A Preliminary Study on Identifying the Predator Community of Invasive Bactericera cockerelli (Hemiptera: Triozidae) and Developing Molecular Identification Tools for Testing Field Predation
Simple Summary
Abstract
1. Introduction
2. Material and Methods
2.1. Field Sampling of Predators and Identification
2.2. Development of a PCR Assay for Predators of B. cockerelli
2.3. Molecular Analysis of Field-Collected Predators
3. Results
3.1. Identification of Predators
3.2. Laboratory Study: Predator Feeding Trials
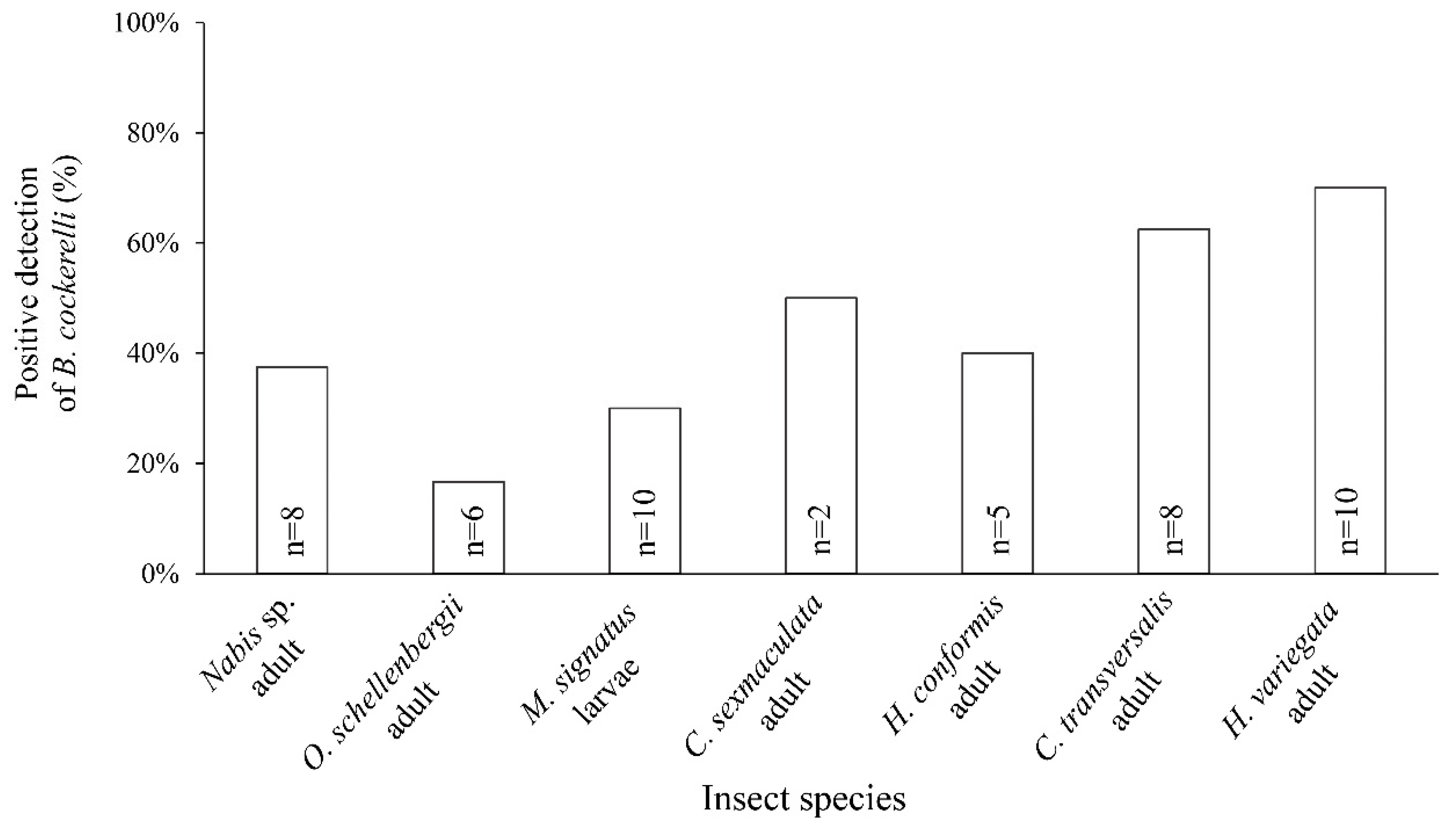
3.3. Molecular Analysis of Field-Collected Predators
4. Discussion
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rosson, P.; Niemeyer, M.; Palma, M.; Ribera, L. Economic Impacts of Zebra Chips on the Texas Potato Industry; Centre for North American Studies, Department of Agricultural Economics, Texas A&M University, Texas Agrilife Extension Publications: College Station, TX, USA, 2006; Volume 2. [Google Scholar]
- Yang, X.B.; Liu, T.X. Life history and life tables of Bactericera cockerelli (Homoptera: Psyllidae) on eggplant and bell pepper. Environ. Entomol. 2009, 38, 1661–1667. [Google Scholar] [CrossRef] [PubMed]
- Munyaneza, J.E. Zebra chip disease of potato: Biology, epidemiology, and management. Am. J. Potato Res. 2012, 89, 329–350. [Google Scholar] [CrossRef]
- Mustafa, T.; Alvarez, J.M.; Munyaneza, J.E. Effect of cyantraniliprole on probing behavior of potato psyllid, Bactericera cockerelli (Hemiptera: Triozidae), as measured by the electrical penetration graph technique. J. Econ. Entomol. 2015, 108, 2529–2535. [Google Scholar] [CrossRef]
- Šulc, K. Trioza cockerelli n. sp., a novelty from North America, being also of economic importance. Acta Soc. Entomol. Bohem. 1909, 6, 102–108. [Google Scholar]
- Department for Primary Industries and Regional Development (DPIRD). Tomato Potato Psyllid. Available online: https://www.agric.wa.gov.au/tomato-potato-psyllid-tpp (accessed on 14 April 2023).
- Munyaneza, J.E.; Crosslin, J.M.; Upton, J.E. Association of Bactericera cockerelli (Homoptera: Psyllidae) with “zebra chip,” a new potato disease in southwestern United States and Mexico. J. Econ. Entomol. 2007, 100, 656–663. [Google Scholar] [CrossRef]
- Goolsby, J.A.; Adamczyk, J.; Bextine, B.; Lin, D.; Munyaneza, J.E.; Bester, G. Development of an IPM program for management of the potato psyllid to reduce incidence of zebra chip disorder in potatoes. Subtrop. Plant Sci. 2007, 59, 85–94. [Google Scholar]
- Olaniyan, O.; Rodríguez-Gasol, N.; Cayla, N.; Michaud, E.; Wratten, S.D. Bactericera cockerelli (Šulc), a potential threat to China’s potato industry. J. Integr. Agric. 2020, 19, 338–349. [Google Scholar] [CrossRef]
