Morphological Changes in Thoracic Internal Structures of Asiophrida xanthospilota (Coleoptera: Chrysomelidae) During Pupal Period
Simple Summary
Abstract
1. Introduction
2. Materials and Methods

3. Results
3.1. Thoracic Skeletons
3.1.1. D1

3.1.2. D2

3.1.3. D4
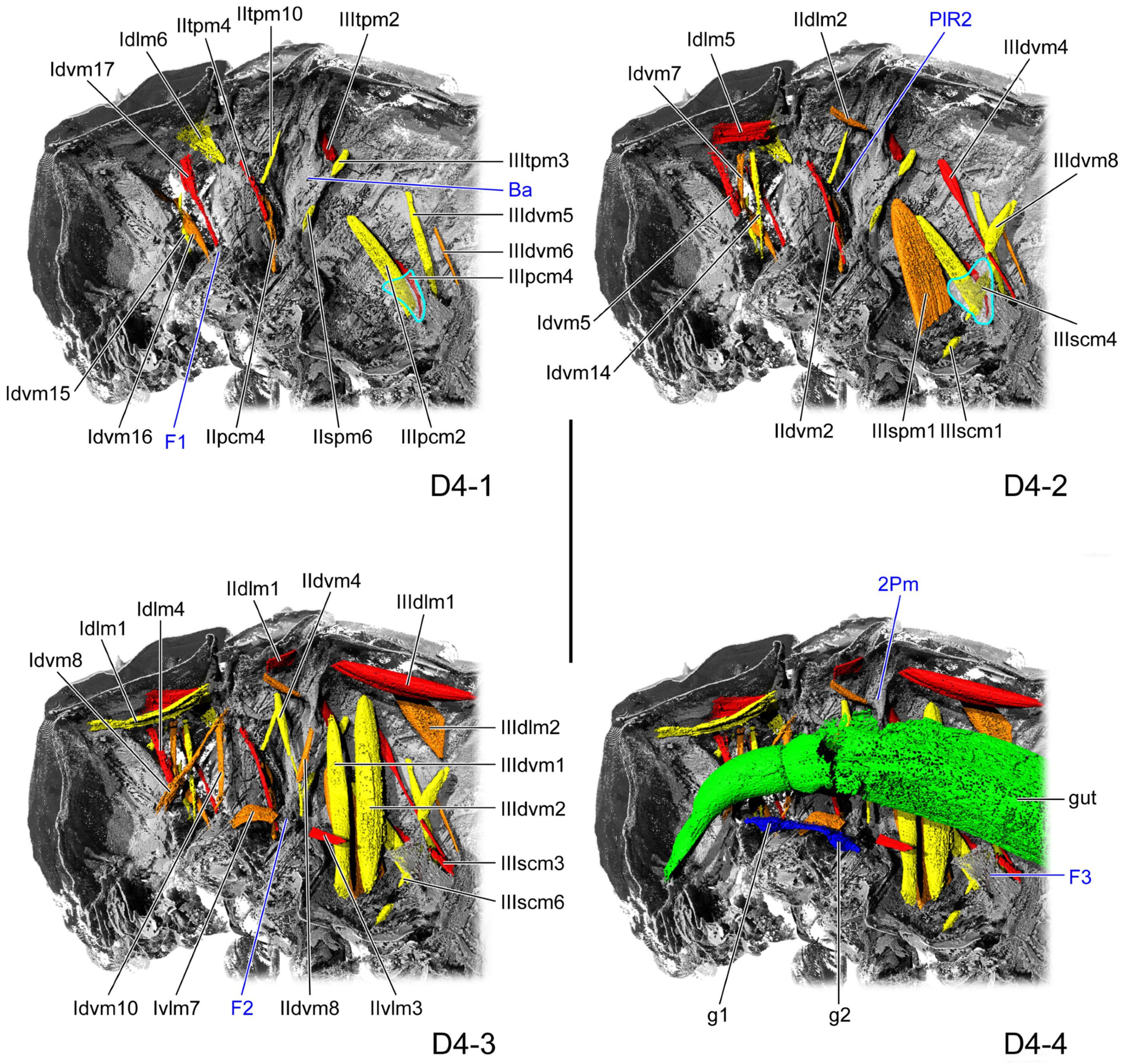


3.1.4. D6
3.1.5. D8

3.1.6. D10

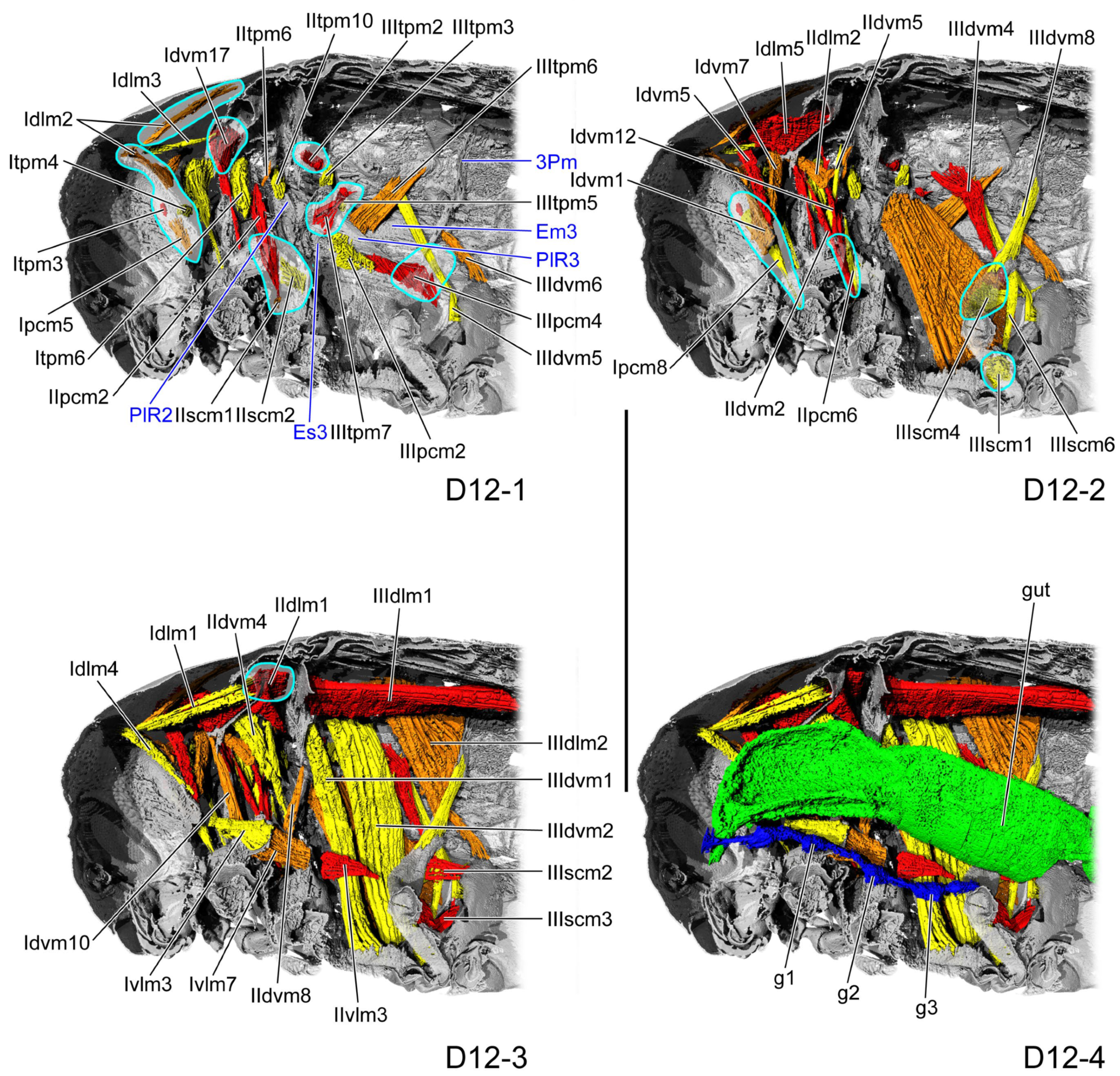
3.1.7. D12
3.1.8. Measurement
3.2. Thoracic Muscles
3.2.1. Prothoracic Muscles
- Idlm1 M. prophragma-occipitalis
- Idlm2 M. pronoto-occipitalis
- Idlm3 M. prophragma-cervicalis
- Idlm4 M. cervico-occipitalis dorsalis
- Idlm5 M. pronoto-phragmalis anterior
- Idlm6 M. pronoto-phragmalis posterior
- Idvm1 M. cervico-occipitalis anterior
- Idvm5 M. pronoto-cervicalis anterior
- Idvm7 M. pronoto-cervicalis posterior
- Idvm8 M. prophragma-tentorialis
- Idvm9 M. profurca-occipitalis
- Idvm10 M. profurca-phragmalis
- Idvm12 M. profurca-mesonotalis
- Idvm14 M. pronoto-trochantinalis posterior
- Idvm15 M. pronoto-trochantinocoxalis
- Idvm16 M. pronoto-coxalis anterior
- Idvm17 M. pronoto-coxalis posterior
- Itpm3 M. pronoto-pleualis anterior
- Itpm4 M. pronoto-apodemalis anterior
- Itpm6 M. pronoto-intersegmentalis
- Ipcm5 M. propleuro-coxalis inferior
- Ipcm8 M. propleuro-trochanteralis
- Ivlm3 M. profurca-tentorialis
- Ivlm7 M. profurca-mesofurcalis
- Iscm4 M. profurca-coxalis lateralis
3.2.2. Mesothoracic Muscles
- IIdlm1 M. prophragma-mesophragmalis
- IIdlm2 M. mesonoto-phragmalis
- IIdvm2 M. mesonoto-trochantinalis anterior
- IIdvm4 M. mesonoto-coxalis anterior
- IIdvm5 M. mesonoto-coxalis posterior
- IIdvm8 M. mesofurca-phragmalis
- IItpm4 M. mesonoto-pleuralis anterior
- IItpm6 M. mesonoto-pleuralis posterior
- IItpm7 M. mesanepisterno-axillaris
- IItpm10 M. mesepimeron-subalaris
- IIspm2 M. mesofurca-pleuralis
- IIspm6 M. mesofurca-metaepisternalis
- IIpcm2 M. mesobasalare-trochantinalis
- IIpcm4 M. mesanepisterno-coxalis posterior
- IIpcm6 M. mesopleura-trochanteralis
- IIvlm3 M. mesofurca-metafurcalis
- IIscm1 M. mesofurca-coxalis anterior
- IIscm2 M. mesofurca-coxalis posterior
3.2.3. Metathoracic Muscles
- IIIdlm1 M. mesophragma-metaphragmalis
- IIIdlm2 M. metanoto-phragmalis
- IIIdvm1 M. metanoto-sternalis
- IIIdvm2 M. metanoto-trochantinalis anterior
- IIIdvm4 M. metanoto-coxalis anterior
- IIIdvm5 M. metanoto-coxalis posterior
- IIIdvm6 M. metacoxal-subalaris
- IIIdvm8 M. metafurca-phragmalis
- IIItpm2 M. metapleural-praealaris
- IIItpm3 M. metanoto-basalaris
- IIItpm5 M. metanoto-pleuralis medialis
- IIItpm6 M. metanoto-pleuralis posterior
- IIItpm7 M. metanepisterno-axillaris
- IIIspm1 M. metapleural-sternalis
- IIIpcm2 M. metabasalar sclerite-trochantinalis
- IIIpcm3 M. metanepisterno-coxalis anterior
- IIIpcm4 M. metanepisterno-coxalis posterior
- IIIscm1 M. metafurca-coxalis anterior
- IIIscm2 M. metafurca-coxalis posterior
- IIIscm3 M. metafurca-coxalis medialis
- IIIscm4 M. metafurca-coxalis lateralis
