Conservation Genetics of the Endangered Danube Clouded Yellow Butterfly Colias myrmidone (Esper, 1780) in the Last Central European Stronghold: Diversity, Wolbachia Infection and Balkan Connections
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
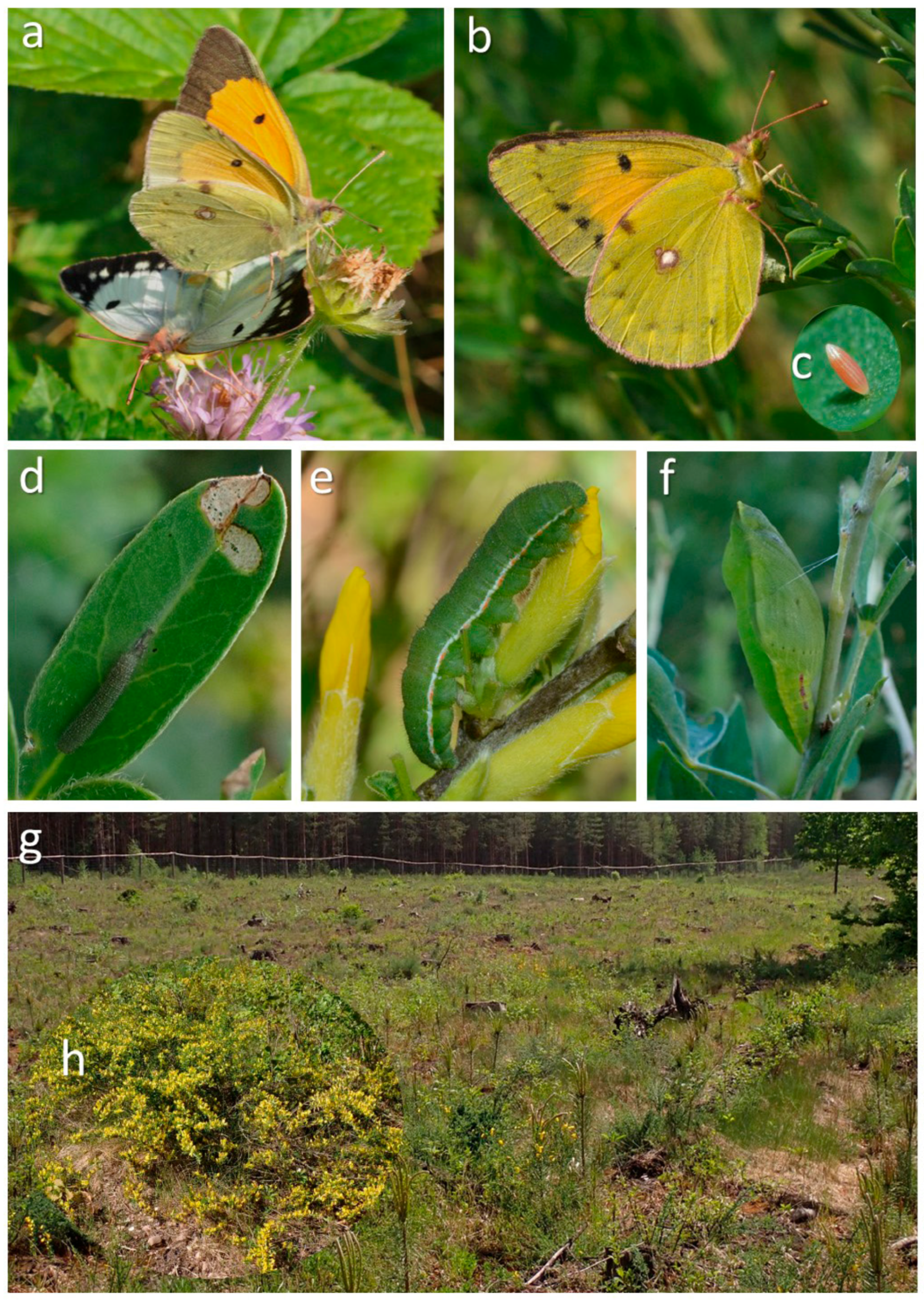
2.1. Study Species
2.2. Sampling for Genetic Studies
2.3. Laboratory Procedures
2.4. Statistical Analysis
3. Results
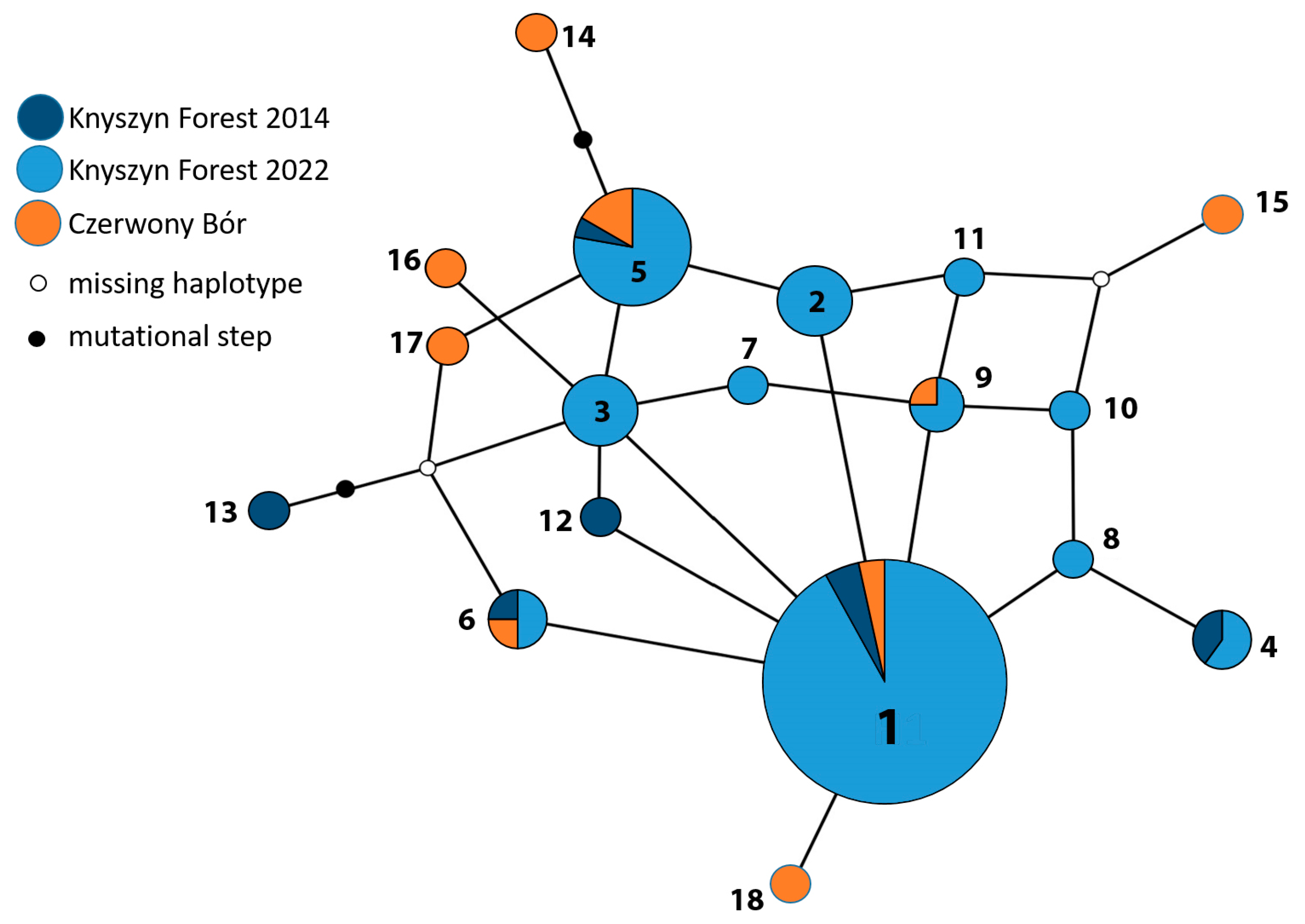
3.1. Cytochrome Oxidase Subunit 1
3.2. Elongation Factor EF-1α
3.3. Wolbachia Infection
4. Discussion
4.1. Genetic Diversity of the Last Remaining Polish Population
4.2. Wolbachia in Colias Myrmidone
4.3. Differentiation Between Colias myrmidone and Colias caucasica
4.4. Conservation Implications
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Van Swaay, C.A.M.; Warren, M.S. Red Data Book of European Butterflies (Rhopalocera); Nature and Environment No. 99; Council of Europe: Strasbourg, France, 1999. [Google Scholar]
- Maes, D.; Verovnik, R.; Wiemers, M.; Brosens, D.; Beshkov, S.; Bonelli, S.; Buszko, J.; Cantú-Salazar, L.; Cassar, L.-F.; Collins, S.; et al. Integrating national Red Lists for prioritising conservation actions for European butterflies. J. Insect Conserv. 2019, 23, 301–330. [Google Scholar] [CrossRef]
- Van Swaay, C.A.M.; Cuttelod, A.; Collins, S.; Maes, D.; López Munguira, M.; Šašić, M.; Settele, J.; Verovnik, R.; Verstrael, T.; Warren, M.; et al. European Red List of Butterflies; Publications Office of the European Union: Luxembourg, 2010. [Google Scholar]
- Sielezniew, M.; Jaworski, T.; Sielezniew, I.; Deoniziak, K.; Bystrowski, C.; Hilszczański, J.; Nowicki, P. Clear-cuts support the metapopulation of a critically endangered butterfly. For. Ecol. Manag. 2024, 562, 121939. [Google Scholar] [CrossRef]
- Loos, J.; Schröer, C.; Becker, T.; Kastal, A.; Kortmann, E.; Dolek, M. Mosaic landscapes provide conservation pockets for an endangered species: Colias myrmidone in Romania. Insect Conserv. Divers. 2022, 15, 359–369. [Google Scholar] [CrossRef]
- Kudrna, O.; Mayer, L. Grundlagen zu einem Artenhilfsprogramm für Colias myrmidone (Esper, 1780) in Bayern. Oedippus 1990, 1, 1–46. [Google Scholar]
- Freese, A.; Dolek, M.; Geyer, A.; Stetter, H. Biology, distribution, and extinction of Colias myrmidone (Lepidoptera, Pieridae) in Bavaria and its situation in other European countries. J. Res. Lepid. 2005, 38, 51–58. [Google Scholar] [CrossRef]
- Konvička, M.; Benes, J.; Cizek, O.; Kopecek, F.; Konvicka, O.; Vitaz, L. How too much care kills species: Grassland reserves, agri-environmental schemes and extinction of Colias myrmidone (Lepidoptera: Pieridae) from its former stronghold. J. Insect Conserv. 2008, 12, 519–525. [Google Scholar] [CrossRef]
- Marhoul, P.; Dolek, M. Action Plan for the Conservation of the Danube Clouded Yellow Colias myrmidone in the European Union; European Commission: Brussels, Belgium, 2012. [Google Scholar]
- Szentirmai, I.; Mesterházy, A.; Varga, I.; Schubert, Z.; Sándor, L.C.; Ábrahám, L.; Kőrösi, Á. Habitat use and population biology of the Danube Clouded Yellow butterfly Colias myrmidone (Lepidoptera: Pieridae) in Romania. J. Insect Conserv. 2014, 18, 417–425. [Google Scholar] [CrossRef]
- Bálint, Z.; Sáfián, S. Narancsszínű kéneslepke Colias myrmidone (Esper 1781). In Natura 2000 Fajok és Élőhelyek Magyarországon; Haraszthy, L., Ed.; Pro Vértes Közalapítvány: Csákvár, Hungary, 2014; pp. 306–307. [Google Scholar]
- Settele, J.; Kudrna, O.; Harpke, A.; Kühn, I.; van Swaay, C.; Verovnik, R.; Warren, M.; Wiemers, M.; Hanspach, J.; Hickler, T.; et al. Climatic Risk Atlas of European Butterflies; Pensoft: Sofia, Bulgaria, 2008. [Google Scholar]
- Steiner, C.F.; Asgari, M. Habitat isolation reduces intra- and interspecific biodiversity and stability. R. Soc. Open Sci. 2022, 9, 211309. [Google Scholar] [CrossRef]