- Butler, C.D.; Trumble, J.T. The potato psyllid, Bactericera cockerelli (Šulc) (Hemiptera: Triozidae): Life history, relationship to plant diseases, and management strategies. Terr. Arthropod Rev. 2012, 5, 87–111. [Google Scholar] [CrossRef]
- EPPO. Bactericera cockerelli. EPPO Bull. 2013, 43, 202–208. [Google Scholar] [CrossRef]
- Butler, C.D.; Trumble, J.T. Identification and Impact of Natural Enemies of Bactericera cockerelli (Hemiptera: Triozidae) in Southern California. J. Econ. Entomol. 2012, 105, 1509–1519. [Google Scholar] [CrossRef] [PubMed]
- Guenthner, J.; Goolsby, J.; Greenway, G. Use and cost of insecticides to control potato psyllids and zebra chip on potatoes. Southwest. Entomol. 2012, 37, 263–270. [Google Scholar] [CrossRef]
- Jorgensen, N.; Butler, R.C.; Vereijssen, J. Biorational insecticides for control of the tomato potato psyllid. N. Z. Plant Prot. 2013, 66, 333–340. [Google Scholar] [CrossRef]
- Dich, J.; Zahm, S.H.; Hanberg, A.; Adami, H.O. Pesticides and cancer. Cancer Causes Control 1997, 8, 420–443. [Google Scholar] [CrossRef]
- Geiger, F.; Bengtsson, J.; Berendse, F.; Weisser, W.W.; Emmerson, M.; Morales, M.B.; Ceryngier, P.; Liira, J.; Tscharntke, T.; Winqvist, C.; et al. Persistent negative effects of pesticides on biodiversity and biological control potential on European farmland. Basic Appl. Ecol. 2010, 11, 97–105. [Google Scholar] [CrossRef]
- Liu, D.; Trumble, J.T. Comparative fitness of invasive and native populations of the potato psyllid (Bactericera cockerelli). Entomol. Exp. Appl. 2007, 123, 35–42. [Google Scholar] [CrossRef]
- Vega-Gutierrez, M.T.; Rodríguez-Maciel, J.C.; Diaz-Gomez, O.; Bujanos-Muniz, R.; Mota-Sanchez, D.; Martínez-Carrillo, J.L.; Lagunes-Tejeda, A.; Garzon-Tiznado, J.A. Susceptibility to insecticides in two Mexican populations of tomato-potato psyllid, Bactericera cockerelli (Šulc) (Hemiptera: Triozidae). Agrociencia 2008, 42, 463–471. [Google Scholar]
- Prager, S.M.; Vindiola, B.; Kund, G.S.; Byrne, F.J.; Trumble, J.T. Considerations for the use of neonicotinoid pesticides in management of Bactericera cockerelli (Šulk) (Hemiptera: Triozidae). Crop Prot. 2013, 54, 84–91. [Google Scholar] [CrossRef]
- Cerna, E.; Ochoa, Y.; Aguirre, L.A.; Flores, M.; Landeros, J. Determination of insecticide resistance in four populations of potato psyllid Bactericera cockerelli (Šulc) (Hemiptera: Triozidae). Phyton 2013, 82, 63–68. [Google Scholar]
- Szczepaniec, A.; Varela, K.A.; Kiani, M.; Paetzold, L.; Rush, C.M. Incidence of resistance to neonicotinoid insecticides in Bactericera cockerelli across Southwest U.S. Crop Prot. 2019, 116, 188–195. [Google Scholar] [CrossRef]
