Bacteria Derived from Diamondback Moth, Plutella xylostella (L.) (Lepidoptera: Plutellidae), Gut Regurgitant Negatively Regulate Glucose Oxidase-Mediated Anti-Defense Against Host Plant
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Insect Strains
2.2. Host Plants
2.3. Defense Responses of the Host Plants to Different Treatments
2.4. Isolation of Culturable Bacteria from Larval Gut Regurgitant
2.5. Sequence Alignment and Phylogenetic Analysis
2.6. RNA Extraction, cDNA Synthesis, and qRT-PCR
2.7. Protein Extraction and Western Blot (WB)
2.8. Effects of Gut RB on Plant Defense Responses
2.9. Fitness Comparison After Inoculation of Gut RB
2.10. Statistical Analysis
3. Results
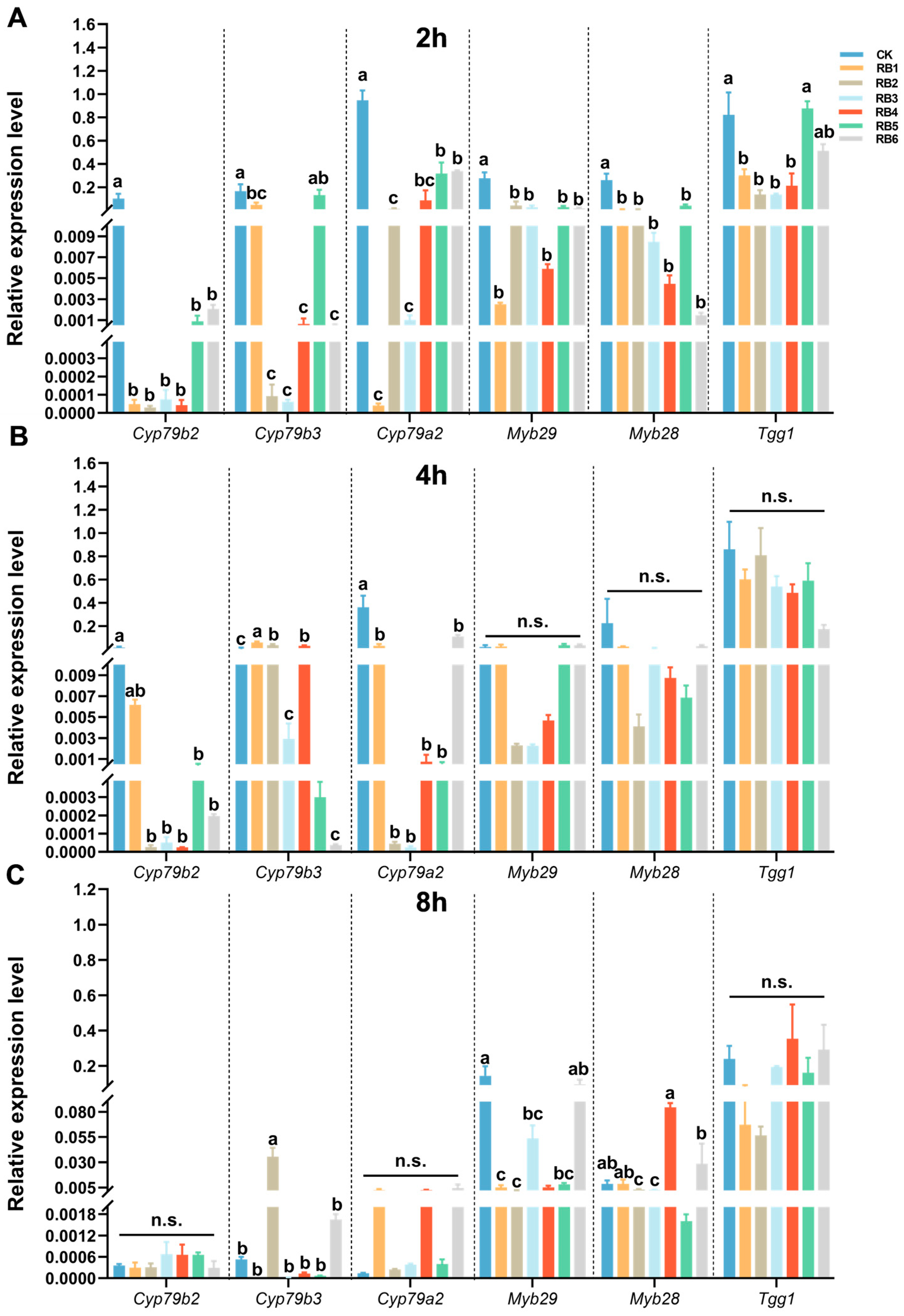
3.1. Suppression of Gut Homogenate Containing RB of DBM on the Defense Responses of Host Plant
3.2. Isolation and Identification of Culturable Gut RB from DBM
3.3. Gut RB from DBM Suppress Defense-Related Gene Expression of Host Plant
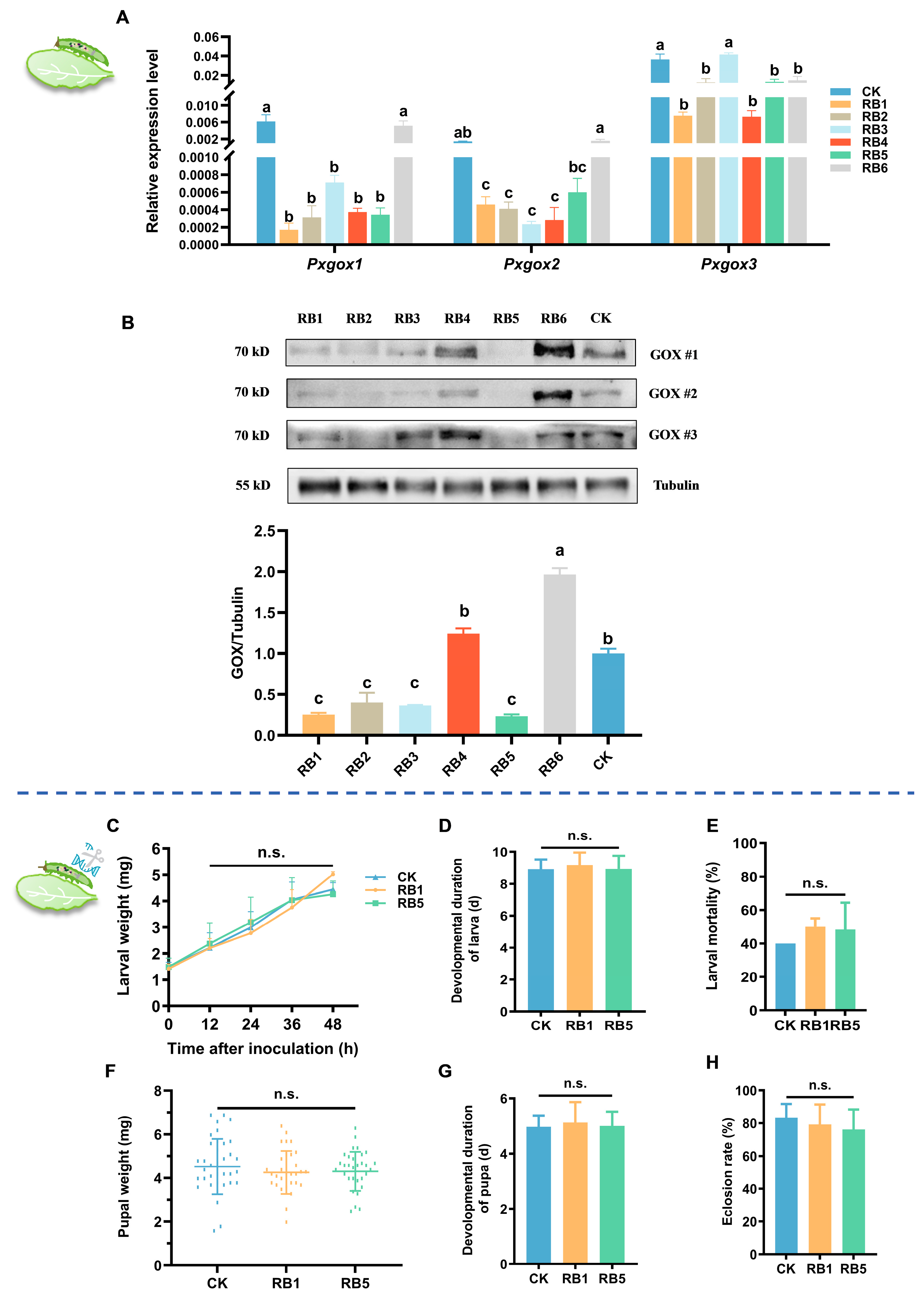
3.4. RB1 and RB5 Effectively Suppress DBM Performance on Host Plants
3.5. RB1 and RB5 Negatively Regulate GOX-Mediated DBM Host Adaptability
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Acevedo, F.E.; Rivera-Vega, L.J.; Chung, S.H.; Ray, S.; Felton, G.W. Cues from chewing insects-the intersection of DAMPs, HAMPs, MAMPs and effectors. Curr. Opin. Plant Biol. 2015, 26, 80–86. [Google Scholar] [CrossRef] [PubMed]
- Erb, M.; Reymond, P. Molecular interactions between plants and insect herbivores. Annu. Rev. Plant Biol. 2019, 70, 527–557. [Google Scholar] [CrossRef] [PubMed]
- Terra, W.R.; Dias, R.O.; Oliveira, P.L.; Ferreira, C.; Venancio, T.M. Transcriptomic analyses uncover emerging roles of mucins, lysosome/secretory addressing and detoxification pathways in insect midguts. Curr. Opin. Insect Sci. 2018, 29, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Opitz, S.E.W.; Müller, C. Plant chemistry and insect sequestration. Chemoecology 2009, 19, 117–154. [Google Scholar] [CrossRef]
- Rivera-Vega, L.J.; Acevedo, F.E.; Felton, G.W. Genomics of Lepidoptera saliva reveals function in herbivory. Curr. Opin. Insect Sci. 2017, 19, 61–69. [Google Scholar] [CrossRef]
- Chen, C.Y.; Mao, Y.B. Research advances in plant-insect molecular interaction. F1000 Res. 2020, 9, 198. [Google Scholar] [CrossRef]
- Eichenseer, H.; Mathews, M.C.; Powell, J.S.; Felton, G.W. Survey of a salivary effector in caterpillars: Glucose oxidase variation and correlation with host range. J. Chem. Ecol. 2010, 36, 885–897. [Google Scholar] [CrossRef]