- Spielman, D.; Brook, B.W.; Frankham, R. Most species are not driven to extinction before genetic factors impact them. Proc. Natl. Acad. Sci. USA 2004, 101, 15261–15264. [Google Scholar] [CrossRef]
- Frankham, R. Genetics and extinction. Biol. Conserv. 2005, 126, 131–140. [Google Scholar] [CrossRef]
- Kardos, M.; Armstrong, E.E.; Fitzpatrick, S.W.; Hauser, S.; Hedrick, P.W.; Miller, J.M.; Tallmon, D.A.; Funk, W.C. The crucial role of genome-wide genetic variation in conservation. Proc. Natl. Acad. Sci. USA 2021, 118, e2104642118. [Google Scholar] [CrossRef] [PubMed]
- Keyghobadi, N.; Roland, J.E.N.S.; Strobeck, C. Genetic differentiation and gene flow among populations of the alpine butterfly, Parnassius smintheus, vary with landscape connectivity. Mol. Ecol. 2005, 14, 1897–1909. [Google Scholar] [CrossRef]
- Lynch, M.; Lande, R. Evolution and extinction in response to environmental change. In Biotic Interactions and Global Change; Kareiva, P.M., Kingsolver, J.G., Huey, R.B., Eds.; Sinauer: Sunderland, MA, USA, 1993; pp. 234–250. [Google Scholar]
- Smith, M.A.; Bertrand, C.; Crosby, K.; Eveleigh, E.S.; Fernandez-Triana, J.; Fisher, B.L.; Gibbs, J.; Hajibabaei, M.; Hallwachs, W.; Hind, K.; et al. Wolbachia and DNA barcoding insects: Patterns, potential, and problems. PLoS ONE 2012, 7, e36514. [Google Scholar] [CrossRef] [PubMed]
- Habel, J.C.; Schmitt, T. The burden of genetic diversity. Biol. Conserv. 2012, 147, 270–274. [Google Scholar] [CrossRef]
- Petit-Marty, N.; Vázquez-Luis, M.; Hendriks, I.E. Use of the nucleotide diversity in COI mitochondrial gene as an early diagnostic of conservation status of animal species. Conserv. Lett. 2021, 14, e12756. [Google Scholar] [CrossRef]
- DiLeo, M.F.; Nair, A.; Kardos, M.; Husby, A.; Saastamoinen, M. Demography and environment modulate the effects of genetic diversity on extinction risk in a butterfly metapopulation. Proc. Natl. Acad. Sci. USA 2024, 121, e2309455121. [Google Scholar] [CrossRef]
- Alves, F.; Banks, S.C.; Edworthy, M.; Stojanovic, D.; Langmore, N.E.; Heinsohn, R. Using conservation genetics to prioritise management options for an endangered songbird. Heredity 2023, 130, 289–301. [Google Scholar] [CrossRef]
- van der Valk, T.; Dalèn, L. From genomic threat assessment to conservation action. Cell 2024, 187, 1038–1041. [Google Scholar] [CrossRef]
- Mo, S.; Zhu, Y.; Braga, M.P.; Lohman, D.J.; Nylin, S.; Moumou, A.; Wheat, C.W.; Wahlberg, N.; Wang, M.; Ma, F.; et al. Rapid evolution of host repertoire and geographic range in a young and diverse genus of montane butterflies. Syst. Biol. 2024, 74, 141–157. [Google Scholar] [CrossRef]
- Dincă, V.; Bálint, Z.; Vodă, R.; Dapporto, L.; Hebert, P.D.; Vila, R. Use of genetic, climatic, and microbiological data to inform reintroduction of a regionally extinct butterfly. Conserv. Biol. 2018, 32, 828–837. [Google Scholar] [CrossRef]
- Hebert, P.D.; Cywinska, A.; Ball, S.L.; DeWaard, J.R. Biological identifications through DNA barcodes. Proc. Roy. Soc. Lond. B Biol. Sci. 2003, 270, 313–321. [Google Scholar] [CrossRef] [PubMed]
- Dincă, V.; Zakharov, E.V.; Hebert, P.D.; Vila, R. Complete DNA barcode reference library for a country’s butterfly fauna reveals high performance for temperate Europe. Proc. Roy. Soc. Lond. B Biol. Sci. 2011, 278, 347–355. [Google Scholar] [CrossRef] [PubMed]
- Dincă, V.; Dapporto, L.; Somervuo, P.; Vodă, R.; Cuvelier, S.; Gascoigne-Pees, M.; Huemer, P.; Mutanen, M.; Hebert, P.D.N.; Vila, R. High resolution DNA barcode library for European butterflies reveals continental patterns of mitochondrial genetic diversity. Commun. Biol. 2021, 4, 315. [Google Scholar] [CrossRef]
- Kulak, A.W. Colias myrmidone (Esper, 1771). In Krasnaya kniga Respubliki Belarusʹ. Zhivotnyye, 4th ed.; Katchanovski, I.M., Nikiforov, M.E., Parfienov, W.I., Eds.; Belorusskaya Entsiklopediya im. Petrusja Brovki: Minsk, Belarus, 2015; pp. 208–209. [Google Scholar]
