Physiological and Putative Organic Cation Transporter Expression Response to Alizarin Dye Exposure in Aedes aegypti Mosquitoes
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Identification of Putative Organic Cation Transporters in Aedes aegypti
2.2. Aedes aegypti Colony
2.3. Mosquito Injections
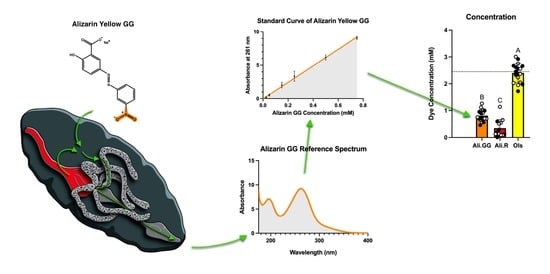
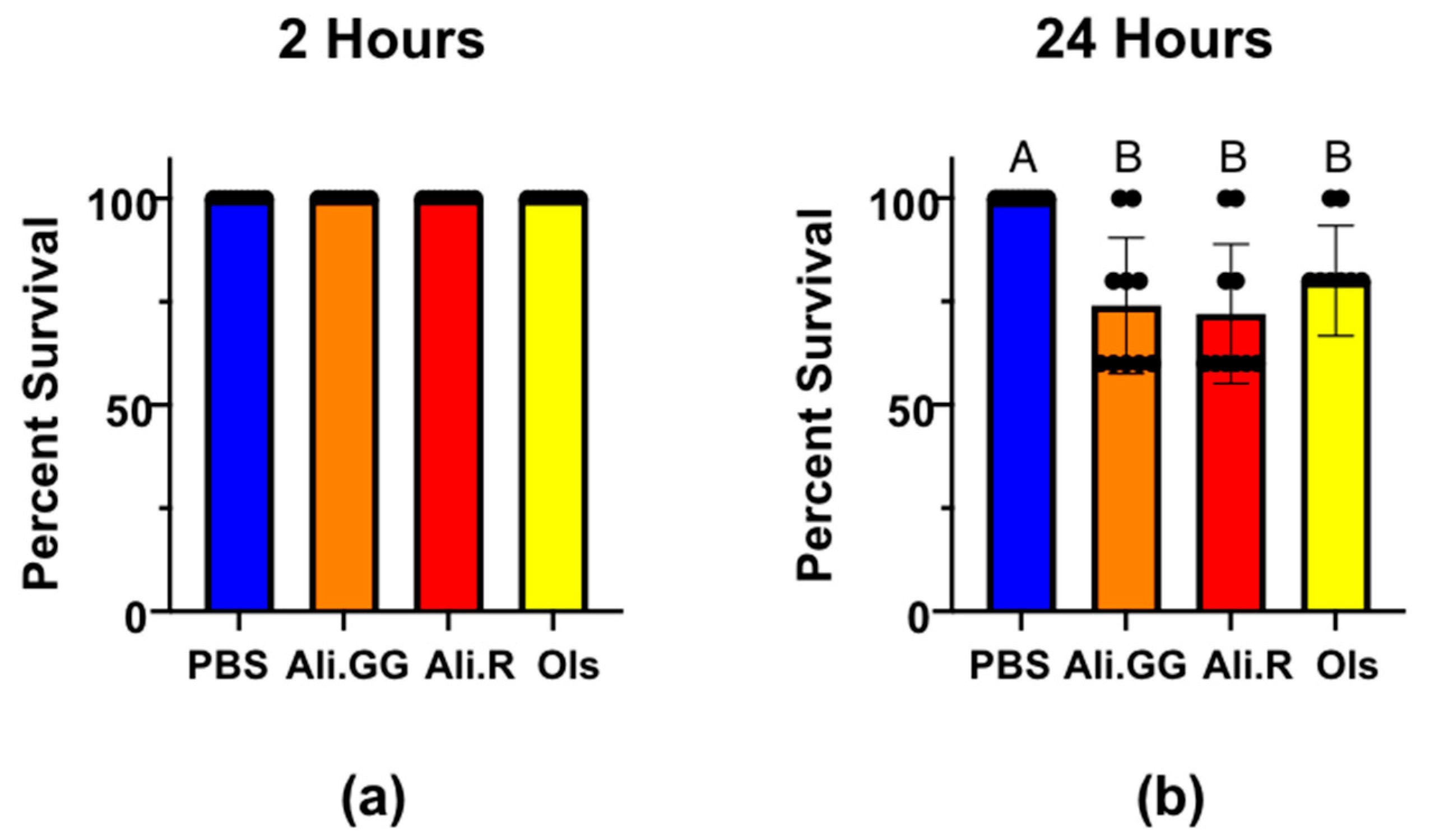
2.4. Dye Toxicity Assay and Urinalysis
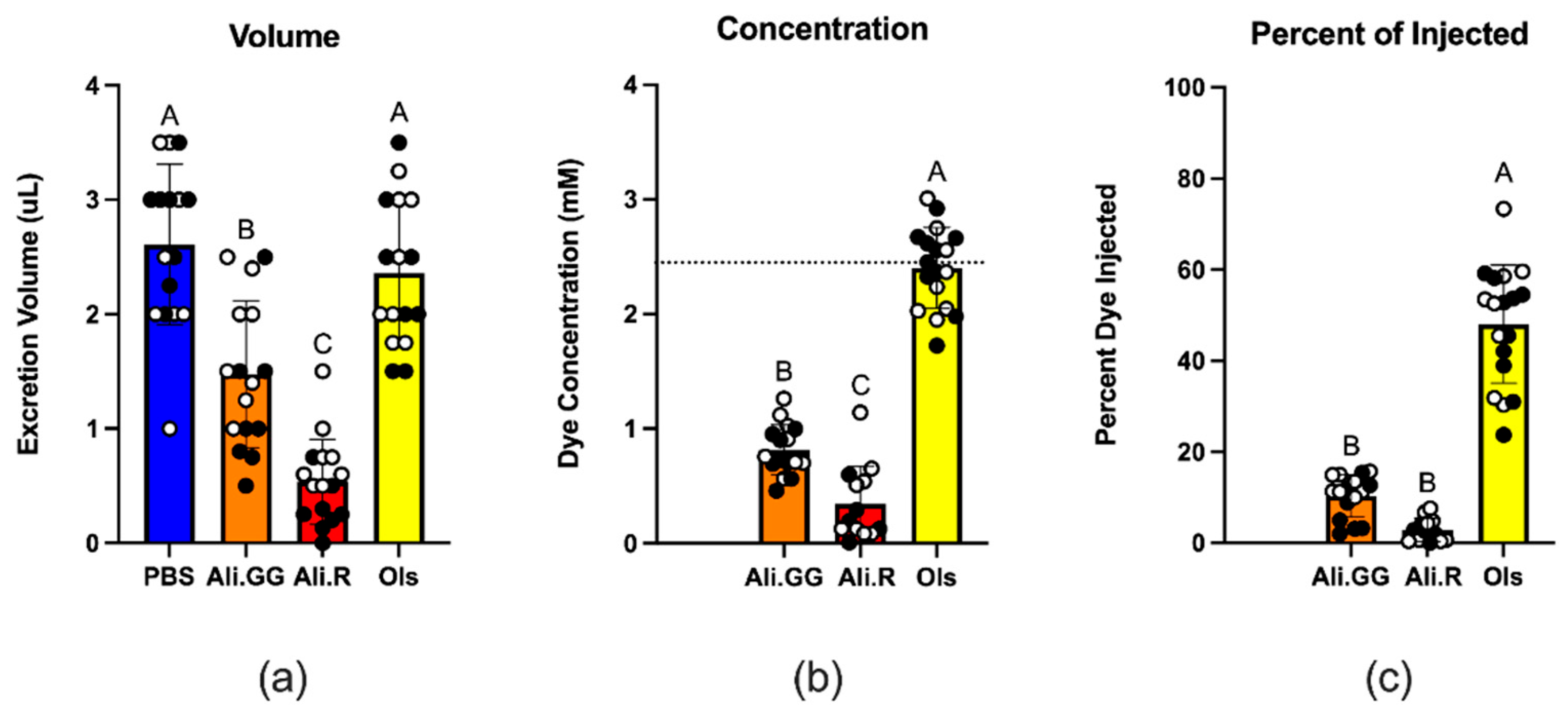
2.5. Measurement of Dye Clearance
2.6. RNA Extraction and cDNA Synthesis
2.7. Primer Design
2.8. Real-Time qPCR
3. Results
3.1. Identification of Putative Genes for Xenobiotic Transporters
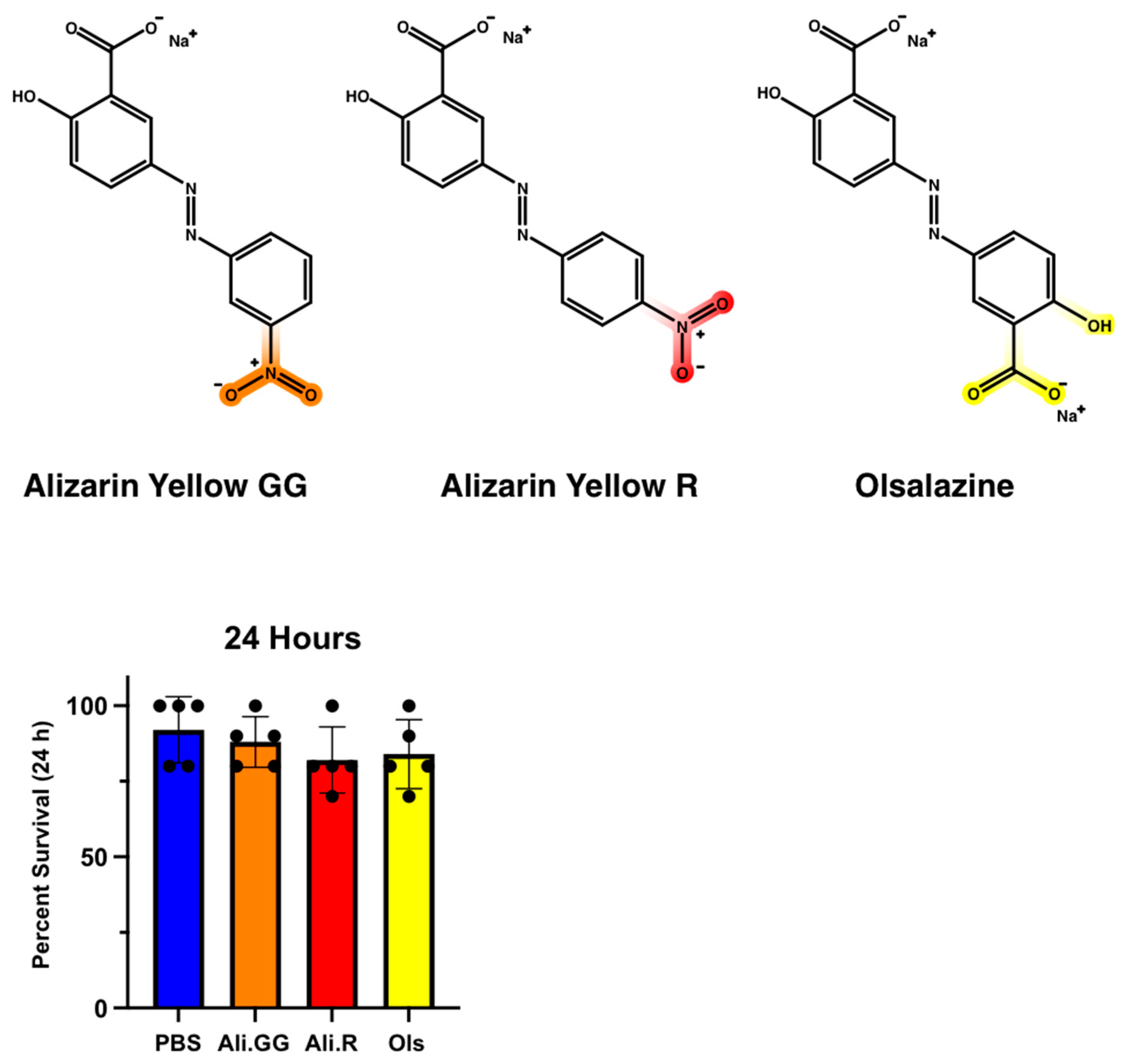
3.2. Establishing a Set of Xenobiotics for Gene Expression
3.3. Urinalysis
3.4. Gene Response to Dye/Volume—Challenge
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jones, R.T.; Ant, T.H.; Cameron, M.M.; Logan, J.G. Novel Control Strategies for Mosquito-Borne Diseases. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2021, 376, 20190802. [Google Scholar] [CrossRef] [PubMed]
- Smith, L.B.; Kasai, S.; Scott, J.G. Pyrethroid Resistance in Aedes aegypti and Aedes albopictus: Important Mosquito Vectors of Human Diseases. Pestic. Biochem. Physiol. 2016, 133, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Laporta, G.Z.; Potter, A.M.; Oliveira, J.F.A.; Bourke, B.P.; Pecor, D.B.; Linton, Y.-M. Global Distribution of Aedes aegypti and Aedes albopictus in a Climate Change Scenario of Regional Rivalry. Insects 2023, 14, 49. [Google Scholar] [CrossRef]
- Iwamura, T.; Guzman-Holst, A.; Murray, K.A. Accelerating Invasion Potential of Disease Vector Aedes aegypti under Climate Change. Nat. Commun. 2020, 11, 2130. [Google Scholar] [CrossRef]
- Sadasivaiah, S.; Tozan, Y.; Breman, J.G. Dichlorodiphenyltrichloroethane (DDT) Indoor Residual Spraying Africa: How Can Used Malaria Control? Defining Defeating Intolerable Burden Malaria III: Progress Perspectives: Supplement Volume. Am. J. Trop. Med. Hyg. 2007, 77. [Google Scholar] [CrossRef]
