Predicted Global Redistribution of Lagria nigricollis (Coleoptera: Tenebrionidae) Under Future Climate Change
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
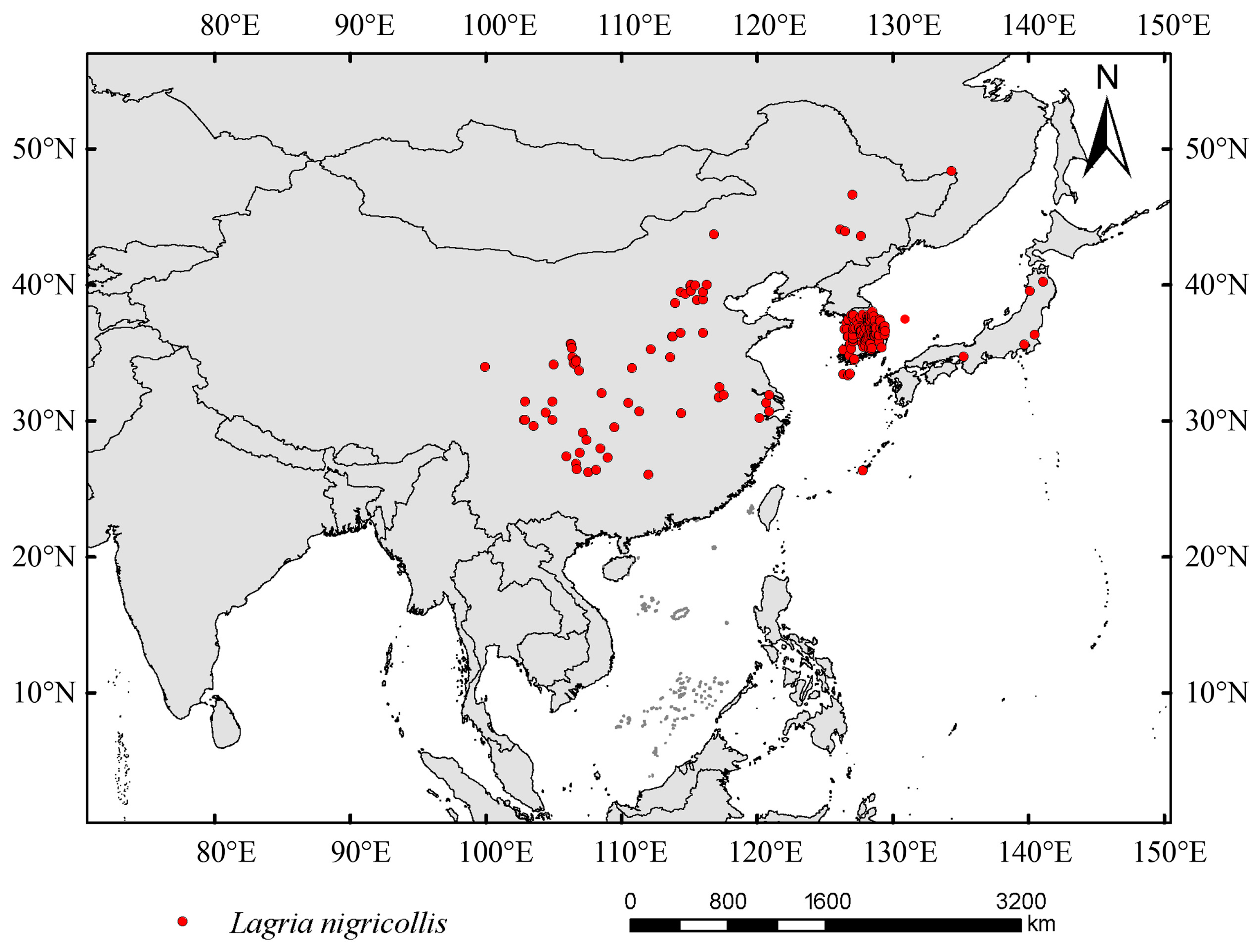
2.1. Obtaining and Processing Occurrence Data
2.2. Environmental Variables
2.3. Modeling Methods
3. Results
3.1. Evaluation of the MaxEnt Model and Dominant Environmental Variables
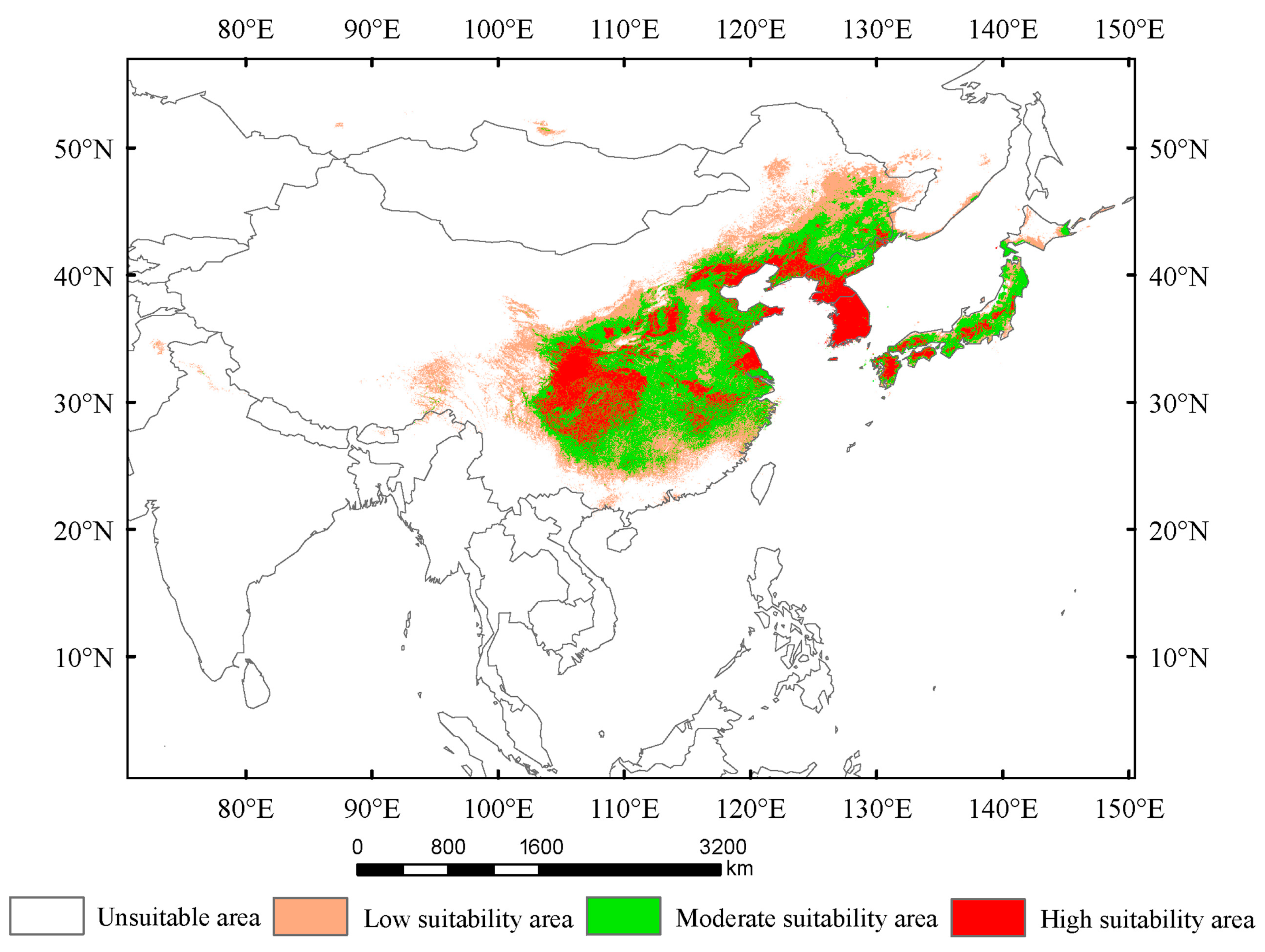
3.2. Suitability Area Under Current Condition
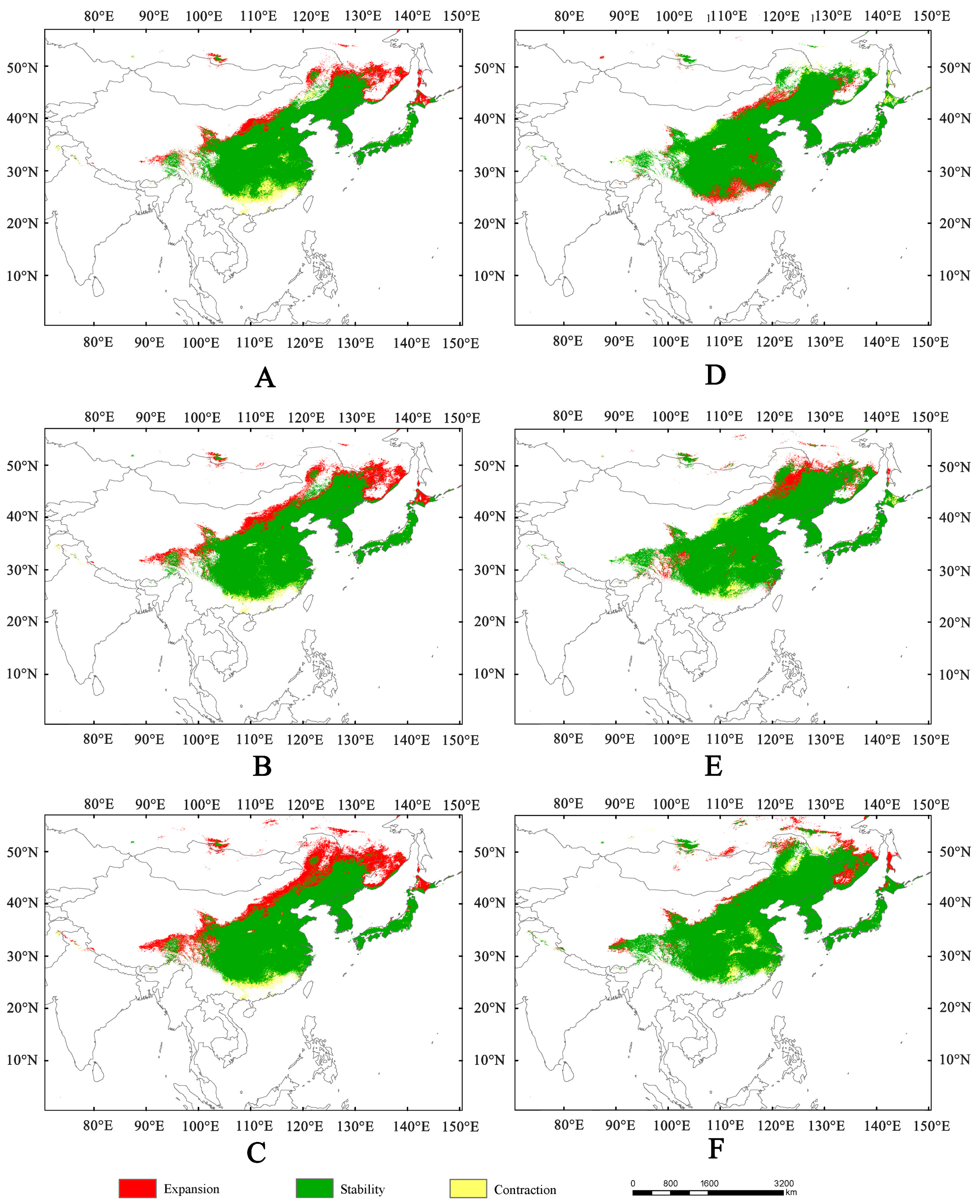
3.3. Suitable Area Under Future Climate Change
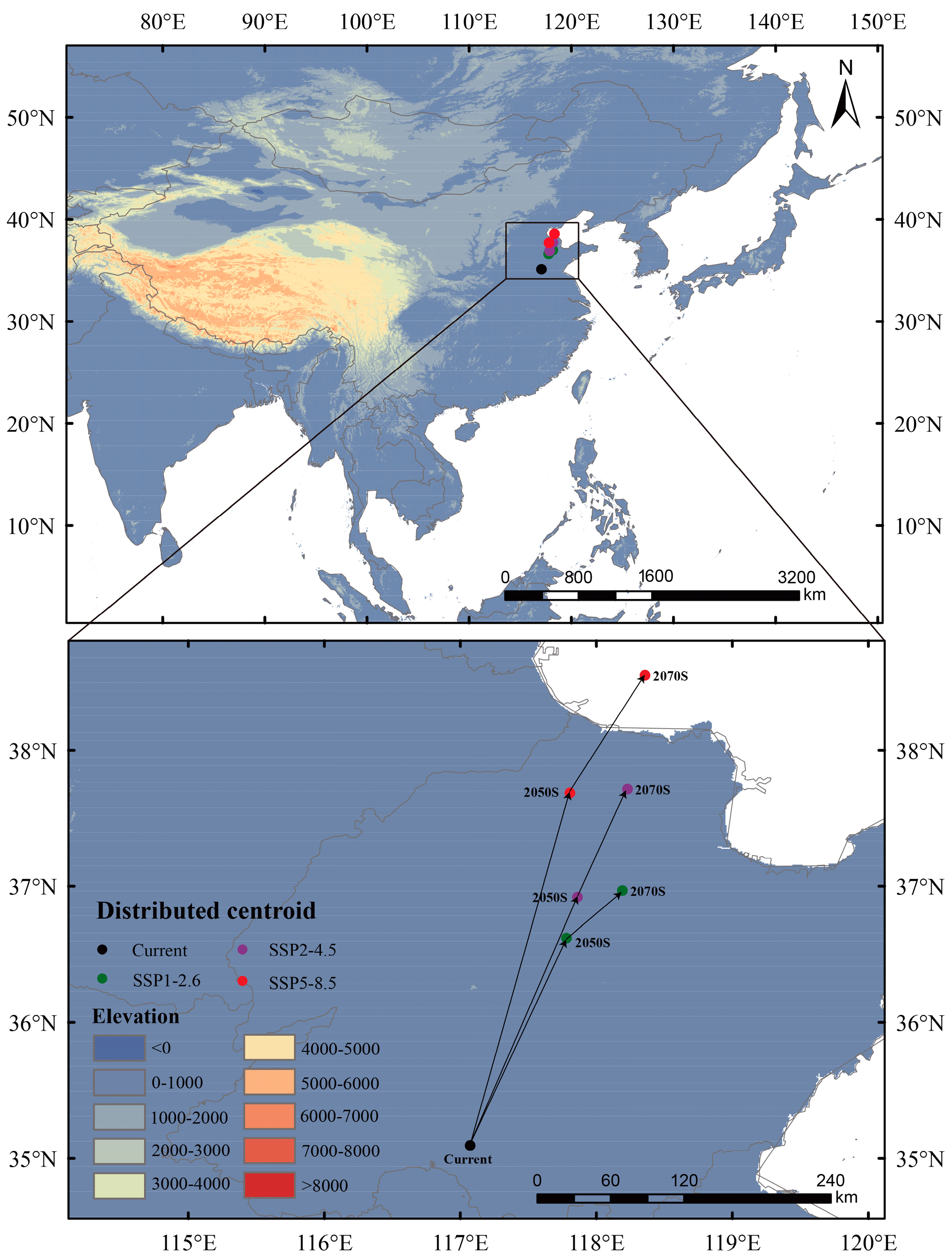
3.4. Shift in the Centroids
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| SDMs | species distribution models |
| L. nigricollis | Lagria nigricollis |
| MaxEnt | maximum entropy |
| ROC | receiver operating characteristic |
| AUC | area under the curve |
References
- Pecl, G.T.; Araújo, M.B.; Bell, J.D.; Blanchard, J.; Bonebrake, T.C.; Chen, I.C.; Clark, T.D.; Colwell, R.K.; Danielsen, F.; Evengård, B.; et al. Biodiversity redistribution under climate change: Impacts on ecosystems and human well-being. Science 2017, 355, eaai9214. [Google Scholar] [CrossRef]
- Urban, M.C. Accelerating extinction risk from climate change. Science 2015, 348, 571–573. [Google Scholar] [CrossRef]
- Storch, D.; Šímová, I.; Smyčka, J.; Bohdalková, E.; Toszogyova, A.; Okie, J.G. Biodiversity dynamics in the Anthropocene: How human activities change equilibria of species richness. Ecography 2022, 4, e05778. [Google Scholar] [CrossRef]
- Ren, G.D.; Li, X. Advances in classification of the storage tenebrionid beetles in the world. J. Hebei Univ. (Nat. Sci.) 2020, 40, 49–56. [Google Scholar]
- Gao, R.T.; Li, J.H.; Wang, Z.S.; Rao, G.D. Pest Investigation and control in Olea europaea. For. Sci. Technol. China 2018, 2018, 32–35. [Google Scholar] [CrossRef]