- IIIscm6 M. metafurca-trochanteralis
3.3. Digestive System and Nervous System
4. Discussion

Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Beutel, R.G.; Friedrich, F.; Ge, S.Q.; Yang, X.K. Insect Morphology and Phylogeny, 1st ed.; Walter de Gruyter GmbH: Berlin, Germany, 2014. [Google Scholar]
- Ellington, C.P. Power and efficiency of insect flight muscle. J. Exp. Biol. 1985, 115, 293. [Google Scholar] [CrossRef] [PubMed]
- Brodsky, A.K. The Evolution of Insect Flight, 1st ed.; Oxford University Press: New York, NY, USA, 1994. [Google Scholar]
- Bullard, B.; Pastore, A. Regulating the contraction of insect flight muscle. J. Muscle Res. Cell Motil. 2011, 32, 303–313. [Google Scholar] [CrossRef] [PubMed]
- Berry, R.P.; Ibbotson, M.R. A three-dimensional atlas of the honeybee neck. PLoS ONE 2010, 5, e10771. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Koenderink, J.J.; van Doorn, A. Facts on optic flow. Biol. Cybern. 1987, 56, 247–254. [Google Scholar] [CrossRef]
- Taylor, G.K.; Krapp, H.G. Sensory systems and flight stability: What do insects measure and why? Adv. Insect Physiol. 2007, 34, 231–316. [Google Scholar]
- Graham, D. Pattern and control of walking in insects. Adv. Insect Physiol. 1985, 18, 31–140. [Google Scholar]
- Burrows, M.; Meinertzhagen, I.A.; Bräunig, P. Slowly contracting muscles power the rapid jumping of planthopper insects (Hemiptera, Issidae). Cell Tissue Res. 2014, 355, 213–222. [Google Scholar] [CrossRef]
- Liu, S.P.; Wipfler, B.; Beutel, R.G. The unique locomoter apparatus of whirligig beetles of the tribe Orectochilini (Gyrinidae, Coleoptera). J. Zool. Syst. Evol. Res. 2018, 56, 196–208. [Google Scholar] [CrossRef]
- Truman, J.W.; Riddiford, L.M. The origins of insects metamorphosis. Nature 1999, 401, 447–452. [Google Scholar] [CrossRef]
- Erezyilmaz, D.F. Imperfect eggs and oviform nymphs: A history of idea about the origins of insect metamorphsis. Integr. Comp. Biol. 2006, 46, 795–807. [Google Scholar] [CrossRef]
- Johnston, P.R.; Reynolds, S.E.; Rolff, J. (Eds.) The Evolution of Complete Metamorphosis; Royal Society: London, UK, 2019; ISSN 0962-8436. [Google Scholar]
- Rolff, J.; Johnston, P.R.; Reynolds, S. Complete metamorphosis of insects. Philos. Trans. R. Soc. Lond. B. 2019, 374, 20190063. [Google Scholar] [CrossRef] [PubMed]
- Dudley, R. The Biomechanics of Insect Flight: Form, Function and Evolution; Princeton University Press: Princeton, NJ, USA, 2000. [Google Scholar]
- Hedrick, T.L.; Combes, S.A.; Miller, L.A. Recent developments in the study of insect flight. Can. J. Zool. 2015, 93, 925–943. [Google Scholar] [CrossRef]
- Liu, H.; Ravi, S.; Kolomenskiy, D.; Tanaka, H. Biomechanics and biomimetics in insect-inspired flight systems. Philos. Trans. R. Soc. Lond. B: Biol. Sci. 2016, 371, 390. [Google Scholar] [CrossRef]
