Assessing the Chronic Effects of Dietary Aluminum on Fitness Traits, Acetylcholinesterase Activity and Locomotion in Lymantria dispar L. Larvae
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Fitness Traits
2.2. Enzyme Detection
2.3. Locomotion Monitoring
2.4. Statistical Analysis
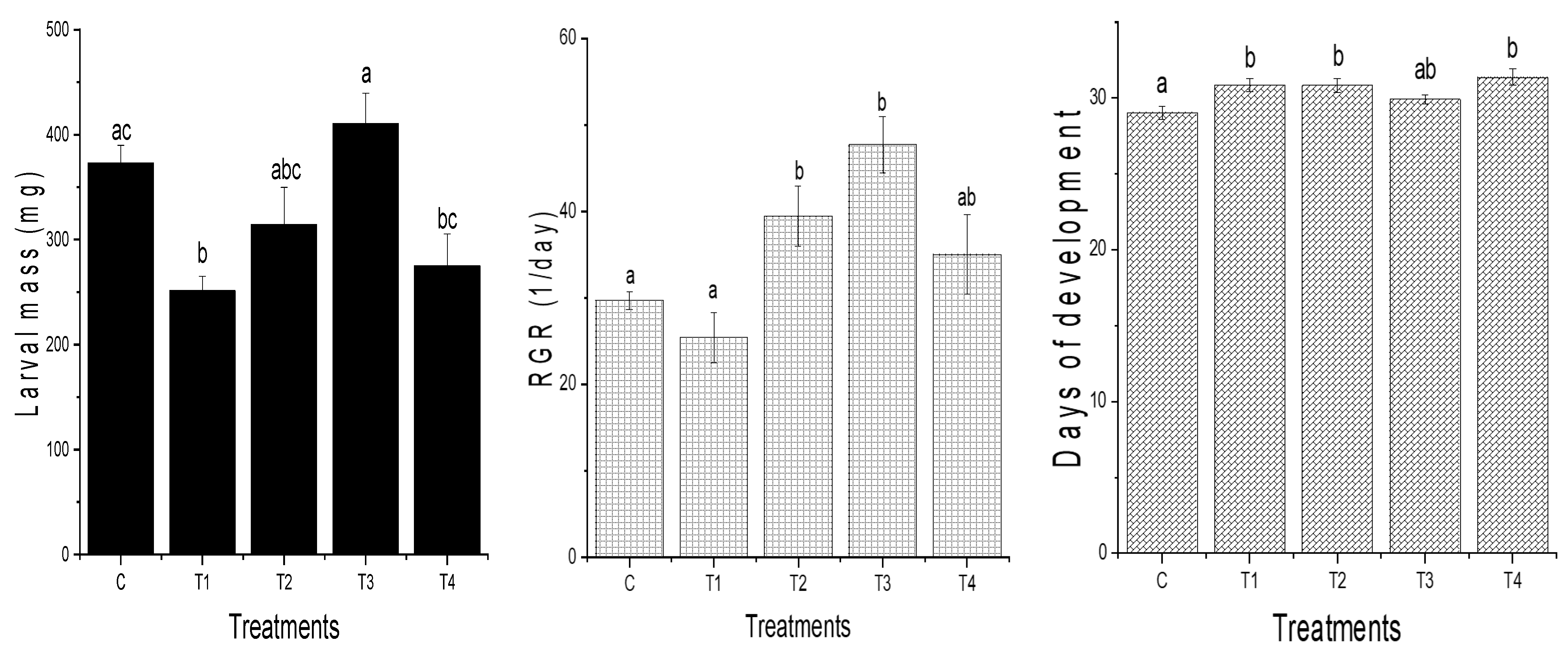
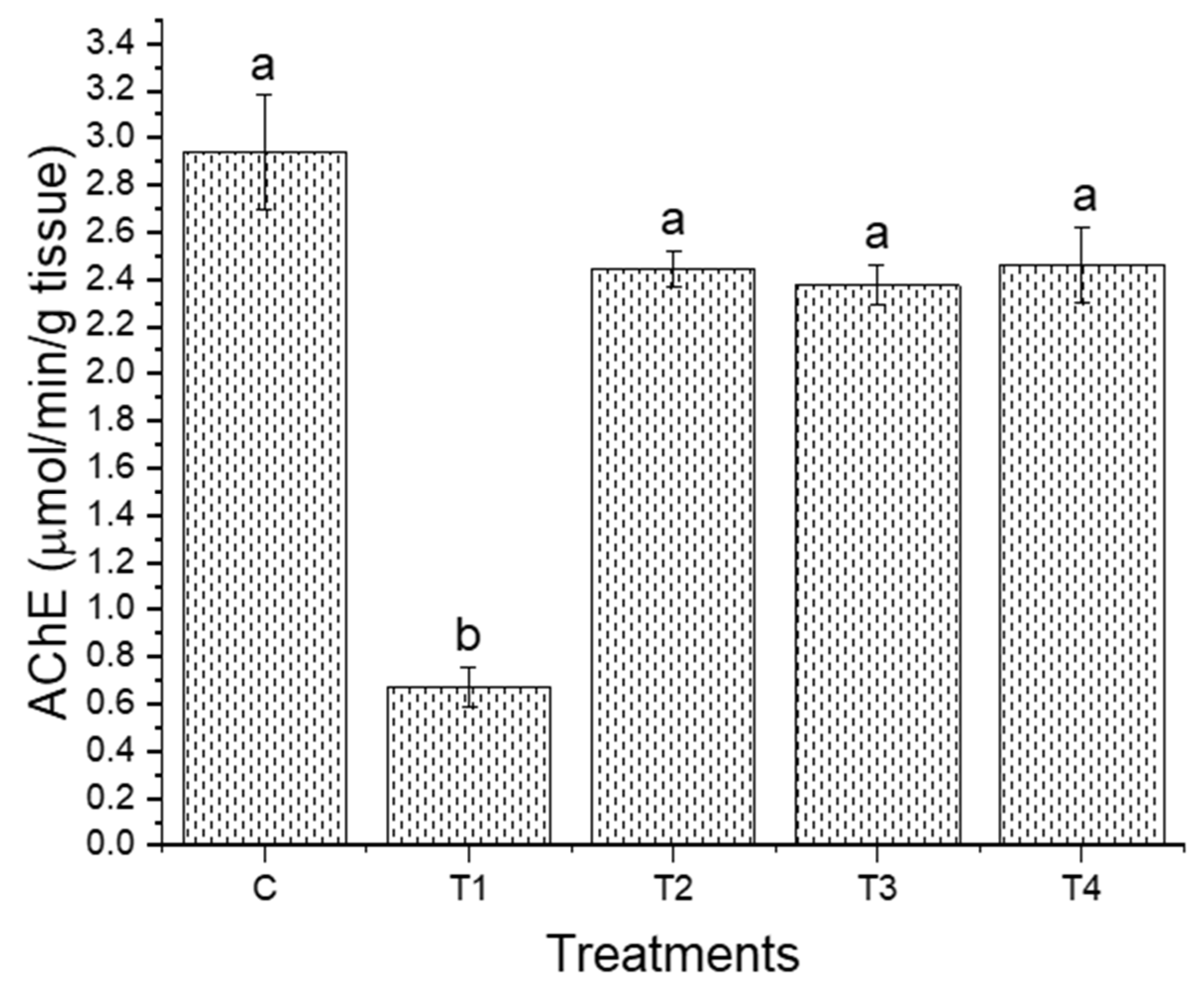
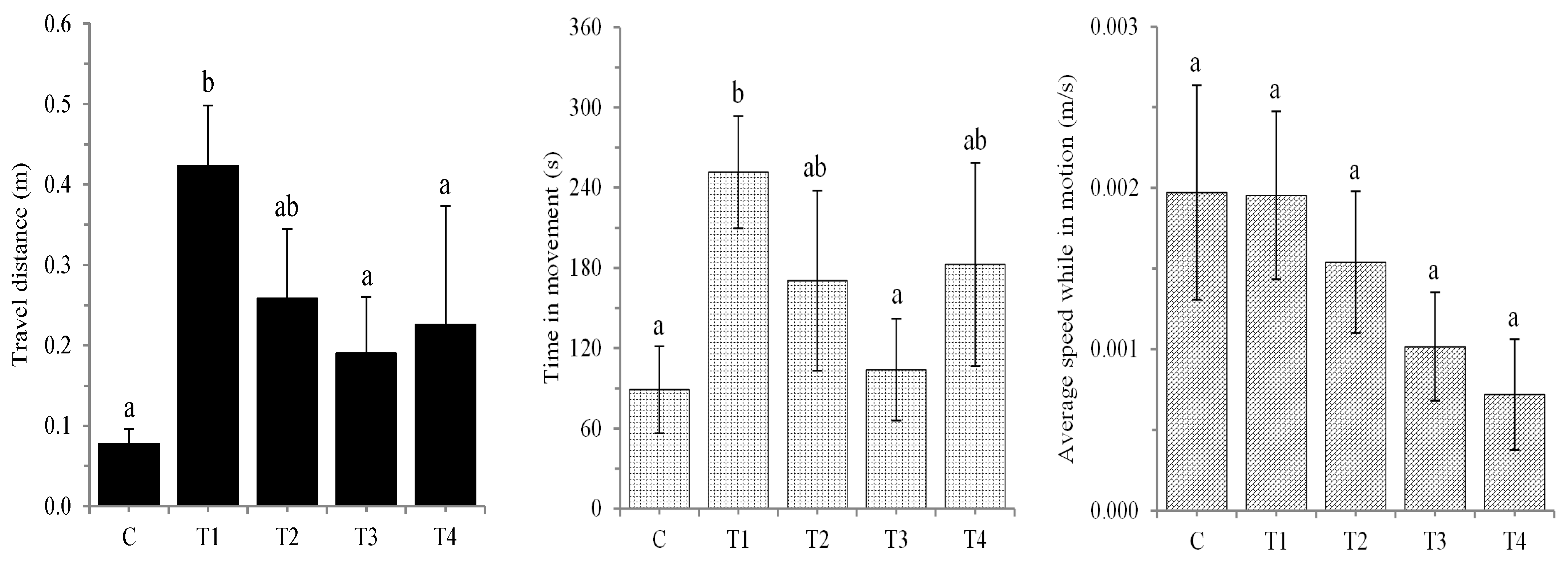
3. Results
4. Discussion
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Namieśnik, J.; Rabajczyk, A. The Speciation of Aluminum in Environmental Samples. Crit. Rev. Anal. Chem. 2010, 40, 68–88. [Google Scholar] [CrossRef]
- Rondeau, V.; Jacqmin-Gadda, H.; Commenges, D.; Helmer, C.; Dartigues, J.-F. Aluminum and Silica in Drinking Water and the Risk of Alzheimer’s Disease or Cognitive Decline: Findings from 15-Year Follow-up of the PAQUID Cohort. Am. J. Epidemiol. 2009, 169, 489–496. [Google Scholar] [CrossRef]
- Fekete, V.; Vandevijvere, S.; Bolle, F.; Van Loco, J. Estimation of Dietary Aluminum Exposure of the Belgian Adult Population: Evaluation of Contribution of Food and Kitchenware. Food Chem. Toxicol. 2013, 55, 602–608. [Google Scholar] [CrossRef]
- McFarland, G.; La Joie, E.; Thomas, P.; Lyons-Weiler, J. Acute Exposure and Chronic Retention of Aluminum in Three Vaccine Schedules and Effects of Genetic and Environmental Variation. J. Trace Elem. Med. Biol. 2020, 58, 126444. [Google Scholar] [CrossRef]
- Reinke, C.M.; Breitkreutz, J.; Leuenberger, H. Aluminium in Over-the-Counter Drugs: Risks Outweigh Benefits? Drug Saf. 2003, 26, 1011–1025. [Google Scholar] [CrossRef]
- Alasfar, R.H.; Isaifan, R.J. Aluminum Environmental Pollution: The Silent Killer. Environ. Sci. Pollut. Res. 2021, 28, 44587–44597. [Google Scholar] [CrossRef]
- Mold, M.; Cottle, J.; Exley, C. Aluminium in Brain Tissue in Epilepsy: A Case Report from Camelford. Int. J. Environ. Res. Public Health 2019, 16, 2129. [Google Scholar] [CrossRef]
- Mold, M.; Linhart, C.; Gómez-Ramírez, J.; Villegas-Lanau, A.; Exley, C. Aluminum and Amyloid-β in Familial Alzheimer’s Disease. J. Alzheimer’s Dis. 2020, 73, 1627–1635. [Google Scholar] [CrossRef]
- Mold, M.; Cottle, J.; King, A.; Exley, C. Intracellular Aluminium in Inflammatory and Glial Cells in Cerebral Amyloid Angiopathy: A Case Report. Int. J. Environ. Res. Public Health 2019, 16, 1459. [Google Scholar] [CrossRef] [PubMed]
- Yellamma, K.; Saraswathamma, S.; Kumari, B.N. Cholinergic System under Aluminium Toxicity in Rat Brain. Toxicol. Int. 2010, 17, 106. [Google Scholar] [CrossRef]