- Ge, X.Z.; He, S.Y.; Zhu, C.Y.; Wang, T.; Xu, Z.C.; Zong, S.X. Projecting the current and future potential global distribution of Hyphantria cunea (Lepidoptera: Arctiidae) using CLIMEX. Pest Manag. Sci. 2019, 75, 160–169. [Google Scholar] [CrossRef] [PubMed]
- Corsi, F.; Dupre, E.; Boitani, L. A large-scale model of wolf’ distribution in Italy for conservation planning. Conserv. Biol. 1999, 13, 150–159. [Google Scholar] [CrossRef]
- Peterson, A.T.; Soberon, J.; Sanchez-Cordero, V. Conservatism of ecological niches in evolutionary time. Science 1999, 285, 1265–1267. [Google Scholar] [CrossRef]
- Wang, S.Y.; Lu, Y.Y.; Han, M.Y.; Li, L.L.; He, P.; Shi, A.M.; Bai, M. Using MaxEnt model to predict the potential distribution of three potentially invasive scarab beetles in China. Insects 2023, 14, 239. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.L.; Li, Z.Q. Predicting the Potential Distribution of Cheirotonus jansoni (Coleoptera: Scarabaeidae) Under Climate Change. Insects 2024, 15, 1012. [Google Scholar] [CrossRef]
- Phillips, S.J.; Anderson, R.P.; Schapire, R.E. Maximum entropy modeling of species geographic distributions. Ecol. Model. 2006, 190, 231–259. [Google Scholar] [CrossRef]
- Mondanaro, A.; Di Febbraro, M.; Castiglione, S.; Melchionna, M.; Serio, C.; Girardi, G.; Belfiore, A.M.; Raia, P. ENphylo: A new method to model the distribution of extremely rare species. Methods Ecol. Evol. 2023, 14, 911–922. [Google Scholar] [CrossRef]
- Booth, T.H.; Nix, H.A.; Busby, J.R.; Hutchinson, M.F. BIOCLIM: The first species distribution modelling package, its early applications and relevance to most current MaxEnt studies. Divers. Distrib. 2014, 20, 1–9. [Google Scholar] [CrossRef]
