Orientation and Oviposition by Female Plodia interpunctella (Lepidoptera: Pyralidae) in Response to Volatiles from Varieties of Peanuts
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Insects
2.2. Peanuts
2.3. VOC Collection from Peanut Samples
2.4. VOCs Analyzed by GC-MS
2.5. Chemicals
2.6. Electroantennogram Bioassay
2.7. Y-Tube Olfactometer Bioassays
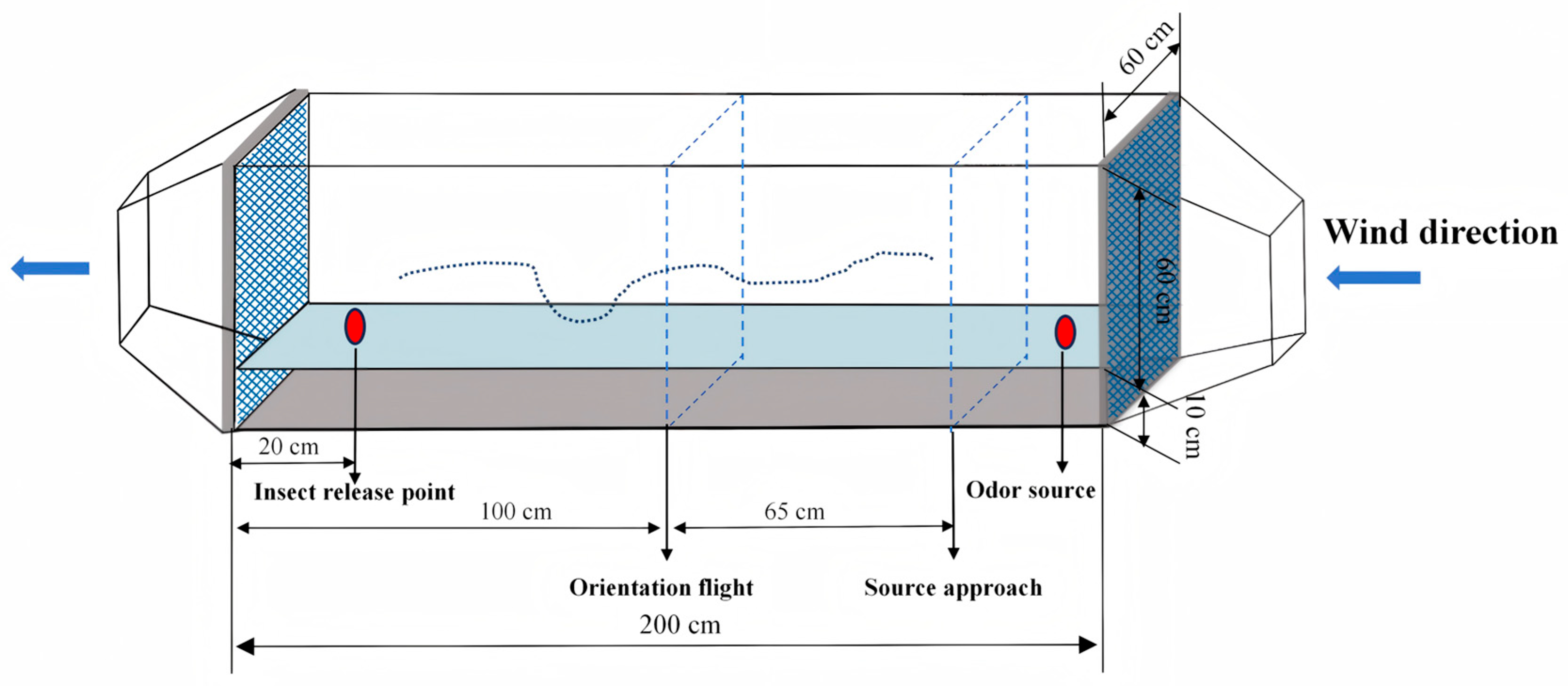
2.8. Wind Tunnel Bioassays
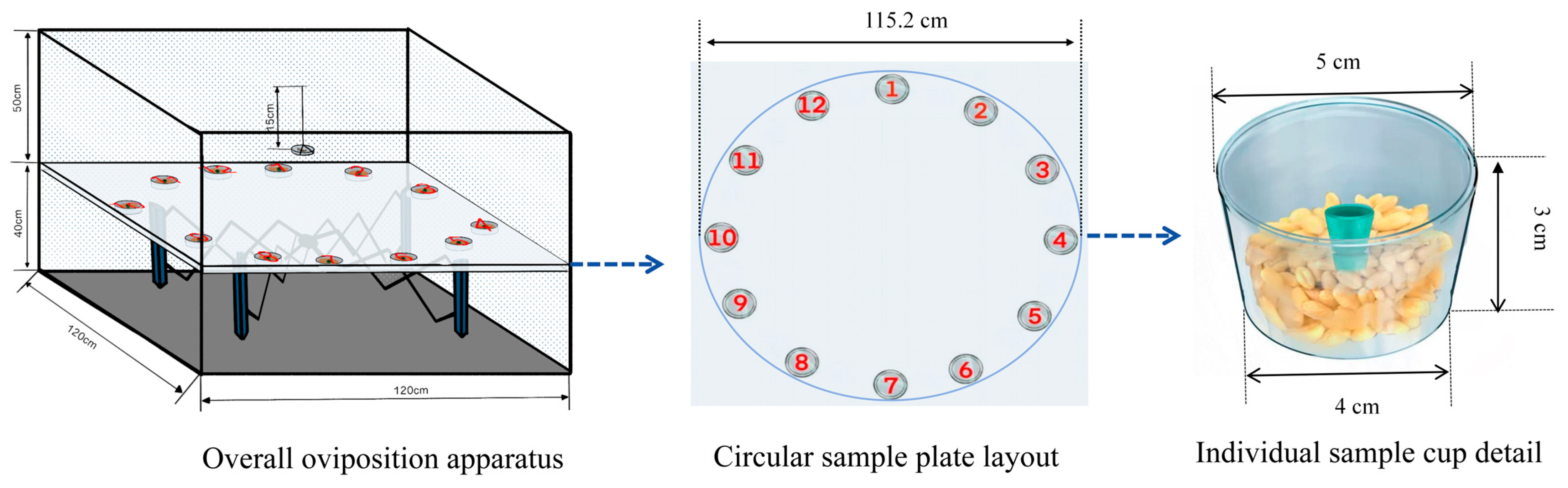
2.9. Oviposition Bioassay
2.10. Statistical Analysis
3. Results
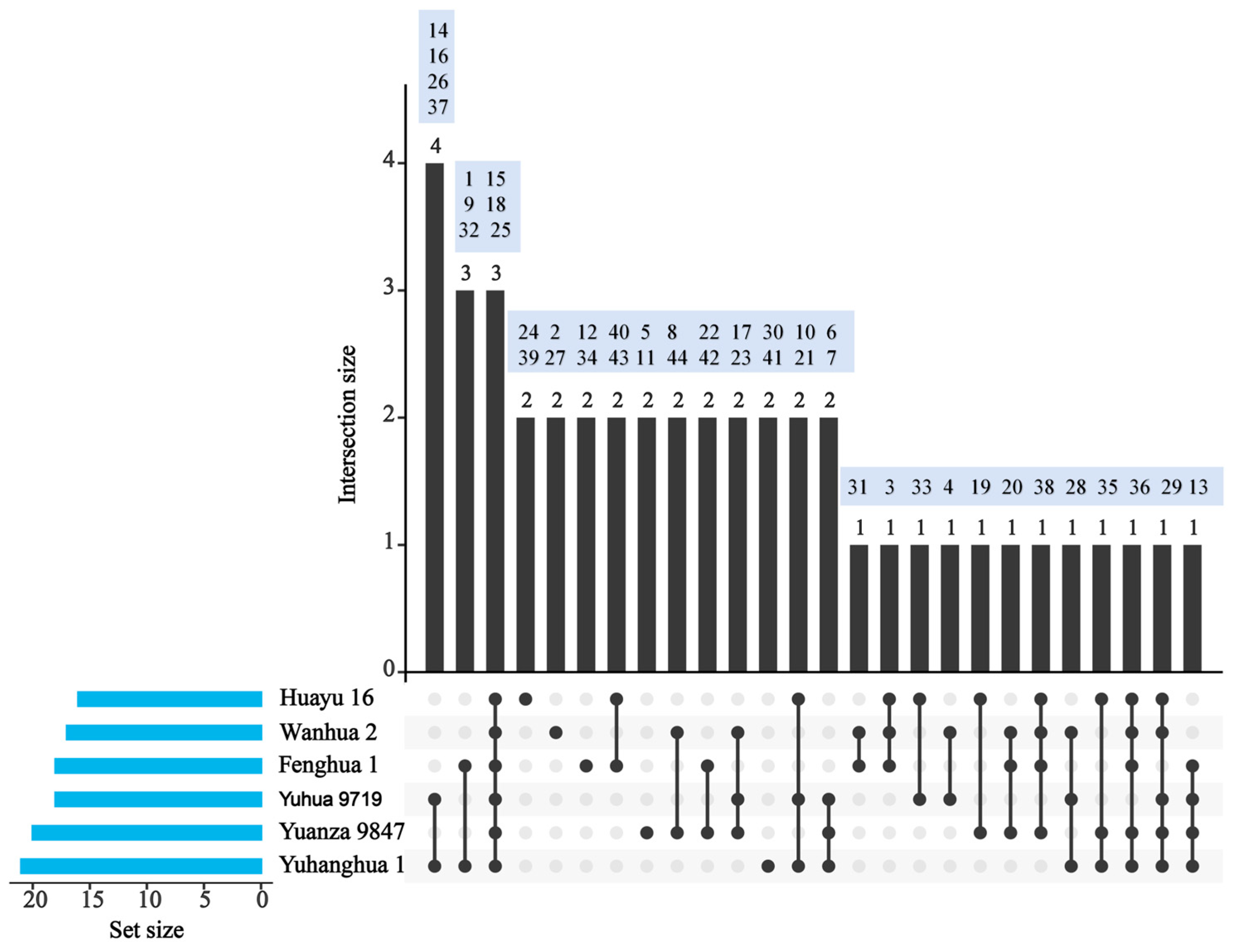
3.1. Special VOCs in Tested Peanut Varieties
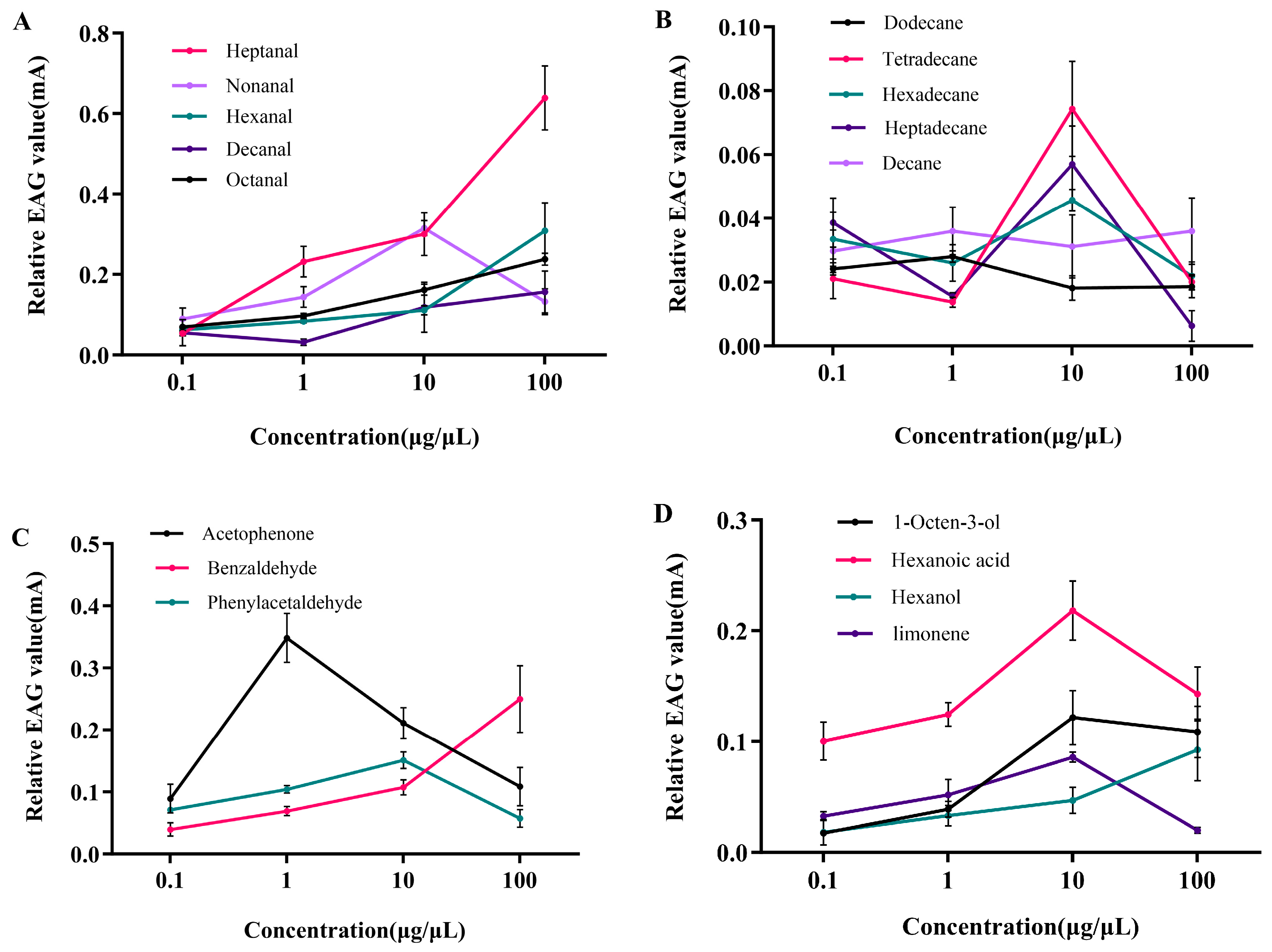
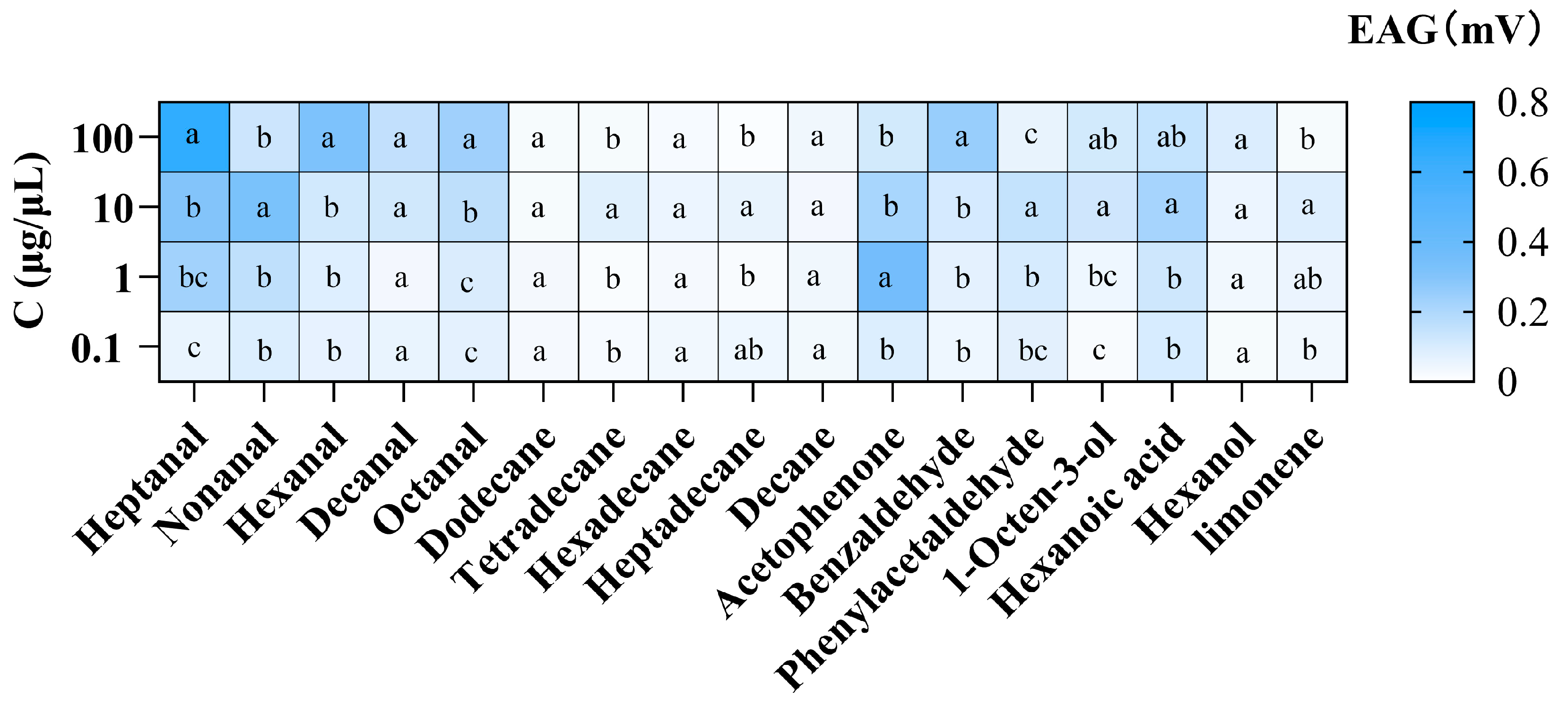
3.2. Electrophysiological Responses of P. interpunctella Females to Seventeen VOCs
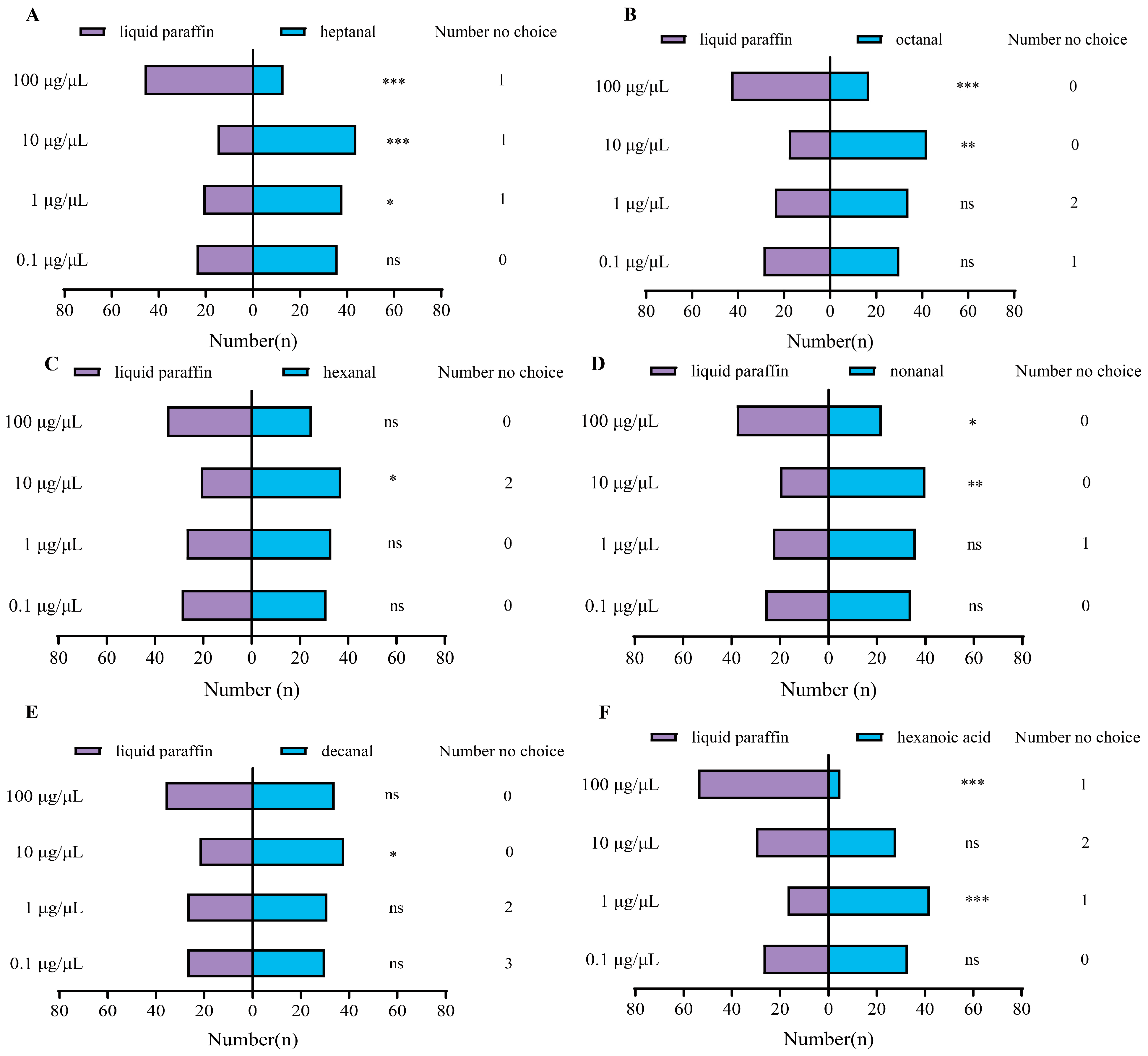
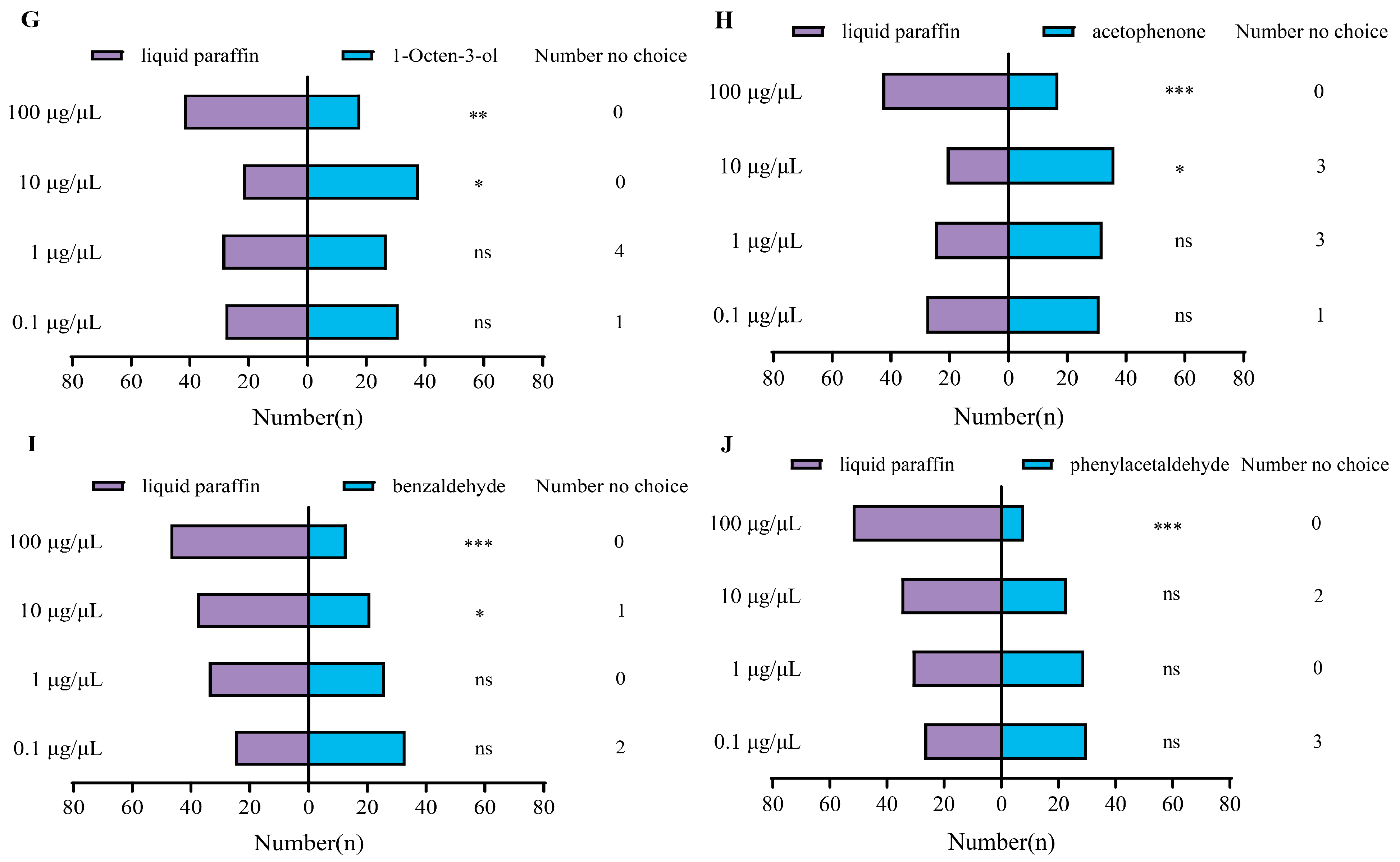
3.3. Behavior Response of P. interpunctella Female to VOCs in Y-Tube Olfactometer Assay
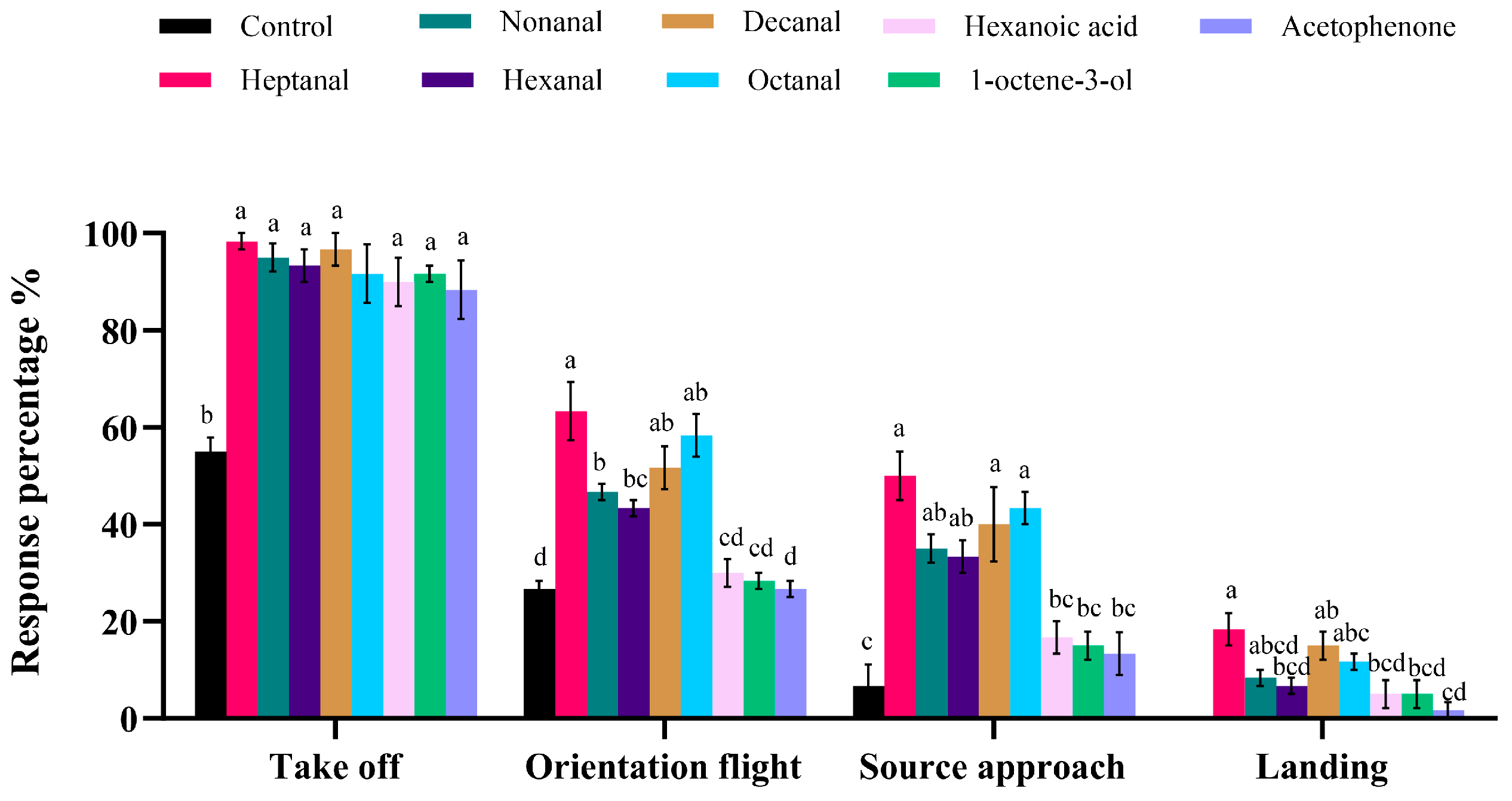
3.4. Behavior Response of the Females to VOCs in Wind Tunnel Measurement
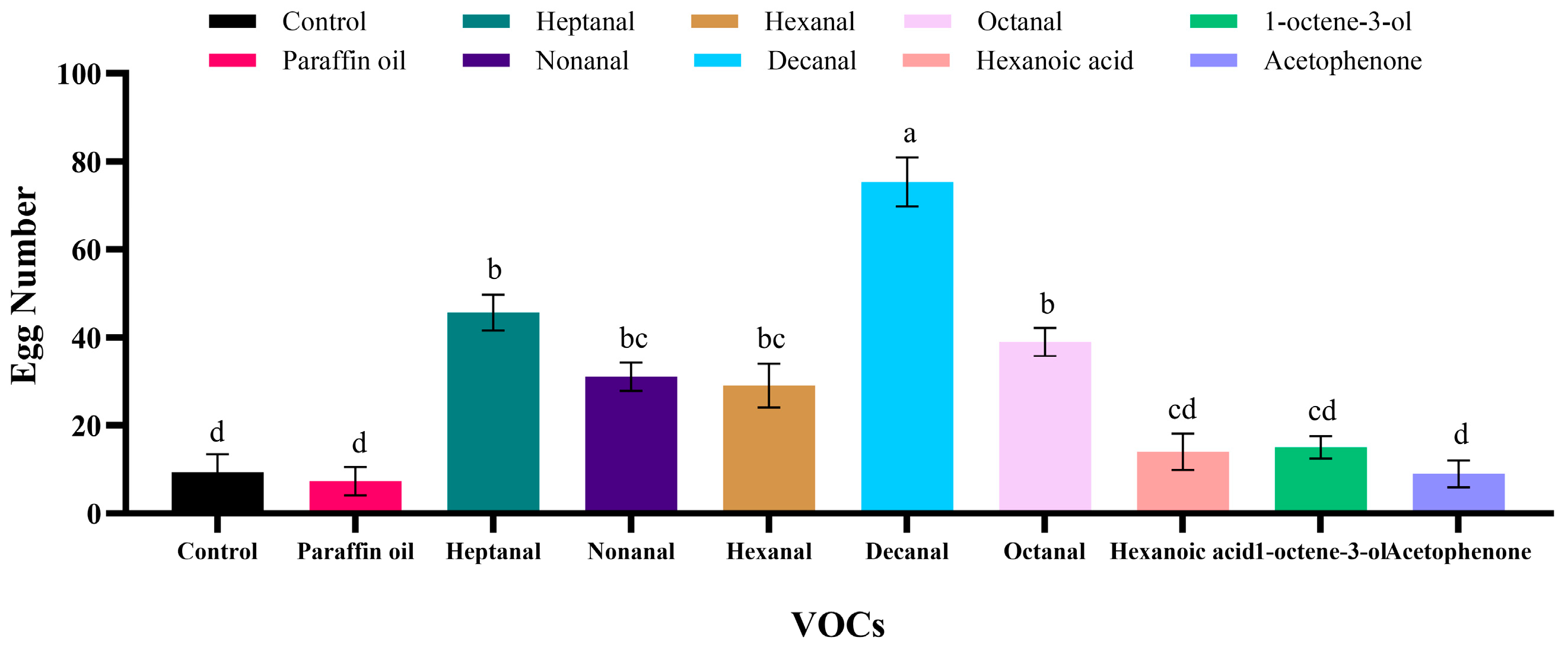
3.5. The Oviposition Laid on Wheat with Different VOCs
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Gvozdenac, S.M.; Prvulović, D.M.; Lozanov-Crvenković, Z.; Štajner-Papuga, I.V.; Ovuka, J.S.; Krstić, M.V.; Tanasković, S.T.; Vukajlović, F.N. Thermal treatments in controlling Plodia interpunctella (Lepidoptera: Pyralidae) on sunflower seeds and their effect on seed vitality. J. Stored Prod. Res. 2024, 108, 102384. [Google Scholar] [CrossRef]
- Guru, P.N.; Mridula, D.; Dukare, A.S.; Ghodki, B.M.; Paschapur, A.U.; Samal, I.; Raj, M.N.; Padala, V.K.; Rajashekhar, M.; Subbanna, A.R.N.S. A comprehensive review on advances in storage pest management: Current scenario and future prospects. Front. Sustain. Food Syst. 2022, 6, 993341. [Google Scholar] [CrossRef]
- Berhe, M.; Subramanyam, B.; Chichaybelu, M.; Demissie, G.; Abay, F.; Harvey, J. Post-Harvest insect pests and their management practices for major food and export crops in east Africa: An Ethiopian case study. Insects 2022, 13, 1068. [Google Scholar] [CrossRef]
- Agrafioti, P.; Kaloudis, E.; Bantas, S.; Sotiroudas, V.; Athanassiou, C.G. Phosphine distribution and insect mortality in commercial metal shipping containers using wireless sensors and CFD modeling. Comput. Electron. Agric. 2021, 184, 106087. [Google Scholar] [CrossRef]
- Somiahnadar, R. Insect Pest Management in Stored Products. Outlooks Pest Manag. 2020, 31, 24–35. [Google Scholar] [CrossRef]
- Saha, T.; Chandran, N. Chemical ecology and pest management: A review. Int. J. Chem. Stud. 2017, 5, 618–621. [Google Scholar]