- Diezel, C.; von Dahl, C.C.; Gaquerel, E.; Baldwin, I.T. Different lepidopteran elicitors account for cross-talk in herbivory-induced phytohormone signaling. Plant Physiol. 2009, 150, 1576–1586. [Google Scholar] [CrossRef]
- Chen, C.; Liu, Y.; Song, W.; Chen, D.; Chen, F.; Chen, X.; Chen, Z.; Ge, S.; Wang, C.; Zhan, S.; et al. An effector from cotton bollworm oral secretion impairs host plant defense signaling. Proc. Natl. Acad. Sci. USA 2019, 116, 14331–14338. [Google Scholar] [CrossRef]
- Felton, G.W.; Chung, S.H.; Hernandez, M.G.E.; Louis, J.; Peiffer, M.; Tian, D. Herbivore oral secretions are the first line of protection against plant-induced defences. In Annual Plant Reviews, Volume 47: Insect-Plant Interactions, 1st ed.; Voelckel, C., Jander, G., Eds.; John Wiley & Sons, Ltd.: Chichester, UK, 2014; Volume 47, pp. 37–76. [Google Scholar]
- Chung, S.H.; Rosa, C.; Scully, E.D.; Peiffer, M.; Tooker, J.F.; Hoover, K.; Luthe, D.S.; Felton, G.W. Herbivore exploits orally secreted bacteria to suppress plant defenses. Proc. Natl. Acad. Sci. USA 2013, 110, 15728–15733. [Google Scholar] [CrossRef]
- Schmelz, E.A. Impacts of insect oral secretions on defoliation-induced plant defense. Curr. Opin. Insect Sci. 2015, 9, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Jing, M.; Shen, D.; Wang, C.; Zhang, M.; Liang, D.; Nyawira, K.T.; Xia, Q.; Zuo, K.; Wu, S.; et al. The mirid bug Apolygus lucorum deploys a glutathione peroxidase as a candidate effector to enhance plant susceptibility. J. Exp. Bot. 2020, 71, 2701–2712. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Zhao, P.; Ma, Y.; Yao, X.; Sun, Y.; Huang, X.; Jin, J.; Zhang, Y.; Zhu, C.; Fang, R.; et al. A whitefly effector Bsp9 targets host immunity regulator WRKY33 to promote performance. Philos. Trans. R. Soc. B. 2019, 374, 20180313. [Google Scholar] [CrossRef] [PubMed]
- Tian, D.; Peiffer, M.; Shoemaker, E.; Tooker, J.; Haubruge, E.; Francis, F.; Luthe, D.S.; Felton, G.W.; Bonaventure, G. Salivary glucose oxidase from caterpillars mediates the induction of rapid and delayed-induced defenses in the tomato plant. PLoS ONE 2012, 7, e36168. [Google Scholar] [CrossRef]
- Zong, N.; Wang, C. Induction of nicotine in tobacco by herbivory and its relation to glucose oxidase activity in the labial gland of three noctuid caterpillars. Chin. Sci. Bull. 2004, 49, 1596–1601. [Google Scholar] [CrossRef]