- Sutherst, R.W.; Maywald, G.F. A climate-envelope model for assessing the distribution and abundance of an insect pest in a changing climate. Environ. Entomol. 1999, 28, 1045–1059. [Google Scholar]
- Townsend, P.A.; Papes, M.; Eaton, M. Transferability and model evaluation in ecological niche modeling; a comparison of GARP and MaxEnt. Ecography 2007, 30, 550–560. [Google Scholar] [CrossRef]
- Merow, C.; Smith, M.J.; Silander, A. A practical guide to Maxent for modeling species’ distributions: What it does, and why inputs and settings matter. Ecography 2013, 36, 1058–1069. [Google Scholar] [CrossRef]
- Phillips, S.J.; Anderson, R.P.; Dudík, M.; Schapire, R.E.; Blair, M.E. Opening the black box: An open-source release of MaxEnt. Ecography 2017, 40, 887–893. [Google Scholar] [CrossRef]
- Hasan, E.L.; Gorman, K.B.; Coletti, H.A.; Konar, B. Species distribution modeling of northern sea otters (Enhydra lutris kenyoni) in a data-limited ecosystem. Ecol. Evol. 2024, 14, e11118. [Google Scholar] [CrossRef]
- Sun, X.; Long, Z.X.; Jia, J.B. A multi-scale Maxent approach to model habitat suitability for the giant pandas in the Qionglai mountain, China. Glob. Ecol. Conserv. 2021, 30, e01766. [Google Scholar] [CrossRef]
- Wei, J.; Gao, G.; Wei, J.F. Potential global distribution of Daktulosphaira vitifoliae under climate change based on MaxEnt. Insects 2021, 12, 347. [Google Scholar] [CrossRef]
- He, Y.L.; Ma, J.M.; Chen, G.S. Potential geographical distribution and its multi-factor analysis of Pinus massoniana in China based on the MaxEnt model. Ecol. Indic. 2023, 154, 110790. [Google Scholar] [CrossRef]
- Wei, B.; Wang, R.L.; Hou, K.; Wang, X.Y.; Wu, W. Predicting the current and future cultivation regions of Carthamus tinctorius L. using MaxEnt model under climate change in China. Glob. Ecol. Conserv. 2018, 16, e00477. [Google Scholar] [CrossRef]