- Miller, J.R.; Gut, L.J.; de Lame, F.M.; Stelinski, L.L. Differentiation of competitive vs. non-competitive mechanisms mediating disruption of moth sexual communication by point sources of sex pheromone (Part 2): Case studies. J. Chem. Ecol. 2006, 32, 2115–2143. [Google Scholar] [CrossRef] [PubMed]
- Miller, J.R.; Gut, L.J. Mating disruption for the 21st century: Matching technology with mechanism. Environ. Entomol. 2015, 44, 427–453. [Google Scholar] [CrossRef]
- Lindenmayer, J.C.; Campbell, J.F.; Miller, J.F.; Gerken, A.R. Evaluation of microencapsulated liquid pheromone for the control of Indian meal moth (Plodia interpunctella) in a retail environment. J. Stored Prod. Res. 2024, 110, 102479. [Google Scholar] [CrossRef]
- Ryne, C.; Svensson, G.P.; Anderbrant, O.; Löfstedt, C. Evaluation of long-term mating disruption of Ephestia kuehniella and Plodia interpunctella (Lepidoptera: Pyralidae) in indoor storage facilities by pheromone traps and monitoring of relative aerial concentrations of pheromone. J. Econ. Entomol. 2007, 100, 1017–1025. [Google Scholar] [CrossRef]
- Trematerra, P.; Athanassiou, C.; Stejskal, V.; Sciarretta, A.; Kavallieratos, N.; Palyvos, N. Large-scale mating disruption of Ephestia spp. and Plodia interpunctella in Czech Republic, Greece and Italy. J. Appl. Entomol. 2011, 135, 749–762. [Google Scholar] [CrossRef]
- Amoah, B.A.; Mahroof, R.M.; Gerken, A.R.; Campbell, J.F. Effect of delayed mating on longevity and reproductive performance of Lasioderma serricorne (Coleoptera: Anobiidae). J. Econ. Entomol. 2019, 112, 475–484. [Google Scholar] [CrossRef]
- Sammani, A.M.P.; Dissanayaka, D.M.S.K.; Wijayaratne, L.K.W.; Morrison, W.R. Effect of pheromone blend components, sex ratio, and population size on the mating of Cadra cautella (Lepidoptera: Pyralidae). J. Insect Sci. 2020, 20, 30. [Google Scholar] [CrossRef] [PubMed]
- Burks, C.S.; McLaughlin, J.R.; Miller, J.R.; Brandl, D.G. Mating disruption for control of Plodia interpunctella (Hübner) (Lepidoptera: Pyralidae) in dried beans. J. Stored Prod. Res. 2011, 47, 216–221. [Google Scholar] [CrossRef]
- Xu, H.; Turlings, T.C.J. Plant volatiles as mate-finding cues for insects. Trends Plant Sci. 2018, 23, 100–111. [Google Scholar] [CrossRef] [PubMed]
- Shrivastava, G.; Rogers, M.; Wszelaki, A.; Panthee, D.R.; Chen, F. plant volatiles-based insect pest management in organic farming. Crit. Rev. Plant Sci. 2010, 29, 123–133. [Google Scholar] [CrossRef]