- Wahlberg, N.; Brower, A.V.Z.; Nylin, S. Phylogenetic relationships and historical biogeography of tribes and genera in the subfamily Nymphalinae (Lepidoptera: Nymphalidae). Biol. J. Linn. Soc. 2005, 86, 227–251. [Google Scholar] [CrossRef]
- Kodandaramaiah, U.; Wahlberg, N. Phylogeny and biogeography of Coenonympha butterflies (Nymphalidae: Satyrinae)—Patterns of colonization in the Holarctic. Syst. Entomol. 2009, 34, 315–323. [Google Scholar] [CrossRef]
- Dapporto, L.; Cini, A.; Vodă, R.; Dincă, V.; Wiemers, M.; Menchetti, M.; Magini, G.; Talavera, G.; Shreeve, T.; Bonelli, S.; et al. Integrating three comprehensive data sets shows that mitochondrial DNA variation is linked to species traits and paleogeographic events in European butterflies. Mol. Ecol. Resour. 2019, 19, 1623–1636. [Google Scholar] [CrossRef] [PubMed]
- Hilgenboecker, K.; Hammerstein, P.; Schlattmann, P.; Telschow, A.; Werren, J.H. How many species are infected with Wolbachia?—A statistical analysis of current data. FEMS Microbiol. Lett. 2008, 281, 215–220. [Google Scholar] [CrossRef]
- Charlat, S.; Hurst, G.D.D.; Merçot, H. Evolutionary consequences of Wolbachia infection. Trends Genet. 2003, 19, 217–223. [Google Scholar] [CrossRef]
- Werren, J.H.; Baldo, L.; Clark, M.E. Wolbachia: Master manipulators of invertebrate biology. Nat. Rev. Microbiol. 2008, 6, 741–751. [Google Scholar] [CrossRef]
- Wendt, M.; Kulanek, D.; Varga, Z.; Rákosy, L.; Schmitt, T. Pronounced mito-nuclear discordance and various Wolbachia infections in the water ringlet Erebia pronoe have resulted in a complex phylogeographic structure. Sci. Rep. 2022, 12, 5175. [Google Scholar] [CrossRef]
- Bartoňová, A.S.; Konvička, M.; Marešová, J.; Wiemers, M.; Ignatev, N.; Wahlberg, N.; Schmitt, T.; Fric, Z.F. Wolbachia affects mitochondrial population structure in two systems of closely related Palaearctic blue butterflies. Sci. Rep. 2021, 11, 3019. [Google Scholar] [CrossRef]
- Ritter, S.; Michalski, S.G.; Settele, J.; Wiemers, M.; Fric, Z.F.; Sielezniew, M.; Šašić, M.; Rozier, Y.; Durka, W. Wolbachia infections mimic cryptic speciation in two parasitic butterfly species, Phengaris teleius and P. nausithous (Lepidoptera: Lycaenidae). PLoS ONE 2013, 8, e78107. [Google Scholar] [CrossRef] [PubMed]
- Nice, C.C.; Gompert, Z.; Forister, M.L.; Fordyce, J.A. An unseen foe in arthropod conservation efforts: The case of Wolbachia infections in the Karner blue butterfly. Biol. Conserv. 2009, 142, 3137–3146. [Google Scholar] [CrossRef]
- Hamm, C.A.; Handley, C.A.; Pike, A.; Forister, M.L.; Fordyce, J.A.; Nice, C.C. Wolbachia infection and Lepidoptera of conservation concern. J. Insect Sci. 2014, 14, 6. [Google Scholar] [CrossRef] [PubMed]
- Buszko, J. Colias myrmidone (Esper 1780)—Szlaczkoń szafraniec. In Polish Red Data Book of Animals. Invertebrates; Głowaciński, Z., Nowacki, J., Eds.; Institute of Nature Conservation PAS: Kraków, Poland, 2004; pp. 243–244. [Google Scholar]
- Marschalek, D.A.; Jesu, J.A.; Berres, M.E. Impact of non-lethal genetic sampling on the survival, longevity and behaviour of the Hermes copper (Lycaena hermes) butterfly. Insect Conserv. Divers. 2013, 6, 658–662. [Google Scholar] [CrossRef]
- Folmer, O.; Black, M.; Hoeh, W.; Lutz, R.; Vrijenhoek, R. DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Mol. Mar. Biol. Biotechnol. 1994, 3, 294–299. [Google Scholar]
- Kim, M.I.; Wan, X.; Kim, M.J.; Jeong, H.C.; Ahn, N.H.; Kim, K.G.; Han, Y.S.; Kim, I. Phylogenetic relationships of true butterflies (Lepidoptera: Papilionoidea) inferred from COI, 16S rRNA and EF-1α sequences. Mol. Cells 2010, 30, 409–425. [Google Scholar] [CrossRef]