- Manikandan, S.; Mathivanan, A.; Bora, B.; Hemaladkshmi, P.; Abhisubesh, V.; Poopathi, S. A Review on Vector Borne Disease Transmission: Current Strategies of Mosquito Vector Control. Indian J. Entomol. 2022, 85, 503–513. [Google Scholar] [CrossRef]
- Mastrantonio, V.; Ferrari, M.; Negri, A.; Sturmo, T.; Favia, G.; Porretta, D.; Epis, S.; Urbanelli, S. Insecticide Exposure Triggers a Modulated Expression of ABC Transporter Genes in Larvae of Anopheles gambiae s.s. Insects 2019, 10, 66. [Google Scholar] [CrossRef]
- Lopez, S.B.G.; Guimarães-Ribeiro, V.; Rodriguez, J.V.G.; Dorand, F.A.P.S.; Salles, T.S.; Sá-Guimarães, T.E.; Alvarenga, E.S.L.; Melo, A.C.A.; Almeida, R.V.; Moreira, M.F. RNAi-Based Bioinsecticide for Aedes Mosquito Control. Sci. Rep. 2019, 9, 4038. [Google Scholar] [CrossRef]
- Sanders, H.R.; Evans, A.M.; Ross, L.S.; Gill, S.S. Blood Meal Induces Global Changes in Midgut Gene Expression in the Disease Vector, Aedes aegypti. Insect Biochem. Mol. Biol. 2003, 33, 1105–1122. [Google Scholar] [CrossRef]
- Alvarez-Jarreta, J.; Amos, B.; Aurrecoechea, C.; Bah, S.; Barba, M.; Barreto, A.; Basenko, E.Y.; Belnap, R.; Blevins, A.; Böhme, U.; et al. VEuPathDB: The Eukaryotic Pathogen, Vector and Host Bioinformatics Resource Center in 2023. Nucleic Acids Res. 2024, 52, D808–D816. [Google Scholar] [CrossRef]
- Madeira, F.; Madhusoodanan, N.; Lee, J.; Eusebi, A.; Niewielska, A.; Tivey, A.R.N.; Lopez, R.; Butcher, S. The EMBL-EBI Job Dispatcher Sequence Analysis Tools Framework in 2024. Nucleic Acids Res. 2024, 52, W521–W525. [Google Scholar] [CrossRef]
- Papadopoulos, J.S.; Agarwala, R. COBALT: Constraint-Based Alignment Tool for Multiple Protein Sequences. Bioinformatics 2007, 23, 1073–1079. [Google Scholar] [CrossRef] [PubMed]
