Bottom-Up and Top-Down Dynamics in the Management of Rosy Apple Aphid
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Apple Seedlings
2.2. Aphids
2.3. Parasitoids
2.4. Experimental Design
2.5. Experiment 1: Single Parasitoid Species with Mono-Cultivar System
2.6. Experiment 2: Single Parasitoid Species with Multi-Cultivar System
2.7. Experiment 3: Mixed Parasitoid Species with Mono-Cultivar System
2.8. Experiment 4: Mixed Parasitoid Species with Multi-Cultivar System
2.9. Data Analysis
3. Results
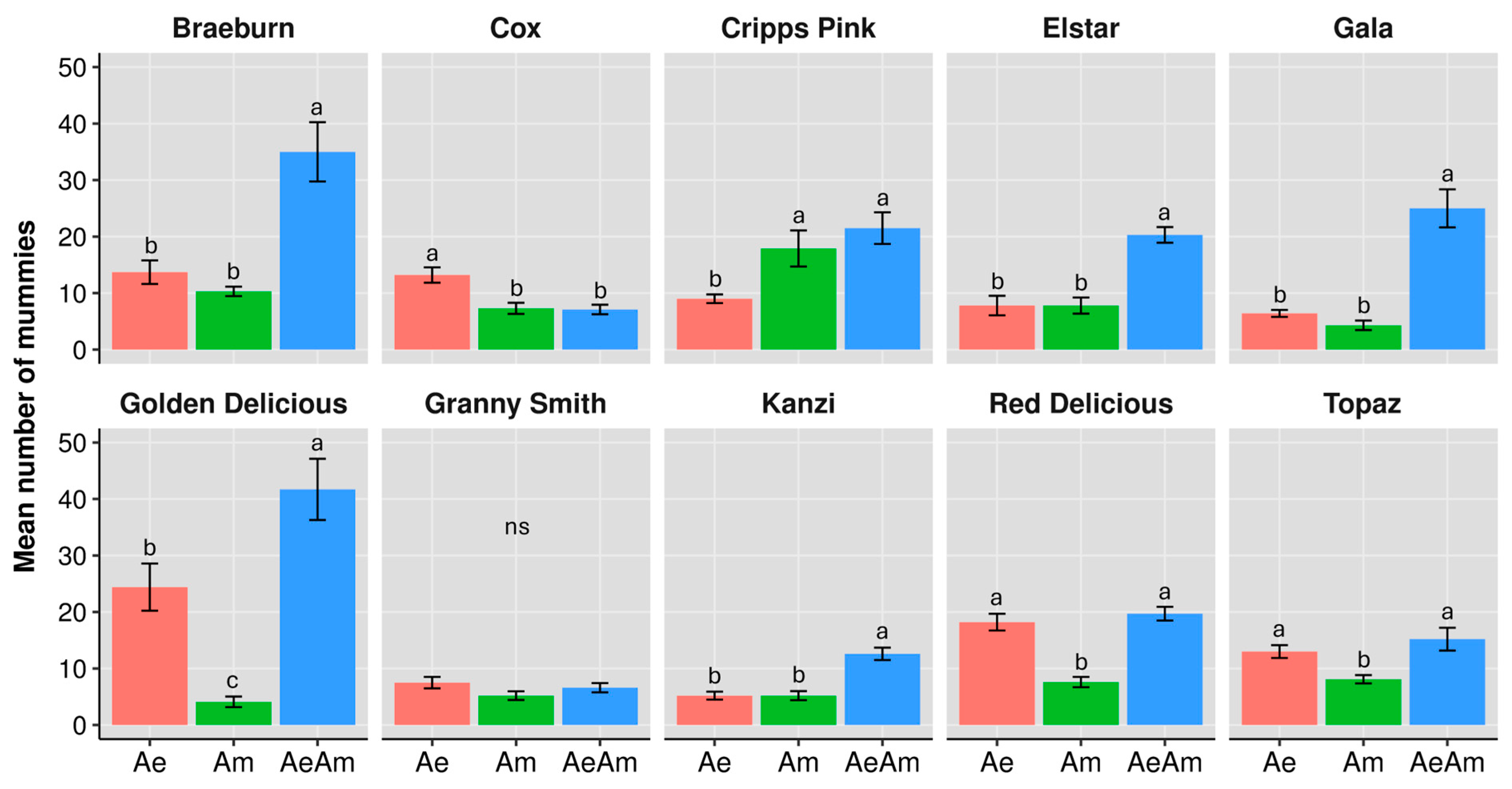
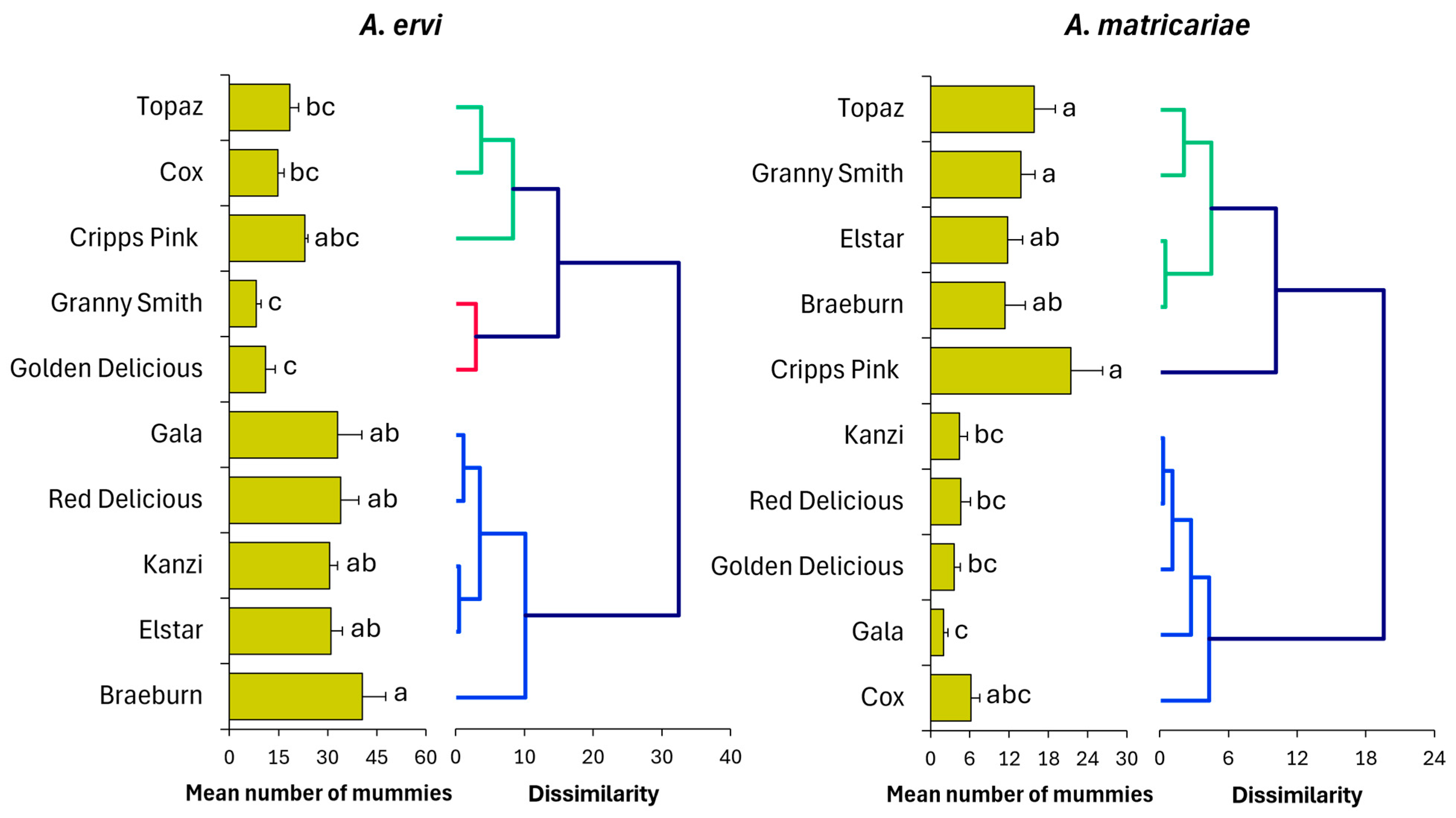
3.1. Mummy Production
3.1.1. Cultivar-Driven Bottom-Up Effects
3.1.2. Interspecific Interaction Effects
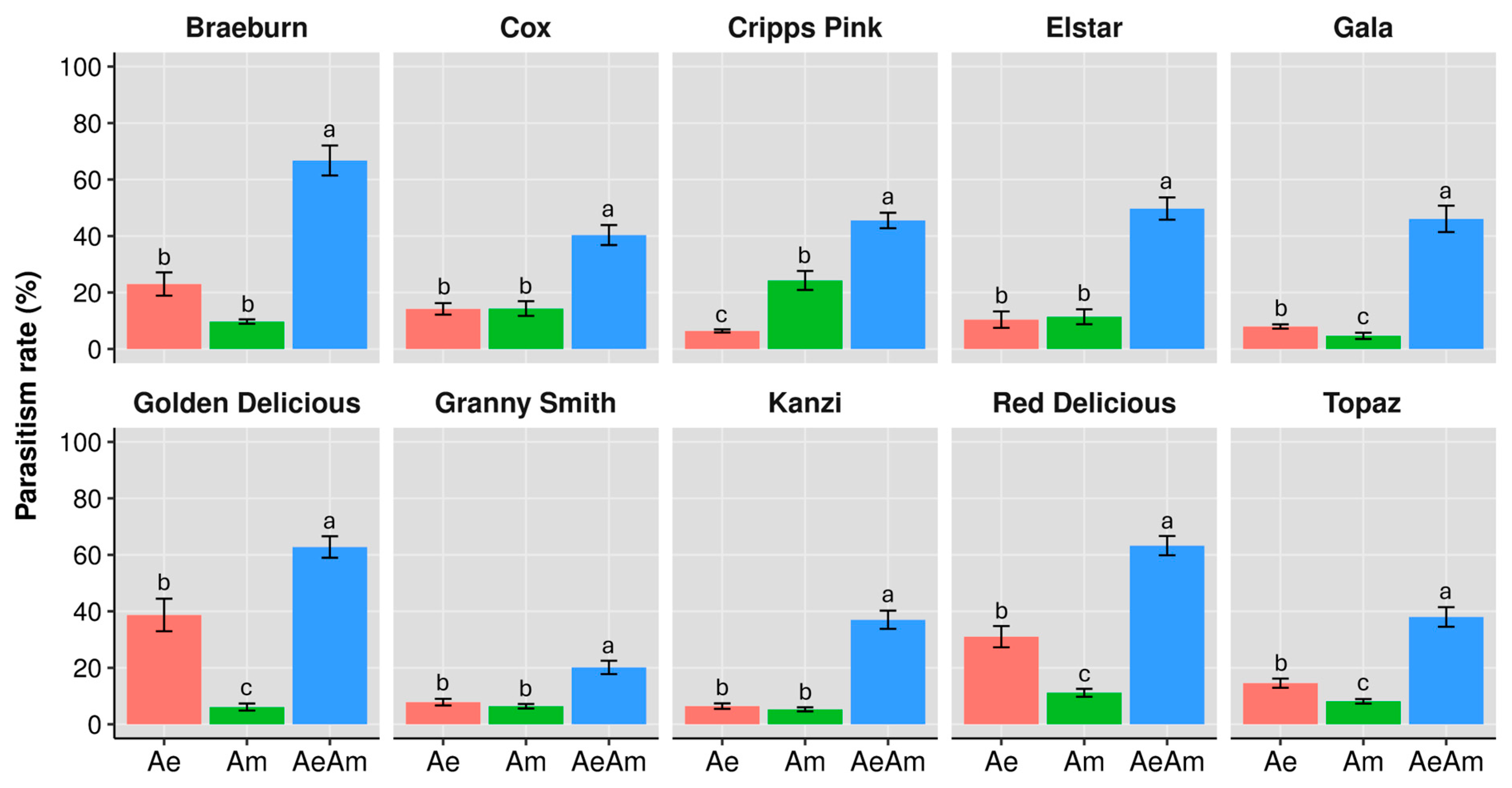
3.2. Parasitism Rate
3.2.1. Cultivar-Driven Bottom-Up Effects
3.2.2. Interspecific Interaction Effects
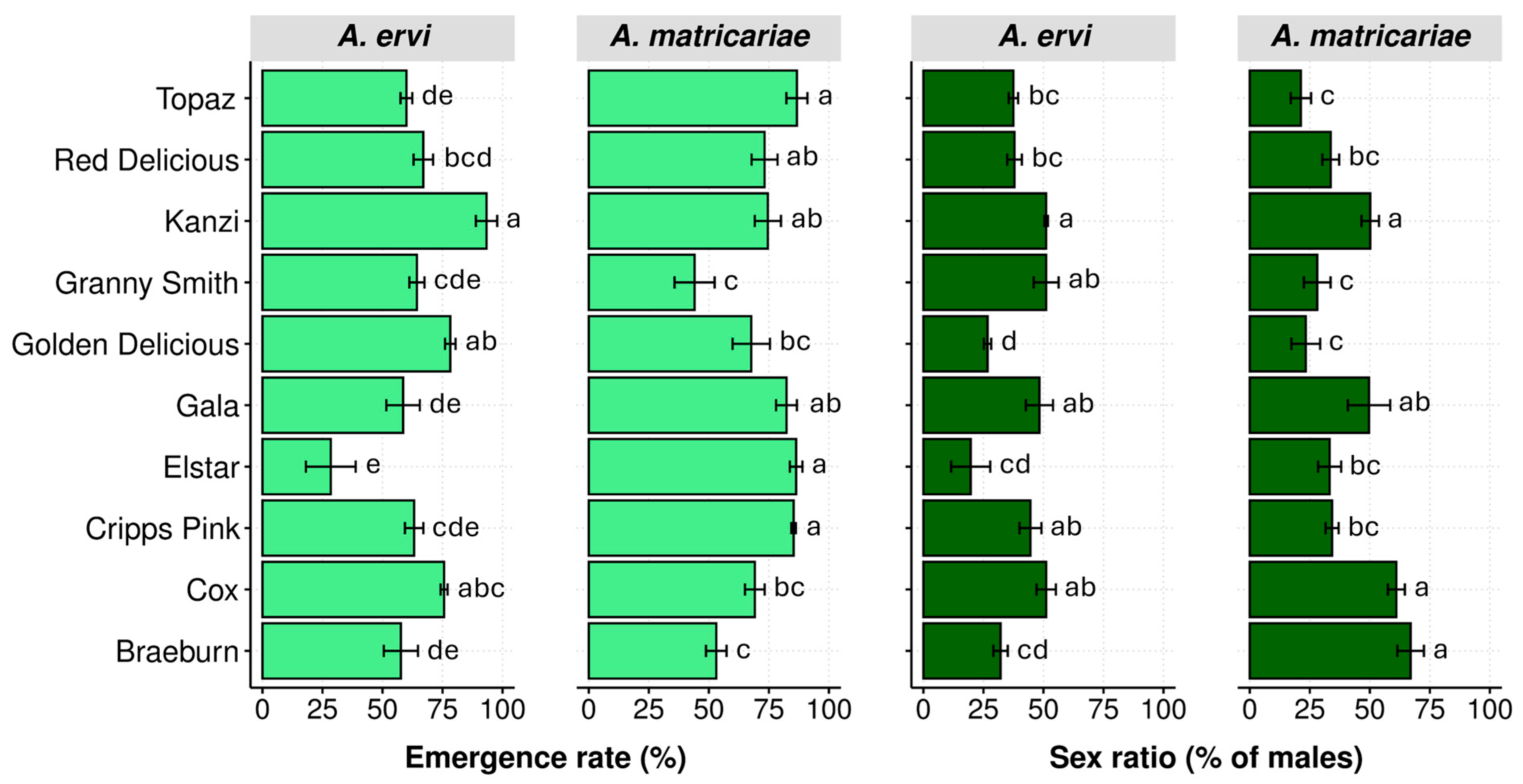
3.3. Emergence Rate
3.4. Sex Ratio
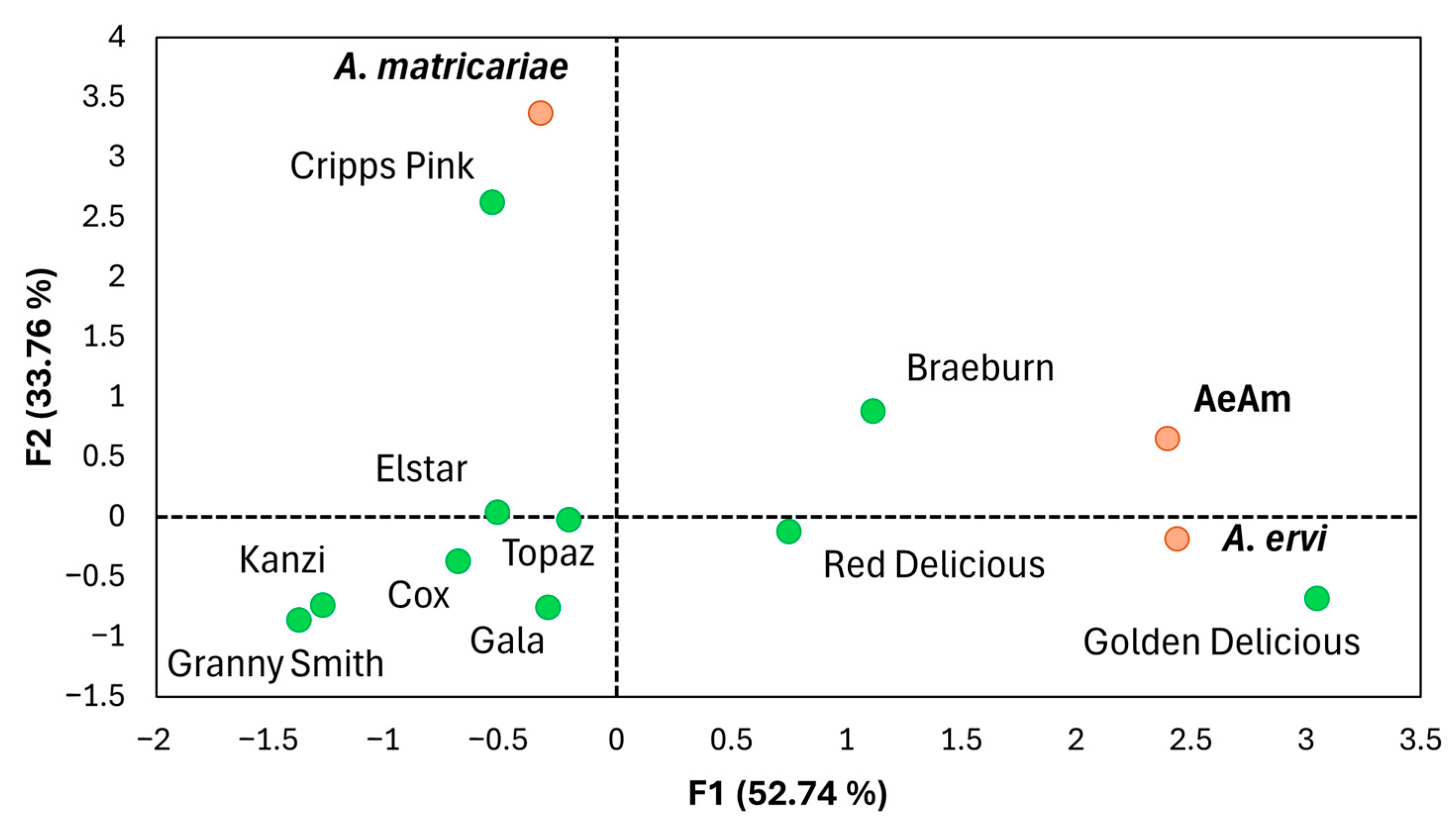
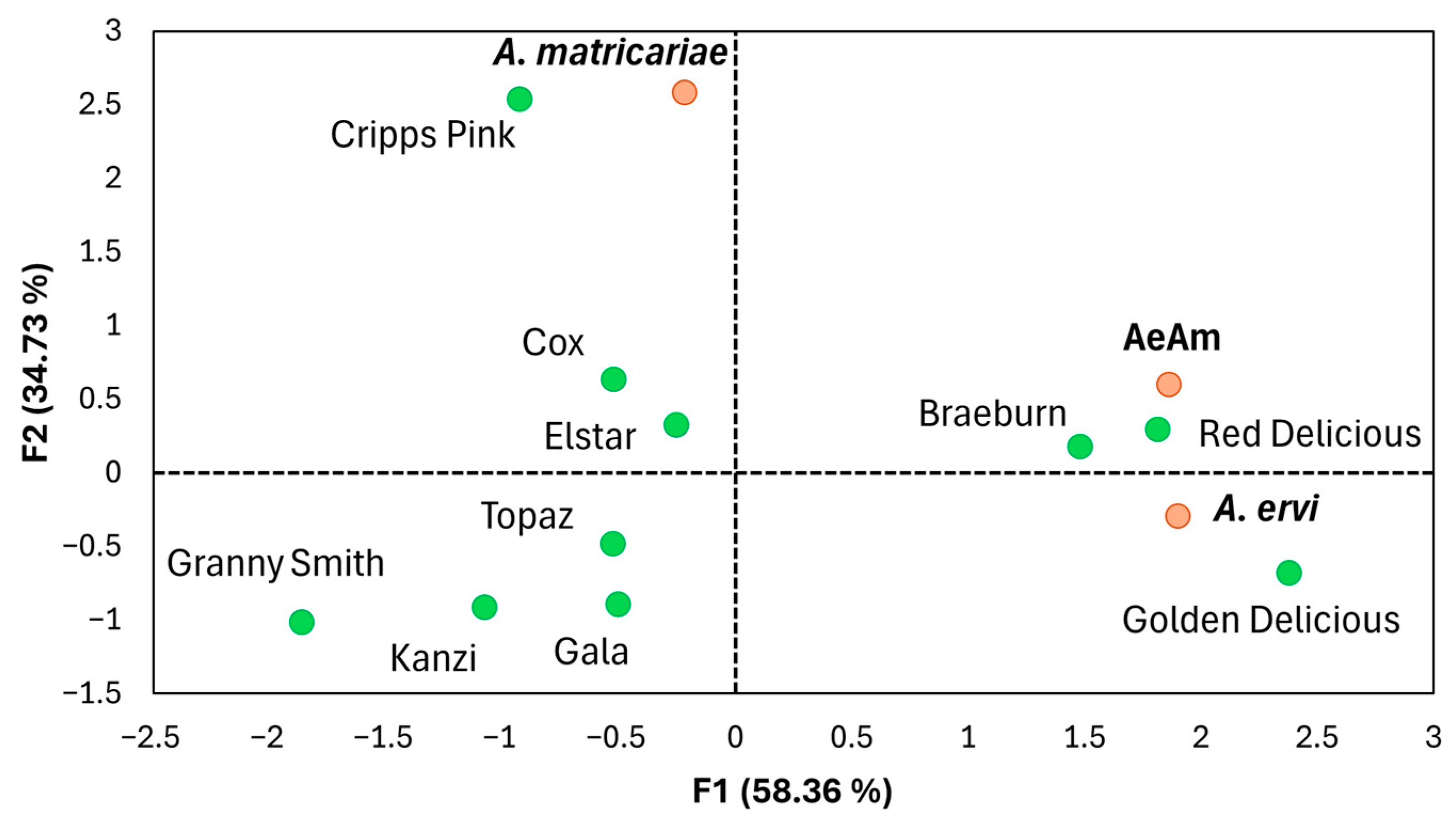
3.5. Parasitoid Preference
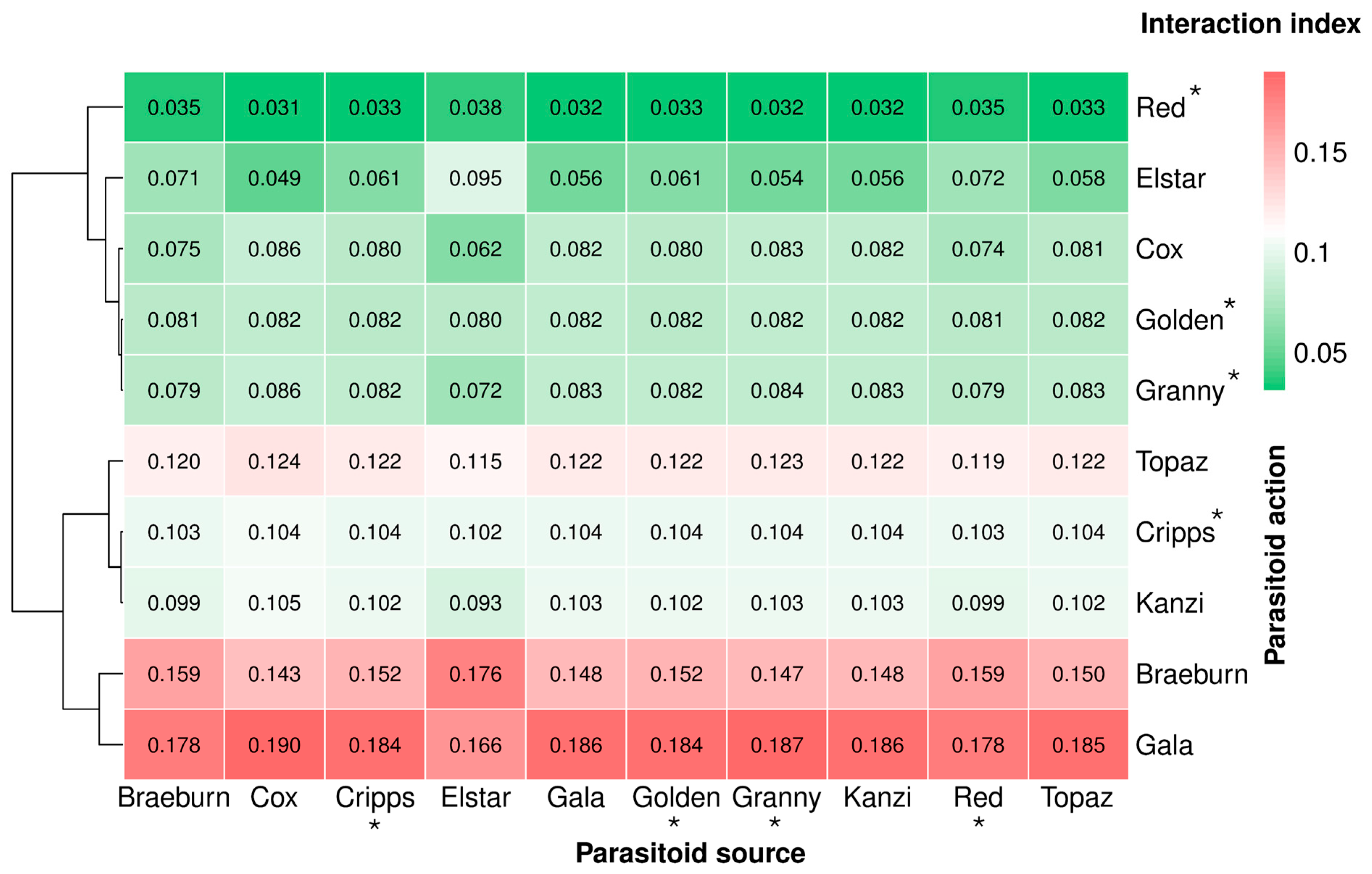
3.6. Parasitoid-Mediated Indirect Interactions
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bribosia, E.; Bylemans, D.; Migon, M.; Impe, G.V. In-Field Production of Parasitoids of Dysaphis plantaginea by Using the Rowan Aphid Dysaphis sorbi as Substitute Host. BioControl 2005, 50, 601–610. [Google Scholar] [CrossRef]
- Bangels, E.; De Schaetzen, C.; Hayen, G.; Paternotte, E.; Gobin, B. The Importance of Arthropod Pests in Belgian Pome Fruit Orchards. Commun. Agric. Appl. Biol. Sci. 2008, 73, 583–588. [Google Scholar]
- Alhmedi, A.; Bylemans, D.; Bangels, E.; Beliën, T. Cultivar-Mediated Effects on Apple–Dysaphis plantaginea Interaction. J. Pest Sci. 2022, 95, 1303–1315. [Google Scholar] [CrossRef]
- Brewer, M.J.; Elliott, N.C. Biological Control of Cereal Aphids in North America and Mediating Effects of Host Plant and Habitat Manipulations. Annu. Rev. Entomol. 2004, 49, 219–242. [Google Scholar] [CrossRef]
- Tentelier, C.; Fauvergue, X. Herbivore-induced Plant Volatiles as Cues for Habitat Assessment by a Foraging Parasitoid. J. Anim. Ecol. 2007, 76, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Albittar, L.; Ismail, M.; Bragard, C.; Hance, T. Host Plants and Aphid Hosts Influence the Selection Behaviour of Three Aphid Parasitoids (Hymenoptera: Braconidae: Aphidiinae). Eur. J. Entomol. 2016, 113, 516–522. [Google Scholar] [CrossRef]
- Mehrparvar, M.; Rajaei, A.; Rokni, M.; Balog, A.; Loxdale, H.D. ‘Bottom-up’ Effects in a Tritrophic Plant–Aphid–Parasitoid System: Why Being the Perfect Host Can Have Its Disadvantages. Bull. Entomol. Res. 2019, 109, 831–839. [Google Scholar] [CrossRef] [PubMed]
- Monticelli, L.S.; Desneux, N.; Heimpel, G.E. Parasitoid-mediated Indirect Interactions between Unsuitable and Suitable Hosts Generate Apparent Predation in Microcosm and Modeling Studies. Ecol. Evol. 2021, 11, 2449–2460. [Google Scholar] [CrossRef]
- Luquet, M.; Peñalver-Cruz, A.; Satour, P.; Anton, S.; Cortesero, A.-M.; Lavandero, B.; Jaloux, B. Aphid Honeydew May Be the Predominant Sugar Source for Aphidius Parasitoids Even in Nectar-Providing Intercrops. Biol. Control 2021, 158, 104596. [Google Scholar] [CrossRef]
- Denoirjean, T.; Engels, C.; Le Goff, G.J.; Dubois, F.; Tougeron, K.; Doury, G.; Ameline, A.; Couty, A. Bottom-up Effects of Apple Cultivars on Parasitoids via Aphid Hosts. Arthropod-Plant Interact. 2024, 18, 181–192. [Google Scholar] [CrossRef]
- Miñarro, M.; Dapena, E. Resistance of Apple Cultivars to Dysaphis plantaginea (Hemiptera: Aphididae): Role of Tree Phenology in Infestation Avoidance. Environ. Entomol. 2007, 36, 1206–1211. [Google Scholar] [CrossRef]