- Ge, D.Y.; Chesters, D.; Gomez-Zurita, J.; Zhang, L.J.; Yang, X.K.; Vogler, A.P. Anti-predator defense drives parallel morphological evolution in flea beetles. Proc. R. Soc. B 2011, 278, 2133–2141. [Google Scholar] [CrossRef]
- Card, G.M. Escape behaviors in insects. Curr. Opin. Neurobiol. 2012, 22, 180–186. [Google Scholar] [CrossRef]
- Chapman, R.F. The Insects Structure and Function, 5th ed.; Cambridge University Press: Cambridge, UK, 2013. [Google Scholar]
- Baly, J.S. Descriptions of new species of Galerucidæ. Trans. R. Entomol. Soc. Lond. 1881, 29, 51–59. [Google Scholar] [CrossRef]
- Ruan, Y.Y.; Konstantinov, A.S.; Shi, G.Y.; Tao, Y.; Li, Y.; Johnson, A.J.; Luo, X.Z.; Zhang, X.Y.; Zhang, M.N.; Wu, J.N.; et al. The jumping mechanism of flea beetles (Coleoptera, Chrysomelidae, Alticini), its application to bionics and preliminary design for a robotic jumping leg. Zookeys 2020, 915, 87–105. [Google Scholar] [CrossRef]
- Zong, L. Genome sequencing and molecular mechanism of jumping behavior of Ophrida xanthospilota and Agasicles hygrophila. Ph.D. Thesis, University of Chinese Academy of Sciences, Beijing, China, 2024. [Google Scholar]
- Richards, C.S.; Simonsen, T.J.; Abel, R.L.; Hall, M.J.R.; Schwyn, D.A.; Wicklein, M. Virtual forensic entomology: Improving estimates of minimum post-mortem interval with 3D micro-computed tomography. Forensic Sci. Int. 2012, 220, 251–264. [Google Scholar] [CrossRef]
- Lowe, T.; Garwood, R.J.; Simonsen, T.J.; Bradley, R.S.; Withers, P.J. Metamorphosis revealed: Time-lapse three-dimensional imaging inside a living chrysalis. J. R. Soc. Interface 2013, 10, 20130304. [Google Scholar] [CrossRef]
- Martín-Vega, D.; Simonsen, T.J.; Hall, M.J.R. Looking into the puparium: Micro-CT visualization of the internal morphological changes during metamorphosis of the blow fly, Calliphora vicina, with the first quantitative analysis of organ development in cyclorrhaphous dipterans. J. Morphol. 2017, 278, 629–651. [Google Scholar] [CrossRef]
- Helm, B.R.; Payne, S.; Rinehart, J.P.; Yocum, G.D.; Bowsher, J.H.; Greenlee, K.J. Micro-computed tomography of pupal metamorphosis in the solitary bee Megachile rotundata. Arthropod. Struct. Dev. 2018, 47, 521–528. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.J.; Ang, Y.C.; Wang, M.Q.; Gao, C.X.; Zhang, K.Y.; Tang, C.F.; Liu, X.Y.; Li, M.; Yang, D.; Meier, R. Contribution to understanding the evolution of holometaboly: Transformation of internal head structures during the metamorphosis in the green lacewing Chrysopa pallens (Neuroptera: Chrysopidae). BMC Evol. Biol. 2020, 20, 79. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.J.; Wang, M.Q.; Gao, C.X.; Li, M.; Zhang, K.Y.; Yang, D.; Liu, X.Y. Evolution of holometaboly revealed by developmental transformation of internal thoracic structures in a green lacewing Chrysopa pallens (Neuroptera: Chrysopidae). Insect Sci. 2022, 29, 767–782. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.P.; Yin, H.D.; Li, W.J.; Qin, Z.H.; Yang, Y.; Huang, Z.Z.; Zong, L.; Liu, X.K.; Du, Z.; Fan, W.L.; et al. The morphological transformation of the thorax during the eclosion of Drosophila melanogaster (Diptera: Drosophilidae). Insects 2023, 14, 893. [Google Scholar] [CrossRef]
