Effects of Trehalose-6-Phosphate Synthase on the Reproduction and Development of Nilaparvata lugens and Its Molecular Mechanism
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Source and Breeding of N. lugens
2.2. Total RNA Isolation and cDNA Synthesis
2.3. Synthesis and Purification of dsRNA
2.4. Microinjection and Post-Injection Rearing of N. lugens
2.5. Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
2.6. Integrated Ovarian Transcriptomic and Metabolomic Profiling
2.7. Detection of the Fecundity of N. lugens
2.8. Determination of Triglyceride Content
2.9. Data Analysis
3. Results
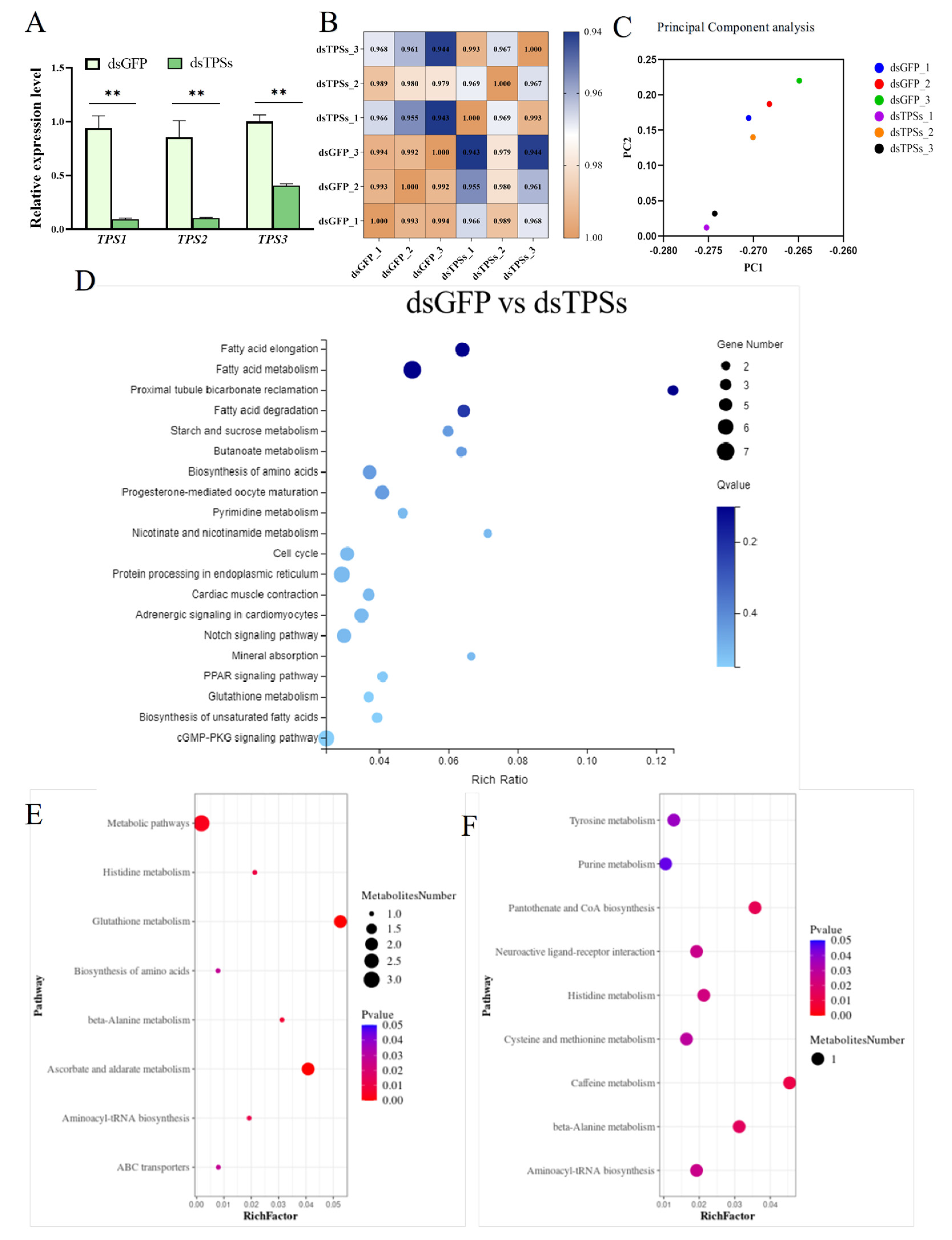
3.1. RNAi Efficacy and Transcriptomic-Metabolomic Analysis
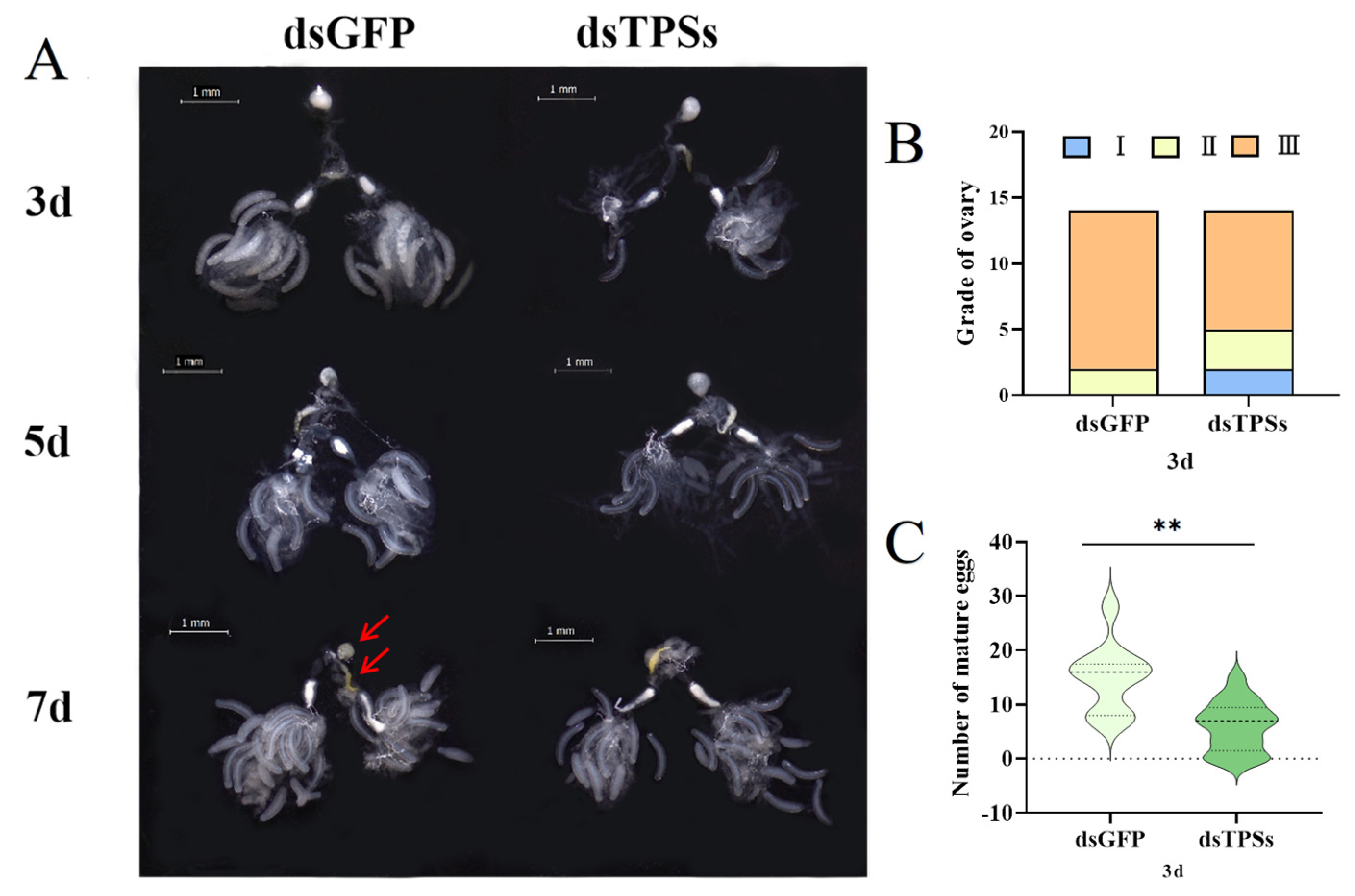
3.2. Impact of Trehalose-6-Phosphate Synthase Gene on Ovarian Development in Female N. lugens
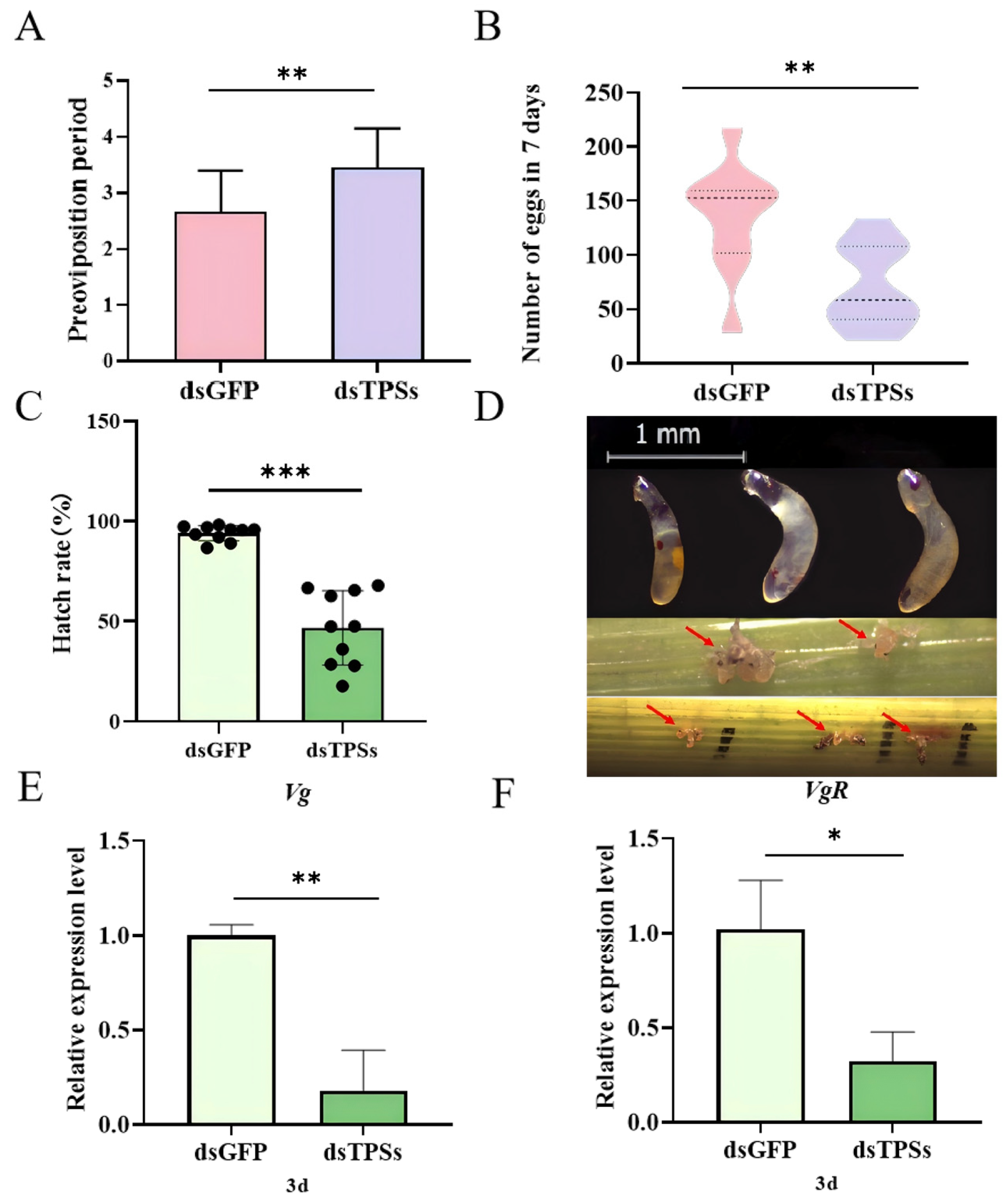
3.3. Impact of Trehalose-6-Phosphate Synthase Gene Silencing on Egg Production and Hatch Rate in Female N. lugens
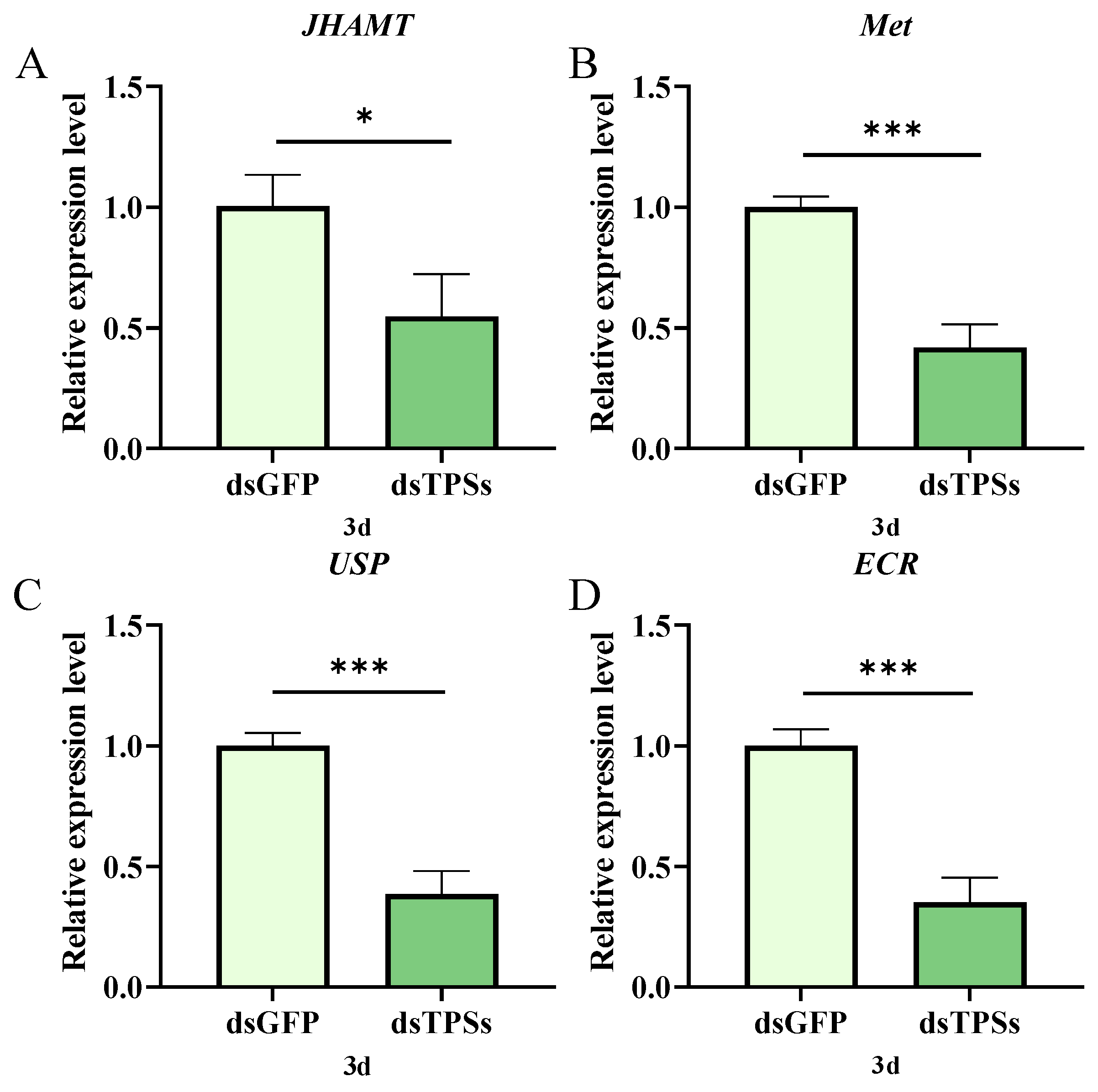
3.4. Impact of Trehalose-6-Phosphate Synthase Gene on JH and 20E Signaling Pathways
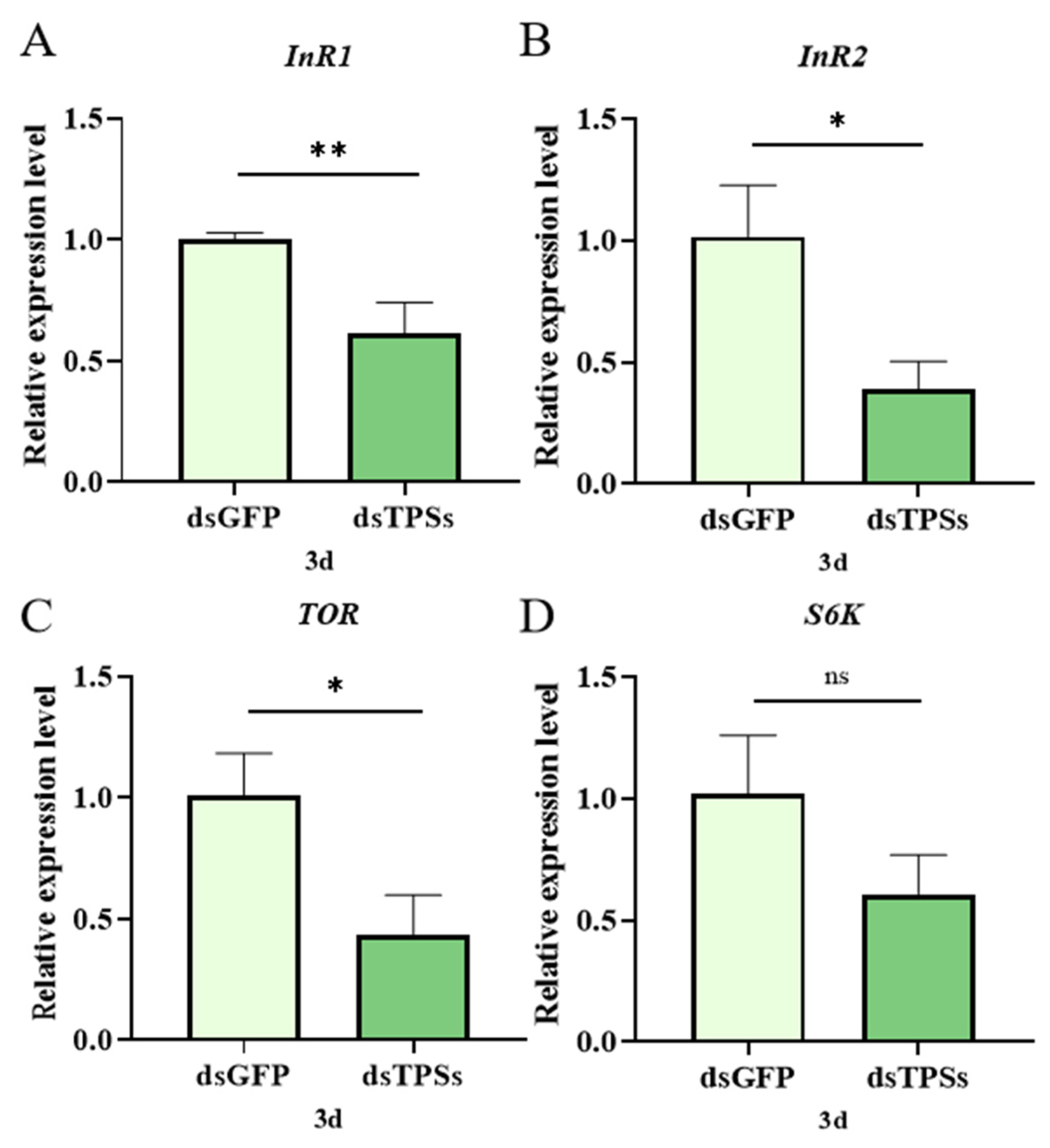
3.5. Impact of Trehalose-6-Phosphate Synthase Gene on Nutrient Signaling Pathways
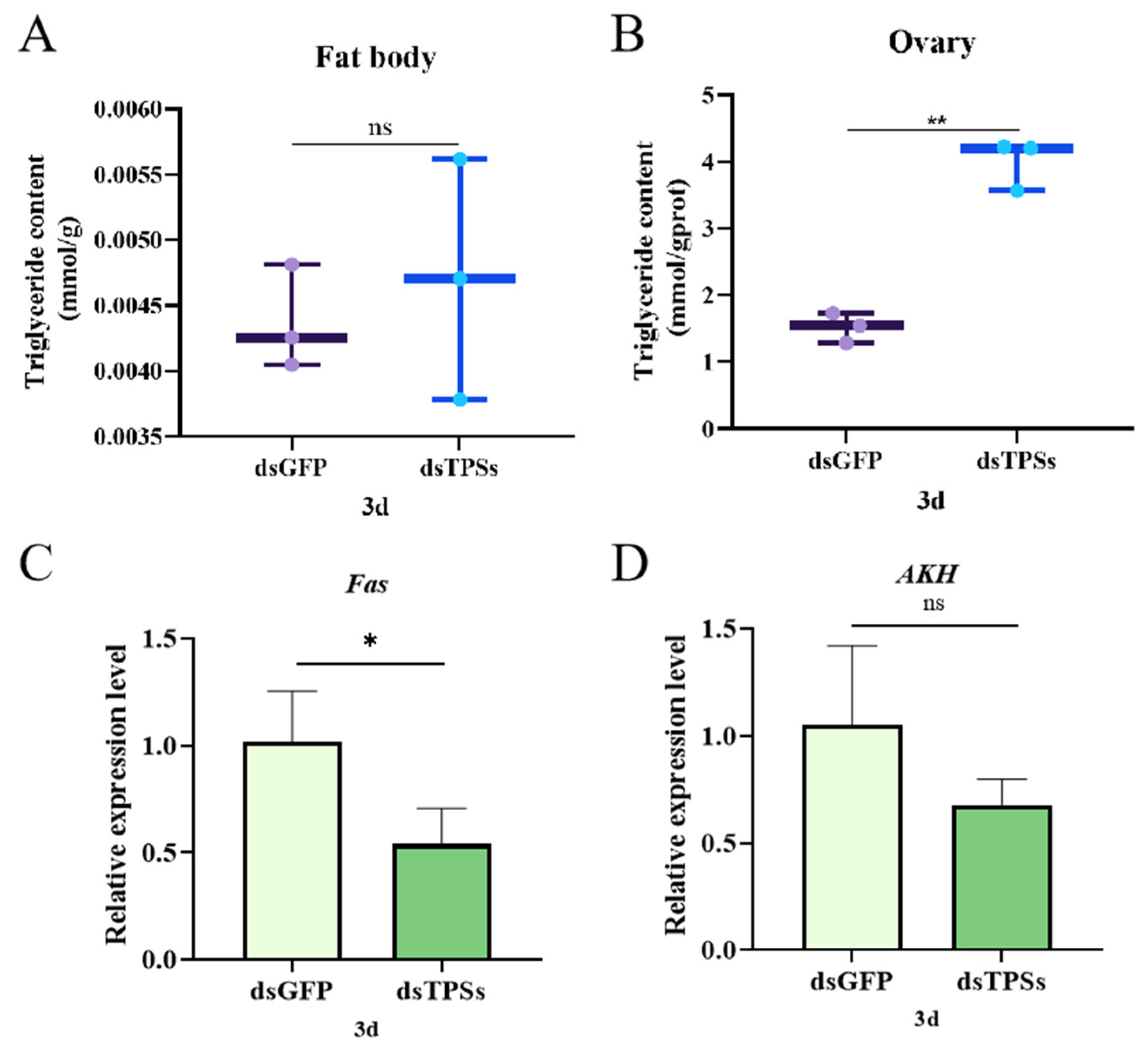
3.6. Impact of Trehalose-6-Phosphate Synthase Gene on Lipid Metabolism
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| TPS | Trehalose-6-phosphate synthase |
| TRE | Trehalose-6-phosphate phosphatase |
| AKH | Adipokinetic hormone |
| JH | Juvenile hormone signaling |
| 20E | 20-hydroxyecdysone pathways |
| IIS | Insulin/IGF signaling |
| TOR | Target of rapamycin cascade |
References
- Haliru, B.S.; Rafii, M.Y.; Mazlan, N.; Ramlee, S.I.; Muhammad, I.; Akos, I.S.; Halidu, J.; Swaray, S.; Bashir, Y.R. Recent strategies for detection and improvement of brown planthopper resistance genes in rice: A Review. Plants 2020, 9, 1202. [Google Scholar] [CrossRef]
- Wang, S.L.; Cheng, R.L.; Lu, J.B.; Yu, X.P.; Zhang, C.X. A Cripavirus in the brown planthopper, Nilaparvata lugens. J. Gen. Virol. 2016, 97, 706–714. [Google Scholar] [CrossRef] [PubMed]
- Ye, Y.; Xiong, S.; Guan, X.; Tang, T.; Zhu, Z.; Zhu, X.; Hu, J.; Wu, J.; Zhang, S. Insight into rice resistance to the brown planthopper: Gene cloning, functional analysis, and breeding applications. Int. J. Mol. Sci. 2024, 25, 13397. [Google Scholar] [CrossRef]
- Anand, R.; Divya, D.; Mazumdar-Leighton, S.; Bentur, J.S.; Nair, S. Expression analysis reveals differentially expressed genes in BPH and WBPH associated with resistance in rice RILs derived from a Cross between RP2068 and TN1. Int. J. Mol. Sci. 2023, 24, 13982. [Google Scholar] [CrossRef]
- Guo, J.P.; Xu, C.X.; Wu, D.; Zhao, Y.; Qiu, Y.; Wang, X.; Ouyang, Y.; Cai, B.; Liu, X.; Jing, S.; et al. Bph6 encodes an exocyst-localized protein and confers broad resistance to planthoppers in rice. Nat. Genet. 2018, 50, 297–306. [Google Scholar] [CrossRef]