- Al Ait, L.; Yamak, Z.; Morgenstern, B. DIALIGN at GOBICS--Multiple Sequence Alignment Using Various Sources of External Information. Nucleic Acids Res. 2013, 41, W3–W7. [Google Scholar] [CrossRef] [PubMed]
- Katoh, K.; Rozewicki, J.; Yamada, K.D. MAFFT Online Service: Multiple Sequence Alignment, Interactive Sequence Choice and Visualization. Brief. Bioinform. 2019, 20, 1160–1166. [Google Scholar] [CrossRef]
- Gabler, F.; Nam, S.-Z.; Till, S.; Mirdita, M.; Steinegger, M.; Söding, J.; Lupas, A.N.; Alva, V. Protein Sequence Analysis Using the MPI Bioinformatics Toolkit. Curr. Protoc. Bioinform. 2020, 72, e108. [Google Scholar] [CrossRef]
- Corpet, F. Multiple Sequence Alignment with Hierarchical Clustering. Nucleic Acids Res. 1988, 16, 10881–10890. [Google Scholar] [CrossRef]
- Dereeper, A.; Guignon, V.; Blanc, G.; Audic, S.; Buffet, S.; Chevenet, F.; Dufayard, J.-F.; Guindon, S.; Lefort, V.; Lescot, M.; et al. Phylogeny.fr: Robust Phylogenetic Analysis for the Non-Specialist. Nucleic Acids Res. 2008, 36, W465–W469. [Google Scholar] [CrossRef] [PubMed]
- Pei, J.; Grishin, N.V. PROMALS: Towards Accurate Multiple Sequence Alignments of Distantly Related Proteins. Bioinformatics 2007, 23, 802–808. [Google Scholar] [CrossRef]
- Notredame, C.; Higgins, D.G.; Heringa, J. T-Coffee: A Novel Method for Fast and Accurate Multiple Sequence Alignment. J. Mol. Biol. 2000, 302, 205–217. [Google Scholar] [CrossRef]
- Löytynoja, A.; Goldman, N. webPRANK: A Phylogeny-Aware Multiple Sequence Aligner with Interactive Alignment Browser. BMC Bioinform. 2010, 11, 579. [Google Scholar] [CrossRef]
- Dobson, L.; Reményi, I.; Tusnády, G.E. CCTOP: A Consensus Constrained TOPology Prediction Web Server. Nucleic Acids Res. 2015, 43, W408–W412. [Google Scholar] [CrossRef] [PubMed]
- Letunic, I.; Bork, P. Interactive Tree of Life (iTOL) v6: Recent Updates to the Phylogenetic Tree Display and Annotation Tool. Nucleic Acids Res. 2024, 52, W78–W82. [Google Scholar] [CrossRef]