- Langellotto, G.A.; Denno, R.F. Responses of Invertebrate Natural Enemies to Complex-Structured Habitats: A Meta-Analytical Synthesis. Oecologia 2004, 139, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Eilers, E.J.; Klein, A.-M. Landscape Context and Management Effects on an Important Insect Pest and Its Natural Enemies in Almond. Biol. Control 2009, 51, 388–394. [Google Scholar] [CrossRef]
- Pocius, V.M.; Kersch-Becker, M.F. Evaluating the Influence of Plant Defenses on Prey Quality as an Opportunity to Enhance Biological Control in Agroecosystems. Biol. Control 2024, 193, 105515. [Google Scholar] [CrossRef]
- Starý, P. Aphid Parasitoids of the Czech Republic: Hymenoptera: Braconidae, Aphidiinae; Academia: Praha, Czech Republic, 2006; ISBN 978-80-200-1384-2. [Google Scholar]
- Alhmedi, A.; Raymaekers, S.; Tomanović, Ž.; Bylemans, D.; Beliën, T. Food Web Structure of Aphids and Their Parasitoids in Belgian Fruit Agroecosystems: Food Webs of Aphids and Parasitoids. Entomol. Sci. 2018, 21, 279–291. [Google Scholar] [CrossRef]
- Alhmedi, A.; Belien, T.; Bylemans, D. Habitat Modification Alters Food Web Interactions with Focus on Biological Control of Aphids in Apple Orchards. Sustainability 2023, 15, 5978. [Google Scholar] [CrossRef]
- Starý, P. Parasites (Hymenoptera, Aphidiidae) of Leaf-Curling Apple Aphids in Csechoslovakia. Acta Entomol. Bohemoslov. 1975, 72, 99–114. [Google Scholar]
- Boivin, G.; Hance, T.; Brodeur, J. Aphid Parasitoids in Biological Control. Can. J. Plant Sci. 2012, 92, 1–12. [Google Scholar] [CrossRef]
- Rezaei, M.; Talebi, A.A.; Fathipour, Y.; Karimzadeh, J.; Mehrabadi, M.; Reddy, G.V.P. Effects of Cold Storage on Life-history Traits of Aphidius matricariae. Entomol. Exp. Appl. 2020, 168, 800–807. [Google Scholar] [CrossRef]
- He, X.Z.; Wang, Q. Host Age Preference in Aphidius ervi (Hymenoptera Aphidiidae). N. Z. Plant Prot. 2006, 59, 195–201. [Google Scholar] [CrossRef]
- Rasool, B.; Mehmood, Z.; Ahmad, M.F.; Iqbal, J.; Younis, T.; Munir, R. Host Instars Preference, Density-Dependent Parasitism and Behavioral Perspective of Parasitoids (Aphidius colemani, Aphidius matricariae and Aphelinus abdominalis) in Aphis glycines and Aphis gossypii. Rev. Bras. Entomol. 2022, 66, e20210045. [Google Scholar] [CrossRef]
- Boivin, G.; Brodeur, J. Intra- and Interspecific Interactions among Parasitoids: Mechanisms, Outcomes and Biological Control. In Trophic and Guild in Biological Interactions Control; Brodeur, J., Boivin, G., Eds.; Progress in Biological Control; Springer: Dordrecht, The Netherlands, 2006; Volume 3, pp. 123–144. ISBN 978-1-4020-4766-4. [Google Scholar]
- Velasco-Hernández, M.C.; Desneux, N.; Ramírez-Martínez, M.M.; Cicero, L.; Ramirez-Romero, R. Host Species Suitability and Instar Preference of Aphidius ervi and Aphelinus abdominalis. Entomol. Gen. 2017, 36, 347–367. [Google Scholar] [CrossRef]
- Zeni, V.; Romano, D.; Kavallieratos, N.G.; Stefanini, C.; Lucchi, A.; Canale, A.; Benelli, G. Tapping for Love: Courtship, Mating, and Behavioral Asymmetry in Two Aphid Parasitoids, Aphidius ervi and Aphidius matricariae (Hymenoptera: Braconidae: Aphidiinae). J. Econ. Entomol. 2024, 117, 1837–1845. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.-B.; Zhang, G.-F.; Liu, W.-X.; Wan, F.-H. Variable Temperatures across Different Stages Have Novel Effects on Behavioral Response and Population Viability in a Host-Feeding Parasitoid. Sci. Rep. 2019, 9, 2202. [Google Scholar] [CrossRef] [PubMed]
- Hatt, S.; Lopes, T.; Boeraeve, F.; Chen, J.; Francis, F. Pest Regulation and Support of Natural Enemies in Agriculture: Experimental Evidence of within Field Wildflower Strips. Ecol. Eng. 2017, 98, 240–245. [Google Scholar] [CrossRef]