- Hawkins, B.A.; Cornell, H.V.; Hochberg, M.E. Predators, parasitoids, and pathogens as mortality agents in phytophagous insect populations. Ecology 1997, 78, 2145–2152. [Google Scholar] [CrossRef]
- Messelink, G.J.; Sabelis, M.W.; Janssen, A. Generalist predators, food web complexities and biological pest control in greenhouse crops. In Integrated Pest Management and Pest Control-Current and Future Tactics; InTech: Rijeka, Croatia, 2012; pp. 191–214. [Google Scholar]
- Veronesi, E.R.; Olaniyan, O.; London, H.; Saville, D.J.; Wratten, S.D. Potential inter-guild interactions to enhance biological control of Bactericera cockerelli on tomatoes: A laboratory and cage study. BioControl 2021, 66, 343–353. [Google Scholar] [CrossRef]
- Shea, K.; Chesson, P. Community ecology theory as a framework for biological invasions. Trends Ecol. Evol. 2002, 17, 170–176. [Google Scholar] [CrossRef]
- Bulgarini, G.; Piemontese, L.; Guidetti, R.; Cesari, M.; di Bella, E.; Maistrello, L. Identification of Predatory Arthropods of the Invasive Halyomorpha halys through Molecular Gut Content Analysis. Agric. For. Entomol. 2021, 24, 219–228. [Google Scholar] [CrossRef]
- Harwood, J.D.; Desneux, N.; Yoo, H.J.; Rowley, D.L.; Greenstone, M.H.; Obrycki, J.J.; O’Neil, R.J. Tracking the role of alternative prey in soybean aphid predation by Orius insidiosus: A molecular approach. Mol. Ecol. 2007, 16, 4390–4400. [Google Scholar] [CrossRef]
- Desneux, N.; O’Neil, R.J. Potential of an alternative prey to disrupt predation of the generalist predator, Orius insidiosus, on the pest aphid, Aphis glycines, via short-term indirect interactions. Bull. Entomol. Res. 2008, 98, 631–639. [Google Scholar] [CrossRef] [PubMed]
- Juen, A.; Hogendoorn, K.; Ma, G.; Schmidt, O.; Keller, M.A. Analysing the diets of invertebrate predators using terminal restriction fragments. J. Pest Sci. 2012, 85, 89–100. [Google Scholar] [CrossRef]
- Chen, X.; Stansly, P.A. Biology of Tamarixia radiata (Hymenoptera: Eulophidae), Parasitoid of the Citrus Greening Disease Vector Diaphorina citri (Hemiptera: Psylloidea): A Mini Review. Fla. Entomol. 2014, 97, 1404–1413. [Google Scholar] [CrossRef]
- Tanaka, S.; Nishida, T.; Ohsaki, N. Sequential rapid adaptation of indigenous parasitoid wasps to the invasive butterfly Pieris brassicae. Evolution 2007, 61, 1791–1802. [Google Scholar] [CrossRef] [PubMed]
- Symondson, W.O.; Sunderland, K.D.; Greenstone, M.H. Can generalist predators be effective biocontrol agents? Annu. Rev. Entomol. 2002, 47, 561–594. [Google Scholar] [CrossRef]