- Sabier, M.; Wang, J.R.; Zhang, T.; Jin, J.D.; Wang, Z.J.; Shen, B.; Deng, J.Y.; Liu, X.Q.; Zhou, G.X. The attractiveness of a food based lure and its component volatiles to the stored-grain pest Oryzaephilus surinamensis (L.). J. Stored Prod. Res. 2022, 98, 102000. [Google Scholar] [CrossRef]
- Cao, Y.; Hu, Q.Q.; Huang, L.J.; Athanassiou, C.G.; Maggi, F.; D’Isita, I.; Liu, Y.Y.; Pistillo, O.M.; Miao, M.Z.; Germinara, G.S.; et al. Attraction of Sitophilus oryzae (L.) (Coleoptera: Curculionidae) to the semiochemical volatiles of stored rice materials. J. Pest Sci. 2024, 97, 73–85. [Google Scholar] [CrossRef]
- Ukeh, D.A.; Birkett, M.A.; Bruce, T.J.A.; Allan, E.J.; Pickett, J.A.; Mordue, A.J. Behavioural responses of the maize weevil, Sitophilus zeamais, to host (stored-grain) and non-host plant volatiles. Pest Manag. Sci. 2010, 66, 44–50. [Google Scholar] [CrossRef]
- Cao, Y.; Jian, L.Y.; Athanassiou, C.G.; Yang, Y.P.; Hu, Q.Q.; Zhang, X.Y.; Dai, F.L.; Maggi, F. Behavioral responses of Rhyzopertha dominica (F.) to volatiles of different stored grains. J. Stored Prod. Res. 2023, 105, 102235. [Google Scholar] [CrossRef]
- Mohandass, S.; Arthur, F.H.; Zhu, K.Y.; Throne, J.E. Biology and management of Plodia interpunctella (Lepidoptera: Pyralidae) in stored products. J. Stored Prod. Res. 2007, 43, 302–311. [Google Scholar] [CrossRef]
- Grieshop, M.J.; Flinn, P.W.; Nechols, J.R. Biological control of Indian meal moth (Lepidoptera: Pyralidae) on finished stored products using egg and larval parasitoids. J. Econ. Entomol. 2006, 99, 1080–1084. [Google Scholar] [CrossRef]
- Scheff, D.S.; Sehgal, B.; Subramanyam, B. Evaluating penetration ability of Plodia interpunctella (Hubner) (Lepidoptera: Pyralidae) larvae into multilayer polypropylene packages. Insects 2018, 9, 42. [Google Scholar] [CrossRef]
- Buda, V.; Apsegaite, V.; Blazyte-Cereskiene, L.; Butkiene, R.; Nedveckyte, I.; Peciulyte, D. Response of moth Plodia interpunctella to volatiles of fungus-infected and uninfected wheat grain. J. Stored Prod. Res. 2016, 69, 152–158. [Google Scholar] [CrossRef]
- Jiang, B.R.; Wang, D.X.; Zhang, L.K.; Chen, L.; Jing, J.G.; Li, Z.H.; Tang, P.A. Comparison on Oviposition Preference of Plodia interpunctella (Hübner) on Grain and Peanut Kernels. J. Henan Univ. Technol. 2019, 40, 86–93. [Google Scholar]
- Wang, C.; Wang, D.X.; Zeng, F.F.; Chen, L. Oviposition preferences of Plodia interpunctella (Hubner)on selected dried fruits and nuts, with the identification of key volatiles. J. Stored Prod. Res. 2025, 114, 102783. [Google Scholar] [CrossRef]
- Talcott, S.T.; Passeretti, S.; Duncan, C.E.; Daniel, W.G. Polyphenolic content and sensory properties of normal and high oleic acid peanuts. Food Chem. 2005, 90, 379–388. [Google Scholar] [CrossRef]