- Ge, S.Q.; Hua, Y.; Ren, J.; Ślipiński, A.; Heming, B.; Beutel, R.G.; Yang, X.K.; Wipfler, B. Transformation of head structures during the metamorphosis of Chrysomela populi (Coleoptera: Chrysomelidae). Arthropod Syst. Phylogeny 2015, 73, 129–152. [Google Scholar] [CrossRef]
- Raś, M.; Iwan, D.; Kaminski, M.J. The tracheal system in post-embryonic development of holometabolous insects: A case study using the mealworm beetle. J. Anat. 2018, 232, 997–1015. [Google Scholar] [CrossRef]
- Vommaro, M.L.; Donato, S.; Caputo, S.; Agostino, R.G.; Montali, A.; Tettamanti, G.; Giglio, A. Anatomical changes of Tenebrio molitor and Tribolium castaneum during complete metamorphosis. Cell Tissue Res. 2024, 396, 19–40. [Google Scholar] [CrossRef]
- Shu, R.G.; Xiao, Y.Q.; Zhang, C.W.; Liu, Y.; Zhou, H.; Li, F. Micro-CT data of complete metamorphosis process in Harmonia axyridis. Sci. Data 2024, 11, 557. [Google Scholar] [CrossRef]
- Liu, S.P.; Liu, X.K.; Zhang, L.J.; Wang, X.S.; Zhang, X.Y.; Zong, L.; Li, W.J.; Huang, Z.Z.; Liu, X.; Ge, S.Q. Transformation of internal thoracic structures of Callobruchus maculatus (Coleoptera: Bruchidae) from larva to adult. Insects 2025, 16, 324. [Google Scholar] [CrossRef]
- Zhang, L.J.; Yang, X.K. Description of the immature stages of Ophrida xanthospilota (Baly) (Coleoptera: Chrysomelidae: Galerucinae) from China. Proc. Entomol. Soc. Wash. 2008, 110, 693–700. [Google Scholar] [CrossRef]
- Ruan, Y.Y.; Zhang, M.N.; Kundrata, R.; Qiu, L.; Ge, S.Q.; Yang, X.K.; Chen, X.Q.; Jiang, S.H. Functional morphology of the thorax of the click beetle Campsosternus auratus (Coleoptera, Elateridae), with an emphasis on its jumping mechanism. Insects 2022, 13, 248. [Google Scholar] [CrossRef]
- Friedrich, F.; Beutel, R.G. The thorax of Zorotypus (Hexapoda, Zoraptera) and a new nomenclature for the musculature of Neoptera. Arthropod. Struct. Dev. 2008, 37, 29–54. [Google Scholar] [CrossRef]
- Hartenstein, V. Atlas of Drosophila development. In The Development of Drosophila melanogaster; Bate, M., Arias, A.M., Eds.; Cold Spring Harbor Press: New York, NY, USA, 1993; Volume 2, pp. 1–53. [Google Scholar]
| D1 | D2 | D4 | D6 | D8 | D10 | D12 | |
|---|---|---|---|---|---|---|---|
| Length | 2.07 | 3.03 | 3.46 | 3.23 | 2.32 | 2.68 | 2.95 |
| 2.05 | 2.92 | 3.35 | 3.17 | 2.33 | 2.66 | 3.00 | |
| 2.06 | 2.94 | 3.39 | 3.13 | 2.34 | 2.63 | 2.96 | |
| Width | 1.83 | 2.21 | 2.50 | 2.42 | 1.59 | 1.97 | 1.80 |
| 1.79 | 2.34 | 2.54 | 2.30 | 1.59 | 2.04 | 1.75 | |
| 1.84 | 2.26 | 2.58 | 2.33 | 1.56 | 2.09 | 1.81 | |
| Heigh | 1.71 | 2.13 | 2.31 | 2.02 | 1.77 | 1.55 | 1.99 |
| 1.66 | 2.21 | 2.35 | 1.91 | 1.66 | 1.55 | 1.86 | |
| 1.67 | 2.26 | 2.31 | 1.92 | 1.73 | 1.56 | 1.94 | |