- Musser, R.O.; Hum-Musser, S.M.; Eichenseer, H.; Peiffer, M.; Ervin, G.; Murphy, J.B.; Felton, G.W. Herbivory: Caterpillar saliva beats plant defences. Nature 2002, 416, 599–600. [Google Scholar] [CrossRef]
- Yang, F.; Jing, X.; Dong, R.; Zhou, L.; Xu, X.; Dong, Y.; Zhang, L.; Zheng, L.; Lai, Y.; Chen, Y.; et al. Glucose oxidase of a crucifer-specialized insect: A potential role in suppressing plant defense via modulating antagonistic plant hormones. J. Agr. Food Chem. 2023, 71, 17469–17483. [Google Scholar] [CrossRef]
- Acevedo, F.E.; Peiffer, M.; Tan, C.W.; Stanley, B.A.; Stanley, A.; Wang, J.; Jones, A.G.; Hoover, K.; Rosa, C.; Luthe, D.; et al. Fall armyworm-associated gut bacteria modulate plant defense responses. Mol. Plant-Microbe Interact. 2017, 30, 127–137. [Google Scholar] [CrossRef]
- Gedling, C.R.; Smith, C.M.; LeMoine, C.M.R.; Cassone, B.J. The Mexican bean beetle (Epilachna varivestis) regurgitome and insights into beetle-borne virus specificity. PLoS ONE 2018, 13, e192003. [Google Scholar] [CrossRef]
- Felton, G.W.; Tumlinson, J.H. Plant-insect dialogs: Complex interactions at the plant-insect interface. Curr. Opin. Plant Biol. 2008, 11, 457–463. [Google Scholar] [CrossRef]
- Zhu, F.; Poelman, E.H.; Dicke, M. Insect herbivore-associated organisms affect plant responses to herbivory. New Phytol. 2014, 204, 315–321. [Google Scholar] [CrossRef]
- Mason, C.J.; Couture, J.J.; Raffa, K.F. Plant-associated bacteria degrade defense chemicals and reduce their adverse effects on an insect defoliator. Oecologia 2014, 175, 901–910. [Google Scholar] [CrossRef] [PubMed]
- Thaler, J.S.; Humphrey, P.T.; Whiteman, N.K. Evolution of jasmonate and salicylate signal crosstalk. Trends Plant Sci. 2012, 17, 260–270. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Peiffer, M.; Hoover, K.; Rosa, C.; Zeng, R.; Felton, G.W. Helicoverpa zea gut-associated bacteria indirectly induce defenses in tomato by triggering a salivary elicitor(s). New Phytol. 2017, 214, 1294–1306. [Google Scholar] [CrossRef]
- Chen, W.; Amir, M.B.; Liao, Y.; Yu, H.; He, W.; Lu, Z. New insights into the Plutella xylostella detoxifying enzymes: Sequence evolution, structural similarity, functional diversity, and application prospects of glucosinolate sulfatases. J. Agr. Food Chem. 2023, 71, 10952–10969. [Google Scholar] [CrossRef]
- Douglas, A.E. Multiorganismal insects: Diversity and function of resident microorganisms. Annu. Rev. Entomol. 2015, 60, 17–34. [Google Scholar] [CrossRef]