- Colovic, M.B.; Krstic, D.Z.; Lazarevic-Pasti, T.D.; Bondzic, A.M.; Vasic, V.M. Acetylcholinesterase Inhibitors: Pharmacology and Toxicology. Curr. Neuropharmacol. 2013, 11, 315–335. [Google Scholar] [CrossRef]
- Williamson, S.M.; Moffat, C.; Gomersall, M.A.E.; Saranzewa, N.; Connolly, C.N.; Wright, G.A. Exposure to Acetylcholinesterase Inhibitors Alters the Physiology and Motor Function of Honeybees. Front. Physiol. 2013, 4, 13. [Google Scholar] [CrossRef]
- Williamson, S.M.; Wright, G.A. Exposure to Multiple Cholinergic Pesticides Impairs Olfactory Learning and Memory in Honeybees. J. Exp. Biol. 2013, 216, 1799–1807. [Google Scholar] [CrossRef]
- Hasselmo, M.E. The Role of Acetylcholine in Learning and Memory. Curr. Opin. Neurobiol. 2006, 16, 710–715. [Google Scholar] [CrossRef]
- Kumar, S. Biphasic Effect of Aluminium on Cholinergic Enzyme of Rat Brain. Neurosci. Lett. 1998, 248, 121–123. [Google Scholar] [CrossRef]
- Hetnarski, B.; Wisniewski, H.M.; Iqbal, K.; Dziedzic, J.D.; Lajtha, A. Central Cholinergic Activity in Aluminum-induced Neurofibrillary Degeneration. Ann. Neurol. 1980, 7, 489–490. [Google Scholar] [CrossRef]
- Zatta, P.; Ibn-Lkhayat-Idrissi, M.; Zambenedetti, P.; Kilyen, M.; Kiss, T. In Vivo and In Vitro Effects of Aluminum on the Activity of Mouse Brain Acetylcholinesterase. Brain Res. Bull. 2002, 59, 41–45. [Google Scholar] [CrossRef]
- Peng, J.-H.F.; Xu, Z.-C.; Xu, Z.-X.; Parker, J.C.; Friedlander, E.R.; Tang, J.-P.; Melethil, S. Aluminum-Induced Acute Cholinergic Neurotoxicity in Rat. Mol. Chem. Neuropathol. 1992, 17, 79–89. [Google Scholar] [CrossRef]
- Chicas-Mosier, A.M.; Black, T.E.; Hester, K.P.; Belzunces, L.P.; Abramson, C.I. Honey Bee (Apis mellifera ligustica) Acetylcholinesterase Enzyme Activity and Aversive Conditioning Following Aluminum Trichloride Exposure. Bmc Zool. 2022, 7, 5. [Google Scholar] [CrossRef]
- Authority, E.F.S. Safety of Aluminium from Dietary Intake—Scientific Opinion of the Panel on Food Additives, Flavourings, Processing Aids and Food Contact Materials (AFC). EFSA J. 2008, 6, 754. [Google Scholar]
- Kawahara, M. Link between Aluminum Neurotoxicity and Neurodegenerative Disorders. Nihon Rinsho 2016, 74, 1176–1185. [Google Scholar]
- Martynov, V.O.; Brygadyrenko, V.V. The Impact of Some Inorganic Substances on Change in Body Mass of Tenebrio molitor (Coleoptera, Tenebrionidae) Larvae in a Laboratory Experiment. Folia Oecologica 2018, 45, 24–32. [Google Scholar] [CrossRef]