- Monteiro, A.; Pierce, N. Phylogeny of Bicyclus (Lepidoptera: Nymphalidae) inferred from COI, COII, and EF1-α gene sequences. Mol. Phylogenet. Evol. 2001, 18, 264–281. [Google Scholar] [CrossRef]
- Braig, H.R.; Zhou, W.; Dobson, S.L.; O’Neill, S.L. Cloning and characterization of a gene encoding the major surface protein of the bacterial endosymbiont Wolbachia pipientis. J. Bacteriol. 1998, 180, 2373–2378. [Google Scholar] [CrossRef]
- Hall, T.A. BioEdit: A user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp. Ser. 1999, 41, 95–98. [Google Scholar]
- Librado, P.; Rozas, J. DnaSP v5: A software for comprehensive analysis of DNA polymorphism data. Bioinformatics 2009, 25, 1451–1452. [Google Scholar] [CrossRef] [PubMed]
- Excoffier, L.; Lischer, H.E.L. Arlequin suite ver 3.5: A new series of programs to perform population genetics analyses under Linux and Windows. Mol. Ecol. Res. 2010, 10, 564–567. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.X. Statistical tests of neutrality of mutations against population growth, hitchhiking and background selection. Genetics 1997, 147, 915–925. [Google Scholar] [CrossRef] [PubMed]
- Tajima, F. Statistical method for testing the neutral mutational hypothesis by DNA polymorphism. Genetics 1989, 123, 585–595. [Google Scholar] [CrossRef]
- Rogers, A.R.; Harpending, H. Population growth makes waves in the distribution of pairwise genetic differences. Mol. Biol. Evol. 1992, 9, 552–569. [Google Scholar]
- Harpending, H.C.; Sherry, S.T.; Rogers, A.R.; Stoneking, M. Genetic structure of ancient human populations. Curr. Anthropol. 1993, 34, 483–496. [Google Scholar] [CrossRef]
- Bandelt, H.J.; Forster, P.; Rohl, A. Median-joining networks for inferring intraspecific phylogenies. Mol. Biol. Evol. 1999, 16, 37–48. [Google Scholar] [CrossRef]
- Posada, D. jModelTest: Phylogenetic model averaging. Mol. Biol. Evol. 2008, 25, 1253–1256. [Google Scholar] [CrossRef]
- Tamura, K.; Stecher, G.; Peterson, D.; Filipski, A.; Kumar, S. MEGA6: Molecular Evolutionary Genetics Analysis version 6.0. Mol. Biol. Evol. 2013, 30, 2725–2729. [Google Scholar] [CrossRef]
- Sucháčková Bartoňová, A.; Konvička, M.; Marešová, J.; Kolev, Z.; Wahlberg, N.; Fric, Z.F. Recently lost connectivity in the Western Palaearctic steppes: The case of a scarce specialist butterfly. Conserv. Gen. 2020, 21, 561–575. [Google Scholar] [CrossRef]
- Dapporto, L.; Menchetti, M.; Vodă, R.; Corbella, C.; Cuvelier, S.; Djemadi, I.; Gascoigne-Pees, M.; Hinojosa, J.C.; Lam, N.T.; Serracanta, M.; et al. The atlas of mitochondrial genetic diversity for Western Palaearctic butterflies. Glob. Ecol. Biogeogr. 2022, 31, 2184–2190. [Google Scholar] [CrossRef]
- Patricelli, D.; Sielezniew, M.; Ponikwicka-Tyszko, D.; Ratkiewicz, M.; Bonelli, S.; Barbero, F.; Witek, M.; Buś, M.M.; Rutkowski, R.; Balletto, E. Contrasting genetic structure of rear edge and continuous range populations of a parasitic butterfly infected by Wolbachia. BMC Evol. Biol. 2013, 13, 14. [Google Scholar] [CrossRef] [PubMed]
- Gratton, P.; Konopiński, M.K.; Sbordoni, V. Pleistocene evolutionary history of the Clouded Apollo (Parnassius mnemosyne): Genetic signatures of climate cycles and a ‘time-dependent’ mitochondrial substitution rate. Mol. Ecol. 2008, 17, 4248–4262. [Google Scholar] [CrossRef] [PubMed]
- Kramp, K.; Cizek, O.; Madeira, P.M.; Ramos, A.A.; Konvicka, M.; Castilho, R.; Schmitt, T. Genetic implications of phylogeographical patterns in the conservation of the boreal wetland butterfly Colias palaeno (Pieridae). Biol. J. Linn. Soc. 2016, 119, 1068–1081. [Google Scholar] [CrossRef]
- Sielezniew, M.; Ponikwicka-Tyszko, D.; Ratkiewicz, M.; Dziekanska, I.; Kostro-Ambroziak, A.; Rutkowski, R. Diverging patterns of mitochondrial and nuclear diversity in the specialized butterfly Plebejus argus (Lepidoptera: Lycaenidae). Eur. J. Entomol. 2011, 108, 537–545. [Google Scholar] [CrossRef]
