Study of Bacterial Communities in Water and Different Developmental Stages of Aedes aegypti from Aquatic Breeding Sites in Leticia City, Colombian Amazon Biome
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Collection Permits
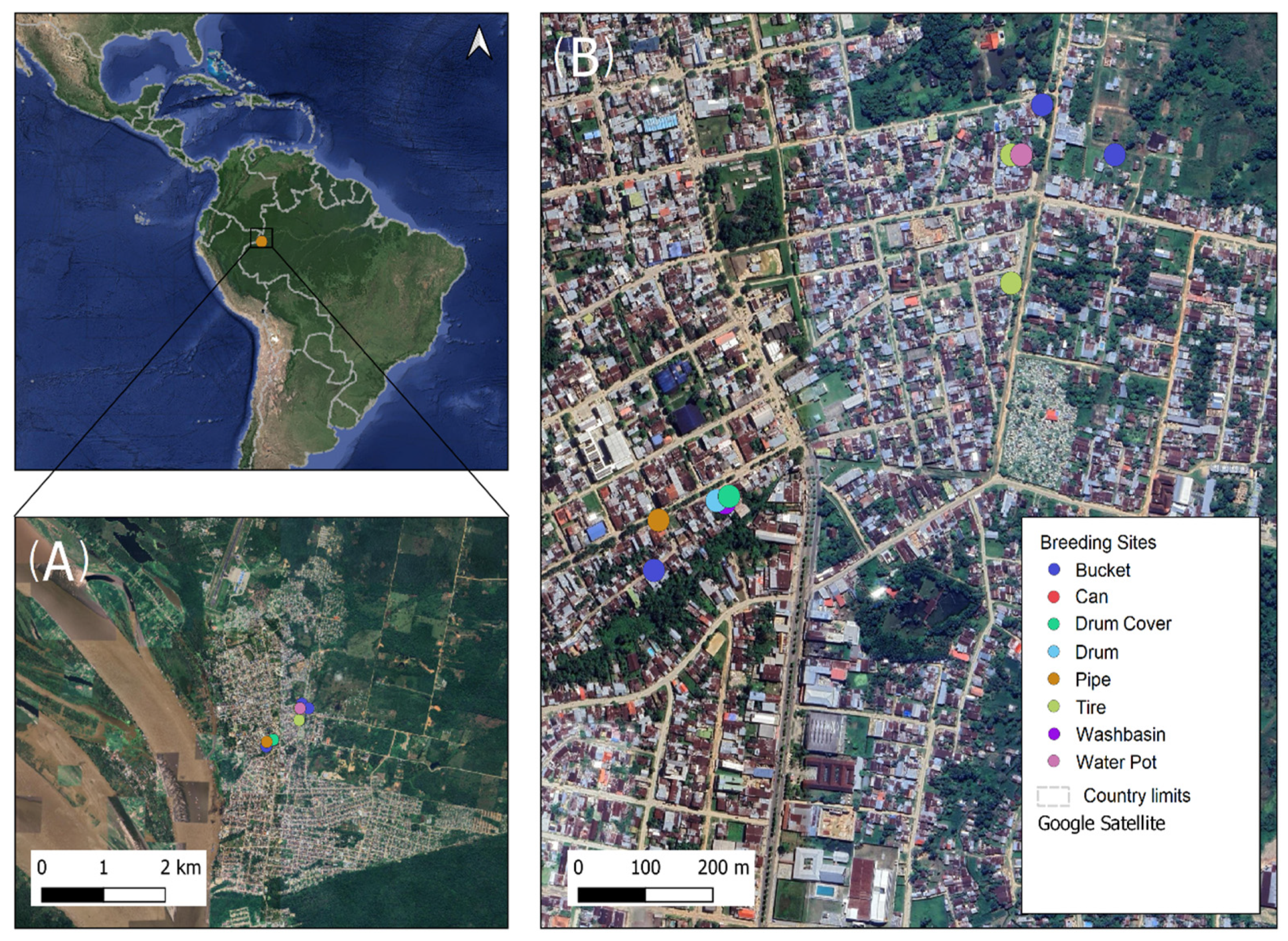
2.2. Collection Area
2.3. Search of Breeding Sites and Physicochemical Analysis of Water Samples Collected at Ae. aegypti Artificial Breeding Sites
2.4. Sample Collection and Total DNA Extraction from Artificial Breeding Site Water for the Study of the Bacterial Communities Associated with the Different Aedes Artificial Breeding Site Types
2.5. Collection of Immature Aedes and Taxonomic Identification of Ae. aegypti Mosquitoes