- Dzaki, N.; Ramli, K.N.; Azlan, A.; Ishak, I.H.; Azzam, G. Evaluation of Reference Genes at Different Developmental Stages for Quantitative Real-Time PCR in Aedes aegypti. Sci. Rep. 2017, 7, srep43618. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- David, J.-P.; Ismail, H.M.; Chandor-Proust, A.; Paine, M.J.I. Role of Cytochrome P450s in Insecticide Resistance: Impact on the Control of Mosquito-Borne Diseases and Use of Insecticides on Earth. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2013, 368, 20120429. [Google Scholar] [CrossRef]
- Gao, L.; Qiao, H.; Wei, P.; Moussian, B.; Wang, Y. Xenobiotic Responses in Insects. Arch. Insect Biochem. Physiol. 2022, 109, e21869. [Google Scholar] [CrossRef] [PubMed]
- Cooper, A.N.; Malmgren, L.; Hawkes, F.M.; Farrell, I.W.; Hien, D.F.D.S.; Hopkins, R.J.; Lefèvre, T.; Stevenson, P.C. Identifying Mosquito Plant Hosts from Ingested Nectar Secondary Metabolites. Sci. Rep. 2025, 15, 6488. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Zheng, J.; Zhang, R.; Wang, H.; Du, J.; Li, J.; Zhou, D.; Sun, Y.; Shen, B. Identification and Functional Analysis of ABC Transporter Genes Related to Deltamethrin Resistance in Culex pipiens pallens. Pest Manag. Sci. 2023, 79, 3642–3655. [Google Scholar] [CrossRef]
- He, Q.; Yan, Z.; Si, F.; Zhou, Y.; Fu, W.; Chen, B. ATP-Binding Cassette (ABC) Transporter Genes Involved in Pyrethroid Resistance in the Malaria Vector Anopheles sinensis: Genome-Wide Identification, Characteristics, Phylogenetics, and Expression Profile. Int. J. Mol. Sci. 2019, 20, 1409. [Google Scholar] [CrossRef]
- Wu, C.; Chakrabarty, S.; Jin, M.; Liu, K.; Xiao, Y. Insect ATP-Binding Cassette (ABC) Transporters: Roles in Xenobiotic Detoxification and Bt Insecticidal Activity. Int. J. Mol. Sci. 2019, 20, 2829. [Google Scholar] [CrossRef]