- Eubanks, M.D.; Denno, R.F. Health food versus fast food: The effects of prey quality and mobility on prey selection by a generalist predator and indirect interactions among prey species. Ecol. Entomol. 2000, 25, 140–146. [Google Scholar] [CrossRef]
- Sarkar, S.C.; Hatt, S.; Philips, A.; Akter, M.; Milroy, S.P.; Xu, W. Tomato Potato Psyllid Bactericera cockerelli (Hemiptera: Triozidae) in Australia: Incursion, Potential Impact and Opportunities for Biological Control. Insects 2023, 14, 263. [Google Scholar] [CrossRef]
- Symondson, W.O.C. Molecular identification of prey in predator diets. Mol. Ecol. 2002, 11, 627–641. [Google Scholar] [CrossRef] [PubMed]
- Greenstone, M.H.; Szendrei, Z.; Payton, M.E.; Rowley, D.L.; Coudron, T.C.; Weber, D.C. Choosing natural enemies for conservation biological control: Use of the prey detectability half-life to rank key predators of Colorado potato beetle. Entomol. Exp. Appl. 2010, 136, 97–107. [Google Scholar] [CrossRef]
- Dhami, M.K.; Dsouza, M.; Waite, D.W.; Anderson, D.; Li, D. Real-Time PCR Assay for the Identification of the Brown Marmorated Stink Bug (Halyomorpha halys). Front. Mol. Biosci. 2016, 3, 5. [Google Scholar] [CrossRef] [PubMed]
- Unruh, T.R.; Miliczky, E.R.; Horton, D.R.; Thomsen-Archer, K.; Rehfield-Ray, L.; Jones, V.P. Gut content analysis of arthropod predators of codling moth in Washington apple orchards. Biol. Control 2016, 102, 85–92. [Google Scholar] [CrossRef]
- Casey, J.M.; Meyer, C.P.; Morat, F.; Brandl, S.J.; Planes, S.; Parravicini, V. Reconstructing Hyperdiverse Food Webs: Gut Content Metabarcoding as a Tool to Disentangle Trophic Interactions on Coral Reefs. Methods Ecol. Evol. 2019, 10, 1157–1170. [Google Scholar] [CrossRef]
- Siegenthaler, A.; Wangensteen, O.S.; Soto, A.Z.; Benvenuto, C.; Corrigan, L.; Mariani, S. Metabarcoding of shrimp stomach content: Harnessing a natural sampler for fish biodiversity monitoring. Mol. Ecol. Resour. 2019, 19, 206–220. [Google Scholar] [CrossRef]
- Bouvet, J.P.; Urbaneja, A.; Pérez-Hedo, M.; Monzó, C. Contribution of Predation to the Biological Control of a Key Herbivorous Pest in Citrus Agroecosystems. J. Anim. Ecol. 2019, 88, 915–926. [Google Scholar] [CrossRef] [PubMed]
- Jacobsen, S.K.; Sigsgaard, L.; Hansen, K.; Harwood, J.D.; Chapman, E.G.; Hurtado, M.A.; Jensen, A.B. Generalist predator contributions to the control of Tetranychus urticae in strawberry crops documented by PCR-based gut content analysis. Exp. Appl. Acarol. 2019, 77, 133–143. [Google Scholar] [CrossRef]
- Hoogendoorn, M.; Heimpel, G.E. PCR-Based Gut Content Analysis of Insect Predators: Using Ribosomal ITS-1 Fragments from Prey to Estimate Predation Frequency. Mol. Ecol. 2001, 10, 2059–2067. [Google Scholar] [CrossRef] [PubMed]