- Exley, C.; Rotheray, E.; Goulson, D. Bumblebee Pupae Contain High Levels of Aluminium. PLoS ONE 2015, 10, e0127665. [Google Scholar] [CrossRef]
- Kijak, E.; Rosato, E.; Knapczyk, K.; Pyza, E. Drosophila melanogaster as a Model System of Aluminum Toxicity and Aging. Insect Sci. 2014, 21, 189–202. [Google Scholar] [CrossRef] [PubMed]
- Clay, R.J. Developmental Toxicity of Aluminium and Silver to Drosophila melanogaster; The University of Manchester: Manchester, UK, 2014; ISBN 1083521373. [Google Scholar]
- Kabata-Pendias, A.; Mukherjee, A.B. Trace Elements of Group 12 (Previously Group IIb). In Trace Elements from Soil to Human; Springer: Berlin/Heidelberg, Germany, 2007; pp. 283–319. [Google Scholar]
- Schmitt, M.; Boras, S.; Tjoa, A.; Watanabe, T.; Jansen, S. Aluminium Accumulation and Intra-Tree Distribution Patterns in Three Arbor aluminosa (Symplocos) Species from Central Sulawesi. PLoS ONE 2016, 11, e0149078. [Google Scholar] [CrossRef] [PubMed]
- Chicas-Mosier, A.M.; Dinges, C.W.; Agosto-Rivera, J.L.; Giray, T.; Oskay, D.; Abramson, C.I. Honey Bees (Apis mellifera Spp.) Respond to Increased Aluminum Exposure in Their Foraging Choice, Motility, and Circadian Rhythmicity. PLoS ONE 2019, 14, e0218365. [Google Scholar] [CrossRef]
- Borowska, S.; Brzóska, M.M. Metals in Cosmetics: Implications for Human Health. J. Appl. Toxicol. 2015, 35, 551–572. [Google Scholar] [CrossRef]
- Aldayel, O.; Hefne, J.; Alharbi, K.N.; Al-Ajyan, T. Heavy Metals Concentration in Facial Cosmetics. Nat. Prod. Chem. Res. 2018, 6, 1–9. [Google Scholar]
- Correa, M.; Coler, R.A.; Yin, C.-M. Changes in Oxygen Consumption and Nitrogen Metabolism in the Dragonfly Somatochlora cingulata Exposed to Aluminum in Acid Waters. Hydrobiologia 1985, 121, 151–156. [Google Scholar] [CrossRef]
- Herrmann, J.; Andersson, K.G. Aluminium Impact on Respiration of Lotic Mayflies at Low PH. Water Air Soil Pollut. 1986, 30, 703–709. [Google Scholar] [CrossRef]
- Odell, T.M.; Bell, R.A.; Mastro, V.C.; Tanner, J.A.; Kennedy, L.F. Production of the Gypsy Moth, Lymantria dispar, for Research and Biological Control. In Advances and Challenges in Insect Rearing; King, E.G., Leppla, N.C., Eds.; USDA Agricultural Research Service (ARS): New Orleans, LA, USA, 1984. [Google Scholar]
- Waldbauer, G.P. The Consumption and Utilization of Food by Insects. In Advances in Insect Physiology; Elsevier: Amsterdam, The Netherlands, 1968; Volume 5, pp. 229–288. ISBN 0065-2806. [Google Scholar]