- Xu, H.X.; He, X.C.; Zheng, X.S.; Yang, Y.J.; Lu, Z.X. Influence of rice black streaked dwarf virus on the ecological fitness of non-vector planthopper Nilaparvata lugens (Hemiptera: Delphacidae). Insect Sci. 2014, 21, 507–514. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.J.; Lu, J.B.; Li, Q.; Bao, Y.Y.; Zhang, C.X. Combined transcriptomic/proteomic analysis of salivary gland and secreted saliva in three planthopper species. J. Proteom. 2018, 172, 25–35. [Google Scholar] [CrossRef]
- Peñalver-Cruz, A.; Horgan, F.G. Interactions between rice resistance to planthoppers and honeydew-related egg parasitism under varying levels of nitrogenous fertilizer. Insects 2022, 13, 251. [Google Scholar] [CrossRef] [PubMed]
- Qian, N.; Zheng, P.; Wang, Y.; Pan, S.; Li, Y.; Zhang, C.; Chen, J.; Teng, J. Characterization of actin and tubulin promoters from two sap-sucking pests, Nilaparvata lugens (Stål) and Nephotettix cincticeps (Uhler). Biochem. Biophys. Res. Commun. 2016, 470, 831–837. [Google Scholar] [CrossRef]
- Garrood, W.T.; Zimmer, C.T.; Gorman, K.J.; Nauen, R.; Bass, C.; Davies, T.G. Field-evolved resistance to imidacloprid and ethiprole in populations of brown planthopper Nilaparvata lugens collected from across South and East Asia. Pest. Manag. Sci. 2016, 72, 140–149. [Google Scholar] [CrossRef]
- Zhu, K.Y.; Palli, S.R. Mechanisms, Applications, and challenges of insect RNA interference. Annu. Rev. Entomol. 2020, 65, 293–311. [Google Scholar] [CrossRef]
- Kunte, N.; McGraw, E.; Bell, S.; Held, D.; Avila, L.A. Prospects, challenges and current status of RNAi through insect feeding. Pest. Manag. Sci. 2020, 76, 26–41. [Google Scholar] [CrossRef] [PubMed]
- Joga, M.R.; Zotti, M.J.; Smagghe, G.; Christiaens, O. RNAi efficiency, systemic properties, and novel delivery methods for pest insect control: What we know so far. Front. Physiol. 2016, 7, 553. [Google Scholar] [CrossRef] [PubMed]
- Yu, N.; Christiaens, O.; Liu, J.S.; Niu, J.Z.; Cappelle, K.; Caccia, S.; Huvenne, H.; Smagghe, G. Delivery of dsRNA for RNAi in insects: An overview and future directions. Insect Sci. 2013, 20, 4–14. [Google Scholar] [CrossRef]
- Wang, G.; Gou, Y.; Guo, S.; Zhou, J.J.; Liu, C. RNA interference of trehalose-6-phosphate synthase and trehalase genes regulates chitin metabolism in two color morphs of Acyrthosiphon pisum Harris. Sci. Rep. 2021, 11, 948. [Google Scholar] [CrossRef]
- Zhao, D.; Liu, X.; Liu, Z.; Han, W.; Lu, X.; Guo, W. Identification and functional analysis of two potential RNAi targets for chitin degradation in Holotrichia parallela Motschulsky (Insecta Coleoptera). Pestic. Biochem. Physiol. 2022, 188, 105257. [Google Scholar] [CrossRef] [PubMed]
- Guo, P.P.; Zhao, X.Y.; Smagghe, G.; Yang, X.B.; Yang, H.; Zeng, Q.H.; Jia, Z.Y.; Jiang, Z.C. RNAi of ago1 and ago2 disrupts molting in the white-backed planthopper (Sogatella furcifera). Arch. Insect Biochem. Physiol. 2025, 119, e70069. [Google Scholar]
- Tang, B.; Wang, S.; Wang, S.G.; Wang, H.J.; Zhang, J.Y.; Cui, S.Y. Invertebrate trehalose-6-phosphate synthase gene: Genetic architecture, biochemistry, physiological function, and potential applications. Front. Physiol. 2018, 9, 30. [Google Scholar] [CrossRef]