- Harwood, J.D.; Yoo, H.J.; Greenstone, M.H.; Rowley, D.L.; O’Neil, R.J. Differential impact of adults and nymphs of a generalist predator on an exotic invasive pest demonstrated by molecular gut-content analysis. Biol. Invasions 2009, 11, 895–903. [Google Scholar] [CrossRef]
- Roubinet, E.; Jonsson, T.; Malsher, G.; Staudacher, K.; Traugott, M.; Ekbom, B.; Jonsson, M. High redundancy as well as complementary prey choice characterize generalist predator food webs in agroecosystems. Sci. Rep. 2018, 8, 8054. [Google Scholar] [CrossRef] [PubMed]
- Rondoni, G.; Athey, K.J.; Harwood, J.D.; Conti, E.; Ricci, C.; Obrycki, J.J. Development and application of molecular gut-content analysis to detect aphid and coccinellid predation by Harmonia axyridis (Coleoptera: Coccinellidae) in Italy. Insect Sci. 2015, 22, 719–730. [Google Scholar] [CrossRef]
- Harper, G.L.; King, R.A.; Dodd, C.S.; Harwood, J.D.; Glen, D.M.; Bruford, M.W.; Symondson, W.O. Rapid screening of invertebrate predators for multiple prey DNA targets. Mol. Ecol. 2005, 14, 819–827. [Google Scholar] [CrossRef]
- Zhang, G.F.; Lü, Z.C.; Wan, F.H. Detection of Bemisia tabaci remains in predator guts using a sequence-characterized amplified region marker. Entomol. Exp. Appl. 2007, 123, 81–90. [Google Scholar] [CrossRef]
- Peterson, J.A.; Burkness, E.C.; Harwood, J.D.; Hutchison, W.D. Molecular gut-content analysis reveals high frequency of Helicoverpa zea (Lepidoptera: Noctuidae) consumption by Orius insidiosus (Hemiptera: Anthocoridae) in sweet corn. BioControl 2018, 121, 1–7. [Google Scholar] [CrossRef]
- Chapman, R.I.; Strube, L.; Bextine, B. Population Genetics of the Potato Psyllid: Impacts on Zebra Chip Epidemiology. In Proceedings of the 10th Annual Zebra Chip Reporting Session, Dallas, TX, USA, 7–10 November 2010; Texas AgriLife: College Station, TX, USA, 2010; pp. 64–68. [Google Scholar]
- Wu, F.; Cen, Y.; Wallis, C.M.; Trumble, J.T.; Prager, S.; Yokomi, R.; Zheng, Z.; Deng, X.; Chen, J.; Liang, G. The complete mitochondrial genome sequence of Bactericera cockerelli and comparison with three other Psylloidea species. PLoS ONE 2016, 11, e0155318. [Google Scholar] [CrossRef]
- Sarkar, S.C.; Milroy, S.P.; Xu, W. Development and reproduction of a native generalist predator, Coccinella transversalis (Coleoptera: Coccinellidae), on the tomato potato psyllid, Bactericera cockerelli, with a greenhouse assay of biocontrol potential. Biol. Control 2022, 176, 105108. [Google Scholar] [CrossRef]