2.6. DNA Extraction from Immature and Adult Aedes from Artificial Breeding Sites for the Analysis of Bacterial Communities
2.7. Amplification, Paired-End Illumina Sequencing, and Bioinformatic and Statistical Analyses
2.8. Study Limitations
3. Results
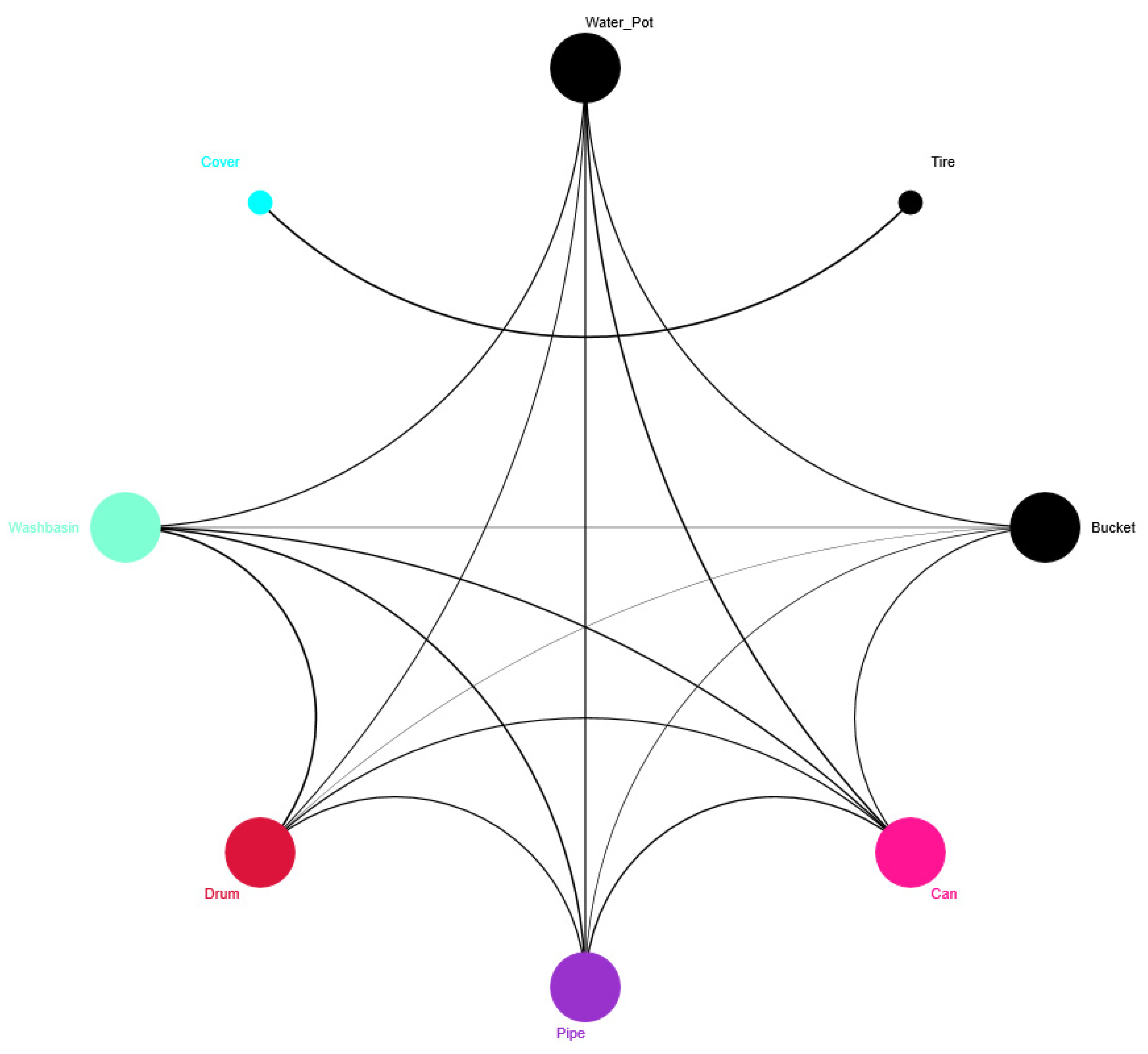
3.1. Description and Classification of Ae. aegypti Artificial Breeding Sites
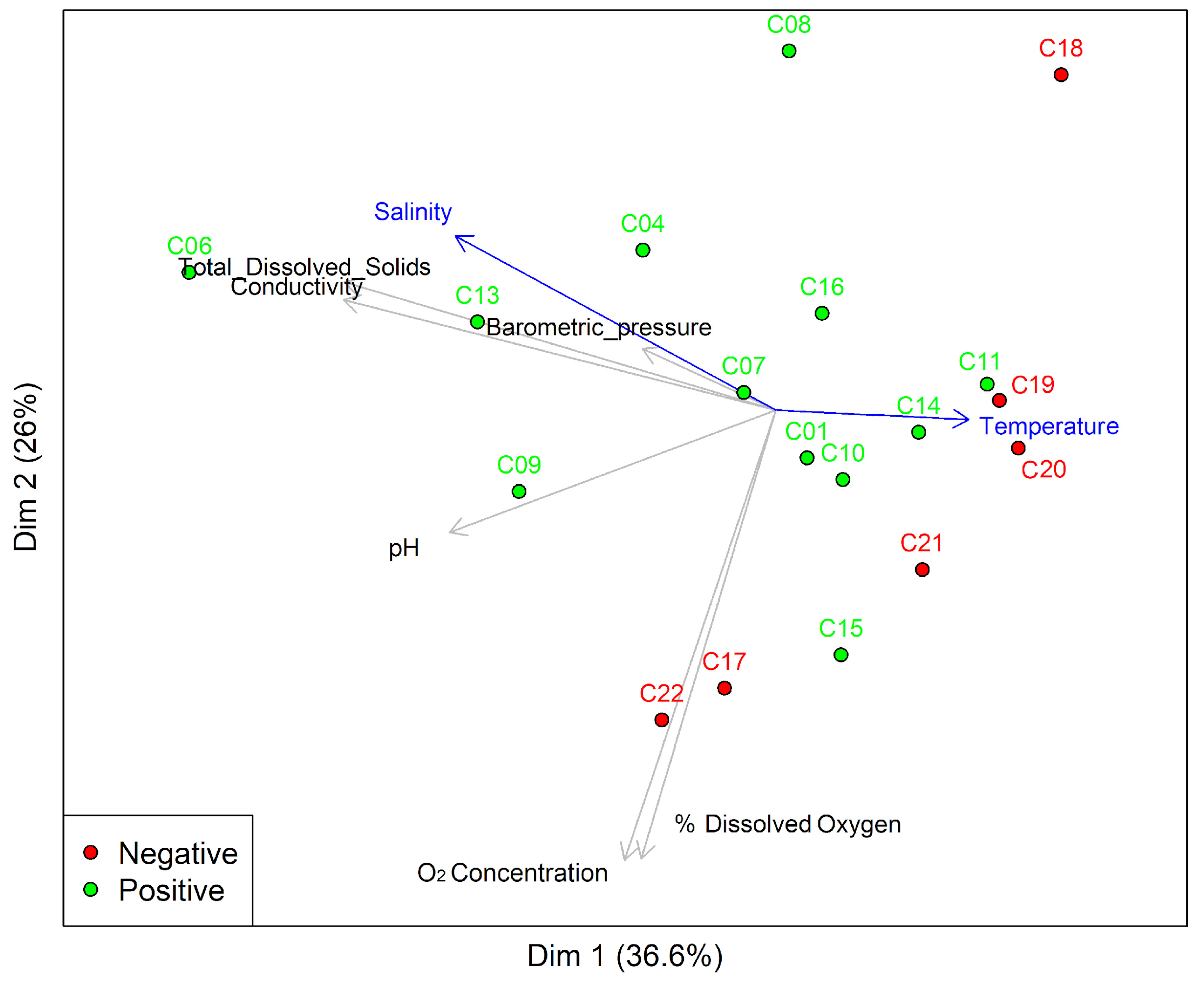
3.2. Characterization of the Physicochemical Variables of Artificial Breeding Sites of Ae. aegypti
3.3. Bacterial Communities of Water Bodies from Artificial Breeding Sites and Developmental Stages of Ae. aegypti
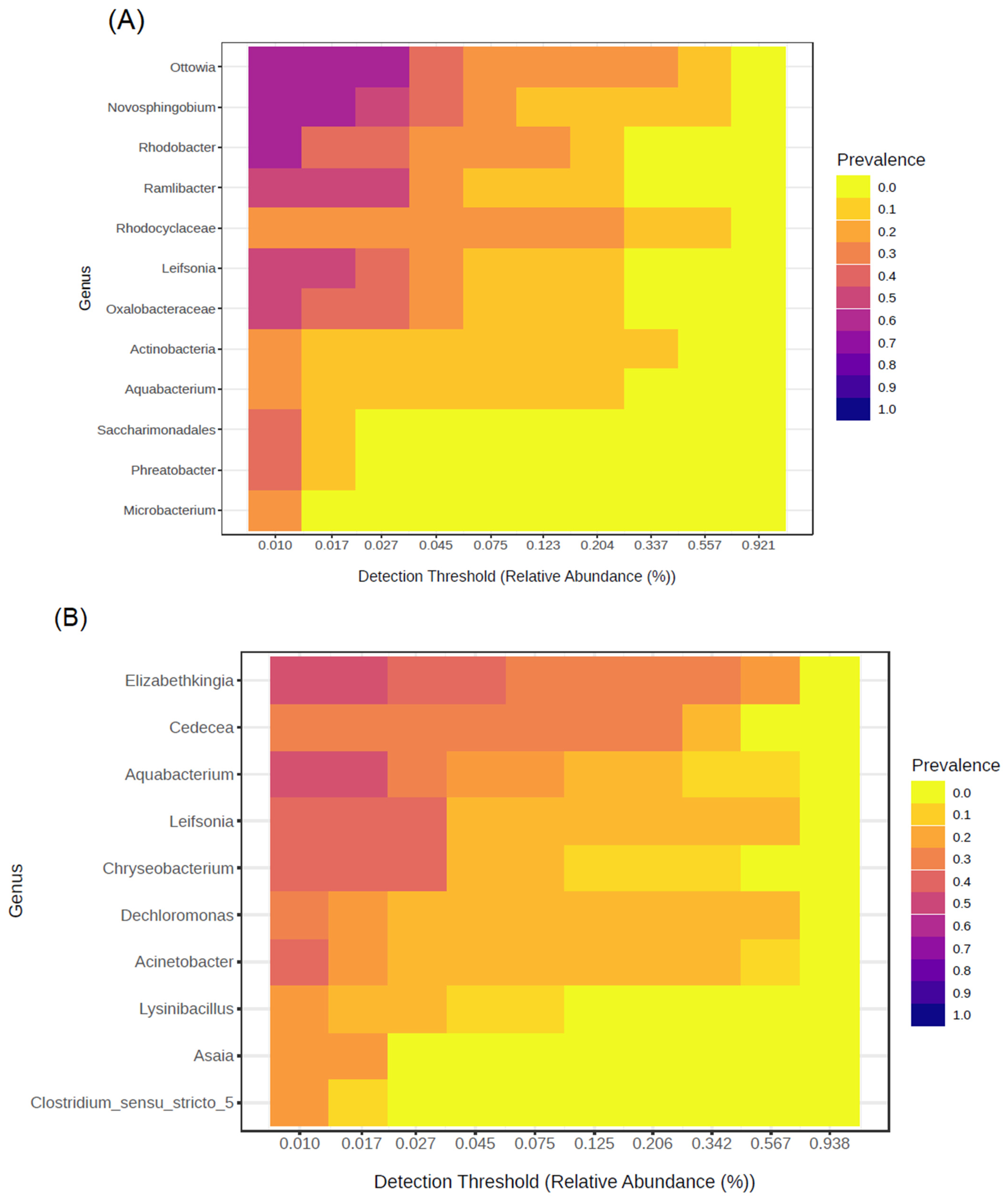