- Ndomo-Moualeu, A.F.; Ulrichs, C.; Ranek, R.; Cornel, A. Structure and distribution of antennal sensilla in the Indianmeal moth, Plodia interpunctella (Hübner, 1813) (Lepidoptera: Pyralidae). J. Stored Prod. Res. 2014, 59, 66–75. [Google Scholar] [CrossRef]
- Liu, X.J.; Jiang, H.X.; Xu, H.Q.; Xu, H.Q.; Shang, S.S.; Wang, D.X.; Bai, C.Q.; Zeng, F.F. Volatile organic compounds as early detection indicators of wheat infected by Sitophilus oryzae. Foods 2024, 13, 3930. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Hu, X.P.; Allan, S.A.; Alborn, H.T.; Bernier, U.R. Electrophysiological and behavioral responses of the kudzu bug, Megacopta cribraria (Hemiptera: Plataspidae), to volatile compounds from kudzu and soybean plants. J. Agric. Food Chem. 2019, 67, 4177–4183. [Google Scholar] [CrossRef]
- Song, C.; Ma, L.; Zhao, J.; Xue, Z.S.; Yan, X.Z.; Hao, C. Electrophysiological and behavioral responses of Plutella xylostella (Lepidoptera: Plutellidae) to volatiles from a non-host plant, Geranium, Pelargonium × hortorum (Geraniaceae). J. Agric. Food. Chem. 2022, 70, 5982–5992. [Google Scholar] [CrossRef]
- Olsson, P.-O.C.; Anderbrant, O.; Löfstedt, C. Flight and oviposition behavior of Ephestia cautella and Plodia interpunctella in response to odors of different chocolate products. J. Insects Behav. 2005, 18, 363–380. [Google Scholar] [CrossRef]
- Bruce, T.J.A.; Wadhams, L.J.; Woodcock, C.M. Insect host location: A volatile situation. Trends Plant Sci. 2005, 10, 269–274. [Google Scholar] [CrossRef]
- Wang, Q.; Xu, P.; Sanchez, S.; Duran, P.; Andreazza, F.; Isaacs, R.; Dong, K. Behavioral and physiological responses of Drosophila melanogaster and D. suzukii to volatiles from plant essential oils. Pest Manag. Sci. 2021, 77, 3698–3705. [Google Scholar] [CrossRef]
- Qian, Q.; Cui, J.R.; Miao, Y.Y.; Xu, X.F.; Gao, H.Y.; Xu, H.X.; Lu, Z.X.; Zhu, P.Y. The plant volatile-sensing mechanism of insects and Its utilization. Plants 2024, 13, 185. [Google Scholar] [CrossRef]
- Noge, K. Hexanal, a major volatile found in fresh peanut seed, elicits foraging behavior in the laboratory-reared brown marmorated stink bug, Halyomorpha halys (Heteroptera: Pentatomidae). J. Pestic. Sci. 2019, 44, 15–19. [Google Scholar] [CrossRef]
- Balakrishnan, K.; Holighaus, G.; Weissbecker, B.; Schütz, S. Electroantennographic responses of red flour beetle Tribolium castaneum Herbst (Coleoptera: Tenebrionidae) to volatile organic compounds. J. Appl. Entomol. 2017, 141, 477–486. [Google Scholar] [CrossRef]
- Beck, J.J.; Light, D.M.; Gee, W.S. Electroantennographic bioassay as a screening tool for host plant volatile. Jove J. Vis. Exp. 2012, 6, e3931. [Google Scholar] [CrossRef]