- Merkl, O. On taxonomy, nomenclature, and distribution of some Palaearctic Lagriini, with description of a new species from Taiwan (Coleoptera: Tenebrionidae). Acta Zool. Acad. Sci. Hung. 2004, 50, 283–305. [Google Scholar]
- Shi, A.M.; Pan, Y.S.; Li, Y.C. A redescription of Lagria nigricollis Hope, 1843 (Coleoptera: Tenebrionidae: Lagriini). Entomotaxonomia 2010, 32, 88–90. [Google Scholar]
- Zhou, Y.; Chen, B. A study on the genus Lagria from China with one new species and new distributional records (Coleoptera: Lagriinae). Zootaxa 2023, 5254, 413–424. [Google Scholar] [CrossRef]
- Wang, J.Q.; Wang, S.Y.; Li, Y.C.; Ding, S.M.; Wei, Z.H.; Shi, A.M.; Yang, D. Distribution pattern and change prediction of Luprops orientalis (Coleoptera: Tenebrionidae) suitable area in East Asia under climate change. Insects 2025, 16, 626. [Google Scholar] [CrossRef]
- GBIF Occurrence Download. Available online: https://doi.org/10.15468/dl.r7amzt (accessed on 10 June 2025).
- Koo, K.S.; Lee, K.-H.; Lee, D.; Jiang, Y. Impact of obscured data on species distribution models. Conserv. Biol. 2025, 39, e70050. [Google Scholar] [CrossRef]
- Wei, J.F.; Peng, L.F.; He, Z.Q.; Lu, Y.; Wang, F. Potential distribution of two invasive pineapple pests under climate change. Pest. Manag. Sci. 2020, 76, 1652–1663. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, T.; Tateishi, R.; Alsaaideh, B.; Sharma, R.C.; Wakaizumi, T.; Miyamoto, D.; Bai, X.; Long, B.D.; Gegentana, G.; Maitiniyazi, A. Production of global land cover data–GLCNMO2013. J. Geogr. Geol. 2017, 9, 1–15. [Google Scholar] [CrossRef]
- Robinson, N.; Regetz, J.; Guralnick, R.P. EarthEnv-DEM90: A nearly-global, void-free, multi-scale smoothed, 90m digital elevation model from fused ASTER and SRTM data. ISPRS J. Photogramm. Remote Sens. 2014, 87, 57–67. [Google Scholar] [CrossRef]
