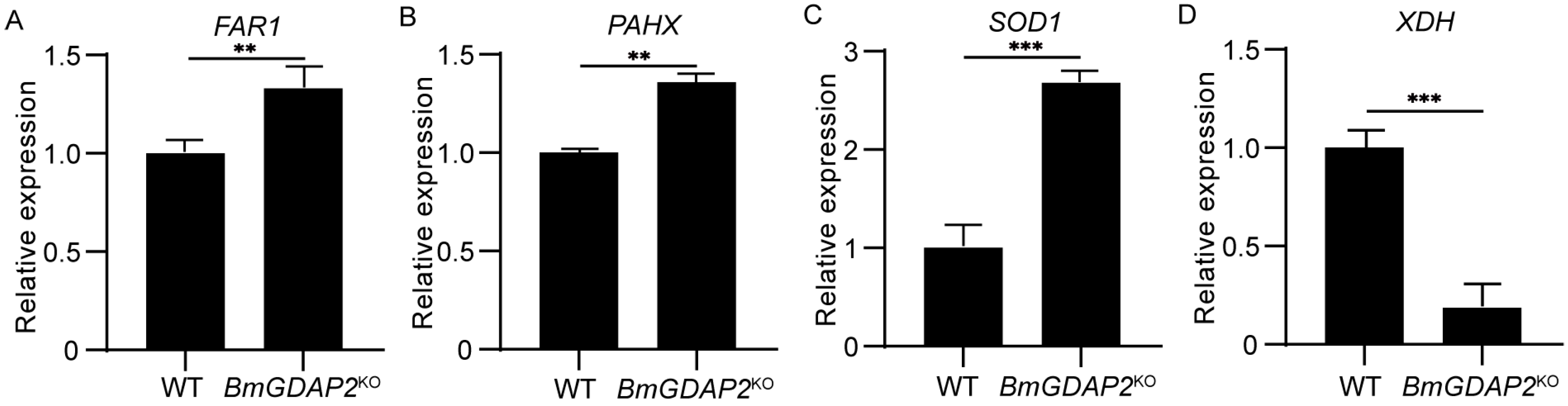
3.5. Candidate DEGs Involved in Larva Development in BmGDAP2KO and Wild-Type Silkworms
3.6. BmGDAP2 Mainly Regulates Development Through the Peroxisome Pathway
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Xu, G.; Yi, Y.; Lyu, H.; Gong, C.; Feng, Q.; Song, Q.; Peng, X.; Liu, L.; Zheng, S. DNA methylation suppresses chitin degradation and promotes the wing development by inhibiting Bmara-mediated chitinase expression in the silkworm, Bombyx mori. Epigenetics Chromatin 2020, 13, 34. [Google Scholar] [CrossRef]
- Wei, D.; Xu, H.-Q.; Chen, D.; Zhang, S.-Y.; Li, W.-J.; Smagghe, G.; Wang, J.-J. Genome-wide gene expression profiling of the melon fly, Zeugodacus cucurbitae, during thirteen life stages. Sci. Data 2020, 7, 45. [Google Scholar] [CrossRef]
- Hiroya, N. Early embryonic development of Bombyx. Dev. Genes Evol. 2021, 231, 95–107. [Google Scholar] [CrossRef]
- Gong, J.; Zheng, X.; Zhao, S.; Yang, L.; Xue, Z.; Fan, Z.; Tang, M. Early Molecular Events during Onset of Diapause in Silkworm Eggs Revealed by Transcriptome Analysis. Int. J. Mol. Sci. 2020, 21, 6180. [Google Scholar] [CrossRef] [PubMed]
- Xu, P.Z.; Zhang, M.R.; Wang, X.Y.; Wu, Y.C. Precocious Metamorphosis of Silkworm Larvae Infected by BmNPV in the Latter Half of the Fifth Instar. Front. Physiol. 2021, 12, 650972. [Google Scholar] [CrossRef]
- Wang, X.; Fan, Y.; Ge, Q.; Xu, J.; Taha, R.H.; Yuan, Y.; Chen, K. Time-Course Transcriptome Analysis Reveals Global Gene Expression Profiling and Dynamic Developmental Signatures across Complete Life Cycle of Bombyx mori. Processes 2021, 9, 1730. [Google Scholar] [CrossRef]
- Ashoka, K.S.; Chaitanya, S.B.M.C. Impact of high temperature on growth and development of Silkworm(Bombyx mori L.). Zenodo (CERN Eur. Organ. Nucl. Res.) 2022, 2, 1819–1821. [Google Scholar] [CrossRef]
- Mei, X.; Huang, T.; Chen, A.; Liu, W.; Jiang, L.; Zhong, S.; Shen, D.; Qiao, P.; Zhao, Q. BmC/EBPZ gene is essential for the larval growth and development of silkworm, Bombyx mori. Front. Physiol. 2024, 15, 1298869. [Google Scholar] [CrossRef]
- Zeng, B.; Huang, Y.; Xu, J.; Shiotsuki, T.; Bai, H.; Palli, S.R.; Huang, Y.; Tan, A. The FOXO transcription factor controls insect growth and development by regulating juvenile hormone degradation in the silkworm, Bombyx mori. J. Biol. Chem. 2017, 292, 11659–11669. [Google Scholar] [CrossRef]
- Wang, Q.; Sun, Z.; Huang, Z.; Ma, S.; Chen, K.; Ju, X. Effects of tolfenpyrad exposure on development and response mechanism in the silkworm, Bombyx mori. Pestic. Biochem. Physiol. 2023, 189, 105280. [Google Scholar] [CrossRef]
- Palmano, K.; Rowan, A.; Guillermo, R.; Guan, J.; Mc Jarrow, P. The Role of Gangliosides in Neurodevelopment. Nutrients 2015, 7, 3891–3913. [Google Scholar] [CrossRef] [PubMed]