- Zhao, J.Y.; Zheng, R.R.; Li, X.F.; Lyu, Z.; Ma, L.; Song, C.F.; Qie, X.T.; Yan, X.Z.; Hao, C. Electrophysiological and behavioral responses of Plodia interpunctella (Hübner) females to aldehyde volatiles from dried fruits. J. Agric. Food Chem. 2023, 71, 17253–17262. [Google Scholar] [CrossRef]
- Mustaparta, H. Innate and changed responses to plant odours in moths and weevils. Chem. Senses 2005, 30, i297–i298. [Google Scholar] [CrossRef] [PubMed]
- Lohonyai, Z.; Vuts, J.; Kárpáti, Z.; Koczor, S.; Domingue, M.J.; Fail, J.; Birkett, M.A.; Tóth, M.; Imrei, Z. Benzaldehyde: An alfalfa-related compound for the spring attraction of the pest weevil Sitona humeralis (Coleoptera: Curculionidae). Pest Manag. Sci. 2019, 75, 3153–3159. [Google Scholar] [CrossRef]
- Wang, P.; Zhang, N.; Zhou, L.L.; Si, S.Y.; Lei, C.L.; Ai, H.; Wang, X.P. Antennal and behavioral responses of female Maruca vitrata to the floral volatiles of Vigna unguiculata and Lablab purpureus. Entomol. Exp. Appl. 2014, 152, 248–257. [Google Scholar] [CrossRef]
- Ma, Y.F.; Xiao, C. Push-pull effects of three plant secondary metabolites on oviposition of the potato tuber moth, Phthorimaea operculella. J. Insect Sci. 2013, 13, 128. [Google Scholar] [CrossRef]
- Olsson, P.O.C.; Anderbrant, O.; Löfstedt, C.; Borg-Karlson, A.K.; Liblikas, I. Electrophysiological and behavioral responses to chocolate volatiles in both sexes of the pyralid moths Ephestia cautella and Plodia interpunctella. J. Chem. Ecol. 2005, 31, 2947–2961. [Google Scholar] [CrossRef]
- Li, P.X.; Wei, Y.; Chen, G.X.; Sattar, A. Perceptual effects of walnut volatiles on the codling moth. Insects 2024, 15, 402. [Google Scholar] [CrossRef] [PubMed]
- Fouad, H.A.; Faroni, L.R.D.; Vilela, E.F.; de Lima, E.R. Flight responses of Sitotroga cerealella (Lepidoptera: Gelechiidae) to corn kernel volatiles in a wind tunnel. Arthropod-Plant Interact. 2013, 7, 651–658. [Google Scholar] [CrossRef]
- Zhang, M.M.; Cui, Z.H.; Zhang, N.; Xie, G.L.; Wang, W.K.; Chen, L. Electrophysiological and behavioral responses of Holotrichia parallela to volatiles from peanut. Insects 2021, 12, 158. [Google Scholar] [CrossRef]
- Silva, F.A.C.; Carrao-Panizzi, M.C.; Blassioli-Moraes, M.C.; Panizzi, A.R. Influence of volatile and nonvolatile secondary metabolites from soybean pods on feeding and on oviposition behavior of Euschistus heros (Hemiptera: Heteroptera: Pentatomidae). Environ. Entomol. 2013, 42, 1375–1382. [Google Scholar] [CrossRef] [PubMed]
- Kumari, A.; Kaushik, N. Oviposition deterrents in herbivorous insects and their potential use in integrated pest management. Indian J. Exp. Biol. 2016, 54, 163–174. [Google Scholar] [PubMed]