- Eyring, V.; Bony, S.; Meehl, G.A.; Senior, C.A.; Stevens, B.; Stouffer, R.J.; Taylor, K.E. Overview of the coupled model intercomparison project phase 6 (CMIP6) experimental design and organization. Geosci. Model Dev. 2016, 9, 1937–1958. [Google Scholar] [CrossRef]
- Riahi, K. The shared socioeconomic pathways and their energy, land use, and greenhouse gas emissions implications: An overview. Glob. Environ. Change 2017, 42, 153–168. [Google Scholar] [CrossRef]
- Worthington, T.A.; Zhang, T.; Logue, D.R.; Mittelstet, A.R.; Brewer, S.K. Landscape and flow metrics affecting the distribution of a federally-threatened fish: Improving management, model fit, and model transferability. Ecol. Model. 2016, 342, 1–18. [Google Scholar] [CrossRef]
- Muscarella, R.; Galante, P.J.; Soley-Guardia, M.; Boria, R.A.; Kass, J.M.; Uriarte, M.; Anderson, R.P. ENMeval: An R package for conducting spatially independent evaluations and estimating optimal model complexity for Maxent ecological niche models. Methods Ecol. Evol. 2014, 5, 1198–1205. [Google Scholar] [CrossRef]
- Yan, H.; Feng, L.; Zhao, Y.; Feng, L.; Di, W.; Zhu, C. Prediction of the Spatial Distribution of Alternanthera philoxeroides in China Based on ArcGIS and MaxEnt. Glob. Ecol. Conserv. 2020, 21, e00856. [Google Scholar] [CrossRef]
- Baek, S.; Kim, M.J.; Lee, J.H. Current and future distribution of Ricania shantungensis (Hemiptera: Ricaniidae) in Korea: Application of spatial analysis to select relevant environmental variables for MaxEnt and CLIMEX modeling. Forests 2019, 10, 490. [Google Scholar] [CrossRef]
- Tang, J.H.; Li, J.H.; Lu, H.; Lu, F.P.; Lu, B.Q. Potential distribution of an invasive pest, Euplatypus parallelus, in China as predicted by Maxent. Pest Manag. Sci. 2019, 75, 1630–1637. [Google Scholar] [CrossRef]