- Claesson, M.J.; Wang, Q.; O’Sullivan, O.; Greene-Diniz, R.; Cole, J.R.; Ross, R.P.; O’Toole, P.W. Comparison of two next-generation sequencing technologies for resolving highly complex microbiota composition using tandem variable 16S rRNA gene regions. Nucleic Acids Res. 2010, 38, e200. [Google Scholar] [CrossRef]
- Lê, K.; Li, Y.; Xu, X.; Yang, W.; Liu, T.; Zhao, X.; Tang, Y.G.; Cai, D.; Go, V.L.W.; Pandol, S.; et al. Alterations in fecal Lactobacillus and Bifidobacterium species in type 2 diabetic patients in Southern China population. Front. Physiol. 2013, 3, 496. [Google Scholar] [CrossRef]
- You, M.; Yue, Z.; He, W.; Yang, X.; Yang, G.; Xie, M.; Zhan, D.; Baxter, S.W.; Vasseur, L.; Gurr, G.M.; et al. A heterozygous moth genome provides insights into herbivory and detoxification. Nat. Genet. 2013, 45, 220–225. [Google Scholar] [CrossRef]
- Huang, Y.; Wang, Y.; Zeng, B.; Liu, Z.; Xu, X.; Meng, Q.; Huang, Y.; Yang, G.; Vasseur, L.; Gurr, G.M.; et al. Functional characterization of Pol III U6 promoters for gene knockdown and knockout in Plutella xylostella. Insect Biochem. Mol. Biol. 2017, 89, 71–78. [Google Scholar] [CrossRef]
- Ehlting, J.; Chowrira, S.G.; Mattheus, N.; Aeschliman, D.S.; Arimura, G.; Bohlmann, J. Comparative transcriptome analysis of Arabidopsis thaliana infested by diamond back moth (Plutella xylostella) larvae reveals signatures of stress response, secondary metabolism, and signalling. Bmc Genom. 2008, 9, 154. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wang, D.; Peng, H.; Chen, X.; Han, X.; Yu, J.; Wang, W.; Liang, L.; Liu, Z.; Zheng, Y.; et al. Epigenetically upregulated oncoprotein PLCE1 drives esophageal carcinoma angiogenesis and proliferation via activating the PI-PLCε-NF-κB signaling pathway and VEGF-C/ Bcl-2 expression. Mol. Cancer 2019, 18, 1. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Saqib, H.S.A.; Chen, J.; Ruan, Q.; Vasseur, L.; He, W.; You, M. Differential profiles of gut microbiota and metabolites associated with host shift of Plutella xylostella. Int. J. Mol. Sci. 2020, 21, 6283. [Google Scholar] [CrossRef] [PubMed]
- Mahala, S.K.; Chandel, R.S.; Verma, K.S.; Ramappa, K.; Walia, A.; Mahala, S.K.; Verma, K.S.; Ramappa, K. The first report of opportunistic human pathogenic bacteria isolated from Brahmina coriacea (Scarabaeidae: Coleoptera) in north-western Himalayas. ESS Open Arch. 2023; in press. [Google Scholar]
