Active Sampling Techniques for Two-Spot Cotton Leafhopper (Amrasca biguttula Ishida) (Hemiptera: Cicadellidae) in Fields with High and Low Populations
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Field Sites
2.2. Sampling Methods
2.3. Sample Processing and Analysis
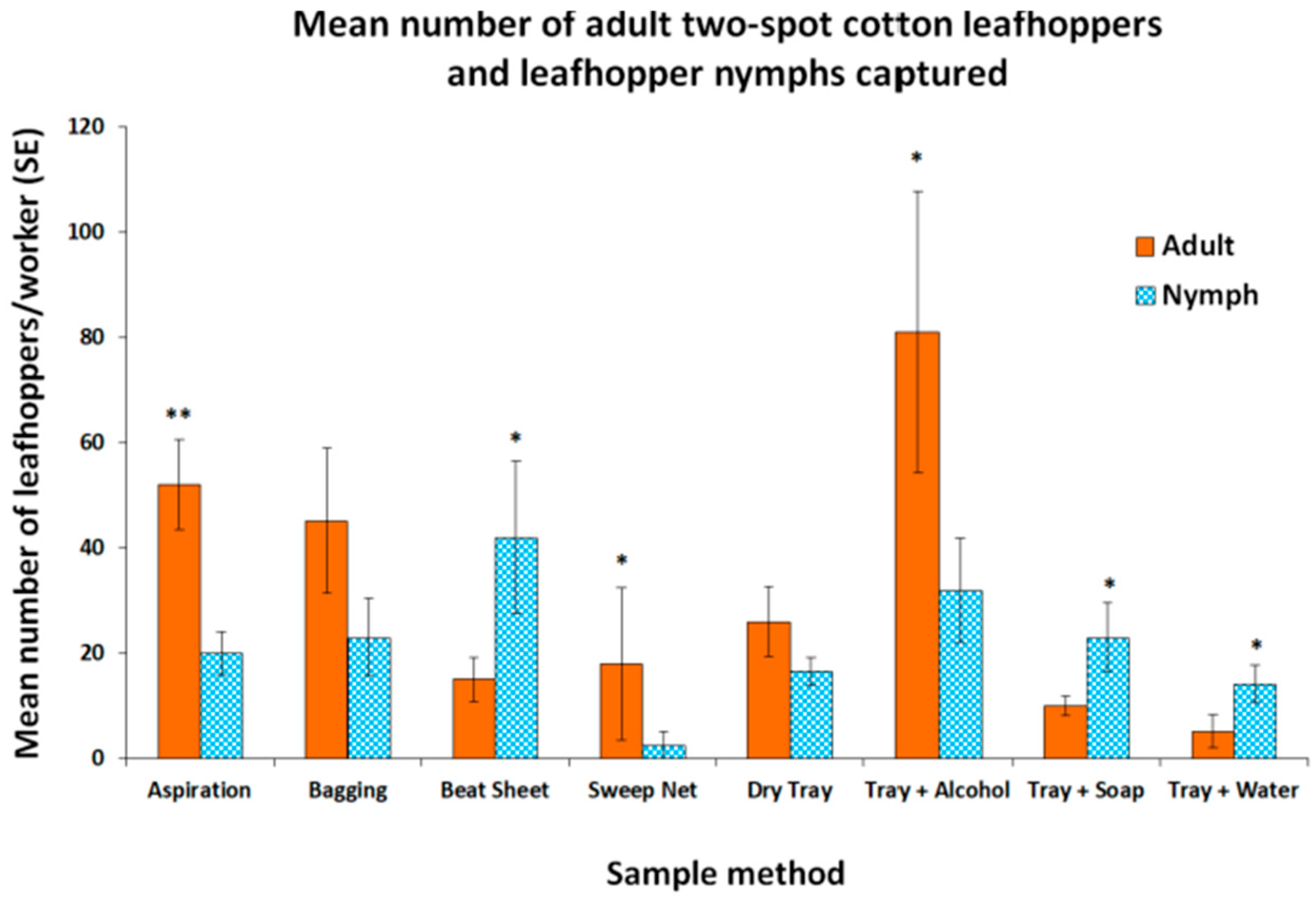
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A. Generalized Linear Mixed Model (GLMM) Using the Negative Binomial Distribution Analysis
References
- Brockerhoff, E.G.; Liebhold, A.M.; Richardson, B.; Suckling, D.M. Eradication of invasive forest insects: Concepts, methods, costs and benefits. N. Z. J. For. Sci. 2010, 40, S117–S135. [Google Scholar]
- Disney, R.H.; Erzinclioglu, Y.Z. Collecting methods and the adequacy of attempted fauna surveys, with reference to the Diptera. Field Stud. 1982, 5, 607–621. [Google Scholar]
- Grootaert, P.; Pollet, M.; Dekoninck, W.; van Achterberg, C. Sampling insects: General techniques, strategies and remarks. In Manual on Field Recording Techniques and Protocols for All Taxa Biodiversity Inventories and Monitoring; Eymann, J., Degreef, J., Hāuser, J., Monje, C., Samyn, Y., Vanden Spiegel, D., Eds.; Abc Taxa: Brussels, Belgium, 2010; pp. 337–399. [Google Scholar]
- Schwertner, C.F.; Carrenho, R.; Moreira, F.F.F.; Cassis, G. Hemiptera Sampling Methods. In Measuring Arthropod Biodiversity; Santos, J.C., Fernandes, G.W., Eds.; Springer: Cham, Switzerland, 2021. [Google Scholar] [CrossRef]
- Ferro, M.L.; Summerlin, M. Developing a standardized list of entomological collection methods for use in databases. ZooKeys 2019, 861, 145. [Google Scholar] [CrossRef] [PubMed]