3.3.1. Richness and Alpha Diversity of the Water Bacterial Community of Artificial Breeding Sites and Developmental Stages of Ae. aegypti
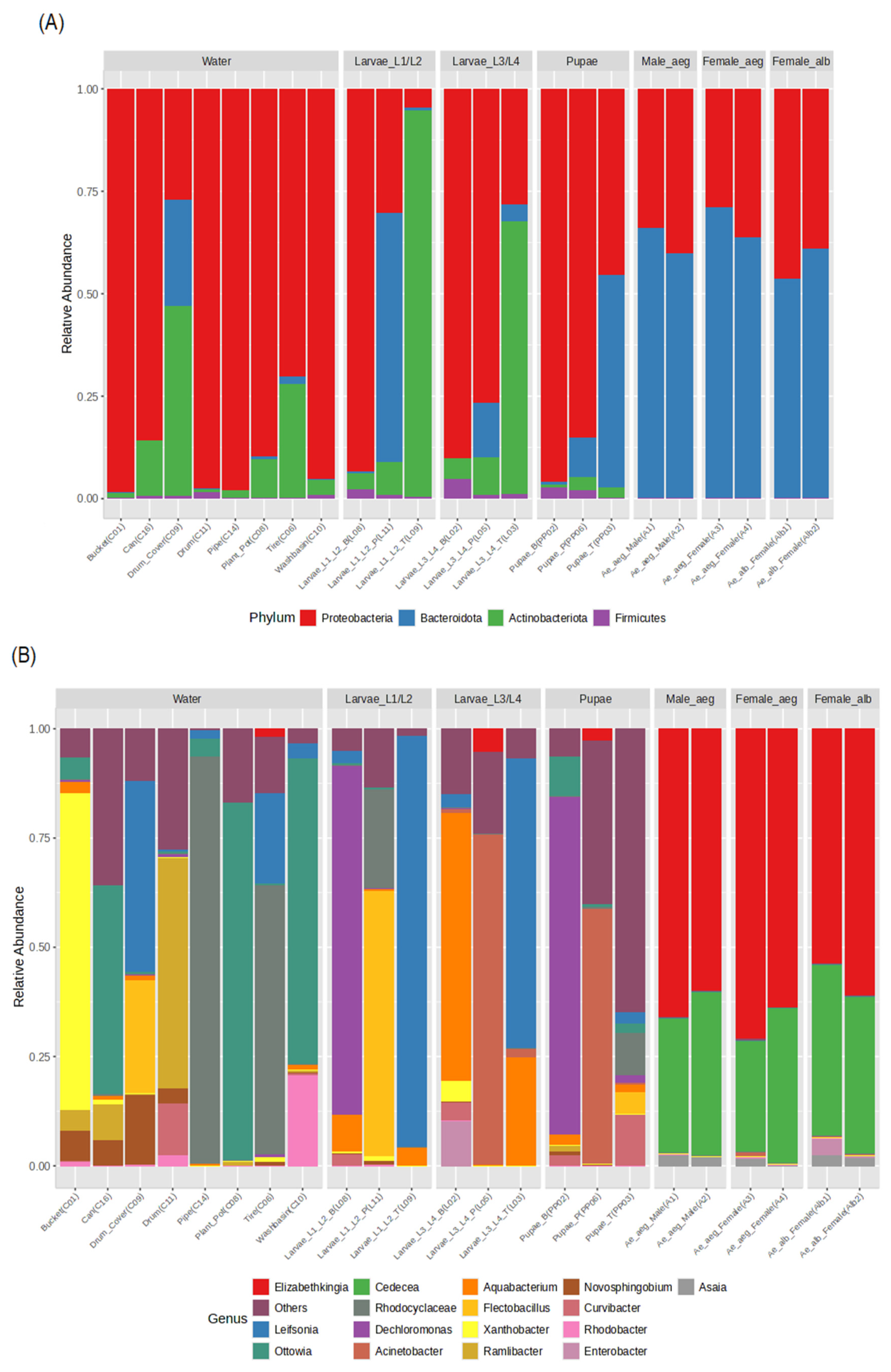
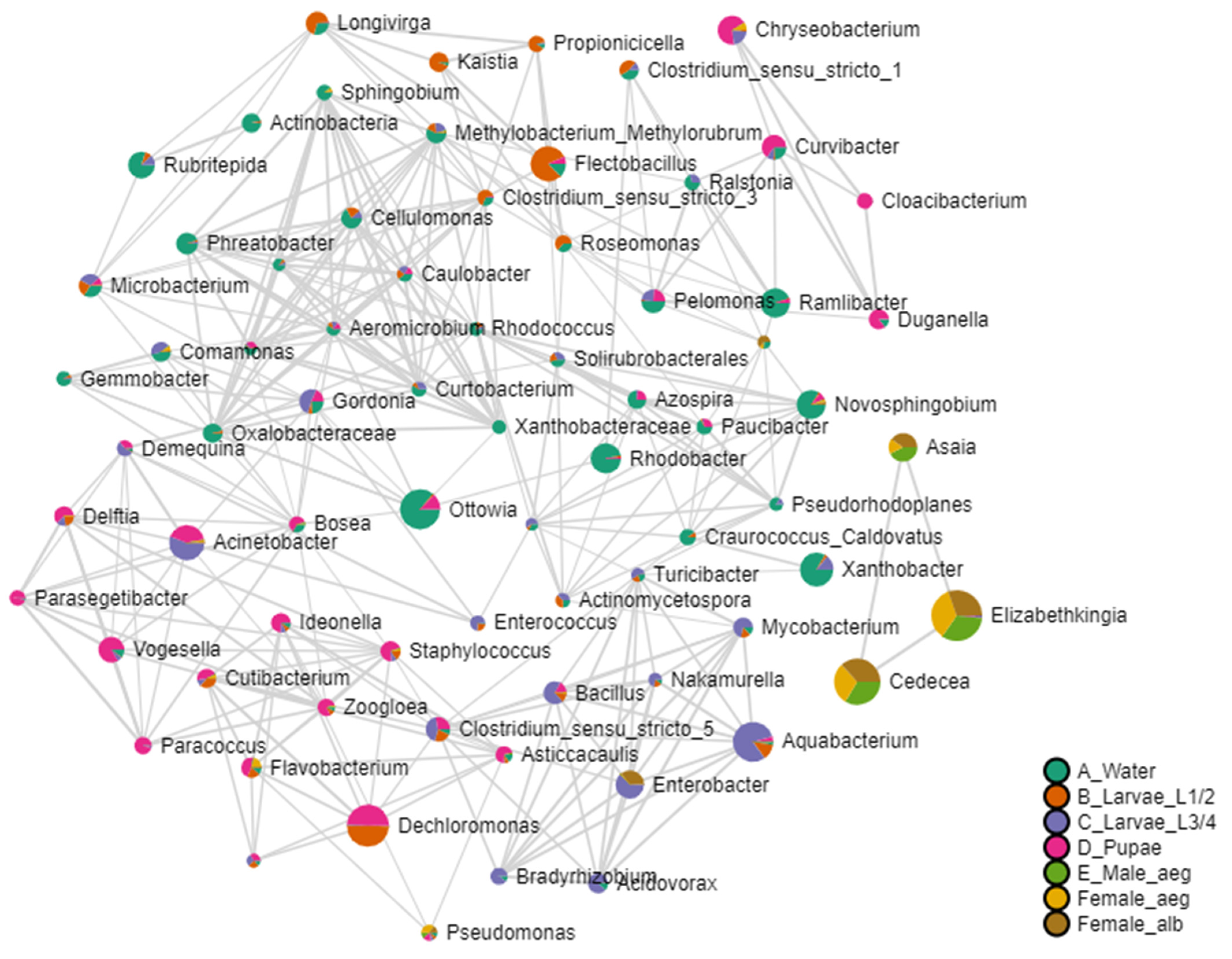
3.3.2. Bacterial Communities from Breeding Site Water and Developmental Stages of Ae. aegypti
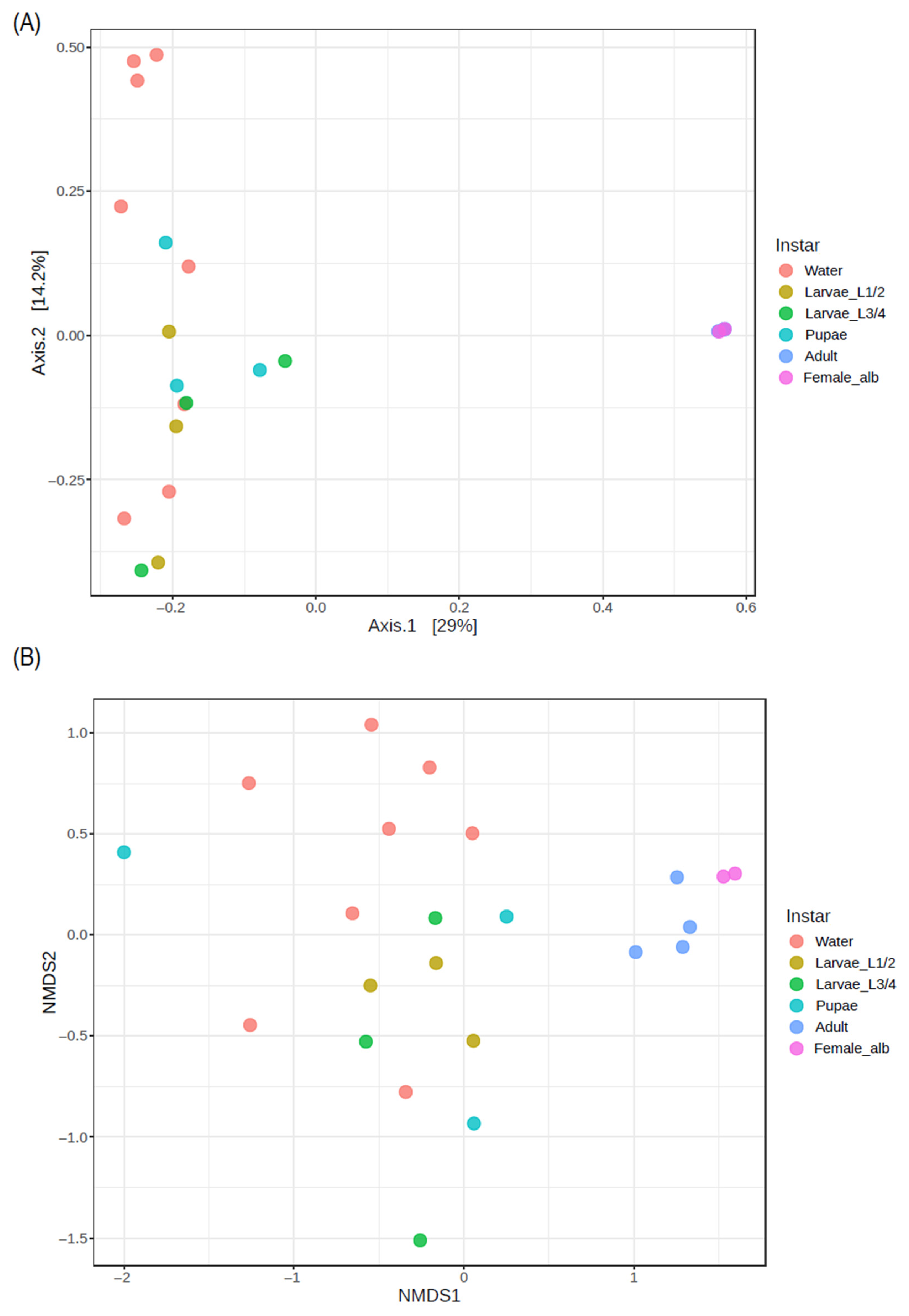
3.3.3. Beta Diversity of Bacterial Communities in Artificial Breeding Water and Immature and Adult Ae. aegypti
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Organización Mundial de la Salud. Enfermedades Transmitidas Por Vectores. Available online: https://www.who.int/es/news-room/fact-sheets/detail/vector-borne-diseases (accessed on 18 September 2024).