- Sarkar, S.C.; Milroy, S.P.; Xu, W. Potential of variegated lady beetle Hippodamia variegata in management of invasive tomato potato psyllid Bactericera cockerelli. Pest Manag. Sci. 2023, 79, 821–832. [Google Scholar] [CrossRef] [PubMed]
- Al-Jabr, A.M. Integrated Pest Management of Tomato Potato Psyllid, Paratrioza cockerelli (Sulc) (Homoptera: Psyllidae) with Emphasis on Its Importance in Greenhouse Grown Tomatoes. Ph.D. Dissertation, Colorado State University, Fort Collins, CO, USA, 1999. [Google Scholar]
- Walker, G.P.; MacDonald, F.H.; Larsen, N.J.; Wallace, A.R. Monitoring Bactericera cockerelli and associated insect populations in potatoes in South Auckland. N. Z. Plant Prot. 2011, 64, 269–275. [Google Scholar] [CrossRef]
- MacDonald, F.H.; Connolly, P.G.; Larsen, N.J.; Walker, G.P. The voracity of five insect predators on Bactericera cockerelli (Sülc) (Hemiptera: Triozidae) (tomato potato psyllid; TPP). N. Z. Entomol. 2016, 39, 15–22. [Google Scholar] [CrossRef]
- Knowlton, G.F.; Allen, M. Three hemipterous predators of the potato psyllid. Proc. Utah Acad. Sci. 1936, 13, 293–294. [Google Scholar]
- Robinson, K.A.; Jonsson, M.; Wratten, S.D.; Wade, M.R.; Buckley, H.L. Implications of floral resources for predation by an omnivorous lacewing. Basic Appl. Ecol. 2008, 9, 172–181. [Google Scholar] [CrossRef]
- Gurr, G.; Wratten, S.; Tylianakis, J.; Kean, J.; Keller, M. Providing plant foods for natural enemies in farming systems: Balancing practicalities and theory. CABI Agric. Biosci. 2005, 1, 140. [Google Scholar]
- Van Rijn, P.C.; Sabelis, M.W. Impact of plant-provided food on herbivore-carnivore dynamics. In Plant-Provided Food and Herbivore–Carnivore Interactions; Wäckers, F.L., van Rijn, P.C.J., Bruin, J., Eds.; Cambridge University Press: Cambridge, UK, 2005; pp. 223–266. [Google Scholar]
- Hayashi, M.; Nomura, M. Eggs of Mallada desjardinsi (Neuroptera: Chrysopidae) are protected by ants: The role of egg stalks in ant-tended aphid colonies. Environ. Entomol. 2014, 43, 1003–1007. [Google Scholar] [CrossRef] [PubMed]
- Gurr, G.M.; Wratten, S.D.; Landis, D.A.; You, M. Habitat management to suppress pest populations: Progress and prospects. Annu. Rev. Entomol. 2017, 62, 91–109. [Google Scholar] [CrossRef] [PubMed]
- Shields, M.W.; Johnson, A.C.; Pandey, S.; Cullen, R.; González-Chang, M.; Wratten, S.D.; Gurr, G.M. History, current situation and challenges for conservation biological control. Biol. Control 2019, 131, 25–35. [Google Scholar] [CrossRef]
- Gardarin, A.; Pigot, J.; Valantin-Morison, M. The hump-shaped effect of plant functional diversity on the biological control of a multi-species pest community. Sci. Rep. 2021, 11, 21635. [Google Scholar] [CrossRef] [PubMed]