- Sielezniew, M.; Rutkowski, R.; Ponikwicka-Tyszko, D.; Ratkiewicz, M.; Dziekańska, I.; Švitra, G. Differences in genetic variability between two ecotypes of endangered myrmecophilous butterfly Phengaris (=Maculinea) alcon—The setting of conservation priorities. Insect Conserv. Divers. 2012, 5, 223–236. [Google Scholar] [CrossRef]
- Czajkowska, M.; Dawidowicz, Ł.; Borkowska, A.; Dziekańska, I.; Sielezniew, M. Population genetic structure and demography of the critically endangered Chequered blue butterfly (Scolitantides orion) in a highly isolated part of its distribution range. Insects 2020, 11, 608. [Google Scholar] [CrossRef]
- Zupan, S.; Jugovic, J.; Čelik, T.; Buzan, E. Population genetic structure of the highly endangered butterfly Coenonympha oedippus (Nymphalidae: Satyrinae) at its southern edge of distribution. Genetica 2021, 149, 21–36. [Google Scholar] [CrossRef]
- Zhang, D.-X.; Hewitt, G.M. Nuclear DNA analyses in genetic studies of populations: Practice, problems and prospects. Mol. Ecol. 2003, 12, 563–584. [Google Scholar] [CrossRef]
- Mackintosh, A.; Laetsch, D.R.; Hayward, A.; Charlesworth, B.; Waterfall, M.; Vila, R.; Lohse, K. The determinants of genetic diversity in butterflies. Nat. Commun. 2019, 10, 3466. [Google Scholar] [CrossRef]
- Robinson, J.A.; Ortega-Del Vecchyo, D.; Fan, Z.; Kim, B.Y.; vonHoldt, B.M.; Marsden, C.D.; Lohmueller, K.E.; Wayne, R.K. Genomic Flatlining in the Endangered Island Fox. Curr. Biol. 2016, 26, 1183–1189. [Google Scholar] [CrossRef] [PubMed]
- Dussex, N.; van der Valk, T.; Morales, H.E.; Wheat, C.W.; Díez-Del-Molino, D.; von Seth, J.; Foster, Y.; Kutschera, V.E.; Guschanski, K.; Rhie, A.; et al. Population genomics of the critically endangered kākāpō. Cell Genom. 2021, 1, 100002. [Google Scholar] [CrossRef] [PubMed]
- Thomas, W.J.W.; Anthony, J.M.; Dobrowolski, M.P.; Krauss, S.L. Optimising the conservation of genetic diversity of the last remaining population of a critically endangered shrub. AoB Plants 2021, 13, plab005. [Google Scholar] [CrossRef] [PubMed]
- Rutkowski, R.; Jagołkowska, P.; Zawadzka, D.; Bogdanowicz, W. Impacts of forest fragmentation and post-glacial colonization on the distribution of genetic diversity in the Polish population of the hazel grouse Terastes bonasia. Eur. J. Wildl. Res. 2016, 62, 293–306. [Google Scholar] [CrossRef]
- Andersen, J.C.; Havill, N.P.; Caccone, A.; Elkinton, J.S. Postglacial recolonization shaped the genetic diversity of the winter moth (Operophtera brumata) in Europe. Ecol. Evol. 2017, 7, 3312–3323. [Google Scholar] [CrossRef]
- Maresova, J.; Habel, J.C.; Neve, G.; Sielezniew, M.; Bartonova, A.; Kostro-Ambroziak, A.; Fric, Z.F. Cross-continental phylogeography of two Holarctic Nymphalid butterflies, Boloria eunomia and Boloria selene. PLoS ONE 2019, 14, e0214483. [Google Scholar] [CrossRef]
- Niedziałkowska, M.; Tarnowska, E.; Babik, W.; Konczal, M.; Gharbi, K.; Cezard, T.; Jędrzejewska, B. Different waves of postglacial recolonisation and genomic structure of bank vole populations in NE Poland. Heredity 2023, 130, 269–277. [Google Scholar] [CrossRef]
- Schwenk, K.; Brede, N.; Streit, B. Introduction. Extent, processes and evolutionary impact of interspecific hybridization in animals. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2008, 363, 2805–2811. [Google Scholar] [CrossRef]
- Jangjoo, M.; Matter, S.F.; Roland, J.; Keyghobadi, N. Connectivity rescues genetic diversity after a demographic bottleneck in a butterfly population network. Proc. Natl. Acad. Sci. USA 2016, 113, 10914–10919. [Google Scholar] [CrossRef]
- Schmitt, T.; Hewitt, G.M. The genetic pattern of population threat and loss: A case study of butterflies. Mol. Ecol. 2004, 13, 21–31. [Google Scholar] [CrossRef]