- Cutillo, G.; Saariaho, A.-H.; Meri, S. Physiology of gangliosides and the role of antiganglioside antibodies in human diseases. Cell. Mol. Immunol. 2020, 17, 313–322. [Google Scholar] [CrossRef]
- Inci, O.K.; Basırlı, H.; Can, M.; Yanbul, S.; Seyrantepe, V. Gangliosides as Therapeutic Targets for Neurodegenerative Diseases. J. Lipids 2024, 2024, 4530255. [Google Scholar] [CrossRef]
- Dong, H.-L.; Cheng, H.-L.; Bai, G.; Shen, Y.; Wu, Z.-Y. Novel GDAP2 pathogenic variants cause autosomal recessive spinocerebellar ataxia-27 (SCAR27) in a Chinese family. Brain 2020, 143, e50. [Google Scholar] [CrossRef]
- Breza, M.; Bourinaris, T.; Efthymiou, S.; Maroofian, R.; Athanasiou-Fragkouli, A.; Tzartos, J.; Velonakis, G.; Karavasilis, E.; Angelopoulou, G.; Kasselimis, D.; et al. A homozygous GDAP2 loss-of-function variant in a patient with adult-onset cerebellar ataxia. Brain 2020, 143, e49. [Google Scholar] [CrossRef] [PubMed]
- Hu, D.; Guo, Y.; Wu, M.; Ma, Y.; Jing, W. GDAP2 Overexpression Affects the Development of Neurons and Dysregulates Neuronal Excitatory Synaptic Transmission. Neuroscience 2022, 488, 32–43. [Google Scholar] [CrossRef] [PubMed]
- Eidhof, I.; Baets, J.; Kamsteeg, E.-J.; Deconinck, T.; van Ninhuijs, L.; Martin, J.-J.; Schüle, R.; Züchner, S.; De Jonghe, P.; Schenck, A.; et al. GDAP2 mutations implicate susceptibility to cellular stress in a new form of cerebellar ataxia. Brain 2018, 141, 2592–2604. [Google Scholar] [CrossRef]
- Zhu, K.; Chen, Y.; Chen, L.; Xiang, H. Comparative Silk Transcriptomics Illuminates Distinctive Impact of Artificial Selection in Silkworm Modern Breeding. Insects 2022, 13, 1163. [Google Scholar] [CrossRef]
- Wang, G.; Xia, Q.; Cheng, D.; Duan, J.; Zhao, P.; Chen, J.; Zhu, L. Reference genes identified in the silkworm Bombyx mori during metamorphism based on oligonucleotide microarray and confirmed by qRT-PCR. Insect Sci. 2008, 15, 405–413. [Google Scholar] [CrossRef]
- Stemmer, M.; Thumberger, T.; del Sol Keyer, M.; Wittbrodt, J.; Mateo, J.L. Correction: CCTop: An Intuitive, Flexible and Reliable CRISPR/Cas9 Target Prediction Tool. PLoS ONE 2017, 12, e0176619. [Google Scholar] [CrossRef]
- Tamura, T.; Thibert, C.; Royer, C.; Kanda, T.; Eappen, A.; Kamba, M.; Kômoto, N.; Thomas, J.-L.; Mauchamp, B.; Chavancy, G.; et al. Germline transformation of the silkworm Bombyx mori L. using a piggyBac transposon-derived vector. Nat. Biotechnol. 2000, 18, 81–84. [Google Scholar] [CrossRef]
- Xu, J.; Chen, R.; Chen, S.; Chen, K.; Tang, L.; Yang, D.; Yang, X.; Zhang, Y.; Song, H.; Huang, Y. Identification of a germline-expression promoter for genome editing in Bombyx mori. Insect Sci. 2019, 26, 991–999. [Google Scholar] [CrossRef] [PubMed]