| Product | 6.48 | 14.3 | 20.0 | 15.8 | 6.53 | 8.18 | 10.6 |
| 6.09 | 15.1 | 20.0 | 13.9 | 6.15 | 8.41 | 9.77 | |
| 6.33 | 15.0 | 20.2 | 14.0 | 6.32 | 8.57 | 10.4 | |
| Mean of products | 6.30 | 14.8 | 20.1 | 14.6 | 6.33 | 8.39 | 10.3 |
| D1 | D2 | D4 | D6 | D8 | D10 | D12 | |
|---|---|---|---|---|---|---|---|
| Prothorax | |||||||
| Idlm1 | + | + | + | + | + | + | + |
| Idlm2 | − | − | − | + | + | + | + |
| Idlm3 | + | − | − | + | − | − | + |
| Idlm4 | + | − | + | + | + | + | + |
| Idlm5 | + | + | + | + | + | + | + |
| Idlm6 | + | + | + | − | + | − | − |
| Idvm1 | + | + | − | + | − | + | + |
| Idvm5 | + | + | + | + | + | + | + |
| Idvm7 | − | − | + | + | − | + | + |
| Idvm8 | + | + | + | − | + | + | − |
| Idvm9 | + | − | − | − | − | − | − |
| Idvm10 | − | + | + | + | + | + | + |
| Idvm12 | − | − | − | − | − | + | + |
| Idvm14 | − | − | + | − | − | − | − |
| Idvm15 | − | − | + | − | − | − | − |
| Idvm16 | + | + | + | + | + | + | + |
| Idvm17 | + | + | + | + | + | + | + |
| Itpm3 | + | − | − | − | + | + | + |
| Itpm4 | + | + | − | − | − | + | + |
| Itpm6 | + | − | − | − | − | − | + |
| Ipcm5 | + | − | − | − | − | + | + |
| Ipcm8 | − | − | − | + | − | + | + |
| Ivlm3 | + | + | − | + | + | + | + |
| Ivlm7 | + | + | + | + | + | + | + |
| Iscm4 | − | + | − | − | − | − | − |
| Sum (25) | 17 | 13 | 13 | 14 | 13 | 18 | 19 |
| Mesothorax | |||||||
| IIdlm1 | + | + | + | − | + | + | + |
| IIdlm2 | + | + | + | − | − | + | + |
| IIdvm2 | + | − | + | − | − | + | + |
| IIdvm4 | + | + | + | + | + | + | + |
| IIdvm5 | + | − | − | − | + | − | + |
| IIdvm8 | + | + | + | + | − | + | + |
| IItpm4 | − | + | + | − | + | − | − |
| Itpm6 | + | − | − | − | + | + | + |
| IItpm7 | − | − | − | − | − | + | − |
| IItpm10 | + | − | + | + | − | + | + |
| IIspm2 | − | − | − | + | − | + | − |
| IIspm6 | + | + | + | + | − | − | − |
| IIpcm2 | + | − | − | + | + | + | + |
| IIpcm4 | − | + | + | + | + | − | − |
| IIpcm6 | − | − | − | − | − | + | + |
| IIvlm3 | + | + | + | + | − | + | + |
| IIscm1 | − | − | − | − | − | + | + |
| IIscm2 | − | − | − | − | − | + | + |
| Sum (18) | 11 | 8 | 10 | 8 | 7 | 14 | 13 |
| Metathorax | |||||||
| IIIdlm1 | + | + | + | + | + | + | + |
| IIIdlm2 | + | + | + | + | + | + | + |
| IIIdvm1 | + | + | + | + | + | + | + |
| IIIdvm2 | + | + | + | + | + | + | + |
| IIIdvm4 | − | + | + | − | + | + | + |
| IIIdvm5 | + | + | + | + | + | + | + |
| IIIdvm6 | − | − | + | − | + | + | + |
| IIIdvm8 | + | + | + | + | + | + | + |
| IIItpm2 | − | + | + | + | + | + | + |
| IIItpm3 | − | + | + | − | − | + | + |
| IIItpm5 | − | − | − | − | − | − | + |
| IIItpm6 | − | + | − | + | − | + | + |
| IIItpm7 | − | − | − | + | + | + | + |
| IIIspm1 | − | + | + | + | + | + | + |
| IIIpcm2 | − | + | + | + | − | − | + |
| IIIpcm3 | − | + | − | − | − | − | − |
| IIIpcm4 | − | − | + | − | − | + | + |
| IIIscm1 | − | − | + | − | + | + | + |
| IIIscm2 | − | − | − | − | − | + | + |
| IIIscm3 | − | + | + | − | + | + | + |