- Chahine, S.; Seabrooke, S.; O’Donnell, M.J. Effects of Genetic Knock-down of Organic Anion Transporter Genes on Secretion of Fluorescent Organic Ions by Malpighian Tubules of Drosophila melanogaster: Genetic Knock-down of Fluorochrome Transporters. Arch. Insect Biochem. Physiol. 2012, 81, 228–240. [Google Scholar] [CrossRef]
- Chahine, S.; Campos, A.; O’Donnell, M.J. Genetic Knockdown of a Single Organic Anion Transporter Alters the Expression of Functionally Related Genes in Malpighian Tubules of Drosophila melanogaster. J. Exp. Biol. 2012, 215, 2601–2610. [Google Scholar] [CrossRef]
- Halberg, K.A.; Møbjerg, N. First Evidence of Epithelial Transport in Tardigrades: A Comparative Investigation of Organic Anion Transport. J. Exp. Biol. 2012, 215, 497–507. [Google Scholar] [CrossRef]
- Tansil, N.C.; Li, Y.; Koh, L.D.; Peng, T.C.; Win, K.Y.; Liu, X.Y.; Han, M.-Y. The Use of Molecular Fluorescent Markers to Monitor Absorption and Distribution of Xenobiotics in a Silkworm Model. Biomaterials 2011, 32, 9576–9583. [Google Scholar] [CrossRef]
- Rossi, M.; De Battisti, D.; Niven, J.E. Transepithelial Transport of P-Glycoprotein Substrate by the Malpighian Tubules of the Desert Locust. PLoS ONE 2019, 14, e0223569. [Google Scholar] [CrossRef]
- Livingston, D.B.H.; Patel, H.; Donini, A.; MacMillan, H.A. Active Transport of Brilliant Blue FCF across the Drosophila Midgut and Malpighian Tubule Epithelia. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2020, 239, 110588. [Google Scholar] [CrossRef]
- Rösner, J.; Tietmeyer, J.; Merzendorfer, H. Organic Anion-Transporting Polypeptides Are Involved in the Elimination of Insecticides from the Red Flour Beetle, Tribolium castaneum. J. Pest Sci. 2021, 94, 1427–1437. [Google Scholar] [CrossRef]
- Drake, L.L.; Boudko, D.Y.; Marinotti, O.; Carpenter, V.K.; Dawe, A.L.; Hansen, I.A. The Aquaporin Gene Family of the Yellow Fever Mosquito, Aedes aegypti. PLoS ONE 2010, 5, e15578. [Google Scholar] [CrossRef]