- Valencia-Marín, B.S.; Gandica, I.D.; Aguirre-Obando, O.A. The Mayaro Virus and Its Potential Epidemiological Consequences in Colombia: An Exploratory Biomathematics Analysis. Parasit Vectors 2020, 13, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Organización Mundial de la Salud. Dengue y Dengue Grave. Available online: https://www.who.int/es/news-room/fact-sheets/detail/dengue-and-severe-dengue (accessed on 18 September 2024).
- Organización Panamericana de la Salud; Organización Mundial de la Salud. Oficina Regional para las Américas. Casos Reportados de Dengue En Las Américas Por País o Territorio Casos Acumulados. Available online: https://www3.paho.org/data/index.php/es/temas/indicadores-dengue/dengue-nacional/9-dengue-pais-ano.html (accessed on 18 September 2024).
- Instituto Nacional de Salud. Boletín Epidemiológico Semanal 52 de 2021. In Boletín Epidemiológico Semanal; Instituto Nacional de Salud: Bogotá, Colombia, 2021; pp. 1–29. [Google Scholar] [CrossRef]
- Instituto Nacional de Salud. Boletín Epidemiológico Semanal 52 de 2022. In Boletín Epidemiológico Semanal; Instituto Nacional de Salud: Bogotá, Colombia, 2022; pp. 1–36. [Google Scholar] [CrossRef]
- Instituto Nacional de Salud. Boletín Epidemiológico Semanal 52 de 2023. In Boletín Epidemiológico Semanal; Instituto Nacional de Salud: Bogotá, Colombia, 2023; pp. 1–35. [Google Scholar] [CrossRef]
- Instituto Nacional de Salud. Boletín Epidemiológico Semanal Semana Epidemiológica 06 al 12 de Octubre de 2024; Instituto Nacional de Salud: Bogotá, Colombia, 2024; pp. 1–40. [Google Scholar] [CrossRef]
- Instituto Nacional de Salud. Boletín Epidemiológico Semanal Semana Epidemiológica 11 al 17 de Agosto de 2024; Instituto Nacional de Salud: Bogotá, Colombia, 2024; pp. 1–40. [Google Scholar] [CrossRef]
- Gómez, M.; Martinez, D.; Muñoz, M.; Ramírez, J.D. Aedes aegypti and Ae. albopictus Microbiome/Virome: New Strategies for Controlling Arboviral Transmission? Parasit Vectors 2022, 15, 287. [Google Scholar] [CrossRef] [PubMed]
- Lowe, R.; Lee, S.; Martins Lana, R.; Torres Codeço, C.; Castro, M.C.; Pascual, M. Emerging Arboviruses in the Urbanized Amazon Rainforest. BMJ 2020, 371, m4385. [Google Scholar] [CrossRef]
- Aponte, A.; Penilla, R.P.; Rodríguez, A.D.; Ocampo, C.B. Mechanisms of Pyrethroid Resistance in Aedes (Stegomyia) aegypti from Colombia. Acta Trop. 2019, 191, 146–154. [Google Scholar] [CrossRef]
- McGregor, B.L.; Connelly, C.R. A Review of the Control of Aedes aegypti (Diptera: Culicidae) in the Continental United States. J. Med. Entomol. 2021, 58, 10–25. [Google Scholar] [CrossRef]
- Weeratunga, P.; Rodrigo, C.; Fernando, S.D.; Rajapakse, S. Control Methods for Aedes albopictus and Aedes aegypti. Cochrane Database Syst. Rev. 2017, 2017, 12759. [Google Scholar] [CrossRef]
- Inesfly corporation Control de Los Mosquitos Del Género Aedes Dengue | Fiebre Amarilla | Chikungunya | Zika. Available online: https://inesfly.com/wp-content/uploads/2020/12/SOLUCIONES-INESFLY-PARA-MOSQUITOS-GENERO-AEDES.pdf (accessed on 1 October 2024).
- Instituto Departamental de Salud de Norte de Santander. Con Pintura Se Busca Prevenir Dengue, Zika Y Chikungunya. Available online: https://ids.gov.co/comunicados-de-prensa/con-pintura-se-busca-prevenir-dengue-zika-y-chikungunya/ (accessed on 1 October 2024).
- Valderrama, Y. Piloto Con ‘Pintura Insecticida’ Para Eliminar Larvas Del Mosquito Transmisor de Dengue. Available online: https://www.cali.gov.co/salud/publicaciones/154621/piloto-con-pintura-insecticida-para-eliminar-larvas-del-mosquito-transmisor-de-dengue/ (accessed on 1 October 2024).
- Ministerio De Salud y Protección Social Dirección de Promoción Y Prevención Subdirección de Enfermedades Transmisibles. Lineamientos para la Implementación Toldillos como Estrategia de Prevención ante la Presencia de Aedes aegypti en Colombia. 2016. Available online: https://www.minsalud.gov.co/sites/rid/Lists/BibliotecaDigital/RIDE/VS/PP/ET/lineamientos-implementacion-toldillos-prevencion-aedes-aegypti-colombia-2016.pdf/ (accessed on 1 October 2024).