- Gayatri Priya, N.; Ojha, A.; Kajla, M.K.; Raj, A.; Rajagopal, R. Host plant induced variation in gut bacteria of Helicoverpa armigera. PLoS ONE 2012, 7, e30768. [Google Scholar] [CrossRef]
- Adams, A.S.; Currie, C.R.; Cardoza, Y.; Klepzig, K.D.; Raffa, K.F. Effects of symbiotic bacteria and tree chemistry on the growth and reproduction of bark beetle fungal symbionts. Can. J. For. Res. 2009, 39, 1133–1147. [Google Scholar] [CrossRef]
- Saxena, S.; Bahadur, J.; Varma, A. Production and localisation of carboxymethylcellulase, xylanase and β-glucosidase from Cellulomonas and Micrococcus spp. Appl. Microbiol. Biot. 1991, 34, 668–670. [Google Scholar] [CrossRef]
- Tang, X.; Freitak, D.; Vogel, H.; Ping, L.; Shao, Y.; Cordero, E.A.; Andersen, G.; Westermann, M.; Heckel, D.G.; Boland, W. Complexity and variability of gut commensal microbiota in polyphagous lepidopteran larvae. PLoS ONE 2012, 7, e36978. [Google Scholar] [CrossRef]
- Pan, Q.; Shikano, I.; Liu, T.; Felton, G.W. Helicoverpa zea-Associated gut bacteria as drivers in shaping plant anti-herbivore defense in tomato. Microb. Ecol. 2023, 86, 2173–2182. [Google Scholar] [CrossRef]
- Zhang, P.; Li, W.; Huang, F.; Zhang, J.; Xu, F.; Lu, Y. Feeding by Whiteflies suppresses downstream jasmonic acid signaling by eliciting salicylic acid signaling. J. Chem. Ecol. 2013, 39, 612–619. [Google Scholar] [CrossRef]
- Simon, J.; D Alençon, E.; Guy, E.; Jacquin-Joly, E.; Jaquiéry, J.; Nouhaud, P.; Peccoud, J.; Sugio, A.; Streiff, R. Genomics of adaptation to host-plants in herbivorous insects. Brief. Funct. Genom. 2015, 14, 413–423. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Qian, L.; Wang, X.; Shao, R.; Hong, Y.; Liu, S.; Wang, X. A salivary effector enables whitefly to feed on host plants by eliciting salicylic acid-signaling pathway. Proc. Natl. Acad. Sci. USA 2019, 116, 490–495. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Dong, Y.; Saqib, H.S.A.; Vasseur, L.; Zhou, W.; Zheng, L.; Lai, Y.; Ma, X.; Lin, L.; Xu, X.; et al. Functions of duplicated glucosinolate sulfatases in the development and host adaptation of Plutella xylostella. Insect Biochem. Mol. Biol. 2020, 119, 103316. [Google Scholar] [CrossRef] [PubMed]
- Bos, J.I.B.; Prince, D.; Pitino, M.; Maffei, M.E.; Win, J.; Hogenhout, S.A. A functional genomics approach identifies candidate effectors from the aphid species Myzus persicae (green peach aphid). PLoS Genet. 2010, 6, e1001216. [Google Scholar] [CrossRef]