- Rouhier, M.F.; Raphemot, R.; Denton, J.S.; Piermarini, P.M. Pharmacological Validation of an Inward-Rectifier Potassium (Kir) Channel as an Insecticide Target in the Yellow Fever Mosquito Aedes aegypti. PLoS ONE 2014, 9, e100700. [Google Scholar] [CrossRef]
- Hine, R.M.; Rouhier, M.F.; Park, S.T.; Qi, Z.; Piermarini, P.M.; Beyenbach, K.W. The Excretion of NaCl and KCl Loads in Mosquitoes. 1. Control Data. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2014, 307, R837–R849. [Google Scholar] [CrossRef]
- Williams, J.C.; Hagedorn, H.; Beyenbach, K. Dynamic Changes in Flow Rate and Composition of Urine during the Post-Bloodmeal Diuresis in Aedes aegypti (L.). J. Comp. Physiol. 1983, 153, 257–265. [Google Scholar] [CrossRef]
- Sajadi, F.; Paluzzi, J.-P.V. Molecular Characterization, Localization, and Physiological Roles of ITP and ITP-L in the Mosquito, Aedes aegypti. Front. Insect Sci. 2024, 4, 1374325. [Google Scholar] [CrossRef]
- Piermarini, P.M. Renal Excretory Processes in Mosquitoes. In Advances in Insect Physiology; Raikhel, A.S., Ed.; Advances in insect physiology; Elsevier: Amsterdam, The Netherlands, 2016; Volume 51, pp. 393–433. ISBN 9780128024577. [Google Scholar]
- Townson, H. The Biology of Mosquitoes. Volume 1. Development, Nutrition and Reproduction. By A.N. Clements. (London: Chapman & Hall, 1992). Viii + 509 pp. Hard Cover £50. ISBN 0-412-40180-0. Bull. Entomol. Res. 1993, 83, 307–308. [Google Scholar] [CrossRef]
- Drake, L.L.; Rodriguez, S.D.; Hansen, I.A. Functional Characterization of Aquaporins and Aquaglyceroporins of the Yellow Fever Mosquito, Aedes aegypti. Sci. Rep. 2015, 5, 7795. [Google Scholar] [CrossRef]
- Pinch, M.; Mitra, S.; Rodriguez, S.D.; Li, Y.; Kandel, Y.; Dungan, B.; Holguin, F.O.; Attardo, G.M.; Hansen, I.A. Fat and Happy: Profiling Mosquito Fat Body Lipid Storage and Composition Post-Blood Meal. Front. Insect Sci. 2021, 1, 693168. [Google Scholar] [CrossRef] [PubMed]