- Zhao, Z.X.; Yang, L.; Chen, X.S. Globally suitable areas for Lycorma delicatula based on an optimized MaxEnt model. Ecol. Evol. 2024, 14, e70252. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, H.; Jiamahate, A.; Yang, H.; Cao, L.; Dang, Y.; Lu, Z.; Yang, Z.; Bozorov, T.A.; Wang, X. Potential Ecological Distribution of the Beetle Agrilus mali Matsumura (Coleoptera: Buprestidae) in China under Three Climate Change Scenarios, with Consequences for Commercial and Wild Apple Forests. Biology 2024, 13, 803. [Google Scholar] [CrossRef]
- Karuppannasamy, A.; Azrag, A.G.A.; Vellingiri, G.; Kennedy, J.S.; Ganapati, P.S.; Subramanian, S.; Venkatasamy, B. Forecasting the future of Fall armyworm Spodoptera frugiperda (J. E. Smith) (Lepidoptera: Noctuidae) in India using ecological niche model. Int. J. Biometeorol. 2024, 68, 1871–1884. [Google Scholar] [CrossRef] [PubMed]
- Syfert, M.M.; Joppa, L.; Smith, M.J.; Coomes, D.A.; Bachman, S.P.; Brummitt, N.A. Using species distribution models to inform IUCN Red List assessments. Biol. Conserv. 2014, 177, 174–184. [Google Scholar] [CrossRef]
- Hu, S.J.; Xing, D.H.; Gong, Z.X.; Hu, J.M. Projecting suitability and climate vulnerability of Bhutanitis thaidina (Blanchard) (Lepidoptera: Papilionidae) with conservation implications. Sci. Rep. 2019, 9, 15384. [Google Scholar] [CrossRef]
- Kwon, T.-S.; Lee, C.M.; Kim, S.-S. Prediction of abundance of beetles according to climate warming in South Korea. J. Asia-Pac. Biodivers. 2015, 8, 7–30. [Google Scholar] [CrossRef]
- Fu, C.; Wen, X.Y.; Huang, T.T.; Wang, Y.L.; Liu, X.; Jiang, N.; Wang, R.L.; Zhao, J.P. Comparison of GARP and MaxEnt in modeling current and future geographic distribution of Ceracris nigricornis Walker (Acrididae, Orthoptera) in China. Ecol. Evol. 2024, 14, e70439. [Google Scholar] [CrossRef]