| IIIscm4 | + | + | + | − | + | + | + |
| IIIscm6 | − | − | + | − | − | + | + |
| Sum (22) | 7 | 15 | 17 | 11 | 14 | 19 | 21 |
| Total number of thoracic muscles | |||||||
| 65 | 35 | 36 | 40 | 33 | 34 | 51 | 53 |
| D1 | D2 | D4 | D6 | D8 | D10 | D12 | |
|---|---|---|---|---|---|---|---|
| Prothorax | |||||||
| Idlm1 | 4.355 | 4.739 | 12.98 | 33.82 | 12.77 | 18.05 | 24.06 |
| Idlm2 | − | − | − | 4.919 | 8.458 | 4.349 | 19.30 |
| Idlm3 | 2.150 | − | − | 7.747 | − | − | 4.972 |
| Idlm4 | 6.661 | − | 1.135 | 8.356 | 5.321 | 15.64 | 9.480 |
| Idlm5 | 10.06 | 17.09 | 8.952 | 12.76 | 13.37 | 55.76 | 63.00 |
| Idlm6 | 16.20 | 25.65 | 8.939 | − | 11.35 | − | − |
| Idvm1 | 7.646 | 5.957 | − | 6.727 | − | 15.84 | 25.34 |
| Idvm5 | 12.23 | 8.209 | 4.142 | 9.838 | 11.78 | 12.36 | 22.98 |
| Idvm7 | − | − | 2.715 | 9.282 | − | 37.12 | 44.19 |
| Idvm8 | 12.71 | 14.61 | 9.162 | − | 1.576 | 2.059 | − |
| Idvm9 | 0.9397 | − | − | − | − | − | − |
| Idvm10 | − | 7.095 | 4.265 | 7.347 | 5.944 | 14.12 | 18.29 |
| Idvm12 | − | − | − | − | − | 21.79 | 4.886 |
| Idvm14 | − | − | 3.558 | − | − | − | − |
| Idvm15 | − | − | 9.892 | − | − | − | − |
| Idvm16 | 5.248 | 20.91 | 1.858 | 8.031 | 23.87 | 14.44 | 46.74 |
| Idvm17 | 5.116 | 15.43 | 7.365 | 20.97 | 25.16 | 7.789 | 40.07 |
| Itpm3 | 1.219 | − | − | − | 1.340 | 3.250 | 1.332 |
| Itpm4 | 10.34 | 3.067 | − | − | − | 8.961 | 4.787 |
| Itpm6 | 1.866 | − | − | − | − | − | 18.57 |
| Ipcm5 | 1.986 | − | − | − | − | 12.48 | 6.129 |
| Ipcm8 | − | − | − | 18.08 | − | 22.90 | 32.22 |
| Ivlm3 | 25.67 | 35.60 | − | 5.646 | 5.404 | 29.12 | 31.79 |
| Ivlm7 | 44.66 | 12.90 | 9.056 | 15.88 | 7.227 | 32.16 | 49.69 |
| Iscm4 | − | 4.319 | − | − | − | − | − |
| Mesothorax | |||||||
| IIdlm1 | 8.394 | 4.847 | 3.422 | − | 10.31 | 47.91 | 50.54 |
| IIdlm2 | 0.5124 | 5.537 | 2.662 | − | − | 8.570 | 19.50 |
| IIdvm2 | 10.02 | − | 1.571 | − | − | 23.56 | 8.732 |
| IIdvm4 | 6.964 | 11.72 | 5.748 | 11.07 | 12.72 | 24.41 | 35.56 |
| IIdvm5 | 14.97 | − | − | − | 3.913 | − | 8.828 |
| IIdvm8 | 8.294 | 7.860 | 2.609 | 6.572 | − | 6.094 | 7.309 |
| IItpm4 | − | 4.681 | 1.725 | − | 4.531 | − | − |
| Itpm6 | 3.307 | − | − | − | 3.284 | 1.244 | 0.9824 |
| IItpm7 | − | − | − | − | − | 5.512 | − |
| IItpm10 | 1.998 | − | 1.263 | 2.876 | − | 6.477 | 10.53 |
| IIspm2 | − | − | − | 7.861 | − | 7.056 | − |
| IIspm6 | 3.758 | 10.73 | 1.996 | 4.395 | − | − | − |
| IIpcm2 | 6.280 | − | − | 12.29 | 1.245 | 12.41 | 20.84 |
| IIpcm4 | − | 1.055 | 4.753 | 6.886 | 3.796 | − | − |
| IIpcm6 | − | − | − | − | − | 11.70 | 9.947 |
| IIvlm3 | 64.14 | 18.43 | 2.411 | 0.8039 | − | 10.66 | 12.43 |
| IIscm1 | − | − | − | − | − | 12.98 | 2.762 |
| IIscm2 | − | − | − | − | − | 12.62 | 13.00 |
| Metathorax | |||||||
| IIIdlm1 | 12.84 | 61.92 | 60.87 | 61.37 | 98.42 | 173.0 | 252.7 |
| IIIdlm2 | 11.75 | 28.74 | 39.40 | 50.82 | 59.92 | 256.3 | 238.3 |
| IIIdvm1 | 32.36 | 45.54 | 51.52 | 34.05 | 89.32 | 157.3 | 198.7 |