- Habel, J.C.; Husemann, M.; Finger, A.; Danley, P.D.; Zachos, F.E. The relevance of time series in molecular ecology and conservation biology. Biol. Rev. 2014, 89, 484–492. [Google Scholar] [CrossRef] [PubMed]
- Sielezniew, M.; Deoniziak, K.; Dziekańska, I.; Nowicki, P. Dispersal in a metapopulation of the critically endangered Danube Clouded Yellow butterfly Colias myrmidone: Implications for conservation. J. Insect Conserv. 2019, 23, 291–300. [Google Scholar] [CrossRef]
- Duffy, E.; Archer, C.R.; Sharma, M.D.; Prus, M.; Joag, R.A.; Radwan, J.; Wedell, N.; Hosken, D.J. Wolbachia infection can bias estimates of intralocus sexual conflict. Ecol. Evol. 2019, 9, 328–338. [Google Scholar] [CrossRef] [PubMed]
- Shapoval, N.A.; Kir’yanov, A.V.; Krupitsky, A.V.; Yakovlev, R.V.; Romanovich, A.E.; Zhang, J.; Grishin, N.V.; Kovalenko, M.G.; Shapoval, G.N. Phylogeography of Two Enigmatic Sulphur Butterflies, Colias mongola Alphéraky, 1897 and Colias tamerlana Staudinger, 1897 (Lepidoptera, Pieridae), with Relations to Wolbachia Infection. Insects 2023, 14, 943. [Google Scholar] [CrossRef]
- Dzurinka, M.; Šemeláková, M.; Panigaj, L. Taxonomy of hybridizing Colias croceus (Geoffroy, 1785) and Colias erate (Esper, 1805) (Lepidoptera, Pieridae) in light of mitochondrial and nuclear DNA, with occurrence and effects of Wolbachia infection. Zool. Anz. 2022, 299, 73–811. [Google Scholar] [CrossRef]
- Funkhouser-Jones, L.J.; Sehnert, S.R.; Martínez-Rodríguez, P.; Toribio-Fernández, R.; Pita, M.; Bella, J.L.; Bordenstein, S.R. Wolbachia co-infection in a hybrid zone: Discovery of horizontal gene transfers from two Wolbachia supergroups into an animal genome. PeerJ 2015, 7, e1479. [Google Scholar] [CrossRef]
- Kemp, D.J.; Thomson, F.E.; Edwards, W.; Iturbe-Ormaetxe, I. Incomplete offspring sex bias in Australian populations of the butterfly Eurema hecabe. Heredity 2017, 118, 284–292. [Google Scholar] [CrossRef]
- Narita, S.; Nomura, M.; Kageyama, D. A natural population of the butterfly Eurema hecabe with Wolbachia-induced female-biased sex ratio not by feminization. Genome 2007, 50, 365–372. [Google Scholar] [CrossRef]
- Charlat, S.; Hornett, E.A.; Dyson, E.A.; Ho, P.P.Y.; Loc, N.T.; Schilthuizen, M.; Davies, N.; Roderick, G.K.; Hurst, G.D.D. Prevalence and penetrance variation of male-killing Wolbachia across Indo-Pacific populations of the butterfly Hypolimnas bolina. Mol. Ecol. 2005, 14, 3525–3530. [Google Scholar] [CrossRef]
- Kern, P.; Cook, J.M.; Kageyama, D.; Riegler, M. Double trouble: Combined action of meiotic drive and Wolbachia feminization in Eurema butterflies. Biol. Lett. 2015, 11, 20150095. [Google Scholar] [CrossRef]
- Kageyama, D.; Ohno, M.; Sasaki, T.; Yoshido, A.; Konagaya, T.; Jouraku, A.; Kuwazaki, S.; Kanamori, H.; Katayose, Y.; Narita, S.; et al. Feminizing Wolbachia endosymbiont disrupts maternal sex chromosome inheritance in a butterfly species. Evol. Lett. 2017, 1, 232–244. [Google Scholar] [CrossRef]
- Després, L.; Henniaux, C.; Rioux, D.; Capblancq, T.; Zupan, S.; Čelik, T.; Sielezniew, M.; Bonato, L.; Ficetola, G.F. Inferring the biogeography and demographic history of an endangered butterfly in Europe from multilocus markers. Biol. J. Linn. Soc. 2019, 126, 95–113. [Google Scholar] [CrossRef]
- Toews, D.P.; Mandic, M.; Richards, J.G.; Irwin, D.E. Migration, mitochondria, and the yellow-rumped warbler. Evolution 2014, 68, 241–255. [Google Scholar] [CrossRef] [PubMed]
- Bazin, E.; Glémin, S.; Galtier, N. Population size does not influence mitochondrial genetic diversity in animals. Science 2006, 312, 570–572. [Google Scholar] [CrossRef] [PubMed]
- Narita, S.; Shimajiri, Y.; Nomura, M. Strong cytoplasmic incompatibility and high vertical transmission rate can explain the high frequencies of Wolbachia infection in Japanese populations of Colias erate poliographus (Lepidoptera: Pieridae). Bull. Entom. Res. 2009, 99, 385–391. [Google Scholar] [CrossRef]