- Zhao, Z.X.; Feng, X.L.; Wang, Y.J.; Zhou, Z.X.; Zhang, Y.B. Potential suitability areas of Sitobion miscanthi in China based on the MaxEnt model: Implications for management. Crop Prot. 2024, 183, 106755. [Google Scholar] [CrossRef]
- Hong, J.; Lee, M.; Kim, Y.; Lee, Y.S.; Wee, J.; Park, J.J.; Lee, W.K.; Song, Y.; Cho, K. Potential range shift of a long-distance migratory rice pest, Nilaparvata lugens, under climate change. Sci. Rep. 2024, 14, 11531. [Google Scholar] [CrossRef]
- Aidoo, O.F.; Souza, P.G.C.; da Silva, R.S.; Santana, P.A.; Picanco, M.C.; Kyerematen, R.; Sètamou, M.; Elkesi, S.; Borgemeister, C. Climate-induced range shifts of invasive species (Diaphorina citri Kuwayama). Pest Manag. Sci. 2022, 78, 2534–2549. [Google Scholar] [CrossRef] [PubMed]
- Poloni, R.; Iannella, M.; Fusco, G.; Fattorini, S. Conservation biogeography of high-altitude longhorn beetles under climate change. Insect Conserv. Diver. 2022, 15, 429–444. [Google Scholar] [CrossRef]
- Zhao, Z.X.; Yang, L.; Long, J.K.; Chang, Z.M.; Chen, X.S. Predicting suitable areas for Metcalfa pruinosa (Hemiptera: Flatidae) under climate change and implications for management. J. Insect Sci. 2024, 24, 7. [Google Scholar] [CrossRef]
- Yang, M.S.; Wang, Y.; Ding, W.L.; Li, H.H.; Zhang, A.B. Predicting habitat suitability for the soybean pod borer Leguminivora glycinivorella (Matsumura) using optimized MaxEnt models with multiple variables. J. Econ. Entomol. 2024, 117, 1796–1808. [Google Scholar] [CrossRef] [PubMed]
- Ullah, F.; Zhang, Y.; Gul, H.; Hafeez, M.; Desneux, N.; Qin, Y.J. Potential economic impact of Bactrocera dorsalis on Chinese citrus based on simulated geographical distribution with MaxEnt and CLIMEX models. Entomol. Gen. 2023, 43, 821–830. [Google Scholar] [CrossRef]