| IIIdvm2 | 15.36 | 77.63 | 94.67 | 145.7 | 166.5 | 327.0 | 483.7 |
| IIIdvm4 | − | 11.62 | 6.704 | − | 38.04 | 70.03 | 38.06 |
| IIIdvm5 | 7.978 | 8.574 | 10.50 | 11.06 | 10.65 | 18.25 | 42.03 |
| IIIdvm6 | − | − | 1.643 | − | 14.24 | 38.87 | 10.71 |
| IIIdvm8 | 6.792 | 19.64 | 8.207 | 14.73 | 23.22 | 12.57 | 24.08 |
| IIItpm2 | − | 6.726 | 3.808 | 5.266 | 6.320 | 6.202 | 8.134 |
| IIItpm3 | − | 4.431 | 2.120 | − | − | 10.20 | 6.736 |
| IIItpm5 | − | − | − | − | − | − | 2.759 |
| IIItpm6 | − | 5.960 | − | 11.77 | − | 12.71 | 23.69 |
| IIItpm7 | − | − | − | 7.150 | 3.235 | 13.84 | 9.411 |
| IIIspm1 | − | 65.29 | 72.67 | 123.5 | 133.2 | 313.2 | 474.6 |
| IIIpcm2 | − | 16.73 | 21.97 | 47.22 | − | − | 17.46 |
| IIIpcm3 | − | 6.115 | − | − | − | − | − |
| IIIpcm4 | − | − | 3.528 | − | − | 35.51 | 38.81 |
| IIIscm1 | − | − | 3.208 | − | 9.336 | 22.33 | 32.23 |
| IIIscm2 | − | − | − | − | − | 47.24 | 5.493 |
| IIIscm3 | − | 5.039 | 1.336 | − | 10.15 | 30.39 | |
| IIIscm4 | 1.507 | 9.527 | 5.420 | − | 2.760 | 24.44 | 28.48 |
| IIIscm6 | − | − | 2.209 | − | − | 18.61 | 14.38 |
| D1 | D2 | D4 | D6 | D8 | D10 | D12 | |
|---|---|---|---|---|---|---|---|
| Prothorax | |||||||
| Idlm1 | 6.91 | 3.20 | 6.46 | 23.2 | 2.02 | 21.5 | 23.4 |
| Idlm2 | − | − | − | 3.37 | 13.4 | 5.18 | 18.7 |
| Idlm3 | 3.41 | − | − | 5.31 | − | − | 4.83 |
| Idlm4 | 10.6 | − | 0.565 | 5.72 | 8.41 | 18.6 | 9.20 |
| Idlm5 | 16.0 | 11.5 | 4.45 | 8.74 | 21.1 | 66.5 | 61.2 |
| Idlm6 | 25.7 | 17.3 | 4.45 | − | 17.9 | − | − |
| Idvm1 | 12.1 | 4.03 | − | 4.61 | − | 18.9 | 24.6 |
| Idvm5 | 19.4 | 5.55 | 2.06 | 6.74 | 18.6 | 14.7 | 22.3 |
| Idvm7 | − | − | 1.35 | 6.36 | 44.2 | 42.9 | |
| Idvm8 | 20.2 | 9.87 | 4.56 | − | 2.49 | 2.45 | − |
| Idvm9 | 1.49 | − | − | − | − | − | − |
| Idvm10 | − | 4.79 | 2.12 | 5.03 | 9.39 | 16.8 | 17.8 |
| Idvm12 | − | − | − | − | − | 26.0 | 4.74 |
| Idvm14 | − | − | 1.77 | − | − | − | − |
| Idvm15 | − | − | 4.92 | − | − | − | − |
| Idvm16 | 8.33 | 14.1 | 0.924 | 5.50 | 37.7 | 17.2 | 45.4 |
| Idvm17 | 8.12 | 10.4 | 3.66 | 14.4 | 39.7 | 9.28 | 38.9 |
| Itpm3 | 1.93 | − | − | − | 2.12 | 3.87 | 1.29 |
| Itpm4 | 16.4 | 2.07 | − | − | − | 10.7 | 4.65 |
| Itpm6 | 2.96 | − | − | − | − | − | 18.0 |
| Ipcm5 | 3.15 | − | − | − | − | 14.9 | 5.95 |
| Ipcm8 | − | − | − | 12.4 | − | 27.3 | 31.3 |
| Ivlm3 | 40.7 | 24.1 | − | 3.87 | 8.54 | 34.7 | 30.9 |
| Ivlm7 | 70.9 | 8.72 | 4.51 | 10.9 | 11.4 | 38.3 | 48.2 |
| Iscm4 | − | 2.92 | − | − | − | − | − |
| Mesothorax | |||||||
| IIdlm1 | 13.3 | 3.28 | 1.70 | − | 16.3 | 57.1 | 49.1 |
| IIdlm2 | 0.813 | 3.74 | 1.32 | − | − | 10.2 | 18.9 |
| IIdvm2 | 15.9 | − | 0.782 | − | − | 28.1 | 8.48 |
| IIdvm4 | 11.1 | 7.92 | 2.86 | 7.58 | 20.1 | 29.1 | 34.5 |
| IIdvm5 | 23.8 | − | − | − | 6.18 | − | 8.57 |
| IIdvm8 | 13.2 | 5.31 | 1.30 | 4.50 | − | 7.26 | 7.10 |
| IItpm4 | − | 3.16 | 0.858 | − | 7.16 | − | − |
| Itpm6 | 5.25 | − | − | − | 5.19 | 1.48 | 0.954 |