- Schoville, S.D.; Stuckey, M.; Roderick, G.K. Pleistocene origin and population history of a neoendemic alpine butterfly. Mol. Ecol. 2011, 20, 1233–1247. [Google Scholar] [CrossRef]
- Karaçetin, E.; Welch, H.J. Red Book of Butterflies in Turkey; Nature Conservation Center: Ankara, Turkey, 2011. [Google Scholar]
- Nekrutenko, Y.P. Butterflies of the Caucasus: Keys to Their Identification (Papilionidae, Pieridae, Satyridae, Danidae); Naukova Dumka: Kiev, Ukraine, 1990. [Google Scholar]
- Pamperis, L.N. The Butterflies of Greece; Editions Pamperis: Athens, Greece, 2009. [Google Scholar]
- Verovnik, R.; Micevski, B.; Đurić, M.; Jakšić, P.; Keymeulen, A.; Van Swaay, C.; Veling, K. Contribution to the knowledge of the butterfly fauna of the Republic of Macedonia (Lepidoptera: Papilionoidea & Hesperioidea). Acta Entomol. Slov. 2010, 18, 31–46. [Google Scholar] [CrossRef]
- Tvrtković, N.; Mihoci, I.; Šašić, M. Colias caucasica balcanica Rebel, 1901 (Pieridae) u Hrvatskoj–najzapadnija točka rasprostranjenja. Nat. Croat. Period. Musei Hist. Nat. Croat. 2011, 20, 375–385. [Google Scholar]
- Nahirnić, A.; Jakšić, P.; Viborg, A.L. Colias caucasica balcanica (Pieridae) rediscovered in Montenegro, with additional new records for Serbia. Phegea 2015, 43, 6–10. [Google Scholar]
- Tot, I.S.; Slacki, A.; Đurić, M.; Popović, M. Butterflies of the Vlasina region in southeast Serbia (Lepidoptera: Papilionoidea). Acta Entomol. Serbica 2015, 20, 117–135. [Google Scholar]
- Hristova, H.O.; Beshkov, S. Checklist of the superfamilies Hesperioidea and Papilionoidea (Insecta: Lepidoptera) of Bulgaria, with application of the IUCN Red List criteria at national level. Acta Zool. Bulg. 2017, 69, 105–114. [Google Scholar]
- Cuvelier, S.; Parmentier, L.; Paparisto, A.; Couckuyt, J. Butterflies of Albania–Fluturat e Shqipërisë: New surveys, new species and a new checklist (Lepidoptera: Papilionoidea). Phegea 2018, 46, 48–69. [Google Scholar] [CrossRef]
- Wiemers, M.; Balletto, E.; Dincă, V.; Fric, Z.F.; Lamas, G.; Lukhtanov, V.; Munguira, M.L.; van Swaay, C.A.M.; Vila, R.; Vliegenthart, A.; et al. An updated checklist of the European butterflies (Lepidoptera, Papilionoidea). ZooKeys 2018, 811, 9. [Google Scholar] [CrossRef][Green Version]
- Wagener, S. Colias caucasica balcanica Rebel, 1901 (comb. nov.; stat. nov.)(Lepidoptera: Pieridae). Phegea 1990, 18, 59–63. [Google Scholar]
- Tolman, T.; Lewington, R. Collins Butterfly Guide: The Most Complete Field Guide to the Butterflies of Britain and Europe; Harper Collins: London, UK, 2009. [Google Scholar]
- Stella, D.; Fric, F.Z.; Rindoš, M.; Kleisner, K.; Pecháček, P. Distribution of ultraviolet ornaments in Colias butterflies (Lepidoptera: Pieridae). Environ. Entomol. 2018, 47, 1344–1354. [Google Scholar] [CrossRef]
- Grieshuber, J.; Worthy, B.; Lamas, G. The Genus Colias Fabricius, 1807. Jan Haugum’s Annotated Catalogue of the Old World Colias (Lepidoptera, Pieridae); Tshikolovets Publications: Pardubice, Czech Republic, 2012. [Google Scholar]
- Grieshuber, J. Guide to the Butterflies of the Palearctic Region; Pieridae Part II; Omnes Artes: Milano, Italy, 2014. [Google Scholar]
- Grieshuber, J.; Lamas, G. A synonymic list of the genus Colias Fabricius, 1807 (Lepidoptera: Pieridae). Mitt. Münch. Ent. Ges. 2007, 97, 131–171. [Google Scholar]
- Gaunet, A.; Dincă, V.; Dapporto, L.; Montagud, S.; Vodă, R.; Schär, S.; Badiane, A.; Font, E.; Vila, R. Two consecutive Wolbachia-mediated mitochondrial introgressions obscure taxonomy in Palearctic swallowtail butterflies (Lepidoptera, Papilionidae). Zool. Scr. 2019, 48, 507–519. [Google Scholar] [CrossRef]
- Miyata, M.N.; Nomura, M.; Kageyama, D. Wolbachia have made it twice: Hybrid introgression between two sister species of Eurema butterflies. Ecol. Evol. 2020, 10, 8323–8330. [Google Scholar] [CrossRef]