- Rebholz, Z.; Shewade, L.; Kaler, K.; Larose, H.; Schubot, F.; Tholl, D.; Morozov, A.V.; O’Maille, P.E. Emergence of terpene chemical communication in insects: Evolutionary recruitment of isoprenoid metabolism. Protein Sci. 2023, 32, e4634. [Google Scholar] [CrossRef]
- Lü, X.; Han, S.C.; Li, Z.G.; Li, L.Y.; Li, J. Gene characterization and enzymatic activities related to trehalose metabolism of in vitro reared Trichogramma dendrolimi Matsumura (Hymenoptera: Trichogrammatidae) under sustained cold stress. Insects 2020, 11, 767. [Google Scholar] [CrossRef]
- Wang, G.; Zhou, J.J.; Li, Y.; Gou, Y.; Quandahor, P.; Liu, C. Trehalose and glucose levels regulate feeding behavior of the phloem-feeding insect, the pea aphid Acyrthosiphon pisum Harris. Sci. Rep. 2021, 11, 15864. [Google Scholar] [CrossRef]
- Ekta, S.; Thorat, L.J.; Nath, B.B.; Gaikwad, S.M. Insect trehalase: Physiological significance and potential applications. Glycobiology 2014, 25, 357–367. [Google Scholar] [CrossRef]
- Elbein, A.D. New insights on trehalose: A multifunctional molecule. Glycobiology 2003, 13, 17R–27R. [Google Scholar] [CrossRef] [PubMed]
- Jin, M.; North, H.L.; Peng, Y.; Liu, H.; Liu, B.; Pan, R.; Zhou, Y.; Zheng, W.; Liu, K.; Yang, B.; et al. Adaptive evolution to the natural and anthropogenic environment in a global invasive crop pest, the cotton bollworm. Innovation 2023, 4, 100454. [Google Scholar] [CrossRef]
- Tellis, M.B.; Chaudhari, B.Y.; Deshpande, S.V.; Nikam, S.V.; Barvkar, V.T.; Kotkar, H.M.; Joshi, R.S. Trehalose transporter-like gene diversity and dynamics enhances stress response and recovery in Helicoverpa armigera. Gene 2023, 862, 147259. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Hong, Y. Regulation of hemolymph trehalose level by an insulin-like peptide through diel feeding rhythm of the beet armyworm, Spodoptera exigua. Peptides 2015, 68, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhu, H.D.; Wang, Y.X.; Guo, Z.X.; Liu, Y.X.; Huang, Z.H.; Zhu, L.B.; Liu, M.H.; Liu, S.H.; Xu, J.P. Trehalose hydrolysis and transport-related genes promote Bombyx mori nucleopolyhedrovirus proliferation through the phosphoinositide 3-kinase-Akt signalling pathway in BmN cell. Dev. Comp. Immunol. 2023, 140, 104625. [Google Scholar] [CrossRef]
- Chen, J.X.; Lyu, Z.H.; Wang, C.Y.; Cheng, J.; Lin, T. RNA interference of a trehalose-6-phosphate synthase gene reveals its roles in the biosynthesis of chitin and lipids in Heortia vitessoides (Lepidoptera: Crambidae). Insect Sci. 2020, 27, 212–223. [Google Scholar] [CrossRef]
- Chen, Q.W.; Jin, S.; Zhang, L.; Shen, Q.D.; Wei, P.; Wei, Z.M.; Wang, S.G.; Tang, B. Regulatory functions of trehalose-6-phosphate synthase in the chitin biosynthesis pathway in Tribolium castaneum (Coleoptera: Tenebrionidae) revealed by RNA interference. Bull. Entomol. Res. 2018, 108, 388–399. [Google Scholar] [CrossRef]
- Wang, S.S.; Li, G.Y.; Liu, Y.K.; Luo, Y.J.; Xu, C.D.; Li, C.; Tang, B. Regulation of Carbohydrate Metabolism by Trehalose-6-Phosphate Synthase 3 in the Brown Planthopper, Nilaparvata lugens. Front. Physiol. 2020, 11, 575485. [Google Scholar] [CrossRef]
- Liu, Y.K.; Zhu, Y.; Wan, S.J.; Wang, X.Z.; Guan, L.W.; Xu, C.D.; Xie, B.H.; Wang, S.G.; Sun, S.S.; Tang, B. Trehalase regulates ovarian maturation and egg hatchability of Nilaparvata lugens. J. Pest. Sci. 2025, 98, 1645–1654. [Google Scholar] [CrossRef]