- Kessler, A.; Baldwin, I.T. Defensive Function of Herbivore-Induced Plant Volatile Emissions in Nature. Science 2001, 291, 2141–2144. [Google Scholar] [CrossRef]
- Yan, H.; Guo, H.; Sun, Y.; Ge, F. Plant Phenolics Mediated Bottom-up Effects of Elevated CO2 on Acyrthosiphon pisum and Its Parasitoid Aphidius avenae. Insect Sci. 2020, 27, 170–184. [Google Scholar] [CrossRef] [PubMed]
- Hågvar, E.B.; Hofsvang, T. Interspecific Competition between the Aphid Parasitoids Aphidius colemani Viereck and Ephedrus cerasicola Starý (Hym., Aphidiidae). J. Appl. Entomol. 1988, 106, 62–71. [Google Scholar] [CrossRef]
- Hawkins, B.A.; Cornell, H.V.; Hochberg, M.E. Predators, Parasitoids, and Pathogens as Mortality Agents in Phytophagous Insect Populations. Ecology 1997, 78, 2145–2152. [Google Scholar] [CrossRef]
- Harvey, J.A.; Poelman, E.H.; Tanaka, T. Intrinsic Inter- and Intraspecific Competition in Parasitoid Wasps. Annu. Rev. Entomol. 2013, 58, 333–351. [Google Scholar] [CrossRef]
- Cusumano, A.; Peri, E.; Colazza, S. Interspecific Competition/Facilitation among Insect Parasitoids. Curr. Opin. Insect Sci. 2016, 14, 12–16. [Google Scholar] [CrossRef]
- Turlings, T.C.J.; Erb, M. Tritrophic Interactions Mediated by Herbivore-Induced Plant Volatiles: Mechanisms, Ecological Relevance, and Application Potential. Annu. Rev. Entomol. 2018, 63, 433–452. [Google Scholar] [CrossRef] [PubMed]
- Cuny, M.A.C.; Poelman, E.H. Evolution of Koinobiont Parasitoid Host Regulation and Consequences for Indirect Plant Defence. Evol. Ecol. 2022, 36, 299–319. [Google Scholar] [CrossRef]
- Ahmed, Q.; Agarwal, M.; Alobaidi, R.; Zhang, H.; Ren, Y. Response of Aphid Parasitoids to Volatile Organic Compounds from Undamaged and Infested Brassica Oleracea with Myzus persicae. Molecules 2022, 27, 1522. [Google Scholar] [CrossRef] [PubMed]
- Cascone, P.; Vuts, J.; Birkett, M.A.; Rasmann, S.; Pickett, J.A.; Guerrieri, E. Small Volatile Lipophilic Molecules Induced Belowground by Aphid Attack Elicit a Defensive Response in Neighbouring Un-Infested Plants. Front. Plant Sci. 2023, 14, 1154587. [Google Scholar] [CrossRef] [PubMed]
- Holt, R.D.; Lawton, J.H. The Ecological Consequences of Shared Natural Enemies. Annu. Rev. Ecol. Syst. 1994, 25, 495–520. [Google Scholar] [CrossRef]
- Müller, C.B.; Godfray, H.C.J. Apparent Competition between Two Aphid Species. J. Anim. Ecol. 1997, 66, 57–64. [Google Scholar] [CrossRef]
- Müller, C.B.; Godfray, H.C.J. Indirect Interactions in Aphid–Parasitoid Communities. Popul. Ecol. 1999, 41, 93–106. [Google Scholar] [CrossRef]
- Morris, R.J.; Lewis, O.T.; Godfray, H.C.J. Experimental Evidence for Apparent Competition in a Tropical Forest Food Web. Nature 2004, 428, 310–313. [Google Scholar] [CrossRef]
- Frost, C.M.; Peralta, G.; Rand, T.A.; Didham, R.K.; Varsani, A.; Tylianakis, J.M. Apparent Competition Drives Community-Wide Parasitism Rates and Changes in Host Abundance across Ecosystem Boundaries. Nat. Commun. 2016, 7, 12644. [Google Scholar] [CrossRef]
- Van Veen, F.J.F.; Müller, C.B.; Pell, J.K.; Godfray, H.C.J. Food Web Structure of Three Guilds of Natural Enemies: Predators, Parasitoids and Pathogens of Aphids. J. Anim. Ecol. 2008, 77, 191–200. [Google Scholar] [CrossRef]
- Alhmedi, A.; Haubruge, E.; D’Hoedt, S.; Francis, F. Quantitative Food Webs of Herbivore and Related Beneficial Community in Non-Crop and Crop Habitats. Biol. Control 2011, 58, 103–112. [Google Scholar] [CrossRef]
- Müller, C.B.; Adriaanse, I.C.T.; Belshaw, R.; Godfray, H.C.J. The Structure of an Aphid–Parasitoid Community. J. Anim. Ecol. 1999, 68, 346–370. [Google Scholar] [CrossRef]