- Hogenhout, S.A.; Bos, J.I.B. Effector proteins that modulate plant-insect interactions. Curr. Opin. Plant Biol. 2011, 14, 422–428. [Google Scholar] [CrossRef]
- Wang, W.; Dai, H.; Zhang, Y.; Chandrasekar, R.; Luo, L.; Hiromasa, Y.; Sheng, C.; Peng, G.; Chen, S.; Tomich, J.M.; et al. Armet is an effector protein mediating aphid-plant interactions. FASEB J. 2015, 29, 2032–2045. [Google Scholar] [CrossRef]
- Cui, N.; Lu, H.; Wang, T.; Zhang, W.; Kang, L.; Cui, F. Armet, an aphid effector protein, induces pathogen resistance in plants by promoting the accumulation of salicylic acid. Philos. Trans. R. Soc. B 2019, 374, 20180314. [Google Scholar] [CrossRef]
- Wittstock, U.; Agerbirk, N.; Stauber, E.J.; Olsen, C.E.; Hippler, M.; Mitchell-Olds, T.; Gershenzon, J.; Vogel, H. Successful herbivore attack due to metabolic diversion of a plant chemical defense. Proc. Natl. Acad. Sci. USA 2004, 101, 4859–4864. [Google Scholar] [CrossRef]
- Chen, W.; Saqib, H.S.A.; Xu, X.; Dong, Y.; Zheng, L.; Lai, Y.; Jing, X.; Lu, Z.; Sun, L.; You, M.; et al. Glucosinolate sulfatases–sulfatase-modifying factors system enables a crucifer-specialized moth to pre-detoxify defensive glucosinolate of the host plant. J. Agric. Food Chem. 2022, 70, 11179–11191. [Google Scholar] [CrossRef]
- Beckers, G.J.; Spoel, S.H. Fine-tuning plant defence signalling: Salicylate versus jasmonate. Plant Biol. 2006, 8, 1–10. [Google Scholar] [CrossRef]
- Sakthi, A.R.; Selvi, C.; Poorninammal, R. Role of phytohormones in plant defence against insects: Signalling and crosstalk. In Plant-Pest Interactions: From Molecular Mechanisms to Chemical Ecology; Singh, I.K., Singh, A., Eds.; Springer Nature Singapore Pte Ltd.: Singapore, 2021; pp. 215–232. ISBN 978-981-15-2467-7. [Google Scholar]
- Park, S.W.; Kaimoyo, E.; Kumar, D.; Mosher, S.; Klessig, D.F. Methyl salicylate is a critical mobile signal for plant systemic acquired resistance. Science 2007, 318, 113–116. [Google Scholar] [CrossRef] [PubMed]
- Baccelli, I.; Lombardi, L.; Luti, S.; Bernardi, R.; Picciarelli, P.; Scala, A.; Pazzagli, L. Cerato-platanin induces resistance in Arabidopsis leaves through stomatal perception, overexpression of salicylic acid- and ethylene-signalling genes and camalexin biosynthesis. PLoS ONE 2014, 9, e100959. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Wang, H.; Xie, J.; Lv, J.; Zhang, G.; Hu, L.; Luo, S.; Li, L.; Yu, J. The roles of cruciferae glucosinolates in disease and pest resistance. Plants 2021, 10, 1097. [Google Scholar] [CrossRef] [PubMed]