- Li, H.; Zaihui, Z.; Hongxia, H.; Weihua, M. Comparative transcriptome analysis of defense response of rice to Nilaparvata lugens and Chilo suppressalis infestation. Int. J. Biol. Macromol. 2020, 163, 2270–2285. [Google Scholar] [CrossRef] [PubMed]
- Shang, F.; Niu, J.Z.; Ding, B.Y.; Zhang, Q.; Ye, C.; Zhang, W.; Smagghe, G.; Wang, J.J. Vitellogenin and its receptor play essential roles in the development and reproduction of the brown citrus aphid, Aphis (Toxoptera) citricidus. Insect Mol. Biol. 2018, 27, 221–233. [Google Scholar] [CrossRef]
- Lu, K.; Shu, Y.; Zhou, J.; Zhang, X.; Zhang, X.; Chen, M.; Yao, Q.; Zhou, Q.; Zhang, Q. Molecular characterization and RNA interference analysis of vitellogenin receptor from Nilaparvata lugens (Stål). J. Insect Physiol. 2015, 73, 20–29. [Google Scholar] [CrossRef]
- Kamruzzaman, A.S.M.; Mikani, A.; Mohamed, A.A.; Elgendy, A.M.; Takeda, M. Crosstalk among indoleamines, neuropeptides and JH/20E in regulation of reproduction in the American cockroach, Periplaneta americana. Insects 2020, 11, 155. [Google Scholar] [CrossRef]
- Song, J.; Zhou, S. Post-transcriptional regulation of insect metamorphosis and oogenesis. Cell Mol. Life Sci. 2020, 77, 1893–1909. [Google Scholar] [CrossRef] [PubMed]
- Tufail, M.; Nagaba, Y.; Elgendy, A.M.; Takeda, M. Regulation of vitellogenin genes in insects. Entomol. Sci. 2014, 17, 269–282. [Google Scholar] [CrossRef]
- Yang, M.M.; Zhao, L.N.; Shen, Q.D.; Xie, G.Q.; Wang, S.G.; Tang, B. Knockdown of two trehalose-6-phosphate synthases severely affects chitin metabolism gene expression in the brown planthopper Nilaparvata lugens. Pest. Manag. Sci. 2017, 73, 206–216. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Yang, M.; Shen, Q.; Liu, X.; Shi, Z.; Wang, S.; Tang, B. Functional characterization of three trehalase genes regulating the chitin metabolism pathway in rice brown planthopper using RNA interference. Sci. Rep. 2016, 6, 27841. [Google Scholar] [CrossRef]
- Tang, B.; Yang, M.M.; Shen, Q.D.; Xu, Y.X.; Wang, H.J.; Wang, S.G. Suppressing the activity of trehalase with validamycin disrupts the trehalose and chitin biosynthesis pathways in the rice brown planthopper, Nilaparvata lugens. Pestic. Biochem. Physiol. 2017, 137, 81–90. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Anders, S.; Huber, W. Differential expression analysis for sequence count data. Genome Biol. 2010, 11, 10. [Google Scholar] [CrossRef]
- Lu, F.; Qi, J.G.; Qin, R.R. The processes of morphological change and grading criteria for ovarian development in the brown planthopper. Chin. Bull. Entomol. 2011, 48, 1394–1400. [Google Scholar]
- Yang, X.; Liu, S.; Lu, W.; Du, M.; Qiao, Z.; Liang, Z.; An, Y.; Gao, J.; Li, X. Delta and jagged are candidate target genes of RNAi biopesticides for the control of Nilaparvata lugens. Front. Bioeng. Biotechnol. 2022, 10, 1023729. [Google Scholar] [CrossRef] [PubMed]