- Ortiz-Martínez, S.; Pierre, J.-S.; Van Baaren, J.; Le Lann, C.; Zepeda-Paulo, F.; Lavandero, B. Interspecific Competition among Aphid Parasitoids: Molecular Approaches Reveal Preferential Exploitation of Parasitized Hosts. Sci. Rep. 2019, 9, 19641. [Google Scholar] [CrossRef]
- Langer, A.; Hance, T. Enhancing Parasitism of Wheat Aphids through Apparent Competition: A Tool for Biological Control. Agric. Ecosyst. Environ. 2004, 102, 205–212. [Google Scholar] [CrossRef]
- Chailleux, A.; Mohl, E.K.; Teixeira Alves, M.; Messelink, G.J.; Desneux, N. Natural Enemy-mediated Indirect Interactions among Prey Species: Potential for Enhancing Biocontrol Services in Agroecosystems. Pest Manag. Sci. 2014, 70, 1769–1779. [Google Scholar] [CrossRef]
- Desneux, N.; Kaplan, I.; Yoo, H.J.S.; Wang, S.; O’Neil, R.J. Temporal Synchrony Mediates the Outcome of Indirect Effects between Prey via a Shared Predator. Entomol. Gen. 2019, 39, 127–136. [Google Scholar] [CrossRef]
- Holt, R.D.; Kotler, B.P. Short-Term Apparent Competition. Am. Nat. 1987, 130, 412–430. [Google Scholar] [CrossRef]
- Prado, S.G.; Frank, S. Optimal Foraging by an Aphid Parasitoid Affects the Outcome of Apparent Competition. Ecol. Entomol. 2014, 39, 236–244. [Google Scholar] [CrossRef]
- Von Burg, S.; Ferrari, J.; Müller, C.B.; Vorburger, C. Genetic Variation and Covariation of Susceptibility to Parasitoids in the Aphid Myzus persicae: No Evidence for Trade-Offs. Proc. R. Soc. B Biol. Sci. 2008, 275, 1089–1094. [Google Scholar] [CrossRef]
- Jarrett, B.J.M.; Linder, S.; Fanning, P.D.; Isaacs, R.; Szűcs, M. Experimental Adaptation of Native Parasitoids to the Invasive Insect Pest, Drosophila suzukii. Biol. Control 2022, 167, 104843. [Google Scholar] [CrossRef]
- Tomanović, Ž.; Starý, P.; Kavallieratos, N.G.; Gagić, V.; Plećaš, M.; Janković, M.; Rakhshani, E.; Ćetković, A.; Petrović, A. Aphid parasitoids (Hymenoptera: Braconidae: Aphidiinae) in wetland habitats in western Palaearctic: Key and associated aphid parasitoid guilds. Ann. Soc. Entomol. Fr. NS 2012, 48, 189–198. [Google Scholar] [CrossRef]
- Rakhshani, E.; Barahoei, H.; Ahmad, Z.; Starý, P.; Ghafouri-Moghaddam, M.; Mehrparvar, M.; Kavallieratos, N.G.; Čkrkić, J.; Tomanović, Ž. Review of Aphidiinae Parasitoids (Hymenoptera: Braconidae) of the Middle East and North Africa: Key to Species and Host Associations. Eur. J. Taxon. 2019, 552, 1–132. [Google Scholar] [CrossRef]
- Minitab Inc. Minitab Statistical Software, release 18; State College: Pennsylvania, PA, USA, 2017.
- Addinsoft XLSTAT Statistical and Data Analysis Solution. 2019. Available online: https://www.xlstat.com/en/ (accessed on 7 February 2021).
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2024. [Google Scholar]
- Wolfram Research Inc. Mathematica, version 5.0; Wolfram Research Inc.: Champaign, IL, USA, 2003.
- Angeli, G.; Simoni, S. Apple Cultivars Acceptance by Dysaphis plantaginea Passerini (Homoptera: Aphididae). J. Pest Sci. 2006, 79, 175–179. [Google Scholar] [CrossRef]
- Dib, H.; Sauphanor, B.; Capowiez, Y. Effect of Management Strategies on Arthropod Communities in the Colonies of Rosy Apple Aphid, Dysaphis plantaginea Passerini (Hemiptera: Aphididae) in South-Eastern France. Agric. Ecosyst. Environ. 2016, 216, 203–206. [Google Scholar] [CrossRef]
- Hunter, M.D.; Price, P.W. Playing Chutes and Ladders: Heterogeneity and the Relative Roles of Bottom-Up and Top-Down Forces in Natural Communities. Ecology 1992, 73, 724–732. [Google Scholar] [CrossRef]