- Lu, F.; Wei, Z.; Luo, Y.; Guo, H.; Zhang, G.; Xia, Q.; Wang, Y. SilkDB 3.0: Visualizing and exploring multiple levels of data for silkworm. Nucleic Acids Res. 2019, 48, D749–D755. [Google Scholar] [CrossRef]
- Langmead, B.; Trapnell, C.; Pop, M.; Salzberg, S.L. Ultrafast and memory-efficient alignment of short DNA sequences to the human genome. Genome Biol. 2009, 10, R25. [Google Scholar] [CrossRef] [PubMed]
- Naito, Y.; Hino, K.; Bono, H.; Ui-Tei, K. CRISPRdirect: Software for designing CRISPR/Cas guide RNA with reduced off-target sites. Bioinformatics 2014, 31, 1120–1123. [Google Scholar] [CrossRef]
- Wang, Y. Progress in the research of autophagy-related molecule Atg8 in lepidopteran insects. Acta Entomol. Sin. 2015, 58, 210–216. [Google Scholar]
- Li, Z.; Lyu, Z.; Ye, Q.; Cheng, J.; Wang, C.; Lin, T. Cloning, Expression Analysis, 20-Hydroxyecdysone Induction, and RNA Interference Study of Autophagy-Related Gene 8 from Heortia vitessoides Moore. Insects 2020, 11, 245. [Google Scholar] [CrossRef]
- Fu, B.; Ma, R.; Liu, F.; Chen, X.; Teng, X.; Yang, P.; Liu, J.; Zhao, D.; Sun, L. Ginsenosides improve reproductive capability of aged female Drosophila through mechanism dependent on ecdysteroid receptor (ECR) and steroid signaling pathway. Front. Endocrinol. 2022, 13, 964069. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, X.; Shiotsuki, T.; Wang, Z.; Xu, X.; Huang, Y.; Li, M.; Li, K.; Tan, A. Depletion of juvenile hormone esterase extends larval growth in Bombyx mori. Insect Biochem. Mol. Biol. 2017, 81, 72–79. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, Y.; Chen, X.; Tian, D.; Xu, X.; Li, K.; Huang, Y.; He, L. CRISPR/Cas9-mediated Tyrosine hydroxylase knockout resulting in larval lethality in Agrotis ipsilon. Insect Sci. 2018, 25, 1017–1024. [Google Scholar] [CrossRef]
- Hebishy, M.; Shintouo, C.M.; Dufait, I.; Debacq-Chainiaux, F.; Bautmans, I.; Njemini, R. Heat shock proteins and cellular senescence in humans: A systematic review. Arch. Gerontol. Geriatr. 2023, 113, 105057. [Google Scholar] [CrossRef] [PubMed]
- Busacca, S.; O’Regan, L.; Singh, A.; Sharkey, A.J.; Dawson, A.G.; Dzialo, J.; Parsons, A.; Kumar, N.; Schunselaar, L.M.; Guppy, N.; et al. BRCA1/MAD2L1 Deficiency Disrupts the Spindle Assembly Checkpoint to Confer Vinorelbine Resistance in Mesothelioma. Mol. Cancer Ther. 2021, 20, 379–388. [Google Scholar] [CrossRef]
- Li, Q.; Tong, D.; Jing, X.; Ma, P.; Li, F.; Jiang, Q.; Zhang, J.; Wen, H.; Cui, M.; Huang, C.; et al. MAD2L1 is transcriptionally regulated by TEAD4 and promotes cell proliferation and migration in colorectal cancer. Cancer Gene Ther. 2023, 30, 727–737. [Google Scholar] [CrossRef] [PubMed]
- Ryu, J.; Pyo, J.; Lee, C.; Kim, J. An Aurora kinase inhibitor, AMG900, inhibits glioblastoma cell proliferation by disrupting mitotic progression. Cancer Med. 2018, 7, 5589–5603. [Google Scholar] [CrossRef]