- Ellman, G.L.; Courtney, K.D.; Andres, V., Jr.; Featherstone, R.M. A New and Rapid Colorimetric Determination of Acetylcholinesterase Activity. Biochem. Pharmacol. 1961, 7, 88–95. [Google Scholar] [CrossRef]
- Trudeau, S.; Cartier, G.S. Biochemical Methods to Determine Cholinesterase Activity in Wildlife Exposed to Pesticides; Canadian Wildlife Service Hull: Hull, QC, Canada, 2000; ISBN 0662280253. [Google Scholar]
- Scharf, I.; Hanna, K.; Gottlieb, D. Experimental Arena Settings Might Lead to Misinterpretation of Movement Properties. Insect Sci. 2023, 31, 271–284. [Google Scholar] [CrossRef]
- Liu, L.; Qian, X.; Chao, M.; Zhao, Y.; Huang, J.; Wang, T.; Sun, F.; Ling, E.; Song, H. Aluminum Toxicity Related to SOD and Expression of Presenilin and CREB in Bombyx mori. Arch. Insect Biochem. Physiol. 2018, 99, e21480. [Google Scholar] [CrossRef]
- Casas, J.; Body, M.; Gutzwiller, F.; Giron, D.; Lazzari, C.R.; Pincebourde, S.; Richard, R.; Llandres, A.L. Increasing Metabolic Rate despite Declining Body Weight in an Adult Parasitoid Wasp. J. Insect Physiol. 2015, 79, 27–35. [Google Scholar] [CrossRef]
- Van der Have, T.M.; De Jong, G. Adult Size in Ectotherms: Temperature Effects on Growth and Differentiation. J. Theor. Biol. 1996, 183, 329–340. [Google Scholar] [CrossRef]
- Vlahović, M.; Ilijin, L.; Lazarević, J.; Mrdaković, M.; Gavrilović, A.; Matić, D.; Mataruga, V.P. Cadmium-Induced Changes of Gypsy Moth Larval Mass and Protease Activity. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2014, 160, 9–14. [Google Scholar] [CrossRef]
- Matić, D.; Vlahović, M.; Grčić, A.; Filipović, A.; Ilijin, L.; Mrdaković, M.; Mutić, J.; Đurđić, S.; Perić-Mataruga, V. Antioxidative Enzymes, Alkaline Phosphatases and Hsp70 Expression in Larvae of Lymantria dispar (Lepidoptera: Erebidae) from Unpolluted and Polluted Forests after Chronic Cadmium Treatment. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2023, 273, 109721. [Google Scholar] [CrossRef]
- Grčić, A.; Ilijin, L.; Filipović, A.; Matić, D.; Mrdaković, M.; Todorović, D.; Vlahović, M.; Perić-Mataruga, V. Digestive Enzyme Activity and Macromolecule Content in the Hemolymph of Differentially Adapted Lymantria dispar L. Populations after Short-Term Increases in Ambient Temperature. Environ. Res. 2023, 236, 116461. [Google Scholar] [CrossRef]
- Vlahović, M.; Matić, D.; Mutić, J.; Trifković, J.; Đurđić, S.; Perić Mataruga, V. Influence of Dietary Cadmium Exposure on Fitness Traits and Its Accumulation (with an Overview on Trace Elements) in Lymantria dispar Larvae. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2017, 200, 27–33. [Google Scholar] [CrossRef]