- Young, O.P.; Lockley, T.C. The striped lynx spider, Oxyopes salticus (Araneae: Oxyopidae), in agroecosystems. Entomophaga 1985, 30, 329–346. [Google Scholar] [CrossRef]
- Nyffeler, M.; Dean, D.A.; Sterling, W.L. Evaluation of the importance of the striped lynx spider, Oxyopes salticus (Araneae: Oxyopidae), as a predator in Texas cotton. Environ. Entomol. 1987, 16, 1114–1123. [Google Scholar] [CrossRef]
- Michalko, R.; Pekár, S.; Entling, M.H. An updated perspective on spiders as generalist predators in biological control. Oecologia 2019, 189, 21–36. [Google Scholar] [CrossRef] [PubMed]
- Eitzinger, B.; Unger, E.M.; Traugott, M.; Scheu, S. Effects of prey quality and predator body size on prey DNA detection success in a centipede predator. Mol. Ecol. 2014, 23, 3767–3776. [Google Scholar] [CrossRef] [PubMed]
- Eitzinger, B.; Rall, B.C.; Traugott, M.; Scheu, S. Testing the validity of functional response models using molecular gut content analysis for prey choice in soil predators. Oikos 2018, 127, 915–926. [Google Scholar] [CrossRef]
- Symondson, W.O.C. The molecular revolution: Using polymerase chain reaction-based methods to explore the role of predators in terrestrial food webs. Biodivers. Insect Pests Key Issues Sustain. Manag. 2012, 166–184. [Google Scholar]
- Greenstone, M.H.; Payton, M.E.; Weber, D.C.; Simmons, A.M. The detectability half-life in arthropod predator-prey research: What it is, why we need it, how to measure it, and how to use it. Mol. Ecol. 2014, 23, 3799–3813. [Google Scholar] [CrossRef] [PubMed]
- Rutledge, C.E.; O’Neil, R.J.; Fox, T.B.; Landis, D.A. Soybean aphid predators and their use in Integrated Pest Management. Ann. Entomol. Soc. Am. 2004, 97, 240–248. [Google Scholar] [CrossRef]


| Order | Family | Genus/Species | 2021 | 2022 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Site-1 | Total | Site-1 | Site-2 | Site-3 | Site-4 | Site-5 | Total | |||||||||
| Capsicum | Capsicum | Potato | Potato | Potato | Potato | |||||||||||
| 1st Date (15.3) | 2nd Date (22.3) | 3rd Date (29.3) | 1st Date (25.3) | 2nd Date (1.4) | 3rd Date (8.4) | 4th Date (15.4) | 1st Date (23.3) | 1st Date (13.4) | 2nd Date (11.11) | 1st Date (11.11) | 1st Date (11.11) | |||||
| Araneae | Araneidae | Argiope protensa | 2 | 3 | 5 | 1 | 1 | 2 | ||||||||
| Backobourkia | 1 | 1 | ||||||||||||||
| Oxyopidae | Oxyopes | 3 | 24 | 1 | 28 | 2 | 4 | 1 | 9 | 3 | 2 | 1 | 22 | |||
| Salticidae | 1 | 1 | 1 | 3 | 4 | |||||||||||
| Tetragnathidae | Tetragnatha | 1 | 1 | 1 | 1 | |||||||||||
| Theridiidae | Euryopes | 3 | 3 | |||||||||||||
| Thomisidae | Thomisus spectabilis | 1 | 1 | 1 | 1 | |||||||||||