- Dissanayake, S.N.; Ribeiro, J.M.C.; Wang, M.-H.; Dunn, W.A.; Yan, G.; James, A.A.; Marinotti, O. aeGEPUCI: A Database of Gene Expression in the Dengue Vector Mosquito, Aedes aegypti. BMC Res. Notes 2010, 3, 248. [Google Scholar] [CrossRef]
- Hixson, B.; Bing, X.-L.; Yang, X.; Bonfini, A.; Nagy, P.; Buchon, N. A Transcriptomic Atlas of Aedes aegypti Reveals Detailed Functional Organization of Major Body Parts and Gut Regional Specializations in Sugar-Fed and Blood-Fed Adult Females. eLife 2022, 11, e76132. [Google Scholar] [CrossRef] [PubMed]
- Tsujimoto, H.; Adelman, Z.N. An 11-Point Time Course Midgut Transcriptome across 72 H after Bloodfeeding Provides Detailed Temporal Resolution of Transcript Expression in the Arbovirus vector, Aedes aegypti. Genome Res. 2023, 33, 1638–1648. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kennel, N.R.; Rouhier, M.F. Physiological and Putative Organic Cation Transporter Expression Response to Alizarin Dye Exposure in Aedes aegypti Mosquitoes. Insects 2025, 16, 1196. https://doi.org/10.3390/insects16121196
Kennel NR, Rouhier MF. Physiological and Putative Organic Cation Transporter Expression Response to Alizarin Dye Exposure in Aedes aegypti Mosquitoes. Insects. 2025; 16(12):1196. https://doi.org/10.3390/insects16121196
Chicago/Turabian StyleKennel, Naomi R., and Matthew F. Rouhier. 2025. "Physiological and Putative Organic Cation Transporter Expression Response to Alizarin Dye Exposure in Aedes aegypti Mosquitoes" Insects 16, no. 12: 1196. https://doi.org/10.3390/insects16121196
APA StyleKennel, N. R., & Rouhier, M. F. (2025). Physiological and Putative Organic Cation Transporter Expression Response to Alizarin Dye Exposure in Aedes aegypti Mosquitoes. Insects, 16(12), 1196. https://doi.org/10.3390/insects16121196