| Knyszyn Forest | Czerwony Bór | All | |||
|---|---|---|---|---|---|
| Year | 2014 | 2022 | 2014–2022 | 2014 | 2014–2022 |
| N | 12 | 125 | 137 | 22 | 159 |
| H | 1 | 4 | 4 | 1 | 4 |
| h | 0.00 | 0.276 | 0.255 | 0.00 | 0.224 |
| π | 0.00 | 0.00556 | 0.00514 | 0.00 | 0.00452 |
| k | 0.00 | 3.262 | 3.018 | 0.00 | 2.652 |
| Tajima’s D test | |||||
| D | – | 0.470 | 0.280 | – | -0.0062 |
| p | – | 0.651 | 0.583 | – | 0.514 |
| Fu’s Fs test | |||||
| Fs | – | 8.544 | 8.062 | – | 7.277 |
| p | – | 0.999 | 0.990 | – | 0.985 |
| Raggedness index | |||||
| r | – | 0.6035 | 0.6191 | – | 0.6466 |
| p | – | 0.556 | 0.602 | – | 0.658 |
| SSD | |||||
| SSD | – | 0.0838 | 0.0724 | – | 0.0566 |
| p | – | 0.035 | 0.034 | – | 0.019 |
| Frequency | |||||
| CmCOI-1 | 1.000 | 0.840 | 0.854 | 1.000 | 0.87 |
| CmCOI-2 | – | 0.144 | 0.131 | – | 0.11 |
| CmCOI-3 | – | 0.008 | 0.007 | – | 0.01 |
| CmCOI-4 | – | 0.008 | 0.007 | – | 0.01 |
| Locality | Knyszyn Forest | Czerwony Bór | All | ||
|---|---|---|---|---|---|
| Year | 2014 | 2022 | 2014–2022 | 2014 | 2014–2022 |
| N | 11 | 124 | 135 | 16 | 151 |
| H | 6 | 11 | 14 | 9 | 18 |
| h | 0.855 | 0.571 | 0.600 | 0.925 | 0.646 |
| π | 0.00363 | 0.00157 | 0.00175 | 0.00485 | 0.00210 |
| k | 2.10909 | 0.91303 | 1.01438 | 2.81667 | 1.21996 |
| Tajima’s D test | |||||
| D | −0.94257 | −0.38639 | −0.91187 | −0.24378 | −1.33313 |
| p | 0.359 | 0.338 | 0.269 | 0.421 | 0.085 |
| Fu’s Fs test | |||||
| Fs | −1.419 | −6.502 | −8.109 | −2.779 | −14.751 |
| p | 0.119 | 0.001 | 0.001 | 0.066 | 0.001 |
| Raggedness index | |||||
| r | 0.0614 | 0.0728 | 0.0621 | 0.042 | 0.0483 |
| p | 0.658 | 0.765 | 0.757 | 0.468 | 0.791 |
| SSD | |||||
| SSD | 0.0096 | 0.0077 | 0.0074 | 0.0061 | 0.0068 |
| p | 0.623 | 0.555 | 0.569 | 0.482 | 0.594 |
| Frequency | |||||
| CmEF-1 | 0.364 | 0.645 | 0.622 | 0.188 | 0.58 |
| CmEF-2 | 0.065 | 0.059 | 0.05 | ||
| CmEF-3 | 0.073 | 0.067 | 0.06 | ||
| CmEF-4 | 0.182 | 0.024 | 0.037 | 0.03 | |
| CmEF-5 | 0.091 | 0.113 | 0.111 | 0.188 | 0.12 |
| CmEF-6 | 0.091 | 0.016 | 0.022 | 0.063 | 0.02 |
| CmEF-7 | 0.008 | 0.007 | 0.01 | ||
| CmEF-8 | 0.016 | 0.015 | 0.01 | ||
| CmEF-9 | 0.024 | 0.022 | 0.063 | 0.03 | |
| CmEF-10 | 0.008 | 0.007 | 0.01 | ||
| CmEF-11 | 0.008 | 0.007 | 0.01 | ||
| CmEF-12 | 0.182 | 0.015 | 0.01 | ||
| CmEF-13 | 0.091 | 0.007 | 0.01 | ||
| CmEF-14 | 0.063 | 0.01 | |||
| CmEF-15 | 0.125 | 0.01 | |||
| CmEF-16 | 0.125 | 0.01 | |||
| CmEF-17 | 0.125 | 0.01 | |||
| CmEF-18 | 0.063 | 0.01 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gwiazdowska, A.; Rutkowski, R.; Sielezniew, M. Conservation Genetics of the Endangered Danube Clouded Yellow Butterfly Colias myrmidone (Esper, 1780) in the Last Central European Stronghold: Diversity, Wolbachia Infection and Balkan Connections. Insects 2025, 16, 220. https://doi.org/10.3390/insects16020220
Gwiazdowska A, Rutkowski R, Sielezniew M. Conservation Genetics of the Endangered Danube Clouded Yellow Butterfly Colias myrmidone (Esper, 1780) in the Last Central European Stronghold: Diversity, Wolbachia Infection and Balkan Connections. Insects. 2025; 16(2):220. https://doi.org/10.3390/insects16020220
Chicago/Turabian StyleGwiazdowska, Aleksandra, Robert Rutkowski, and Marcin Sielezniew. 2025. "Conservation Genetics of the Endangered Danube Clouded Yellow Butterfly Colias myrmidone (Esper, 1780) in the Last Central European Stronghold: Diversity, Wolbachia Infection and Balkan Connections" Insects 16, no. 2: 220. https://doi.org/10.3390/insects16020220
APA StyleGwiazdowska, A., Rutkowski, R., & Sielezniew, M. (2025). Conservation Genetics of the Endangered Danube Clouded Yellow Butterfly Colias myrmidone (Esper, 1780) in the Last Central European Stronghold: Diversity, Wolbachia Infection and Balkan Connections. Insects, 16(2), 220. https://doi.org/10.3390/insects16020220