- Prendergast, K.S.; Menz, M.H.; Dixon, K.W.; Bateman, P.W. The relative performance of sampling methods for native bees: An empirical test and review of the literature. Ecosphere 2020, 11, e03076. [Google Scholar] [CrossRef]
- Duan, M.C.; Qin, R.X.; Zhang, H.B.; Chen, B.X.; Jin, B.; Zhang, S.B.; Ren, S.; Ji, S.; Zhu, S.-H.; Yu, Z.; et al. Comprehensive comparison of different sampling methods for arthropod diversity in farmland. Biodivers. Sci. 2021, 29, 477–487. [Google Scholar] [CrossRef]
- Cabrera-Asencio, I.; Dietrich, C.H.; Zahniser, J.N. A new invasive pest in the Western Hemisphere: Amrasca biguttula (Hemiptera: Cicadellidae). Fla. Entomol. 2023, 106, 263–266. [Google Scholar] [CrossRef]
- Franç, Z.; Moussa, O.Z.; Atta, S.; Leyo, I.H.; Guimbo, I.D. Cotton leafhoppers, Amrasca biguttula (Ishida, 1913) (Hemiptera: Cicadellidae), identified as a new species on okra and guinea sorrel in Niger. Adv. Èntomol. 2024, 12, 183–194. [Google Scholar] [CrossRef]
- Liburd, O.E.; Halbert, S.E.; Samuel, N.; Dreves, A.J. Pest Alert. Two-Spot Cotton Leafhopper, Hemiptera: Cicadellidae, Typhlocybinae, Empoascini; Amrasca biguttula (Ishida)—A Serious Pest of Cotton, Okra, and Eggplant That Has Become Established in the Caribbean Basin; Florida Department of Agriculture and Consumer Services, Division of Plant Industry, Pest Alert 2024, FDACS-P-02229, pp. 1–5. Available online: https://ccmedia.fdacs.gov/content/download/117692/file/two-spot-cotton-leaf-hopper-pest-alert.pdf (accessed on 2 October 2025).
- Halbert, S.E.; Moore, M.R. Amrasca biguttula (Ishida), two-spot cotton leafhopper, a new Continental USA record. Tri-Ology 2025, 63, 9. Available online: https://ccmedia.fdacs.gov/content/download/118539/file/tri-ology-vol-63-no-4.pdf (accessed on 2 October 2025).