- Bottrell, D.G.; Barbosa, P.; Gould, F. Manipulating Natural Enemies by Plant Variety Selection and Modification: A Realistic Strategy? Annu. Rev. Entomol. 1998, 43, 347–367. [Google Scholar] [CrossRef]
- Ode, P.J. Plant Chemistry and Natural Enemy Fitness: Effects on Herbivore and Natural Enemy Interactions. Annu. Rev. Entomol. 2006, 51, 163–185. [Google Scholar] [CrossRef] [PubMed]
- Mumm, R.; Dicke, M. Variation in Natural Plant Products and the Attraction of Bodyguards Involved in Indirect Plant defenseThe Present Review Is One in the Special Series of Reviews on Animal–Plant Interactions. Can. J. Zool. 2010, 88, 628–667. [Google Scholar] [CrossRef]
- Chen, Y.H.; Gols, R.; Benrey, B. Crop Domestication and Its Impact on Naturally Selected Trophic Interactions. Annu. Rev. Entomol. 2015, 60, 35–58. [Google Scholar] [CrossRef] [PubMed]
- Kök, Ş.; Tomanović, Ž.; Karabacak, E.; Kasap, İ. Do Primary and Secondary Host Plants Affect Aphid- Parasitoid Interactions in Fruit Orchards? Bull. Entomol. Res. 2023, 113, 326–334. [Google Scholar] [CrossRef]
- Dinant, S.; Bonnemain, J.-L.; Girousse, C.; Kehr, J. Phloem Sap Intricacy and Interplay with Aphid Feeding. C. R. Biol. 2010, 333, 504–515. [Google Scholar] [CrossRef]
- Jakobs, R.; Schweiger, R.; Müller, C. Aphid Infestation Leads to Plant Part-specific Changes in Phloem Sap Chemistry, Which May Indicate Niche Construction. New Phytol. 2019, 221, 503–514. [Google Scholar] [CrossRef] [PubMed]
- Nalam, V.; Louis, J.; Shah, J. Plant Defense against Aphids, the Pest Extraordinaire. Plant Sci. 2019, 279, 96–107. [Google Scholar] [CrossRef] [PubMed]
- Stallmann, J.; Pons, C.A.A.; Schweiger, R.; Müller, C. Time Point- and Plant Part-Specific Changes in Phloem Exudate Metabolites of Leaves and Ears of Wheat in Response to Drought and Effects on Aphids. PLoS ONE 2022, 17, e0262671. [Google Scholar] [CrossRef]
- Turlings, T.C.J.; Benrey, B. Effects of Plant Metabolites on the Behavior and Development of Parasitic Wasps. Écoscience 1998, 5, 321–333. [Google Scholar] [CrossRef]
- Gols, R.; Bukovinszky, T.; Van Dam, N.M.; Dicke, M.; Bullock, J.M.; Harvey, J.A. Performance of Generalist and Specialist Herbivores and Their Endoparasitoids Differs on Cultivated and Wild Brassica Populations. J. Chem. Ecol. 2008, 34, 132–143. [Google Scholar] [CrossRef] [PubMed]
- Dofuor, A.K.; Osei-Owusu, J.; Osabutey, A.F.; Lutuf, H.; Antwi-Agyakwa, A.K.; Andoh-Mensah, S.; Asante, K.; Aidoo, O.F. Plant-Insect Interactions under Agroecosystems: An Overview of Ecological Implications for Future Research. Cogent Food Agric. 2024, 10, 2379606. [Google Scholar] [CrossRef]
- Hassell, M.P. Host–Parasitoid Population Dynamics*. J. Anim. Ecol. 2000, 69, 543–566. [Google Scholar] [CrossRef]
- Miksanek, J.R.; Heimpel, G.E. A Matrix Model Describing Host–Parasitoid Population Dynamics: The Case of Aphelinus Certus and Soybean Aphid. PLoS ONE 2019, 14, e0218217. [Google Scholar] [CrossRef]
- Kruitwagen, A.; Beukeboom, L.W.; Wertheim, B.; Van Doorn, G.S. Evolution of Parasitoid Host Preference and Performance in Response to an Invasive Host Acting as Evolutionary Trap. Ecol. Evol. 2022, 12, e9030. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, F.; Zhang, Y.; Temir, E.; Zhou, X.; Shangguan, Y.; Zhang, D.; Cai, Z. Combination of Functional Plants Conserves Predators, Repels Pests, and Enhances Biological Control of Aphis spiraecola in Apple Orchards. Biol. Control 2024, 192, 105512. [Google Scholar] [CrossRef]
- Ponzio, C.; Cascone, P.; Cusumano, A.; Weldegergis, B.T.; Fatouros, N.E.; Guerrieri, E.; Dicke, M.; Gols, R. Volatile-Mediated Foraging Behaviour of Three Parasitoid Species under Conditions of Dual Insect Herbivore Attack. Anim. Behav. 2016, 111, 197–206. [Google Scholar] [CrossRef]