- Sadler, K.; Lynam, S. The Mineral Content of Some Freshwater Invertebrates in Relation to Stream PH and Calcium Concentration; Central Electricity Generating Board: Solihull, UK, 1985. [Google Scholar]
- Frick, K.G.; Herrmann, J. Aluminum Accumulation in a Lotic Mayfly at Low PH—A Laboratory Study. Ecotoxicol. Environ. Saf. 1990, 19, 81–88. [Google Scholar] [CrossRef]
- McCahon, C.P.; Pascoe, D. Short-Term Experimental Acidification of a Welsh Stream: Toxicity of Different Forms of Aluminium at Low PH to Fish and Invertebrates. Arch. Environ. Contam. Toxicol. 1989, 18, 233–242. [Google Scholar] [CrossRef]
- Zhao, L.; Qiu, J. Aluminum Bioaccumulation in the Earthworm and Acute Toxicity to the Earthworm. In Proceedings of the 2010 4th International Conference on Bioinformatics and Biomedical Engineering, Chengdu, China, 18–20 June 2010; pp. 1–4. [Google Scholar]
- Posthuma, L.; Van Straalen, N.M. Heavy-Metal Adaptation in Terrestrial Invertebrates: A Review of Occurrence, Genetics, Physiology and Ecological Consequences. Comp. Biochem. Physiol. Part C Pharmacol. Toxicol. Endocrinol. 1993, 106, 11–38. [Google Scholar] [CrossRef]
- Donker, M.H. Energy Reserves and Distribution of Metals in Populations of the Isopod Porcellio scaber from Metal-Contaminated Sites. Funct. Ecol. 1992, 6, 445–454. [Google Scholar] [CrossRef]
- Senger, M.R.; Seibt, K.J.; Ghisleni, G.C.; Dias, R.D.; Bogo, M.R.; Bonan, C.D. Aluminum Exposure Alters Behavioral Parameters and Increases Acetylcholinesterase Activity in Zebrafish (Danio rerio) Brain. Cell Biol. Toxicol. 2011, 27, 199–205. [Google Scholar] [CrossRef]
- Gulya, K.; Rakonczay, Z.; Kasa, P. Cholinotoxic Effects of Aluminum in Rat Brain. J. Neurochem. 1990, 54, 1020–1026. [Google Scholar] [CrossRef]
- Kaizer, R.R.; Corrêa, M.C.; Spanevello, R.M.; Morsch, V.M.; Mazzanti, C.M.; Gonçalves, J.F.; Schetinger, M.R.C. Acetylcholinesterase Activation and Enhanced Lipid Peroxidation after Long-Term Exposure to Low Levels of Aluminum on Different Mouse Brain Regions. J. Inorg. Biochem. 2005, 99, 1865–1870. [Google Scholar] [CrossRef]
- Quinn, D.M. Acetylcholinesterase: Enzyme Structure, Reaction Dynamics, and Virtual Transition States. Chem. Rev. 1987, 87, 955–979. [Google Scholar] [CrossRef]
- Huchard, E.; Martinez, M.; Alout, H.; Douzery, E.J.P.; Lutfalla, G.; Berthomieu, A.; Berticat, C.; Raymond, M.; Weill, M. Acetylcholinesterase Genes within the Diptera: Takeover and Loss in True Flies. Proc. R. Soc. B Biol. Sci. 2006, 273, 2595–2604. [Google Scholar] [CrossRef]