- Weeraddana, C.D.S.; Wijesundara, R.; Hillier, W.; Swanburg, T.; Hillier, N.K.; Wang, H.V.; Faraone, N.; Wolfe, S.; McCartney, C.; Wist, T.; et al. Volatile organic compounds mediate host selection of wheat midge, Sitodiplosis Mosellana (Géhin) (Diptera: Cecidomyiidae) between Preanthesis and Postanthesis Stages of Wheat. J. Chem. Ecol. 2024, 50, 237–249. [Google Scholar] [CrossRef]
- Uechia, K.; Matsuyama, S.; Suzuki, T. Oviposition attractants for Plodia interpunctella (Hubner) (Lepidoptera: Pyralidae) in the volatiles of whole wheat flour. J. Stored Prod. Res. 2007, 43, 193–201. [Google Scholar] [CrossRef]
- Li, X.; Zhang, X.G.; Xiao, C.; Guo, E.X.; Dong, W.X. Behavioral responses of potato tuber moth (Phthorimaea operculella) to tobacco plant volatiles. J. Integr. Agric. 2020, 19, 325–332. [Google Scholar] [CrossRef]
| Peanut Variety | Water (%) | Protein (%) | Fat (%) | Carbohydrate (%) | Oleic Acid (%) | Linoleic Acid (%) |
|---|---|---|---|---|---|---|
| Yuhanghua 1 | 3.43 ± 0.24 | 24.91 ± 0.37 | 53.61 ± 0.42 | 13.17 ± 0.75 | 39.57 ± 0.96 | 33.42 ± 0.34 |
| Yuanza 9847 | 5.27 ± 0.18 | 23.94 ± 0.39 | 51.82 ± 0.27 | 14.87 ± 0.59 | 36.87 ± 0.40 | 37.35 ± 0.51 |
| Wanhua 2 | 4.21 ± 0.40 | 25.00 ± 0.22 | 52.82 ± 0.31 | 12.47 ± 0.31 | 39.01 ± 0.43 | 32.90 ± 0.23 |
| Huayu 16 | 5.07 ± 0.12 | 22.55 ± 0.70 | 51.62 ± 0.18 | 15.06 ± 0.60 | 50.06 ± 2.30 | 30.88 ± 0.20 |
| Fenghua 1 | 5.35 ± 0.78 | 23.05 ± 0.28 | 50.19 ± 0.41 | 16.73 ± 0.91 | 58.72 ± 0.89 | 28.87 ± 0.08 |
| Yuhua 9719 | 6.55 ± 0.40 | 24.60 ± 0.16 | 52.00 ± 0.48 | 12.35 ± 0.71 | 37.87 ± 0.59 | 35.80 ± 0.67 |
| No. | VOCs | Retention Time (min) | MS Match Range (%) | RI a | RI b | Quantity (ng/μL) | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Yuhanghua 1 | Yuhua 9719 | Fenghua 1 | Huayu 16 | Wanhua 2 | Yuanza 9847 | ||||||
| 1 | 2,4-dimethyl-heptane | 4.455 | 96–99 | 793 | 788 | 0.84 ± 0.20 | — | 0.33 ± 0.02 | — | — | — |
| 2 | butyl acetate | 4.486 | 98–99 | 791 | 785 | — | — | — | — | 0.46 ± 0.06 | — |
| 3 | Hexanal * | 4.560 | 95–99 | 797 | 806 | — | — | 0.69 ± 0.03 | 0.64 ± 0.06 | 0.61 ± 0.05 | — |
| 4 | 4-methyl-octane | 5.872 | 97–99 | 845 | 852 | — | 0.45 ± 0.03 | — | — | 0.18 ± 0.02 | — |
| 5 | 2,5-dimethyl-heptane | 6.056 | 96–98 | 796 | 790 | — | — | — | — | — | 0.56 ± 0.09 |
| 6 | 1-hexanol * | 6.188 | 95–98 | 859 | 860 | 0.07 ± 0.01 b | 0.51 ± 0.05 a | — | — | — | 0.42 ± 0.03 a |
| 7 | heptanal * | 7.366 | 95–100 | 883 | 905 | 0.44 ± 0.03 a | 0.14 ± 0.00 b | — | — | — | 0.36 ± 0.03 a |
| 8 | (1-methylethyl)-cyclohexane | 7.707 | 98–100 | 912 | 915 | — | — | — | — | 0.20 ± 0.03 | 0.15 ± 0.01 |