- Aartsma, Y. Herbivore-Induced Plant Volatiles and Tritrophic Interactions: From Local to Landscape Scale. Ph.D. Thesis, Wageningen University, Wageningen, The Netherlands, 2018. [Google Scholar]
- Ismail, M.; Zanolli, P.; Muratori, F.; Hance, T. Aphids Facing Their Parasitoids: A First Look at How Chemical Signals May Make Higher Densities of the Pea Aphid Acyrthosiphon pisum Less Attractive to the Parasitoid Aphidius ervi. Insects 2021, 12, 878. [Google Scholar] [CrossRef]
- Vet, L.E.M.; Godfray, H.C.J. Multitrophic Interactions and Parasitoid Behavioral Ecology. In Behavioral Ecology of Insect Parasitoids; Wajnberg, É., Bernstein, C., Van Alphen, J., Eds.; Wiley: Hoboken, NJ, USA, 2008; pp. 229–252. ISBN 978-1-4051-6347-7. [Google Scholar]
- Gontijo, L.M.; Beers, E.H.; Snyder, W.E. Complementary Suppression of Aphids by Predators and Parasitoids. Biol. Control 2015, 90, 83–91. [Google Scholar] [CrossRef]
- Pourtaghi, E.; Shirvani, A.; Rashki, M. Host Stage Preference and Temperature-Dependent Functional Response of Aphidius matricariae (Hymenoptera) on Aphis fabae (Hemiptera). Orient. Insects 2018, 52, 275–285. [Google Scholar] [CrossRef]
- Palmer, T.M.; Stanton, M.L.; Young, T.P. Competition and Coexistence: Exploring Mechanisms That Restrict and Maintain Diversity within Mutualist Guilds. Am. Nat. 2003, 162, S63–S79. [Google Scholar] [CrossRef]
- Lankau, R.A. Rapid Evolutionary Change and the Coexistence of Species. Annu. Rev. Ecol. Evol. Syst. 2011, 42, 335–354. [Google Scholar] [CrossRef]
- Amyntas, A.; Berti, E.; Gauzens, B.; Albert, G.; Yu, W.; Werner, A.S.; Eisenhauer, N.; Brose, U. Niche Complementarity among Plants and Animals Can Alter the Biodiversity–Ecosystem Functioning Relationship. Funct. Ecol. 2023, 37, 2652–2665. [Google Scholar] [CrossRef]
- Pålsson, J.; Porcel, M.; Dekker, T.; Tasin, M. Attract, Reward and Disrupt: Responses of Pests and Natural Enemies to Combinations of Habitat Manipulation and Semiochemicals in Organic Apple. J. Pest Sci. 2022, 95, 619–631. [Google Scholar] [CrossRef]
- Ferrais, L.; Tougeron, K.; Gardin, P.; Hance, T. Assessing the Optimal Frequency of Early Parasitoid Releases in an Apple Orchard to Control Dysaphis plantaginea: A Proof-of-Concept Study. Biol. Agric. Hortic. 2022, 38, 189–201. [Google Scholar] [CrossRef]
- Chesnais, Q.; Ameline, A.; Doury, G.; Le Roux, V.; Couty, A. Aphid Parasitoid Mothers Don’t Always Know Best through the Whole Host Selection Process. PLoS ONE 2015, 10, e0135661. [Google Scholar] [CrossRef]
- Thierry, M.; Pardikes, N.A.; Rosenbaum, B.; Ximénez-Embún, M.G.; Hrček, J. The Presence of Multiple Parasitoids Decreases Host Survival under Warming, but Parasitoid Performance Also Decreases. Proc. R. Soc. B Biol. Sci. 2022, 289, 20220121. [Google Scholar] [CrossRef] [PubMed]
- Pekas, A.; Tena, A.; Peri, E.; Colazza, S.; Cusumano, A. Competitive Interactions in Insect Parasitoids: Effects of Microbial Symbionts across Tritrophic Levels. Curr. Opin. Insect Sci. 2023, 55, 101001. [Google Scholar] [CrossRef] [PubMed]
- Godfray, H.C.J. Parasitoids: Behavioral and Evolutionary Ecology; Princeton University Press: Princeton, NJ, USA, 2019; ISBN 978-0-691-20702-5. [Google Scholar]
- Hopper, K.R. Modeling the Effects of Plant Resistance, Herbivore Virulence, and Parasitism, on the Population Dynamics of Aphids and Parasitoids in Wheat and Soybean in Different Climates. Ecol. Model. 2023, 481, 110376. [Google Scholar] [CrossRef]
- Larocca, A.; Fanti, P.; Romano, V.A.; Marsicovetere, E.; Isidoro, N.; Romani, R.; Ruschioni, S.; Pennacchio, F.; Battaglia, D. Functional Bases of Host-acceptance Behaviour in the Aphid Parasitoid Aphidius ervi. Physiol. Entomol. 2007, 32, 305–312. [Google Scholar] [CrossRef]