| IItpm7 | − | − | − | − | − | 6.60 | − |
| IItpm10 | 3.17 | − | 0.628 | 1.97 | − | 7.72 | 10.2 |
| IIspm2 | − | − | − | 5.38 | − | 8.41 | − |
| IIspm6 | 5.97 | 7.25 | 0.993 | 3.01 | − | − | − |
| IIpcm2 | 9.97 | − | − | 8.42 | 1.97 | 14.8 | 20.2 |
| IIpcm4 | − | 0.713 | 2.36 | 4.72 | 6.00 | − | − |
| IIpcm6 | − | − | − | − | − | 13.9 | 9.66 |
| IIvlm3 | 102 | 12.5 | 1.20 | 0.551 | − | 12.7 | 12.1 |
| IIscm1 | − | − | − | − | − | 15.5 | 2.68 |
| IIscm2 | − | − | − | − | − | 15.0 | 12.6 |
| Metathorax | |||||||
| IIIdlm1 | 20.4 | 41.8 | 30.3 | 42.0 | 155 | 206 | 245 |
| IIIdlm2 | 18.7 | 19.4 | 19.6 | 34.8 | 94.7 | 305 | 231 |
| IIIdvm1 | 51.4 | 30.8 | 25.6 | 23.3 | 141 | 187 | 193 |
| IIIdvm2 | 24.4 | 52.5 | 47.1 | 99.8 | 263 | 390 | 470 |
| IIIdvm4 | − | 7.85 | 3.34 | − | 60.1 | 83.5 | 37.0 |
| IIIdvm5 | 12.7 | 5.79 | 5.22 | 7.58 | 16.8 | 21.8 | 40.8 |
| IIIdvm6 | − | − | 0.817 | − | 22.5 | 46.3 | 10.4 |
| IIIdvm8 | 10.8 | 13.3 | 4.08 | 10.1 | 36.7 | 15.0 | 23.4 |
| IIItpm2 | − | 4.54 | 1.89 | 3.61 | 9.98 | 7.39 | 7.90 |
| IIItpm3 | − | 2.99 | 1.05 | − | − | 12.2 | 6.54 |
| IIItpm5 | − | − | − | − | − | − | 2.68 |
| IIItpm6 | − | 4.03 | − | 8.06 | − | 15.1 | 23.0 |
| IIItpm7 | − | − | − | 4.90 | 5.11 | 16.5 | 9.14 |
| IIIspm1 | − | 44.1 | 36.2 | 84.6 | 210 | 373 | 461 |
| IIIpcm2 | − | 11.3 | 10.9 | 32.3 | − | − | 17.0 |
| IIIpcm3 | − | 4.13 | − | − | − | − | − |
| IIIpcm4 | − | − | 1.76 | − | − | 42.3 | 37.7 |
| IIIscm1 | − | − | 1.60 | − | 14.7 | 26.6 | 31.3 |
| IIIscm2 | − | − | − | − | − | 56.3 | 5.33 |
| IIIscm3 | − | 3.40 | 0.665 | − | 16.0 | 29.5 | |
| IIIscm4 | 2.39 | 6.44 | 2.70 | − | 4.36 | 29.1 | 27.7 |
| IIIscm6 | − | − | 1.10 | − | − | 22.2 | 14.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Haider, I.; Zong, L.; Li, W.; Yang, Y.; Liang, Z.; Zhou, X.; Wang, Y.; Liu, S.; Ge, S. Morphological Changes in Thoracic Internal Structures of Asiophrida xanthospilota (Coleoptera: Chrysomelidae) During Pupal Period. Insects 2025, 16, 1133. https://doi.org/10.3390/insects16111133
Haider I, Zong L, Li W, Yang Y, Liang Z, Zhou X, Wang Y, Liu S, Ge S. Morphological Changes in Thoracic Internal Structures of Asiophrida xanthospilota (Coleoptera: Chrysomelidae) During Pupal Period. Insects. 2025; 16(11):1133. https://doi.org/10.3390/insects16111133
Chicago/Turabian StyleHaider, Irfan, Le Zong, Wenjie Li, Youyou Yang, Zulong Liang, Xinyi Zhou, Yanting Wang, Sipei Liu, and Siqin Ge. 2025. "Morphological Changes in Thoracic Internal Structures of Asiophrida xanthospilota (Coleoptera: Chrysomelidae) During Pupal Period" Insects 16, no. 11: 1133. https://doi.org/10.3390/insects16111133
APA StyleHaider, I., Zong, L., Li, W., Yang, Y., Liang, Z., Zhou, X., Wang, Y., Liu, S., & Ge, S. (2025). Morphological Changes in Thoracic Internal Structures of Asiophrida xanthospilota (Coleoptera: Chrysomelidae) During Pupal Period. Insects, 16(11), 1133. https://doi.org/10.3390/insects16111133