- He, P.; Wei, E.; Wang, R.; Wang, Q.; Zhang, Y.; Tang, X.; Zhu, F.; Shen, Z. The spirotetramat inhibits growth and reproduction of silkworm by interfering with the fatty acid metabolism. Pestic. Biochem. Physiol. 2022, 188, 105282. [Google Scholar] [CrossRef] [PubMed]
- Kômoto, N. A deleted portion of one of the two xanthine dehydrogenase genes causes translucent larval skin in the oq mutant of the silkworm (Bombyx mori). Insect Biochem. Mol. Biol. 2002, 32, 591–597. [Google Scholar] [CrossRef]
- Tower, J. Superoxide Dismutase (SOD) Genes and Aging in Drosophila. Healthy Ageing Longev. 2015. [Google Scholar] [CrossRef]
- Shao, X.; Zhang, M.; Chen, Y.; Sun, S.; Yang, S.; Li, Q. Exosome-mediated delivery of superoxide dismutase for anti-aging studies in Caenorhabditis elegans. Int. J. Pharm. 2023, 641, 123090. [Google Scholar] [CrossRef]
- Li, D.; Dai, Y.; Chen, X.; Wang, X.; Li, Z.; Moussian, B.; Zhang, C. Ten fatty acyl-CoA reductase family genes were essential for the survival of the destructive rice pest, Nilaparvata lugens. Pest Manag. Sci. 2020, 76, 2304–2315. [Google Scholar] [CrossRef]
- Chen, A.; Liao, P.; Li, Q.; Zhao, Q.; Gao, M.; Wang, P.; Liu, Z.; Meng, G.; Dong, Z.; Liu, M. phytanoyl-CoA dioxygenase domain-containing protein 1 plays an important role in egg shell formation of silkworm (Bombyx mori). PLoS ONE 2021, 16, e0261918. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yuan, C.; Zhou, Z.; Guo, Q.; Yang, Y.; Sun, Y.; Liu, Y.; Jia, W.; Fan, S.; Wu, J.; Hua, X.; et al. CRISPR/Cas9-Mediated Knockout of BmGDAP2 in the Silkworm, Bombyx mori: Extended Lifespan and Altered Gene Expression Impacting Developmental Pathways. Insects 2025, 16, 354. https://doi.org/10.3390/insects16040354
Yuan C, Zhou Z, Guo Q, Yang Y, Sun Y, Liu Y, Jia W, Fan S, Wu J, Hua X, et al. CRISPR/Cas9-Mediated Knockout of BmGDAP2 in the Silkworm, Bombyx mori: Extended Lifespan and Altered Gene Expression Impacting Developmental Pathways. Insects. 2025; 16(4):354. https://doi.org/10.3390/insects16040354
Chicago/Turabian StyleYuan, Chaojun, Zichong Zhou, Qifeng Guo, Ying Yang, Yue Sun, Yong Liu, Wenyi Jia, Shuoqi Fan, Jinxin Wu, Xiaoting Hua, and et al. 2025. "CRISPR/Cas9-Mediated Knockout of BmGDAP2 in the Silkworm, Bombyx mori: Extended Lifespan and Altered Gene Expression Impacting Developmental Pathways" Insects 16, no. 4: 354. https://doi.org/10.3390/insects16040354
APA StyleYuan, C., Zhou, Z., Guo, Q., Yang, Y., Sun, Y., Liu, Y., Jia, W., Fan, S., Wu, J., Hua, X., Lin, P., Zhao, P., & Xia, Q. (2025). CRISPR/Cas9-Mediated Knockout of BmGDAP2 in the Silkworm, Bombyx mori: Extended Lifespan and Altered Gene Expression Impacting Developmental Pathways. Insects, 16(4), 354. https://doi.org/10.3390/insects16040354