- Afsheen, S.; Wang, X.; Li, R.; Zhu, C.; Lou, Y. Differential Attraction of Parasitoids in Relation to Specificity of Kairomones from Herbivores and Their By-products. Insect Sci. 2008, 15, 381–397. [Google Scholar] [CrossRef]
- Kansman, J.T.; Jaramillo, J.L.; Ali, J.G.; Hermann, S.L. Chemical Ecology in Conservation Biocontrol: New Perspectives for Plant Protection. Trends Plant Sci. 2023, 28, 1166–1177. [Google Scholar] [CrossRef]
- Frago, E.; Dicke, M.; Godfray, H.C.J. Insect Symbionts as Hidden Players in Insect–Plant Interactions. Trends Ecol. Evol. 2012, 27, 705–711. [Google Scholar] [CrossRef]
- Burghardt, K.T.; Schmitz, O.J. Influence of Plant Defenses and Nutrients on Trophic Control of Ecosystems. In Trophic Ecology; Hanley, T.C., La Pierre, K.J., Eds.; Cambridge University Press: Cambridge, UK, 2015; pp. 203–232. ISBN 978-1-107-07732-4. [Google Scholar]
- Manzano, C.; Fernandez, P.; Hill, J.; Luft Albarracin, E.; Virla, E.; Coll Aráoz, M. Chemical Ecology of the Host Searching Behavior in an Egg Parasitoid: Are Common Chemical Cues Exploited to Locate Hosts in Taxonomically Distant Plant Species? J. Chem. Ecol. 2022, 48, 650–659. [Google Scholar] [CrossRef]
- Hirao, T.; Murakami, M. Quantitative Food Webs of Lepidopteran Leafminers and Their Parasitoids in a Japanese Deciduous Forest. Ecol. Res. 2008, 23, 159–168. [Google Scholar] [CrossRef]
- Moreira, X.; Mooney, K.A. Influence of Plant Genetic Diversity on Interactions between Higher Trophic Levels. Biol. Lett. 2013, 9, 20130133. [Google Scholar] [CrossRef]
- Moreira, X.; Abdala-Roberts, L.; Rasmann, S.; Castagneyrol, B.; Mooney, K.A. Plant Diversity Effects on Insect Herbivores and Their Natural Enemies: Current Thinking, Recent Findings, and Future Directions. Curr. Opin. Insect Sci. 2016, 14, 1–7. [Google Scholar] [CrossRef]
- Namba, T.; Umemoto, A.; Minami, E. The Effects of Habitat Fragmentation on Persistence of Source–Sink Metapopulations in Systems with Predators and Prey or Apparent Competitors. Theor. Popul. Biol. 1999, 56, 123–137. [Google Scholar] [CrossRef] [PubMed]
- Briggs, C.J.; Hoopes, M.F. Stabilizing Effects in Spatial Parasitoid–Host and Predator–Prey Models: A Review. Theor. Popul. Biol. 2004, 65, 299–315. [Google Scholar] [CrossRef] [PubMed]
- Gurr, G.M.; Wratten, S.D.; Landis, D.A.; You, M. Habitat Management to Suppress Pest Populations: Progress and Prospects. Annu. Rev. Entomol. 2017, 62, 91–109. [Google Scholar] [CrossRef]
- Yang, F.; Liu, B.; Zhu, Y.; Wyckhuys, K.A.G.; Van Der Werf, W.; Lu, Y. Species Diversity and Food Web Structure Jointly Shape Natural Biological Control in Agricultural Landscapes. Commun. Biol. 2021, 4, 979. [Google Scholar] [CrossRef]
- Wyckhuys, K.A.G.; Abram, P.K.; Barrios, E.; Cancino, J.; Collatz, J.; Fancelli, M.; Klein, A.-M.; Lindell, C.A.; Osterman, J.; Pinto, M.; et al. Orchard Systems Offer Low-Hanging Fruit for Low-Carbon, Biodiversity-Friendly Farming. BioScience 2025, biae140. [Google Scholar] [CrossRef]
- Tougeron, K.; Ferrais, L.; Renard, M.-E.; Hance, T. Effects of Constant versus Fluctuating Temperatures on Fitness Indicators of the Aphid Dysaphis plantaginea and the Parasitoid Aphidius matricariae. Insects 2021, 12, 855. [Google Scholar] [CrossRef] [PubMed]
- Jerbi-Elayed, M.; Tougeron, K.; Grissa-Lebdi, K.; Hance, T. Effect of Developmental Temperatures on Aphidius colemani Host-Foraging Behavior at High Temperature. J. Therm. Biol. 2022, 103, 103140. [Google Scholar] [CrossRef]