- Roy, D.; Biswas, A.; Sarkar, S.; Chakraborty, G.; Gaber, A.; Kobeasy, M.I.; Hossain, A. Evaluation and characterization of indigenous rice (Oryza sativa L.) landraces resistant to brown planthopper Nilaparvata lugens (Stål.) biotype 4. PeerJ 2022, 10, e14360. [Google Scholar] [CrossRef]
- Kang, K.; Cai, Y.; Yue, L.; Zhang, W. Effects of Different Nutritional Conditions on the Growth and Reproduction of Nilaparvata lugens (Stål). Front. Physiol. 2022, 12, 794721. [Google Scholar] [CrossRef] [PubMed]
- Diaz-Albiter, H.; Mitford, R.; Genta, F.A.; Sant’Anna, M.R.; Dillon, R.J. Reactive oxygen species scavenging by catalase is important for female Lutzomyia longipalpis fecundity and mortality. PLoS ONE 2011, 9, e17486. [Google Scholar]
- Couto, N.; Wood, J.; Barber, J. The role of glutathione reductase and related enzymes on cellular redox homoeostasis network. Free Rad. Biol. Med. 2016, 95, 27–42. [Google Scholar] [CrossRef]
- Bashandy, T.; Guilleminot, J.; Vernoux, T.; Caparros-Ruiz, D.; Ljung, K.; Meyer, Y.; Reichheld, J. Interplay between the NADP-linked thioredoxin and glutathione systems in Arabidopsis auxin signaling. Plant Cell 2010, 22, 376–391. [Google Scholar] [CrossRef]
- Xie, M.; Zhong, Y.; Lin, L.; Zhang, G.; Su, W.; Ni, W.; Qu, M.; Chen, H. Transcriptome analysis of Holotrichia oblita reveals differentially expressed unigenes related to reproduction and development under different photoperiods. Comp. Biochem. Physiol. Part D Genom. Proteom. 2022, 42, 100959. [Google Scholar] [CrossRef]
- Abdelfattah, E.A.; Augustyniak, M.; Yousef, H.A. Stage-, sex- and tissue-related changes in H2O2, glutathione concentration, and glutathione-dependent enzymes activity in Aiolopus thalassinus (Orthoptera: Acrididae) from heavy metal polluted areas. Ecotoxicology 2021, 30, 478–491. [Google Scholar] [CrossRef]
- Zhao, L.; Xue, H.; Elumalai, P.; Zhu, X.; Wang, L.; Zhang, K.; Li, D.; Ji, J.; Luo, J.; Cui, J.; et al. Sublethal acetamiprid affects reproduction, development and disrupts gene expression in Binodoxys communis. Environ. Sci. Pollut. Res. Int. 2024. [Google Scholar] [CrossRef]
- Saranya, M.; Kennedy, J.S.; Anandham, R. Functional characterization of cultivable gut bacterial communities associated with rugose spiralling whitefly, Aleurodicus rugioperculatus Martin. 3 Biotech 2022, 12, 14. [Google Scholar] [CrossRef]
- Enya, S.; Yamamoto, C.; Mizuno, H.; Esaki, T.; Lin, H.K.; Iga, M.; Morohashi, K.; Hirano, Y.; Kataoka, H.; Masujima, T.; et al. Dual Roles of glutathione in ecdysone biosynthesis and antioxidant function during larval development in Drosophila. Genetics 2017, 207, 1519–1532. [Google Scholar] [CrossRef]
- Luck, M.R.; Jeyaseelan, I.; Scholes, R.A. Ascorbic acid and fertility. Biol. Reprod. 1995, 52, 262–266. [Google Scholar] [CrossRef] [PubMed]
- Ishii, N.; Homma, T.; Lee, J.; Mitsuhashi, H.; Yamada, K.I.; Kimura, N.; Yamamoto, Y.; Fujii, A.J. Ascorbic acid and CoQ10 ameliorate the reproductive ability of Superoxide dismutase 1-deficient female mice. Biol. Reprod. 2020, 102, 102–115. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.S.; Dutta, R.K.; Muralidhar, K.; Gupta, R.D. Decreased ascorbic acid biosynthesis in response to PMSG in the pre-pubertal female rat ovary. Res. Vet. Sci. 2020, 131, 15–20. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Shen, L.W.; Xing, X.R.; Xie, Y.Q.; Li, Y.J.; Liu, Z.X.; Wang, J.; Wu, F.A.; Sheng, S. Lipid dynamics, identification, and expression patterns of fatty acid synthase genes in an endoparasitoid, Meteorus pulchricornis (Hymenoptera: Braconidae). Int. J. Mol. Sci. 2020, 21, 6228. [Google Scholar] [CrossRef]