- Lingjærde, O.C.; Stenseth, N.C.; Kristoffersen, A.B.; Smith, R.H.; Jannicke Moe, S.; Read, J.M.; Daniels, S.; Simkiss, K. Exploring the Density-dependent Structure of Blowfly Populations by Nonparametric Additive Modeling. Ecology 2001, 82, 2645–2658. [Google Scholar] [CrossRef]
- Hasan, Z.; Rolle-McFarland, D.; Liu, Y.; Zhou, J.; Mostafaei, F.; Li, Y.; Fan, Q.; Zhou, Y.; Zheng, W.; Nie, L.H. Characterization of Bone Aluminum, a Potential Biomarker of Cumulative Exposure, within an Occupational Population from Zunyi, China. J. Trace Elem. Med. Biol. 2020, 59, 126469. [Google Scholar] [CrossRef]
- Wan, Y.; Liu, J.; Mai, Y.; Hong, Y.; Jia, Z.; Tian, G.; Liu, Y.; Liang, H.; Liu, J. Current Advances and Future Trends of Hormesis in Disease. npj Aging 2024, 10, 26. [Google Scholar] [CrossRef]
- Calabrese, E.J. Hormesis: Why It Is Important to Toxicology and Toxicologists. Environ. Toxicol. Chem. 2008, 27, 1451–1474. [Google Scholar] [CrossRef]
- Calabrese, E.J.; Baldwin, L.A. Defining Hormesis. Hum. Exp. Toxicol. 2002, 21, 91–97. [Google Scholar] [CrossRef]
- Erofeeva, E.A. Environmental Hormesis: From Cell to Ecosystem. Curr. Opin. Environ. Sci. Health 2022, 29, 100378. [Google Scholar] [CrossRef]
- Gunning, R.V.; Moores, G.D. Insensitive Acetylcholinesterase as Sites for Resistance to Organophosphates and Carbamates in Insects: Insensitive Acetylcholinesterase Confers Resistance in Lepidoptera. In Biochemical Sites of Insecticide Action and Resistance; Springer: Berlin/Heidelberg, Germany, 2001; pp. 221–238. [Google Scholar]
- Perić-Mataruga, V.; Petković, B.; Ilijin, L.; Mrdaković, M.; Dronjak Čučaković, S.; Todorović, D.; Vlahović, M. Cadmium and High Temperature Effects on Brain and Behaviour of Lymantria dispar L. Caterpillars Originating from Polluted and Less-Polluted Forests. Chemosphere 2017, 185, 628–636. [Google Scholar] [CrossRef]
- Sabullah, M.K.; Sulaiman, M.R.; Shukor, M.S.; Yusof, M.T.; Johari, W.L.W.; Shukor, M.Y.; Syahir, A. Heavy Metals Biomonitoring via Inhibitive Assay of Acetylcholinesterase from Periophthalmodon schlosseri. Rend. Lincei 2015, 26, 151–158. [Google Scholar] [CrossRef]
- Lukiw, W.J. Evidence Supporting a Biological Role for Aluminum in Chromatin Compaction and Epigenetics. J. Inorg. Biochem. 2010, 104, 1010–1012. [Google Scholar] [CrossRef]
- Olla, B.L. Applicability of Behavioral Measures in Environmental Stress Assessment. Rapp. P.-v. Reun. Cons. Int. Explor. Mer 1980, 179, 162–173. Available online: https://cir.nii.ac.jp/crid/1572261550530261760 (accessed on 6 November 2025).