- Jeschke, V.; Gershenzon, J.; Vassão, D.G. Insect Detoxification of Glucosinolates and Their Hydrolysis Products. In Advances in Botanical Research, 1st ed.; Kopriva, S., Ed.; Academic Press: London, UK, 2016; Volume 80, pp. 199–245. ISBN 0065-2296. [Google Scholar]
- Farrar, R.R.; Shapiro, M.; Shepard, M. Relative activity of baculoviruses of the diamondback moth, Plutella xylostella (L.) (Lepidoptera: Plutellidae). BioControl 2007, 52, 657–667. [Google Scholar] [CrossRef]
- Kazana, E.; Pope, T.W.; Tibbles, L.; Bridges, M.; Pickett, J.A.; Bones, A.M.; Powell, G.; Rossiter, J.T. The cabbage aphid: A walking mustard oil bomb. Proc. R. Soc. B 2007, 274, 2271–2277. [Google Scholar] [CrossRef]
- Glazebrook, J. Contrasting mechanisms of defense against biotrophic and necrotrophic pathogens. Annu. Rev. Phytopathol. 2005, 43, 205–227. [Google Scholar] [CrossRef]
- Adie, B.A.T.; Pérez-Pérez, J.; Pérez-Pérez, M.M.; Godoy, M.; Sánchez-Serrano, J.; Schmelz, E.A.; Solano, R. ABA is an essential signal for plant resistance to pathogens affecting JA biosynthesis and the activation of defenses in Arabidopsis. Plant Cell 2007, 19, 1665–1681. [Google Scholar] [CrossRef]
- Wasternack, C. Jasmonates: An update on biosynthesis, signal transduction and action in plant stress response, growth and development. Ann. Bot. 2007, 100, 681–697. [Google Scholar] [CrossRef]
- Wasternack, C.; Hause, B. Jasmonates: Biosynthesis, perception, signal transduction and action in plant stress response, growth and development. An update to the 2007 review in Annals of Botany. Ann. Bot. 2013, 111, 1021–1058. [Google Scholar] [CrossRef]
- Wang, J.; Yang, M.; Song, Y.; Acevedo, F.E.; Hoover, K.; Zeng, R.; Felton, G.W. Gut-associated bacteria of Helicoverpa zea indirectly trigger plant defenses in Maize. J. Chem. Ecol. 2018, 44, 690–699. [Google Scholar] [CrossRef]
- Leroy, P.D.; Sabri, A.; Heuskin, S.; Thonart, P.; Lognay, G.; Verheggen, F.J.; Francis, F.; Brostaux, Y.; Felton, G.W.; Haubruge, E. Microorganisms from aphid honeydew attract and enhance the efficacy of natural enemies. Nat. Commun. 2011, 2, 348. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, R.; Atamian, H.S.; Shen, Z.; Brigg, S.P.; Kaloshian, I. GroEL from the endosymbiont Buchnera aphidicola betrays the aphid by triggering plant defense. Proc. Natl. Acad. Sci. USA 2014, 111, 8919–8924. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; He, Z.; Wang, E.; Xu, X.; Lei, Z. Application of Beauveria bassiana and Neoseiulus barkeri for improved control of Frankliniella occidentalis in greenhouse cucumber. Crop Prot. 2017, 96, 83–87. [Google Scholar] [CrossRef]