- Esquivel, I.L.; Bryant, T.; Malone, S.; Jacobson, A.L.; Graham, S.H.; Gimenez-Cremonez, P.S.; Roberts, P.; Paula-Moreas, S.; Reisig, D.; Huseth, A.; et al. First Report of Two-Spot Cotton Leafhopper (Amrasca biguttula (Ishida)) (Hemiptera: Cicadellidae) on Commercial Cotton in the Southeastern United States. Insects 2025, 16, 966. [Google Scholar] [CrossRef] [PubMed]
- Mejdalani, G.; Cavichioli, R.R. Notes on Neotropical Proconiini (Insecta: Hemiptera: Cicadellidae). VIII: Morphology of the male and female genitalia of Paraulacizes munda, revalidated from synonymy of P. confusa. Zoologia 2015, 32, 403–408. [Google Scholar] [CrossRef]
- PPQ. Cooperative Agricultural Pest Survey (CAPS) Pest Datasheet for Amrasca biguttula (Cicadellidae): Cotton Jassid; United States Department of Agriculture, Animal and Plant Health Inspection Service, Plant Protection and Quarantine (PPQ): Raleigh, NC, USA, 2025. Available online: https://caps.ceris.purdue.edu/wp-content/uploads/2025/07/Amrasca-biguttula-CAPS-Datasheet-for-PDF.pdf (accessed on 2 October 2025).
- Sagarbarria, M.G.S.; Taylo, L.D.; Hautea, D.M. Morphological and molecular analyses of Leafhopper, Amrasca biguttula (Ishida) (Hemiptera: Cicadellidae) infesting eggplant (Solanum melongena L.) in Luzon Island, Philippines. J. Asia-Pac. Entomol. 2020, 23, 260–268. [Google Scholar] [CrossRef]
- Smith, S.M.; Ellis, C.R. Economic importance of insects on regrowths of established alfalfa fields in Ontario. Can. Entomol. 1983, 115, 859–868. [Google Scholar] [CrossRef]
- Taylor, R.A.J.; Reling, D. Preferred wind direction of long-distance leafhopper (Empoasca fabae) migrants and its relevance to the return migration of small insects. J. Anim. Ecol. 1986, 55, 1103–1114. [Google Scholar] [CrossRef]
- Pavan, F.; Cargnus, E.; Zandigiacomo, P. Use of yellow sticky traps to study daily flight activity and behaviour of sap-sucking insects inhabiting European vineyards. Bull. Insectol. 2023, 76, 101–115. [Google Scholar]




| Sample Method | Adults + Nymphs Leafhoppers/Worker | Adult Leafhoppers/Worker | Male Leafhoppers/Worker |
|---|---|---|---|
| Bagging | 0.4 (0.24) | 0.2 (0.2) | 0 |
| Beat sheet | 0 | 0 | 0 |
| Netting | 0 | 0 | 0 |
| Dry tray | 0 | 0 | 0 |
| Tray + Alcohol | 1 (0.44) | 1 (0.55) | 0.8 (0.37) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zapsas, D.; Halbert, S.E.; Cong, M.Y.; Soto Adames, F.; KC, S.; Seal, D.; Roda, A.L. Active Sampling Techniques for Two-Spot Cotton Leafhopper (Amrasca biguttula Ishida) (Hemiptera: Cicadellidae) in Fields with High and Low Populations. Insects 2025, 16, 1226. https://doi.org/10.3390/insects16121226
Zapsas D, Halbert SE, Cong MY, Soto Adames F, KC S, Seal D, Roda AL. Active Sampling Techniques for Two-Spot Cotton Leafhopper (Amrasca biguttula Ishida) (Hemiptera: Cicadellidae) in Fields with High and Low Populations. Insects. 2025; 16(12):1226. https://doi.org/10.3390/insects16121226
Chicago/Turabian StyleZapsas, Daphne, Susan E. Halbert, Mary Yong Cong, Felipe Soto Adames, Sajan KC, Dakshina Seal, and Amy L. Roda. 2025. "Active Sampling Techniques for Two-Spot Cotton Leafhopper (Amrasca biguttula Ishida) (Hemiptera: Cicadellidae) in Fields with High and Low Populations" Insects 16, no. 12: 1226. https://doi.org/10.3390/insects16121226
APA StyleZapsas, D., Halbert, S. E., Cong, M. Y., Soto Adames, F., KC, S., Seal, D., & Roda, A. L. (2025). Active Sampling Techniques for Two-Spot Cotton Leafhopper (Amrasca biguttula Ishida) (Hemiptera: Cicadellidae) in Fields with High and Low Populations. Insects, 16(12), 1226. https://doi.org/10.3390/insects16121226