- Carrasquilla, M.C.; Ortiz, M.I.; León, C.; Rondón, S.; Kulkarni, M.A.; Talbot, B.; Sander, B.; Vásquez, H.; Cordovez, J.M.; González, C.; et al. Entomological Characterization of Aedes Mosquitoes and Arbovirus Detection in Ibagué, a Colombian City with Co-Circulation of Zika, Dengue and Chikungunya Viruses. Parasit Vectors 2021, 14, 1–14. [Google Scholar] [CrossRef]
- Mains, J.W.; Kelly, P.H.; Dobson, K.L.; Petrie, W.D.; Dobson, S.L. Localized Control of Aedes aegypti (Diptera: Culicidae) in Miami, FL, via Inundative Releases of Wolbachia-Infected Male Mosquitoes. J. Med. Entomol. 2019, 56, 1296–1303. [Google Scholar] [CrossRef]
- Gesto, J.S.M.; Ribeiro, G.S.; Rocha, M.N.; Dias, F.B.S.; Peixoto, J.; Carvalho, F.D.; Pereira, T.N.; Moreira, L.A. Reduced Competence to Arboviruses Following the Sustainable Invasion of Wolbachia into Native Aedes aegypti from Southeastern Brazil. Sci. Rep. 2021, 11, 10039. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, D.; Shi, P.; Li, J.; Niu, J.; Chen, J.; Wang, G.; Wu, L.; Chen, L.; Yang, Z.; et al. A Naturally Isolated Symbiotic Bacterium Suppresses Flavivirus Transmission by Aedes Mosquitoes. Science (1979) 2024, 384, adn9524. [Google Scholar] [CrossRef]
- Egid, B.R.; Coulibaly, M.; Dadzie, S.K.; Kamgang, B.; McCall, P.J.; Sedda, L.; Toe, K.H.; Wilson, A.L. Review of the Ecology and Behaviour of Aedes aegypti and Aedes albopictus in Western Africa and Implications for Vector Control. Curr. Res. Parasitol. Vector-Borne Dis. 2022, 2, 74. [Google Scholar] [CrossRef] [PubMed]
- Hery, L.; Guidez, A.; Durand, A.A.; Delannay, C.; Normandeau-Guimond, J.; Reynaud, Y.; Issaly, J.; Goindin, D.; Legrave, G.; Gustave, J.; et al. Natural Variation in Physicochemical Profiles and Bacterial Communities Associated with Aedes aegypti Breeding Sites and Larvae on Guadeloupe and French Guiana. Microb. Ecol. 2021, 81, 93–109. [Google Scholar] [CrossRef] [PubMed]
- Sánchez Gutierrez, C.; Santamaría, E.; Morales, C.A.; Lesmes, M.C.; Cadena, H.; Avila-Diaz, A.; Fuya, P.; Marceló-Díaz, C. Spatial Patterns Associated with the Distribution of Immature Stages of Aedes aegypti in Three Dengue High-Risk Municipalities of Southwestern Colombia. Subj. Hum. Biomed. Sci. 2023, 2023, 6611. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Kibech, R.; Stone, C.M. Differential Effects of Larval and Adult Nutrition on Female Survival, Fecundity, and Size of the Yellow Fever Mosquito, Aedes aegypti. Front Zool 2021, 18, 1–9. [Google Scholar] [CrossRef]
- Scolari, F.; Sandionigi, A.; Carlassara, M.; Bruno, A.; Casiraghi, M.; Bonizzoni, M. Exploring Changes in the Microbiota of Aedes albopictus: Comparison Among Breeding Site Water, Larvae, and Adults. Front. Microbiol. 2021, 12, 624170. [Google Scholar] [CrossRef]
- Ouédraogo, W.M.; Toé, K.H.; Sombié, A.; Viana, M.; Bougouma, C.; Sanon, A.; Weetman, D.; McCall, P.J.; Kanuka, H.; Badolo, A. Impact of Physicochemical Parameters of Aedes aegypti Breeding Habitats on Mosquito Productivity and the Size of Emerged Adult Mosquitoes in Ouagadougou City, Burkina Faso. Parasit Vectors 2022, 15, 478. [Google Scholar] [CrossRef]
- Amini, M.; Hanafi-Bojd, A.A.; Aghapour, A.A.; Chavshin, A.R. Larval Habitats and Species Diversity of Mosquitoes (Diptera: Culicidae) in West Azerbaijan Province, Northwestern Iran. BMC Ecol. 2020, 20, 1–11. [Google Scholar] [CrossRef]
- Dalpadado, R.; Amarasinghe, D.; Gunathilaka, N. Water Quality Characteristics of Breeding Habitats in Relation to the Density of Aedes aegypti and Aedes albopictus in Domestic Settings in Gampaha District of Sri Lanka. Acta Trop. 2022, 229, 106339. [Google Scholar] [CrossRef]
- Herath, J.M.M.K.; De Silva, W.A.P.P.; Weeraratne, T.C.; Karunaratne, S.H.P.P. Breeding Habitat Preference of the Dengue Vector Mosquitoes Aedes aegypti and Aedes albopictus from Urban, Semiurban, and Rural Areas in Kurunegala District, Sri Lanka. J. Trop. Med. 2024, 2024, 4123543. [Google Scholar] [CrossRef]
- Onyango, G.M.; Bialosuknia, M.S.; Payne, F.A.; Mathias, N.; Ciota, T.A.; Kramer, D.L. Increase in Temperature Enriches Heat Tolerant Taxa in Aedes aegypti Midguts. Sci. Rep. 2020, 10, 19135. [Google Scholar] [CrossRef]
- Ware-Gilmore, F.; Sgrò, C.M.; Xi, Z.; Dutra, H.L.C.; Jones, M.J.; Shea, K.; Hall, M.D.; Thomas, M.B.; McGraw, E.A. Microbes Increase Thermal Sensitivity in the Mosquito Aedes aegypti, with the Potential to Change Disease Distributions. PLoS Negl. Trop. Dis. 2021, 15, e0009548. [Google Scholar] [CrossRef] [PubMed]
- Muturi, E.J.; Dunlap, C.; Smartt, C.T.; Shin, D. Resistance to Permethrin Alters the Gut Microbiota of Aedes aegypti. Sci. Rep. 2021, 11, 14406. [Google Scholar] [CrossRef]
- Gómez-Govea, M.A.; de Ramírez-Ahuja, M.L.; Contreras-Perera, Y.; Jiménez-Camacho, A.J.; Ruiz-Ayma, G.; Villanueva-Segura, O.K.; Trujillo-Rodríguez, G.d.J.; Delgado-Enciso, I.; Martínez-Fierro, M.L.; Manrique-Saide, P.; et al. Suppression of Midgut Microbiota Impact Pyrethroid Susceptibility in Aedes aegypti. Front. Microbiol. 2022, 13, 761459. [Google Scholar] [CrossRef] [PubMed]
- Gusmão, D.S.; Santos, A.V.; Marini, D.C.; Bacci, M.; Berbert-Molina, M.A.; Lemos, F.J.A. Culture-Dependent and Culture-Independent Characterization of Microorganisms Associated with Aedes aegypti (Diptera: Culicidae) (L.) and Dynamics of Bacterial Colonization in the Midgut. Acta Trop. 2010, 115, 275–281. [Google Scholar] [CrossRef]
- Alvarado, W.A.; Agudelo, S.O.; Velez, I.D.; Vivero, R.J. Description of the Ovarian Microbiota of Aedes aegypti (L) Rockefeller Strain. Acta Trop. 2021, 214, 105765. [Google Scholar] [CrossRef] [PubMed]
- Martinez Villegas, L.E.; Radl, J.; Dimopoulos, G.; Short, S.M. Bacterial Communities of Aedes aegypti Mosquitoes Differ between Crop and Midgut Tissues. PLoS Negl. Trop. Dis. 2023, 17, 11218. [Google Scholar] [CrossRef]
- Osei-Poku, J.; Mbogo, C.M.; Palmer, W.J.; Jiggins, F.M. Deep Sequencing Reveals Extensive Variation in the Gut Microbiota of Wild Mosquitoes from Kenya. Mol. Ecol. 2012, 21, 5138–5150. [Google Scholar] [CrossRef]
- Audsley, M.D.; Ye, Y.H.; McGraw, E.A. The Microbiome Composition of Aedes aegypti Is Not Critical for Wolbachia-Mediated Inhibition of Dengue Virus. PLoS Negl. Trop. Dis. 2017, 11, 5426. [Google Scholar] [CrossRef]
- Zouache, K.; Martin, E.; Rahola, N.; Gangue, M.F.; Minard, G.; Dubost, A.; Van, V.T.; Dickson, L.; Ayala, D.; Lambrechts, L.; et al. Larval Habitat Determines the Bacterial and Fungal Microbiota of the Mosquito Vector Aedes aegypti. FEMS Microbiol. Ecol. 2022, 98, 16. [Google Scholar] [CrossRef]
- Louie, W.; Coffey, L.L. Microbial Composition in Larval Water Enhances Aedes aegypti Development but Reduces Transmissibility of Zika Virus. mSphere 2021, 6, e00687-21. [Google Scholar] [CrossRef]
- Ranasinghe, K.; Gunathilaka, N.; Amarasinghe, D.; Rodrigo, W.; Udayanga, L. Diversity of Midgut Bacteria in Larvae and Females of Aedes aegypti and Aedes albopictus from Gampaha District, Sri Lanka. Parasit Vectors 2021, 14, 433. [Google Scholar] [CrossRef]
- de Oliveira, J.C.; de Melo Katak, R.; Muniz, V.A.; de Oliveira, M.R.; Rocha, E.M.; da Silva, W.R.; do Carmo, E.J.; Roque, R.A.; Marinotti, O.; Terenius, O.; et al. Bacteria Isolated from Aedes aegypti with Potential Vector Control Applications. J. Invertebr. Pathol. 2024, 204, 108094. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.; Sun, P.; Nie, K.; Zhu, Y.; Shi, M.; Xiao, C.; Liu, H.; Liu, Q.; Zhao, T.; Chen, X.; et al. A Gut Commensal Bacterium Promotes Mosquito Permissiveness to Arboviruses. Cell Host Microbe 2019, 25, 101–112.e5. [Google Scholar] [CrossRef]
- Scolari, F.; Casiraghi, M.; Bonizzoni, M. Aedes Spp. and Their Microbiota: A Review. Front. Microbiol. 2019, 10, 2036. [Google Scholar] [CrossRef]
- Sim, S.; Jupatanakul, N.; Ramirez, J.L.; Kang, S.; Romero-Vivas, C.M.; Mohammed, H.; Dimopoulos, G. Transcriptomic Profiling of Diverse Aedes aegypti Strains Reveals Increased Basal-Level Immune Activation in Dengue Virus-Refractory Populations and Identifies Novel Virus-Vector Molecular Interactions. PLoS Negl. Trop. Dis. 2013, 7, 2295. [Google Scholar] [CrossRef]
- Coon, K.L.; Brown, M.R.; Strand, M.R. Gut Bacteria Differentially Affect Egg Production in the Anautogenous Mosquito Aedes aegypti and Facultatively Autogenous Mosquito Aedes atropalpus (Diptera: Culicidae). Parasit Vectors 2016, 9, 1–12. [Google Scholar] [CrossRef]
- Apte-Deshpande, A.; Paingankar, M.; Gokhale, M.D.; Deobagkar, D.N. Serratia Odorifera a Midgut Inhabitant of Aedes aegypti Mosquito Enhances Its Susceptibility to Dengue-2 Virus. PLoS ONE 2012, 7, 40401. [Google Scholar] [CrossRef]
- Apte-Deshpande, A.D.; Paingankar, M.S.; Gokhale, M.D.; Deobagkar, D.N. Serratia Odorifera Mediated Enhancement in Susceptibility of Aedes aegypti for Chikungunya Virus. Indian J. Med. Res. 2014, 139, 762–768. [Google Scholar]
- Hughes, G.L.; Rasgon, J.L. Wolbachia Infections in Arthropod Hosts. In Insect Pathology, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2012; pp. 351–366. ISBN 9780123849847. [Google Scholar]
- Utarini, A.; Indriani, C.; Ahmad, R.A.; Tantowijoyo, W.; Arguni, E.; Ansari, M.R.; Supriyati, E.; Wardana, D.S.; Meitika, Y.; Ernesia, I.; et al. Efficacy of Wolbachia-Infected Mosquito Deployments for the Control of Dengue. N. Engl. J. Med. 2021, 384, 2177–2186. [Google Scholar] [CrossRef]
- Diebel, J.; Norda, J.; Kretchmer, O.; Kretchmer, J. Datos Históricos Meteorológicos de 19 de Noviembre de 2023 En El Alfredo Vásquez Cobo International Airport Colombia. Available online: https://es.weatherspark.com/h/d/147330/2023/11/19/Tiempo-hist%C3%B3rico-el-domingo-19-de-noviembre-de-2023-en-Alfredo-V%C3%A1squez-Cobo-International-Airport-Colombia#Figures-Temperature (accessed on 18 September 2024).