| 9 | α-pinene | 8.272 | 95–99 | 957 | 948 | 0.19 ± 0.01 | 0.19 ± 0.01 | — | — | — | |
| 10 | benzaldehyde * | 9.365 | 96–98 | 973 | 982 | 1.24 ± 0.22 a | 1.20 ± 0.13 a | — | 0.77 ± 0.08 b | — | |
| 11 | 1,2,3-trimethyl- benzene | 9.512 | 98–99 | 1011 | 1020 | — | — | — | — | — | 0.10 ± 0.01 |
| 12 | 1-methyl-2-propyl-cyclohexane | 10.159 | 98 | 1026 | 1040 | — | — | 0.28 ± 0.07 | — | — | — |
| 13 | 1-octen-3-ol * | 10.361 | 97–100 | 964 | 969 | 1.02 ± 0.10 ab | 1.32 ± 0.40 a | 0.27 ± 0.06 b | — | — | 0.67 ± 0.06 ab |
| 14 | 2,2,4,6,6-pentamethyl- heptane | 10.447 | 99–100 | 977 | 981 | 0.89 ± 0.06 | 3.07 ± 0.56 | — | — | — | — |
| 15 | decane * | 11.069 | 97–100 | 1007 | 1015 | 0.71 ± 0.08 ab | 0.60 ± 0.19 ab | 0.45 ± 0.05 b | 1.17 ± 0.23 a | 0.85 ± 0.12 ab | 0.57 ± 0.10 ab |
| 16 | hexanoic acid, ethyl ester | 11.178 | 98–100 | 983 | 984 | 0.57 ± 0.05 | 0.52 ± 0.03 | — | — | — | — |
| 17 | octanal * | 11.242 | 98–100 | 1000 | 1007 | — | 0.35 ± 0.03 a | — | — | 0.27 ± 0.03 a | 0.31 ± 0.04 a |
| 18 | limonene * | 12.013 | 99–100 | 1011 | 1018 | 0.66 ± 0.08 b | 0.71 ± 0.09 b | 1.49 ± 0.25 a | 0.20 ± 0.02 b | 0.58 ± 0.04 b | 1.52 ± 0.17 a |
| 19 | 2-ethyl-1-hexanol | 12.345 | 98–100 | 986 | 995 | — | — | — | 0.48 ± 0.09 | — | 0.13 ± 0.01 |
| 20 | phenylacetaldehyde * | 12.749 | 97–99 | 1038 | 1043 | — | — | 0.13 ± 0.01 b | — | 0.32 ± 0.04 a | 0.31 ± 0.06 ab |
| 21 | acetophenone * | 13.029 | 97–100 | 1069 | 1078 | 2.36 ± 0.36 a | 0.65 ± 0.09 b | — | 2.12 ± 0.27 a | — | — |
| 22 | undecane | 13.316 | 95–97 | 1108 | 1115 | — | — | 0.13 ± 0.01 | — | — | 0.31 ± 0.06 |
| 23 | hexanoic acid * | 13.550 | 96–99 | 966 | 974 | — | 0.21 ± 0.01 b | — | — | 0.36 ± 0.04 ab | 0.39 ± 0.05 a |
| 24 | 2-methyl-decane | 14.080 | 98–99 | 1059 | 1051 | — | — | — | 0.19 ± 0.01 | — | — |
| 25 | nonanal * | 15.158 | 99–100 | 1098 | 1104 | 0.70 ± 0.13 b | 0.55 ± 0.05 b | 1.28 ± 0.04 a | 0.56 ± 0.14 b | 0.35 ± 0.01 b | 0.75 ± 0.11 b |
| 26 | 4-methyl-undecane | 15.775 | 96–99 | 1145 | 1150 | 0.44 ± 0.03 | 0.24 ± 0.01 | — | — | — | — |
| 27 | 2-decen-1-ol | 17.535 | 95–97 | 1275 | 1278 | — | — | — | — | 0.34 ± 0.02 | — |
| 28 | dodecane * | 18.571 | 97–99 | 1209 | 1214 | 0.71 ± 0.02 b | 1.18 ± 0.06 ab | — | — | 1.57 ± 0.29 a | — |
| 29 | decanal * | 18.854 | 96–99 | 1197 | 1204 | 0.21 ± 0.05 a | 0.14 ± 0.01 ab | — | 0.15 ± 0.01 ab | 0.19 ± 0.01 a | 0.06 ± 0.01 b |
| 30 | 4-methyl-1-undecene | 21.340 | 97–98 | 1150 | 1140 | 0.16 ± 0.03 | — | — | — | — | — |
| 31 | tridecane | 22.033 | 95–99 | 1298 | 1313 | — | — | 0.31 ± 0.06 | — | 1.13 ± 0.05 | — |