- Ma, L.; Zhang, W.; Liu, C.; Chen, L.; Xu, Y.; Xiao, H.; Liang, G. Methoprene-Tolerant (Met) Is Indispensable for larval metamorphosis and female reproduction in the cotton bollworm Helicoverpa armigera. Front. Physiol. 2018, 9, 1601. [Google Scholar] [CrossRef] [PubMed]
- Gondim, K.C.; Atella, G.C.; Pontes, E.G.; Majerowicz, D. Lipid metabolism in insect disease vectors. Insect Biochem. Mol. Biol. 2018, 101, 108–123. [Google Scholar] [CrossRef]
- Arêdes, D.S.; Rios, T.; Carvalho-Kelly, L.F.; Braz, V.; Araripe, L.O.; Bruno, R.V.; Meyer-Fernandes, J.R.; Ramos, I.; Gondim, K.C. Deficiency of Brummer lipase disturbs lipid mobilization and locomotion, and impairs reproduction due to defects in the eggshell ultrastructure in the insect vector Rhodnius prolixus. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2024, 1869, 159442. [Google Scholar] [CrossRef]
- Arrese, E.L.; Soulages, J.L. Insect fat body: Energy, metabolism, and regulation. Annu. Rev. Entomol. 2010, 55, 207–225. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Fan, H.; Li, Y.; Zhang, T.F.; Liu, Y.H. Trehalose-6-phosphate phosphatases are involved in trehalose synthesis and metamorphosis in Bactrocera minax. Insect Sci. 2022, 29, 1643–1658. [Google Scholar] [CrossRef]
- Magalhães, R.S.S.; De Lima, K.C.; De Almeida, D.S.G.; De Mesquita, J.F.; Eleutherio, E.C.A. Trehalose-6-phosphate as a potential lead candidate for the development of TPS1 inhibitors: Insights from the trehalose biosynthesis pathway in diverse yeast species. Appl. Biochem. Biotechnol. 2017, 181, 914–924. [Google Scholar] [CrossRef]
- Yang, H.J.; Cui, M.Y.; Zhao, X.H.; Zhang, C.Y.; Hu, Y.S.; Fan, D. Trehalose-6-phosphate synthase regulates chitin synthesis in Mythimna separata. Front. Physiol. 2023, 14, 1109661. [Google Scholar] [CrossRef]
- Santos, R.; Alves-Bezerra, M.; Rosas-Oliveira, R.; Majerowicz, D.; Meyer-Fernandes, J.R.; Gondim, K.C. Gene identification and enzymatic properties of a membrane-bound trehalase from the ovary of Rhodnius prolixus. Arch. Insect Biochem. Physiol. 2012, 81, 199–213. [Google Scholar] [CrossRef]
- Tellis, M.B.; Mohite, S.D.; Nair, V.S.; Chaudhari, B.Y.; Ahmed, S.; Kotkar, H.M.; Joshi, R.S. Inhibition of trehalose synthesis in lepidoptera reduces larval fitness. Adv. Biol. 2024, 8, e2300404. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Guo, W.; Yang, L.; He, Q.; Zhou, S. Juvenile hormone promotes locust fat body cell polyploidization and vitellogenesis by activating the transcription of Cdk6 and E2f1. Insect Biochem. Mol. Biol. 2018, 102, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Tufail, M.; Takeda, M. Insect vitellogenin/lipophorin receptors: Molecular structures, role in oogenesis, and regulatory mechanisms. J. Insect Physiol. 2009, 55, 87–103. [Google Scholar] [CrossRef]