| Variable | Description | Contribution (100%) | Permutation Importance (100%) |
|---|---|---|---|
| bio18 | Precipitation of the warmest quarter | 60.7 | 55.4 |
| bio04 | Temperature seasonality | 25.1 | 7.4 |
| bio15 | Precipitation seasonality | 5 | 1.4 |
| bio11 | Mean temperature of coldest quarter | 4.7 | 29.8 |
| bio19 | Precipitation of the coldest quarter | 1.5 | 1.2 |
| gm-lc | Land cover type | 1.4 | 0.4 |
| bio02 | Mean daily temperature range | 0.7 | 1.4 |
| bio08 | Mean temperature of the wettest quarter | 0.5 | 2.4 |
| elev | Elevation | 0.3 | 0.5 |
| gm-ve | Global land vegetation | 0.1 | 0.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, M.; Wang, J.; Liu, F.; Li, Y.; Fei, H.; Wei, Z.; Shi, A. Predicted Global Redistribution of Lagria nigricollis (Coleoptera: Tenebrionidae) Under Future Climate Change. Insects 2025, 16, 1227. https://doi.org/10.3390/insects16121227
Zhao M, Wang J, Liu F, Li Y, Fei H, Wei Z, Shi A. Predicted Global Redistribution of Lagria nigricollis (Coleoptera: Tenebrionidae) Under Future Climate Change. Insects. 2025; 16(12):1227. https://doi.org/10.3390/insects16121227
Chicago/Turabian StyleZhao, Manlu, Jieqiong Wang, Fen Liu, Yunchun Li, Hanlan Fei, Zhonghua Wei, and Aimin Shi. 2025. "Predicted Global Redistribution of Lagria nigricollis (Coleoptera: Tenebrionidae) Under Future Climate Change" Insects 16, no. 12: 1227. https://doi.org/10.3390/insects16121227
APA StyleZhao, M., Wang, J., Liu, F., Li, Y., Fei, H., Wei, Z., & Shi, A. (2025). Predicted Global Redistribution of Lagria nigricollis (Coleoptera: Tenebrionidae) Under Future Climate Change. Insects, 16(12), 1227. https://doi.org/10.3390/insects16121227