- Van Capelleveen, H.E.; Van Straalen, N.M.; Van den Berg, M.; Van Wachem, E. Avoidance as a Mechanism of Tolerance for Lead in Terrestrial Arthropods. In Proceedings of the Third European Congress of Entomology, Amsterdam, The Netherlands, 24–29 August 1986; pp. 251–254. [Google Scholar]
- Gould, S.J.; Lloyd, E.A. Individuality and Adaptation across Levels of Selection: How Shall We Name and Generalize the Unit of Darwinism? Proc. Natl. Acad. Sci. USA 1999, 96, 11904–11909. [Google Scholar] [CrossRef]
- Kumar, V.; Gill, K.D. Oxidative Stress and Mitochondrial Dysfunction in Aluminium Neurotoxicity and Its Amelioration: A Review. Neurotoxicology 2014, 41, 154–166. [Google Scholar] [CrossRef] [PubMed]
- Barabasz, W.; Albinska, D.; Jaskowska, M.; Lipiec, J. Ecotoxicology of Aluminium. Pol. J. Environ. Stud. 2002, 11, 199–204. [Google Scholar]
- Weaver, L.T.; Laker, M.F.; Nelson, R. Intestinal Permeability in the Newborn. Arch. Dis. Child. 1984, 59, 236–241. [Google Scholar] [CrossRef]
- Berthon, G. Aluminium Speciation in Relation to Aluminium Bioavailability, Metabolism and Toxicity. Coord. Chem. Rev. 2002, 228, 319–341. [Google Scholar] [CrossRef]
| Larval Mass | Relative Growth Rate | Development Time | AChE | Travel Distance | Time in Movement | Average Speed in Motion | ||
|---|---|---|---|---|---|---|---|---|
| Larval mass | −0.314 | 0.213 | −0.257 | 0.348 | 0.143 | −0.314 | C | |
| −0.371 | 0.441 | 0.899 | 0.257 | 0.943 | −0.257 | T1 | ||
| 0.300 | 0.564 | −0.300 | −0.300 | −0.300 | 0.100 | T2 | ||
| 0.700 | 0.632 | 0.800 | −0.500 | 0.100 | 0.300 | T3 | ||
| 0.400 | −0.316 | 0.600 | −1.000 | −1.000 | −1.000 | T4 | ||
| Relative growth rate | −0.314 | −0.030 | −0.029 | 0.754 | 0.086 | 0.029 | C | |
| −0.371 | 0.177 | −0.406 | 0.257 | −0.314 | 0.657 | T1 | ||
| 0.300 | −0.205 | 0.800 | −0.700 | −0.700 | −0.600 | T2 | ||
| 0.700 | −0.105 | 0.800 | −0.800 | −0.400 | −0.300 | T3 | ||
| 0.400 | −0.632 | 0.400 | −0.400 | −0.400 | −0.400 | T4 | ||
| Development time | 0.213 | −0.030 | −0.334 | 0.185 | −0.091 | 0.091 | C | |
| 0.441 | 0.177 | 0.448 | 0.088 | 0.530 | 0.177 | T1 | ||
| 0.564 | −0.205 | −0.616 | −0.359 | −0.359 | −0.205 | T2 | ||
| 0.632 | −0.105 | 0.264 | 0.105 | 0.527 | 0.738 | T3 | ||
| −0.316 | −0.632 | 0.316 | 0.316 | 0.316 | 0.316 | T4 | ||
| AChE | −0.257 | −0.029 | −0.334 | −0.029 | 0.257 | 0.029 | C | |
| 0.899 | −0.406 | 0.448 | 0.290 | 0.986 | −0.261 | T1 | ||