- Instituto de Hidrología, Meteorología y Estudios Ambientales; Instituto Nacional de Salud. Boletín de Clima y Salud; Instituto Nacional de Salud: Bogotá, Colombia, 2023. [Google Scholar]
- Williams, K.E.; Huyvaert, K.P.; Piaggio, A.J. No Filters, No Fridges: A Method for Preservation of Water Samples for EDNA Analysis. BMC Res. Notes 2016, 9, 1–5. [Google Scholar] [CrossRef]
- Djurhuus, A.; Port, J.; Closek, C.J.; Yamahara, K.M.; Romero-Maraccini, O.; Walz, K.R.; Goldsmith, D.B.; Michisaki, R.; Breitbart, M.; Boehm, A.B.; et al. Evaluation of Filtration and DNA Extraction Methods for Environmental DNA Biodiversity Assessments across Multiple Trophic Levels. Front. Mar. Sci. 2017, 4, 314. [Google Scholar] [CrossRef]
- Imam, H.; Sofi, G.; Zarnigar; Aziz, S. The Basic Rules and Methods of Mosquito Rearing (Aedes aegypti). Trop. Parasitol. 2014, 4, 53. [Google Scholar] [CrossRef] [PubMed]
- González, C.R.; Rada, V.; Saldarriaga, M.; Pavletic, C.; Parra, A. Manual de Culícidos (DIPTERA: CULICIDAE), 2nd ed.; Instituto de Salud Pública de Chile: Santiago, Chile, 2016; ISBN 9789567770113. [Google Scholar]
- Golczer, G.; Arrivillaga, J. Modificación de Un Protocolo Estándar de Extracción de ADN Para Flebotominos Pequeños (Phlebotominae: Lutzomyia). Rev. Colomb. Entomol. 2008, 34, 199–202. [Google Scholar] [CrossRef]
- Espejo, R.T.; Feijóo, C.G.; Romero, J.; Vásquez, M. PAGE Analysis of the Heteroduplexes Formed between PCR-Amplified 16S RRNA Genes: Estimation of Sequence Similarity and RDNA Complexity. Microbiology 1998, 144, 1611–1617. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Yu, Y.; Lee, C.; Kim, J.; Hwang, S. Group-specific Primer and Probe Sets to Detect Methanogenic Communities Using Quantitative Real-time Polymerase Chain Reaction. Biotechnol. Bioeng. 2005, 89, 670–679. [Google Scholar] [CrossRef] [PubMed]
- Chong, J.; Liu, P.; Zhou, G.; Xia, J. Using MicrobiomeAnalyst for Comprehensive Statistical, Functional, and Meta-Analysis of Microbiome Data. Nat. Protoc. 2020, 15, 799–821. [Google Scholar] [CrossRef]
- Duque-Granda, D.; Vivero-Gómez, R.J.; Junca, H.; Cadavid-Restrepo, G.; Moreno-Herrera, C.X. Interaction and Effects of Temperature Preference under a Controlled Environment on the Diversity and Abundance of the Microbiome in Lutzomyia longipalpis (Diptera: Psychodidae). Biotechnol. Rep. 2024, 44, e00857. [Google Scholar] [CrossRef]
- Tedjou, A.N.; Kamgang, B.; Yougang, A.P.; Wilson-Bahun, T.A.; Njiokou, F.; Wondji, C.S. Patterns of Ecological Adaptation of Aedes aegypti and Aedes albopictus and Stegomyia Indices Highlight the Potential Risk of Arbovirus Transmission in Yaoundé, the Capital City of Cameroon. Pathogens 2020, 9, 491. [Google Scholar] [CrossRef]
- Padonou, G.G.; Ossè, R.; Salako, A.S.; Aikpon, R.; Sovi, A.; Kpanou, C.; Sagbohan, H.; Akadiri, Y.; Lamine, B.-M.; Akogbeto, M.C. Entomological Assessment of the Risk of Dengue Outbreak in Abomey-Calavi Commune, Benin. Trop. Med. Health 2020, 48, 20. [Google Scholar] [CrossRef]
- Simard, F.; Nchoutpouen, E.; Toto, J.C.; Fontenille, D. Geographic Distribution and Breeding Site Preference of Aedes albopictus and Aedes aegypti (Diptera: Culicidae) in Cameroon, Central Africa. J. Med. Entomol. 2005, 42, 726–731. [Google Scholar] [CrossRef]
- Shriram, A.N.; Sivan, A.; Sugunan, A.P. Spatial Distribution of Aedes aegypti and Aedes albopictus in Relation to Geo-Ecological Features in South Andaman, Andaman and Nicobar Islands, India. Bull. Entomol. Res. 2018, 108, 166–174. [Google Scholar] [CrossRef] [PubMed]
- Prasad, P.; Lata, S.; Gupta, S.K.; Kumar, P.; Saxena, R.; Arya, D.K.; Singh, H. Aedes aegypti Container Preference for Oviposition and Its Possible Implications for Dengue Vector Surveillance in Delhi, India. Epidemiol. Health 2023, 45, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Dom, N.C.; Ahmad, P.; Azman, M.; Mokhtar, M.; Rajan, S.; Nazri, C.; Dom, C.; Megat Mokhtar, A. Assessment of Heavy Metal Concentration on Aedes Mosquito Breeding Sites in Urban Area, Malaysia. Int. J. Mosq. Res. 2017, 4, 12–19. [Google Scholar]
- Getachew, D.; Tekie, H.; Gebre-Michael, T.; Balkew, M.; Mesfin, A. Breeding Sites of Aedes aegypti: Potential Dengue Vectors in Dire Dawa, East Ethiopia. Interdiscip. Perspect. Infect. Dis. 2015, 2015, 706276. [Google Scholar] [CrossRef]
- Couret, J.; Benedict, M.Q. A Meta-Analysis of the Factors Influencing Development Rate Variation in Aedes aegypti (Diptera: Culicidae). BMC Ecol. 2014, 14, 3. [Google Scholar] [CrossRef]
- Couret, J.; Dotson, E.; Benedict, M.Q. Temperature, Larval Diet, and Density Effects on Development Rate and Survival of Aedes aegypti (Diptera: Culicidae). PLoS ONE 2014, 9, e87468. [Google Scholar] [CrossRef]
- Bar-Zeev, M. The Effect of Temperature on the Growth Rate and Survival of the Immature Stages of Aëdes aegypti (L.). Bull. Entomol. Res. 1958, 49, 157–163. [Google Scholar] [CrossRef]
- Dennington, N.L.; Grossman, M.K.; Ware-Gilmore, F.; Teeple, J.L.; Johnson, L.R.; Shocket, M.S.; McGraw, E.A.; Thomas, M.B. Phenotypic Adaptation to Temperature in the Mosquito Vector, Aedes aegypti. Glob. Change Biol. 2024, 30, 17041. [Google Scholar] [CrossRef]
- Medeiros-Sousa, A.R.; de Oliveira-Christe, R.; Camargo, A.A.; Scinachi, C.A.; Milani, G.M.; Urbinatti, P.R.; Natal, D.; Ceretti-Junior, W.; Marrelli, M.T. Influence of Water’s Physical and Chemical Parameters on Mosquito (Diptera: Culicidae) Assemblages in Larval Habitats in Urban Parks of São Paulo, Brazil. Acta Trop. 2020, 205, 105394. [Google Scholar] [CrossRef]
- Overgaard, H.J.; Olano, V.A.; Jaramillo, J.F.; Matiz, M.I.; Sarmiento, D.; Stenström, T.A.; Alexander, N. A Cross-Sectional Survey of Aedes aegypti Immature Abundance in Urban and Rural Household Containers in Central Colombia. Parasit. Vectors 2017, 10, 1–12. [Google Scholar] [CrossRef]
- Ricardo Arenas Villamizar, Á.; Alexander Carvajal Pinilla, L.; Investigación, A.D.E.; Recursos, G. Influence of Climate Changes on the Definition of the Sex of Aedes aegypti and Its Impact on Dengue. Epidemics 2012, 4, 11–24. [Google Scholar]