- Toledo, A.V.; de Remes Lenicov, A.M.M.; López Lastra, C.C. Pathogenicity of fungal isolates (Ascomycota: Hypocreales) against Peregrinus maidis, Delphacodes kuscheli (Hemiptera: Delphacidae), and Dalbulus maidis (Hemiptera: Cicadellidae), vectors of corn diseases. Mycopathologia 2007, 163, 225–232. [Google Scholar] [CrossRef]
- Zhang, F.; Sun, X.X.; Zhang, X.C.; Zhang, S.; Lu, J.; Xia, Y.M.; Huang, Y.H.; Wang, X.J. The interactions between gut microbiota and entomopathogenic fungi: A potential approach for biological control of Blattella germanica (L.). Pest. Manag. Sci. 2018, 74, 438–447. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qiao, Q.; Feng, H.; Jiao, L.; Zaheer, U.; Zheng, C.; Zhou, L.; Lin, G.; Xiang, X.; Liao, H.; Li, S.; et al. Bacteria Derived from Diamondback Moth, Plutella xylostella (L.) (Lepidoptera: Plutellidae), Gut Regurgitant Negatively Regulate Glucose Oxidase-Mediated Anti-Defense Against Host Plant. Insects 2024, 15, 1001. https://doi.org/10.3390/insects15121001
Qiao Q, Feng H, Jiao L, Zaheer U, Zheng C, Zhou L, Lin G, Xiang X, Liao H, Li S, et al. Bacteria Derived from Diamondback Moth, Plutella xylostella (L.) (Lepidoptera: Plutellidae), Gut Regurgitant Negatively Regulate Glucose Oxidase-Mediated Anti-Defense Against Host Plant. Insects. 2024; 15(12):1001. https://doi.org/10.3390/insects15121001
Chicago/Turabian StyleQiao, Qingxuan, Huiting Feng, Lu Jiao, Uroosa Zaheer, Chanqin Zheng, Li Zhou, Guifang Lin, Xiujuan Xiang, Huang Liao, Shanyu Li, and et al. 2024. "Bacteria Derived from Diamondback Moth, Plutella xylostella (L.) (Lepidoptera: Plutellidae), Gut Regurgitant Negatively Regulate Glucose Oxidase-Mediated Anti-Defense Against Host Plant" Insects 15, no. 12: 1001. https://doi.org/10.3390/insects15121001
APA StyleQiao, Q., Feng, H., Jiao, L., Zaheer, U., Zheng, C., Zhou, L., Lin, G., Xiang, X., Liao, H., Li, S., Lu, H., Yin, A., Salum, Y. M., Wei, H., Chen, W., He, W., & Yang, F. (2024). Bacteria Derived from Diamondback Moth, Plutella xylostella (L.) (Lepidoptera: Plutellidae), Gut Regurgitant Negatively Regulate Glucose Oxidase-Mediated Anti-Defense Against Host Plant. Insects, 15(12), 1001. https://doi.org/10.3390/insects15121001