- Benrabaa, S.A.M.; Orchard, I.; Lange, A.B. The role of ecdysteroid in the regulation of ovarian growth and oocyte maturation in Rhodnius prolixus, a vector of Chagas disease. J. Exp. Biol. 2022, 1, jeb244830. [Google Scholar] [CrossRef]
- Zheng, H.; Chen, C.; Liu, C.; Song, Q.; Zhou, S. Rhythmic change of adipokinetic hormones diurnally regulates locust vitellogenesis and egg development. Insect Mol. Biol. 2020, 29, 283–292. [Google Scholar] [CrossRef] [PubMed]
- Lu, K.; Wang, Y.; Chen, X.; Zhang, X.; Li, W.; Cheng, Y.; Li, Y.; Zhou, J.; You, K.; Song, Q.; et al. Adipokinetic hormone receptor mediates trehalose homeostasis to promote vitellogenin uptake by oocytes in Nilaparvata lugens. Front. Physiol. 2018, 9, 1904. [Google Scholar] [CrossRef] [PubMed]
- Tang, B.; Han, Y.; Mao, Q.; Fu, H.; Luo, Y.; Hua, L.; Liu, B.; Hu, G.; Wang, S.; Desneux, N.; et al. Regulation of three novel pepper thiothiazolidinones on the fecundity of Spodoptera frugiperda. Pestic. Biochem. Physiol. 2024, 204, 106033. [Google Scholar] [CrossRef] [PubMed]
- Keyes-Scott, N.I.; Swade, K.R.; Allen, L.R.; Vogel, K.J. RNAi-mediated knockdown of two orphan G protein-coupled receptors reduces fecundity in the yellow fever mosquito Aedes aegypti. Front. Insect Sci. 2023, 3, 1197945. [Google Scholar] [CrossRef] [PubMed]
- Vieira, P.H.; Bomfim, L.; Atella, G.C.; Masuda, H.; Ramos, I. Silencing of RpATG6 impaired the yolk accumulation and the biogenesis of the yolk organelles in the insect vector R. prolixus. PLoS Negl. Trop. Dis. 2018, 12, e0006507. [Google Scholar] [CrossRef]
- Jiang, X.; Zheng, S.; Bamu, A.; Dai, H.; Lin, X. Nilaparvata lugens ERR2 regulates moulting and ovary development is related to hormone signalling. Insect Mol. Biol. 2023, 32, 376–386. [Google Scholar] [CrossRef]
- Ruan, Y.; Wong, N.K.; Zhang, X.; Zhu, C.; Wu, X.; Ren, C.; Luo, P.; Jiang, X.; Ji, J.; Wu, X.; et al. Vitellogenin receptor (VgR) mediates oocyte maturation and ovarian development in the pacific white shrimp (Litopenaeus vannamei). Front. Physiol. 2020, 11, 485. [Google Scholar] [CrossRef]
- Gruntenko, N.E.; Rauschenbach, I.Y. Interplay of JH, 20E and biogenic amines under normal and stress conditions and its effect on reproduction. J. Insect Physiol. 2008, 54, 902–908. [Google Scholar] [CrossRef]
- Huangfu, N.; Zhu, X.; Wang, L.; Zhang, K.; Li, D.; Chen, L.; Gao, X.; Niu, L.; Gao, M.; Ji, J.; et al. Insulin Receptor substrate-1 (IRS1) regulates oogenesis and vitellogenesis in Propylea japonica by mediating the FOXO transcription factor expression, independent of JH and 20E signaling pathways. J. Agric. Food Chem. 2023, 71, 300–310. [Google Scholar] [CrossRef]
- Xu, H.J.; Xue, J.; Lu, B.; Zhang, X.C.; Zhuo, J.C.; He, S.F.; Ma, X.F.; Jiang, Y.Q.; Fan, H.W.; Xu, J.Y.; et al. Two insulin receptors determine alternative wing morphs in planthoppers. Nature 2015, 519, 464–467. [Google Scholar] [CrossRef]
- Xue, W.H.; Xu, N.; Chen, S.J.; Liu, X.Y.; Zhang, J.L. Neofunctionalization of a second insulin receptor gene in the wing-dimorphic planthopper, Nilaparvata lugens. PLoS Genet. 2021, 17, e1009653. [Google Scholar] [CrossRef] [PubMed]