- Sasmita, H.I.; Tu, W.C.; Bong, L.J.; Neoh, K.B. Effects of Larval Diets and Temperature Regimes on Life History Traits, Energy Reserves and Temperature Tolerance of Male Aedes aegypti (Diptera: Culicidae): Optimizing Rearing Techniques for the Sterile Insect Programmes. Parasit Vectors 2019, 12, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Hapairai, L.K.; Marie, J.; Sinkins, S.P.; Bossin, H.C. Effect of Temperature and Larval Density on Aedes polynesiensis (Diptera: Culicidae) Laboratory Rearing Productivity and Male Characteristics. Acta Trop. 2014, 132, 24. [Google Scholar] [CrossRef] [PubMed]
- Rodpai, R.; Boonroumkaew, P.; Sadaow, L.; Sanpool, O.; Janwan, P.; Thanchomnang, T.; Intapan, P.M.; Maleewong, W. Microbiome Composition and Microbial Community Structure in Mosquito Vectors Aedes aegypti and Aedes albopictus in Northeastern Thailand, a Dengue-Endemic Area. Insects 2023, 14, 184. [Google Scholar] [CrossRef]
- Yadav, K.K.; Datta, S.; Naglot, A.; Bora, A.; Hmuaka, V.; Bhagyawant, S.; Gogoi, H.K.; Veer, V.; Raju, P.S. Diversity of Cultivable Midgut Microbiota at Different Stages of the Asian Tiger Mosquito, Aedes albopictus from Tezpur, India. PLoS ONE 2016, 11, 167409. [Google Scholar] [CrossRef]
- Díaz, S.; Camargo, C.; Avila, F.W. Characterization of the Reproductive Tract Bacterial Microbiota of Virgin, Mated, and Blood-Fed Aedes aegypti and Aedes albopictus Females. Parasit Vectors 2021, 14, 1–12. [Google Scholar] [CrossRef]
- Oliveira, J.H.M.; Gonçalves, R.L.S.; Lara, F.A.; Dias, F.A.; Gandara, A.C.P.; Menna-Barreto, R.F.S.; Edwards, M.C.; Laurindo, F.R.M.; Silva-Neto, M.A.C.; Sorgine, M.H.F.; et al. Blood Meal-Derived Heme Decreases ROS Levels in the Midgut of Aedes aegypti and Allows Proliferation of Intestinal Microbiota. PLoS Pathog. 2011, 7, e1001320. [Google Scholar] [CrossRef]
- Tokash-Peters, A.G.; Niyonzima, J.D.; Kayirangwa, M.; Muhayimana, S.; Tokash, I.W.; Jabon, J.D.; Lopez, S.G.; Kearns, P.J.; Woodhams, D.C. Mosquito Microbiomes of Rwanda: Characterizing Mosquito Host and Microbial Communities in the Land of a Thousand Hills. Microb. Ecol. 2024, 87, 64. [Google Scholar] [CrossRef]
- Geng, S.; Pan, X.C.; Mei, R.; Wang, Y.N.; Sun, J.Q.; Liu, X.Y.; Tang, Y.Q.; Wu, X.L. Ottowia Shaoguanensis Sp. Nov., Isolated from Coking Wastewater. Curr. Microbiol. 2014, 68, 324–329. [Google Scholar] [CrossRef]
- Cao, J.; Lai, Q.; Liu, Y.; Li, G.; Shao, Z. Ottowia Beijingensis Sp. Nov., Isolated from Coking Wastewater Activated Sludge, and Emended Description of the Genus Ottowia. Int. J. Syst. Evol. Microbiol. 2014, 64, 963–967. [Google Scholar] [CrossRef]
- Vivero, R.J.; Mesa, G.B.; Robledo, S.M.; Herrera, C.X.M.; Cadavid-Restrepo, G. Enzymatic, Antimicrobial, and Leishmanicidal Bioactivity of Gram-Negative Bacteria Strains from the Midgut of Lutzomyia evansi, an Insect Vector of Leishmaniasis in Colombia. Biotechnol. Rep. 2019, 24, e00379. [Google Scholar] [CrossRef] [PubMed]
- Shelomi, M.; Han, C.J.; Chen, W.M.; Chen, H.K.; Liaw, S.J.; Mühle, E.; Clermont, D. Chryseobacterium oryctis Sp. Nov., Isolated from the Gut of the Beetle Oryctes rhinoceros, and Chryseobacterium kimseyorum Sp. Nov., Isolated from a Stick Insect Rearing Cage. Int. J. Syst. Evol. Microbiol. 2023, 73, 5813. [Google Scholar] [CrossRef]
- Alfouzan, W.; Dhar, R.; Al-Hashemi, H.; Al-Sweih, N.; Albert, M.J. Clinical and Microbiological Characteristics of Chryseobacterium Spp. Isolated from Neonates in Kuwait. JMM Case Rep. 2014, 1, 1008. [Google Scholar] [CrossRef]
- Chen, W.M.; Lin, K.R.; Young, C.C.; Sheu, S.Y. Flectobacillus fontis Sp. Nov., Isolated from a Freshwater Spring. Int. J. Syst. Evol. Microbiol. 2017, 67, 336–342. [Google Scholar] [CrossRef]
- Zhao, W.; Bi, X.; Peng, Y.; Bai, M. Research Advances of the Phosphorus-Accumulating Organisms of Candidatus Accumulibacter, Dechloromonas and Tetrasphaera: Metabolic Mechanisms, Applications and Influencing Factors. Chemosphere 2022, 307, 135675. [Google Scholar] [CrossRef]
- Salinero, K.K.; Keller, K.; Feil, W.S.; Feil, H.; Trong, S.; Di Bartolo, G.; Lapidus, A. Metabolic Analysis of the Soil Microbe Dechloromonas aromatica Str. RCB: Indications of a Surprisingly Complex Life-Style and Cryptic Anaerobic Pathways for Aromatic Degradation. BMC Genomics 2009, 10, 1–23. [Google Scholar] [CrossRef]
- Zhang, X.; Li, A.; Szewzyk, U.; Ma, F. Improvement of Biological Nitrogen Removal with Nitrate-Dependent Fe(II) Oxidation Bacterium Aquabacterium parvum B6 in an up-Flow Bioreactor for Wastewater Treatment. Bioresour. Technol. 2016, 219, 624–631. [Google Scholar] [CrossRef]
- Ryu, S.H.; Park, J.H.; Moon, J.C.; Sung, Y.; Lee, S.S.; Jeon, C.O.; Moon, J.C. Flavobacterium resistens Sp. Nov., Isolated from Stream Sediment. Int. J. Syst. Evol. Microbiol. 2008, 58, 2266–2270. [Google Scholar] [CrossRef][Green Version]
- Spring, S.; Jäckel, U.; Wagner, M.; Kämpfer, P. Ottowia thiooxydans Gen. Nov., Sp. Nov., a Novel Facultatively Anaerobic, N2O-Producing Bacterium Isolated from Activated Sludge, and Transfer of Aquaspirillum gracile to Hylemonella gracilis Gen. Nov., Comb. Nov. Int. J. Syst. Evol. Microbiol. 2004, 54, 99–106. [Google Scholar] [CrossRef]
- Suresh, G.; Kumar, D.; Krishnaiah, A.; Sasikala, C.; Ramana, C.V. Rhodobacter sediminicola Sp. Nov., Isolated from a Fresh Water Pond. Int. J. Syst. Evol. Microbiol. 2020, 70, 1294–1299. [Google Scholar] [CrossRef]
- Costa, S.; Ganzerli, S.; Rugiero, I.; Pellizzari, S.; Pedrini, P.; Tamburini, E. Potential of Rhodobacter capsulatus Grown in Anaerobic-Light or Aerobic-Dark Conditions as Bioremediation Agent for Biological Wastewater Treatments. Water 2017, 9, 108. [Google Scholar] [CrossRef]
- Alfano, N.; Tagliapietra, V.; Rosso, F.; Manica, M.; Arnoldi, D.; Pindo, M.; Rizzoli, A. Changes in Microbiota Across Developmental Stages of Aedes koreicus, an Invasive Mosquito Vector in Europe: Indications for Microbiota-Based Control Strategies. Front. Microbiol. 2019, 10, 2832. [Google Scholar] [CrossRef]
- Gibson, L.; Crombie, A.T.; McNamara, N.P.; Murrell, J.C. Isoprene-Degrading Bacteria Associated with the Phyllosphere of Salix Fragilis, a High Isoprene-Emitting Willow of the Northern Hemisphere. Environ. Microbiomes 2021, 16, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Li, L.; Li, F.; Zhang, C.; Lyu, J.J. Mechanism of Carbonate Mineralization Induced by Microbes: Taking Curvibacter lanceolatus Strain HJ-1 as an Example. Micron 2021, 140, 102980. [Google Scholar] [CrossRef] [PubMed]