| Unidentified spider species | 2 | 6 | 2 | 10 | 2 | 5 | 3 | 10 | ||||||||
| Diptera | Syrphidae | Melangyna viridiceps | 1 | 1 | 2 | 1 | 2 | 2 | 5 | |||||||
| Coleoptera | Coccinellidae | Harmonia conformis | 7 | 7 | 1 | 2 | 1 | 1 | 5 | |||||||
| Cheilomenes sexmaculata | 2 | 2 | 3 | 1 | 4 | |||||||||||
| Coccinella transversalis | 2 | 41 | 43 | 2 | 2 | 1 | 1 | 1 | 1 | 8 | ||||||
| Hippodamia variegata | 1 | 9 | 10 | 5 | 2 | 8 | 1 | 2 | 1 | 3 | 22 | |||||
| Hemiptera | Pentatomidae | Oechalia schellenbergii | 2 | 7 | 9 | 2 | 2 | |||||||||
| Miridae | Creontiades sp. | 24 | 52 | 76 | 1 | 1 | ||||||||||
| Nabidae | Nabis sp. | 31 | 31 | |||||||||||||
| Rhyparochromidae Rhyparochromidae sp. | 3 | 3 | ||||||||||||||
| Neuroptera | Chrysopidae | Mallada signatus | Adult: 36 | Adult: 356 | Adult: 43 | 469 | Adult: 38 | Adult: 268 | Adult: 68 | Adult: 300 | Adult: 7 | 859 | ||||
| Larvae: 4 | Larvae: 17 | Larvae: 13 | Larvae: 32 | Larvae: 73 | Larvae: 23 | Larvae: 46 | Larvae: 4 | |||||||||
| Micromus tasmaniae | Adult:1 | Adult: 2 | Adult: 1 | Adult: 1 | 5 | |||||||||||
| Predator Species | Time Since Feeding on B. cockerelli | ||||||
|---|---|---|---|---|---|---|---|
| 0 h | 0.5 h | 1 h | 2 h | 6 h | 12 h | 24 h | |
| Coccinella transversalis (adult) | + | + | + | + | - | - | - |
| Hippodamia variegata (adult) | + | + | + | - | - | - | - |
| Mallada signatus (larvae) | + | + | + | + | + | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sarkar, S.C.; Milroy, S.P.; Xu, W. A Preliminary Study on Identifying the Predator Community of Invasive Bactericera cockerelli (Hemiptera: Triozidae) and Developing Molecular Identification Tools for Testing Field Predation. Insects 2025, 16, 179. https://doi.org/10.3390/insects16020179
Sarkar SC, Milroy SP, Xu W. A Preliminary Study on Identifying the Predator Community of Invasive Bactericera cockerelli (Hemiptera: Triozidae) and Developing Molecular Identification Tools for Testing Field Predation. Insects. 2025; 16(2):179. https://doi.org/10.3390/insects16020179
Chicago/Turabian StyleSarkar, Shovon Chandra, Stephen Paul Milroy, and Wei Xu. 2025. "A Preliminary Study on Identifying the Predator Community of Invasive Bactericera cockerelli (Hemiptera: Triozidae) and Developing Molecular Identification Tools for Testing Field Predation" Insects 16, no. 2: 179. https://doi.org/10.3390/insects16020179
APA StyleSarkar, S. C., Milroy, S. P., & Xu, W. (2025). A Preliminary Study on Identifying the Predator Community of Invasive Bactericera cockerelli (Hemiptera: Triozidae) and Developing Molecular Identification Tools for Testing Field Predation. Insects, 16(2), 179. https://doi.org/10.3390/insects16020179