| −0.300 | 0.800 | −0.616 | −0.500 | −0.500 | −0.600 | T2 | ||
| 0.800 | 0.800 | 0.264 | −0.700 | −0.500 | −0.300 | T3 | ||
| 0.600 | 0.400 | 0.316 | −0.600 | −0.600 | −0.600 | T4 | ||
| Travel distance | 0.348 | 0.754 | 0.185 | −0.029 | 0.290 | −0.232 | C | |
| 0.257 | 0.257 | 0.088 | 0.290 | 0.314 | 0.771 | T1 | ||
| −0.300 | −0.700 | −0.359 | −0.500 | 1.000 | 0.900 | T2 | ||
| −0.500 | −0.800 | 0.105 | −0.700 | 0.600 | 0.300 | T3 | ||
| −1.000 | −0.400 | 0.316 | −0.600 | 1.000 | 1.000 | T4 | ||
| Time movement | 0.143 | 0.086 | −0.091 | 0.257 | 0.290 | −0.943 | C | |
| 0.943 | −0.314 | 0.530 | 0.986 | 0.314 | −0.200 | T1 | ||
| −0.300 | −0.700 | −0.359 | −0.500 | 1.000 | 0.900 | T2 | ||
| 0.100 | −0.400 | 0.527 | −0.500 | 0.600 | 0.900 | T3 | ||
| −1.000 | −0.400 | 0.316 | −0.600 | 1.000 | 1.000 | T4 | ||
| Average speed in motion | −0.314 | 0.029 | 0.091 | 0.029 | −0.232 | −0.943 | C | |
| −0.257 | 0.657 | 0.177 | −0.261 | 0.771 | −0.200 | T1 | ||
| 0.100 | −0.600 | −0.205 | −0.600 | 0.900 | 0.900 | T2 | ||
| 0.300 | −0.300 | 0.738 | −0.300 | 0.300 | 0.900 | T3 | ||
| −1.000 | −0.400 | 0.316 | −0.600 | 1.000 | 1.000 | T4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vlahović, M.; Matić, D.; Todorović, D.; Petković, B.; Ilijin, L.; Mrdaković, M.; Perić-Mataruga, V. Assessing the Chronic Effects of Dietary Aluminum on Fitness Traits, Acetylcholinesterase Activity and Locomotion in Lymantria dispar L. Larvae. Insects 2025, 16, 1146. https://doi.org/10.3390/insects16111146
Vlahović M, Matić D, Todorović D, Petković B, Ilijin L, Mrdaković M, Perić-Mataruga V. Assessing the Chronic Effects of Dietary Aluminum on Fitness Traits, Acetylcholinesterase Activity and Locomotion in Lymantria dispar L. Larvae. Insects. 2025; 16(11):1146. https://doi.org/10.3390/insects16111146
Chicago/Turabian StyleVlahović, Milena, Dragana Matić, Dajana Todorović, Branka Petković, Larisa Ilijin, Marija Mrdaković, and Vesna Perić-Mataruga. 2025. "Assessing the Chronic Effects of Dietary Aluminum on Fitness Traits, Acetylcholinesterase Activity and Locomotion in Lymantria dispar L. Larvae" Insects 16, no. 11: 1146. https://doi.org/10.3390/insects16111146
APA StyleVlahović, M., Matić, D., Todorović, D., Petković, B., Ilijin, L., Mrdaković, M., & Perić-Mataruga, V. (2025). Assessing the Chronic Effects of Dietary Aluminum on Fitness Traits, Acetylcholinesterase Activity and Locomotion in Lymantria dispar L. Larvae. Insects, 16(11), 1146. https://doi.org/10.3390/insects16111146