- Loret, J.F.; Dumoutier, N. Non-Tuberculous Mycobacteria in Drinking Water Systems: A Review of Prevalence Data and Control Means. Int. J. Hyg. Environ. Health 2019, 222, 628–634. [Google Scholar] [CrossRef]
- Dávalos, A.F.; Garcia, P.K.; Montoya-Pachongo, C.; Rengifo, A.; Guerrero, D.; Díaz-Ordoñez, L.; Díaz, G.; Ferro, B.E. Identification of Nontuberculous Mycobacteria in Drinking Water in Cali, Colombia. Int. J. Environ. Res. Public Health 2021, 18, ijerph18168451. [Google Scholar] [CrossRef]
- Erkmen, O. Isolation and Counting of Coliforms and Escherichia coli. In Microbiological Analysis of Foods and Food Processing Environments; Elsevier: Amsterdam, The Netherlands, 2022; pp. 105–140. [Google Scholar]
- Näsström, E.; Vu Thieu, N.T.; Dongol, S.; Karkey, A.; Voong Vinh, P.; Ha Thanh, T.; Johansson, A.; Arjyal, A.; Thwaites, G.; Dolecek, C.; et al. Salmonella typhi and Salmonella paratyphi a Elaborate Distinct Systemic Metabolite Signatures during Enteric Fever. eLife 2014, 3, 3100. [Google Scholar] [CrossRef]
- Baltar, J.M.C.; Pavan, M.G.; Corrêa-Antônio, J.; Couto-Lima, D.; Maciel-de-Freitas, R.; David, M.R. Gut Bacterial Diversity of Field and Laboratory-Reared Aedes Albopictus Populations of Rio de Janeiro, Brazil. Viruses 2023, 15, 1309. [Google Scholar] [CrossRef]
- Gómez, M.; Martínez, D.; Luna, N.; Vega, L.; Yepez-Pérez, Y.; Cantillo-Barraza, O.; Camargo, M.; Patiño, L.H.; Muñoz, M.; Ramírez, J.D. Comparative Analysis of Bacterial Microbiota in Aedes Aegypti (Diptera: Culicidae): Insights from Field and Laboratory Populations in Colombia. J. Med. Entomol. 2025, tjaf002. [Google Scholar] [CrossRef]
- Mosquera, K.D.; Martínez Villegas, L.E.; Rocha Fernandes, G.; Rocha David, M.; Maciel-de-Freitas, R.; Moreira, L.A.; Lorenzo, M.G. Egg-Laying by Female Aedes aegypti Shapes the Bacterial Communities of Breeding Sites. BMC Biol. 2023, 21, 97. [Google Scholar] [CrossRef]
- Roman, A.; Koenraadt, C.J.M.; Raymond, B. Asaia Spp. Accelerate Development of the Yellow Fever Mosquito, Aedes aegypti, via Interactions with the Vertically Transmitted Larval Microbiome. J. Appl. Microbiol. 2024, 135, lxae261. [Google Scholar] [CrossRef] [PubMed]
- Ganley, J.G.; D’Ambrosio, H.K.; Shieh, M.; Derbyshire, E.R. Coculturing of Mosquito-Microbiome Bacteria Promotes Heme Degradation in Elizabethkingia anophelis. ChemBioChem 2020, 21, 1279–1284. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Bagdasarian, M.; Walker, E.D. Elizabethkingia anophelis: Molecular Manipulation and Interactions with Mosquito Hosts. Appl. Environ. Microbiol. 2015, 81, 2233–2243. [Google Scholar] [CrossRef] [PubMed]
- Rossi, P.; Ricci, I.; Cappelli, A.; Damiani, C.; Ulissi, U.; Mancini, M.V.; Valzano, M.; Capone, A.; Epis, S.; Crotti, E.; et al. Mutual Exclusion of Asaia and Wolbachia in the Reproductive Organs of Mosquito Vectors. Parasit Vectors 2015, 8, 278. [Google Scholar] [CrossRef]
- Ilbeigi Khamseh Nejad, M.; Cappelli, A.; Damiani, C.; Falcinelli, M.; Catapano, P.L.; Nanfack-Minkeu, F.; Mayi, M.P.A.; Currà, C.; Ricci, I.; Favia, G. Wolbachia and Asaia Distribution among Different Mosquito Vectors Is Affected by Tissue Localization and Host Species. Microorganisms 2024, 12, 545. [Google Scholar] [CrossRef]
- do Nascimento, R.M.; Campolina, T.B.; Chaves, B.A.; Delgado, J.L.F.; Godoy, R.S.M.; Pimenta, P.F.P.; Secundino, N.F.C. The Influence of Culture-Dependent Native Microbiota in Zika Virus Infection in Aedes aegypti. Parasit Vectors 2022, 15, 57. [Google Scholar] [CrossRef]
- Juma, E.O.; Allan, B.F.; Kim, C.H.; Stone, C.; Dunlap, C.; Muturi, E.J. Effect of Life Stage and Pesticide Exposure on the Gut Microbiota of Aedes albopictus and Culex pipiens L. Sci. Rep. 2020, 10, 9489. [Google Scholar] [CrossRef]
- Dada, N.; Sheth, M.; Liebman, K.; Pinto, J.; Lenhart, A. Whole Metagenome Sequencing Reveals Links between Mosquito Microbiota and Insecticide Resistance in Malaria Vectors. Sci. Rep. 2018, 8, 2084. [Google Scholar] [CrossRef]
- Hegde, S.; Khanipov, K.; Albayrak, L.; Golovko, G.; Pimenova, M.; Saldaña, M.A.; Rojas, M.M.; Hornett, E.A.; Motl, G.C.; Fredregill, C.L.; et al. Microbiome Interaction Networks and Community Structure from Laboratory-Reared and Field-Collected Aedes aegypti, Aedes albopictus, and Culex quinquefasciatus Mosquito Vectors. Front. Microbiol. 2018, 9, 2160. [Google Scholar] [CrossRef]
- Coon, K.L.; Vogel, K.J.; Brown, M.R.; Strand, M.R. Mosquitoes Rely on Their Gut Microbiota for Development. Mol. Ecol. 2014, 23, 2727–2739. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Castañeda-Espinosa, A.; Duque-Granda, D.; Cadavid-Restrepo, G.; Murcia, L.M.; Junca, H.; Moreno-Herrera, C.X.; Vivero-Gómez, R.J. Study of Bacterial Communities in Water and Different Developmental Stages of Aedes aegypti from Aquatic Breeding Sites in Leticia City, Colombian Amazon Biome. Insects 2025, 16, 195. https://doi.org/10.3390/insects16020195
Castañeda-Espinosa A, Duque-Granda D, Cadavid-Restrepo G, Murcia LM, Junca H, Moreno-Herrera CX, Vivero-Gómez RJ. Study of Bacterial Communities in Water and Different Developmental Stages of Aedes aegypti from Aquatic Breeding Sites in Leticia City, Colombian Amazon Biome. Insects. 2025; 16(2):195. https://doi.org/10.3390/insects16020195
Chicago/Turabian StyleCastañeda-Espinosa, Alejandro, Daniela Duque-Granda, Gloria Cadavid-Restrepo, Luz Mila Murcia, Howard Junca, Claudia X. Moreno-Herrera, and Rafael J. Vivero-Gómez. 2025. "Study of Bacterial Communities in Water and Different Developmental Stages of Aedes aegypti from Aquatic Breeding Sites in Leticia City, Colombian Amazon Biome" Insects 16, no. 2: 195. https://doi.org/10.3390/insects16020195
APA StyleCastañeda-Espinosa, A., Duque-Granda, D., Cadavid-Restrepo, G., Murcia, L. M., Junca, H., Moreno-Herrera, C. X., & Vivero-Gómez, R. J. (2025). Study of Bacterial Communities in Water and Different Developmental Stages of Aedes aegypti from Aquatic Breeding Sites in Leticia City, Colombian Amazon Biome. Insects, 16(2), 195. https://doi.org/10.3390/insects16020195