| 32 | dodecanal | 23.585 | 99–100 | 1395 | 1402 | 0.29 ± 0.05 | — | 0.99 ± 0.07 | — | — | — |
| 33 | 3-methyl- tridecane | 24.344 | 95–98 | 1339 | 1349 | — | 0.29 ± 0.03 | — | 0.31 ± 0.07 | — | — |
| 34 | 1,1′-(1,4-phenylene)bis-ethanone | 26.300 | 98–99 | 1375 | 1378 | — | — | 2.77 ± 0.20 | — | — | — |
| 35 | tetradecane * | 28.687 | 98–100 | 1413 | 1413 | 0.55 ± 0.07 b | — | — | 1.26 ± 0.09 a | — | 0.75 ± 0.05 b |
| 36 | hexadecane * | 32.728 | 99–100 | 1609 | 1612 | 1.77 ± 0.15 a | — | 0.38 ± 0.03 c | 0.36 ± 0.02 c | 0.83 ± 0.10 b | 0.72 ± 0.04 bc |
| 37 | 3-methyl-hexadecane | 33.594 | 96–98 | 1645 | 1647 | 0.39 ± 0.05 | 0.13 ± 0.00 | — | — | — | — |
| 38 | heptadecane * | 35.262 | 98–100 | 1695 | 1711 | — | — | 0.53 ± 0.02 b | 2.01 ± 0.27 a | 1.68 ± 0.15 a | 0.40 ± 0.04 b |
| 39 | 6-methyl-octadecane | 35.860 | 97–98 | 1741 | 1740 | — | — | — | 0.70 ± 0.08 | — | — |
| 40 | nonadecane | 36.162 | 98–100 | 1910 | 1910 | — | — | 0.70 ± 0.09 | 0.14 ± 0.02 | — | — |
| 41 | 2,6-dimethyl- heptadecane | 36.329 | 96–97 | 1779 | 1782 | 0.41 ± 0.04 | — | — | — | — | — |
| 42 | 2,6,10-trimethyl-heptadecane | 36.360 | 96–99 | 1877 | 1882 | — | — | 0.25 ± 0.05 | — | — | 0.21 ± 0.07 |
| 43 | 1,2-benzenedicarboxylic acid bis(2-methylpropyl) ester | 41.212 | 97–99 | 1900 | 1908 | — | — | 1.09 ± 0.16 | 0.33 ± 0.04 | — | — |
| 44 | 1,2-benzenedicarboxylic acid, butyl 2-ethylhexyl ester | 44.219 | 98–99 | 2354 | 2370 | — | — | — | — | 1.140 ± 0.21 | 0.66 ± 0.08 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, X.; Wang, D.; Zeng, F.; Chen, L.; Wang, C.; Shang, S.; Guo, Z. Orientation and Oviposition by Female Plodia interpunctella (Lepidoptera: Pyralidae) in Response to Volatiles from Varieties of Peanuts. Insects 2025, 16, 1145. https://doi.org/10.3390/insects16111145
Zhu X, Wang D, Zeng F, Chen L, Wang C, Shang S, Guo Z. Orientation and Oviposition by Female Plodia interpunctella (Lepidoptera: Pyralidae) in Response to Volatiles from Varieties of Peanuts. Insects. 2025; 16(11):1145. https://doi.org/10.3390/insects16111145
Chicago/Turabian StyleZhu, Xi, Dianxuan Wang, Fangfang Zeng, Liang Chen, Chen Wang, Sijia Shang, and Zixin Guo. 2025. "Orientation and Oviposition by Female Plodia interpunctella (Lepidoptera: Pyralidae) in Response to Volatiles from Varieties of Peanuts" Insects 16, no. 11: 1145. https://doi.org/10.3390/insects16111145
APA StyleZhu, X., Wang, D., Zeng, F., Chen, L., Wang, C., Shang, S., & Guo, Z. (2025). Orientation and Oviposition by Female Plodia interpunctella (Lepidoptera: Pyralidae) in Response to Volatiles from Varieties of Peanuts. Insects, 16(11), 1145. https://doi.org/10.3390/insects16111145