- Li, B.; Duan, Y.; Du, Z.; Wang, X.; Liu, S.; Feng, Z.; Tian, L.; Song, F.; Yang, H.; Cai, W.; et al. Natural Selection and Genetic Diversity Maintenance in a Parasitic Wasp during Continuous Biological Control Application. Nat. Commun. 2024, 15, 1379. [Google Scholar] [CrossRef]
- Castelo, M.K.; Crespo, J.E. Habitats and Parasitoid Abundance Influence Spatial Density Dependence Patterns, Rendering an Asilid Fly as a Potential Biological Controller of White Grubs. Front. Agron. 2023, 5, 1029232. [Google Scholar] [CrossRef]
- Nell, L.A.; Kishinevsky, M.; Bosch, M.J.; Sinclair, C.; Bhat, K.; Ernst, N.; Boulaleh, H.; Oliver, K.M.; Ives, A.R. Dispersal Stabilizes Coupled Ecological and Evolutionary Dynamics in a Host-Parasitoid System. Science 2024, 383, 1240–1244. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Dong, Z.; Wu, Q.; Gagic, V.; Tomanovic, Ž.; Zalucki, M.P.; Lu, Z. Landscape Structure and Composition Affect Aphid Biological Control in Alfalfa Fields, but Regional Differences Prevail. Entomol. Gen. 2024, 44, 535–544. [Google Scholar] [CrossRef]
- Van Schalkwyk, J.; Pryke, J.S.; Samways, M.J.; Gaigher, R. Environmental Filtering and Spillover Explain Multi-Species Edge Responses across Agricultural Boundaries in a Biosphere Reserve. Sci. Rep. 2020, 10, 14800. [Google Scholar] [CrossRef]
- Guariento, E.; Obwegs, L.; Anderle, M.; Bellè, A.; Fontana, P.; Paniccia, C.; Plunger, J.; Rüdisser, J.; Stifter, S.; Giombini, V.; et al. Meadow Orchards as a Good Practice Example for Improving Biodiversity in Intensive Apple Orchards. Biol. Conserv. 2024, 299, 110815. [Google Scholar] [CrossRef]
- Ryalls, J.M.W.; Garratt, M.P.D.; Spadaro, D.; Mauchline, A.L. The Benefits of Integrated Pest Management for Apple Depend on Pest Type and Production Metrics. Front. Sustain. Food Syst. 2024, 8, 1321067. [Google Scholar] [CrossRef]
| Cultivars | A. ervi | A. matricariae |
|---|---|---|
| Braeburn | 13.7 ± 2.0 ab | 10.3 ± 0.8 ab |
| Cox | 13.2 ± 1.3 ab | 7.3 ± 0.9 bcd |
| Cripps Pink | 9.0 ± 0.7 bc | 17.9 ± 3.0 a |
| Elstar | 7.8 ± 1.6 c | 7.8 ± 1.4 bcd |
| Gala | 6.4 ± 0.6 c | 4.3 ± 0.8 d |
| Golden Delicious | 24.4 ± 4.0 a | 4.1 ± 0.9 d |
| Granny Smith | 7.5 ± 1.0 bc | 5.2 ± 0.7 cd |
| Kanzi | 5.2 ± 0.7 c | 5.2 ± 0.8 cd |
| Red Delicious | 18.2 ± 1.4 a | 7.6 ± 0.9 bcd |
| Topaz | 13.0 ± 1.1 ab | 8.1 ± 0.7 bc |
| F9,90 | 11.32 | 9.80 |
| p | <0.001 | <0.001 |
| Cultivars | A. ervi | A. matricariae |
|---|---|---|
| Braeburn | 23.0 ± 4.1 ab | 9.7 ± 0.8 bc |
| Cox | 14.2 ± 2.1 bc | 14.3 ± 2.6 ab |
| Cripps Pink | 6.4 ± 0.5 d | 24.3 ± 3.4 a |
| Elstar | 10.4 ± 2.9 cd | 11.4 ± 2.7 bc |
| Gala | 8.0 ± 0.8 cd | 4.7 ± 1.1 e |
| Golden Delicious | 38.7 ± 5.8 a | 6.1 ± 1.3 de |
| Granny Smith | 7.8 ± 1.2 d | 6.4 ± 0.8 cde |
| Kanzi | 6.4 ± 1.0 d | 5.3 ± 0.7 de |
| Red Delicious | 31.0 ± 3.8 a | 11.2 ± 1.4 b |
| Topaz | 14.6 ± 1.6 bc | 8.1 ± 0.8 bcd |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alhmedi, A.; Belien, T.; Bylemans, D. Bottom-Up and Top-Down Dynamics in the Management of Rosy Apple Aphid. Insects 2025, 16, 1134. https://doi.org/10.3390/insects16111134
Alhmedi A, Belien T, Bylemans D. Bottom-Up and Top-Down Dynamics in the Management of Rosy Apple Aphid. Insects. 2025; 16(11):1134. https://doi.org/10.3390/insects16111134
Chicago/Turabian StyleAlhmedi, Ammar, Tim Belien, and Dany Bylemans. 2025. "Bottom-Up and Top-Down Dynamics in the Management of Rosy Apple Aphid" Insects 16, no. 11: 1134. https://doi.org/10.3390/insects16111134
APA StyleAlhmedi, A., Belien, T., & Bylemans, D. (2025). Bottom-Up and Top-Down Dynamics in the Management of Rosy Apple Aphid. Insects, 16(11), 1134. https://doi.org/10.3390/insects16111134