- Oldham, S. Obesity and nutrient sensing TOR pathway in flies and vertebrates: Functional conservation of genetic mechanisms. Trends Endocrinol. Metab. 2011, 22, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.J.; Xi, G.S.; Zhao, J. Vitellogenin regulates estrogen-related receptor expression by crosstalk with the JH and IIS-TOR signaling pathway in Polyrhachis vicina Roger (Hymenoptera, Formicidae). Gen. Comp. Endocrinol. 2021, 310, 113836. [Google Scholar] [CrossRef] [PubMed]
| Primer Name | Primer Sequence (5′-3′) |
|---|---|
| dsNlTPS1-F | ACCAGGAGTTGAAGGAGGAG |
| dsNlTPS1-R | CGATACCCGTGGGACTAG |
| dsNlTPS2-F | CACCAAAGGTCTAAGGCACA |
| dsNlTPS2-R | AGGGATGCTCTAGTTGCTAC |
| dsNlTPS3-F | GAGTCTGACCTGATAGCCTTTA |
| dsNlTPS3-R | TAGCCTCAGGTAAATCAACA |
| dsNlGFP-F | AAGGGCGAGGAGCTGTTCACCG |
| dsNlGFP-R | CAGCAGGACCATGTGATCGCGC |
| Primer Name | Forward Primer (5′-3′) | Reverse Primer (5′-3′) |
|---|---|---|
| QActin | TGGACTTCGAGCAGGAAATGG | ACGTCGCACTTCATGATCGAG |
| QNlTPS1 | AAGACTGAGGCGAATGGT | AAGGTGGAAATGGAATGTG |
| QNlTPS2 | AGAGTGGACCGCAACAACA | TCAACGCCGAGAATGACTT |
| QNlTPS3 | GTGATGCGTCGGTGGCTAT | CCGAACACGGTCCGCATA |
| QNlVg | CACTGCCCGTGCTGTGCTCTA | TGACTTCCTTGCTTTGCTCCC |
| QNlVgR | AGGCAGCCACACAGATAACCGC | AGCCGCTCGCTCCAGAACATT |
| QNlJHAMT | GAACCTGCAGGCCAAACACA | ACCACTCGGTTGGGCTGAAT |
| QNlMet | AGTGGCAGCGAGCGATGATT | TGAGGCGCAGCAAAAAGGAG |
| QNlUSP | GGTGGAGCTGCTGAGGGAGA | AGCACTTGAGGCCGATGGAG |
| QNlEcR | CGAAGCCTGGAAGGTGGAGA | GGCAAAGATTGGCGACGATT |
| QNlInR1 | GAGTGCAACCCGGAGTATGT | TCTTGACGGCACACTTCTTG |
| QNlInR2 | CTCTTGCCGAACAGCCTTAC | GGGTCGTTTAGTGGGTCTGA |
| QNlTOR | GGCTACAGGGATGTCAAA | GAGATAGATTCAAACGGAAAG |
| QNlS6K | AATCGGACGACTTGGAGACAGT | CAGTTTGGAAAGCGTACATCAGG |
| QNlAKH | CCCTTCTGATGGCAGTCCTTTG | ATGGATGCCTTGCAGCCTTCT |
| QNlFas | CGGAGACTCTGCCCTAA | CAGCGACTAATCCAACATC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, Y.; Zhong, F.; Zhang, X.; Zhang, Y.; Zhou, Y.; Guan, L.; Liu, Y.; Zhang, Y.; Zhang, X.; Zhou, M.; et al. Effects of Trehalose-6-Phosphate Synthase on the Reproduction and Development of Nilaparvata lugens and Its Molecular Mechanism. Insects 2025, 16, 1195. https://doi.org/10.3390/insects16121195
Han Y, Zhong F, Zhang X, Zhang Y, Zhou Y, Guan L, Liu Y, Zhang Y, Zhang X, Zhou M, et al. Effects of Trehalose-6-Phosphate Synthase on the Reproduction and Development of Nilaparvata lugens and Its Molecular Mechanism. Insects. 2025; 16(12):1195. https://doi.org/10.3390/insects16121195
Chicago/Turabian StyleHan, Ye, Fan Zhong, Xinyu Zhang, Yuya Zhang, Yanfei Zhou, Liwen Guan, Yongkang Liu, Yi Zhang, Xinyi Zhang, Min Zhou, and et al. 2025. "Effects of Trehalose-6-Phosphate Synthase on the Reproduction and Development of Nilaparvata lugens and Its Molecular Mechanism" Insects 16, no. 12: 1195. https://doi.org/10.3390/insects16121195
APA StyleHan, Y., Zhong, F., Zhang, X., Zhang, Y., Zhou, Y., Guan, L., Liu, Y., Zhang, Y., Zhang, X., Zhou, M., & Tang, B. (2025). Effects of Trehalose-6-Phosphate Synthase on the Reproduction and Development of Nilaparvata lugens and Its Molecular Mechanism. Insects, 16(12), 1195. https://doi.org/10.3390/insects16121195







