Parasitization Activity by Eretmocerus iulii over the Orange Spiny Whitefly, Aleurocanthus spiniferus, in Sicily
Simple Summary
Abstract
1. Introduction
- (i)
- the evolution of its population since the first findings in summer 2023, and
- (ii)
- its potential role as a first step toward developing effective long-term biocontrol strategies against OSW in Sicily.
2. Materials and Methods
2.1. Pest Abundance/Density
2.2. Parasitoid Parasitization Rate
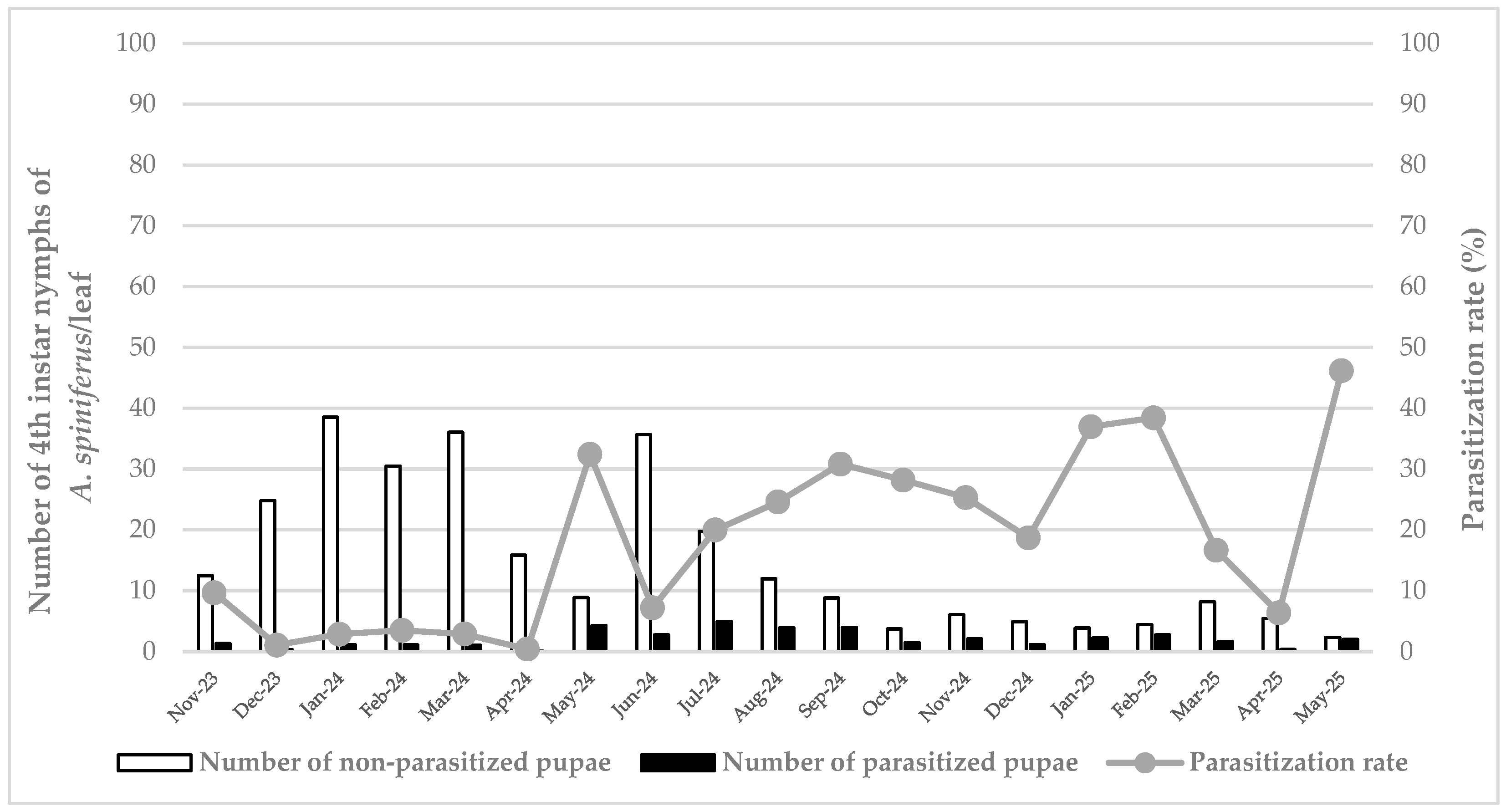
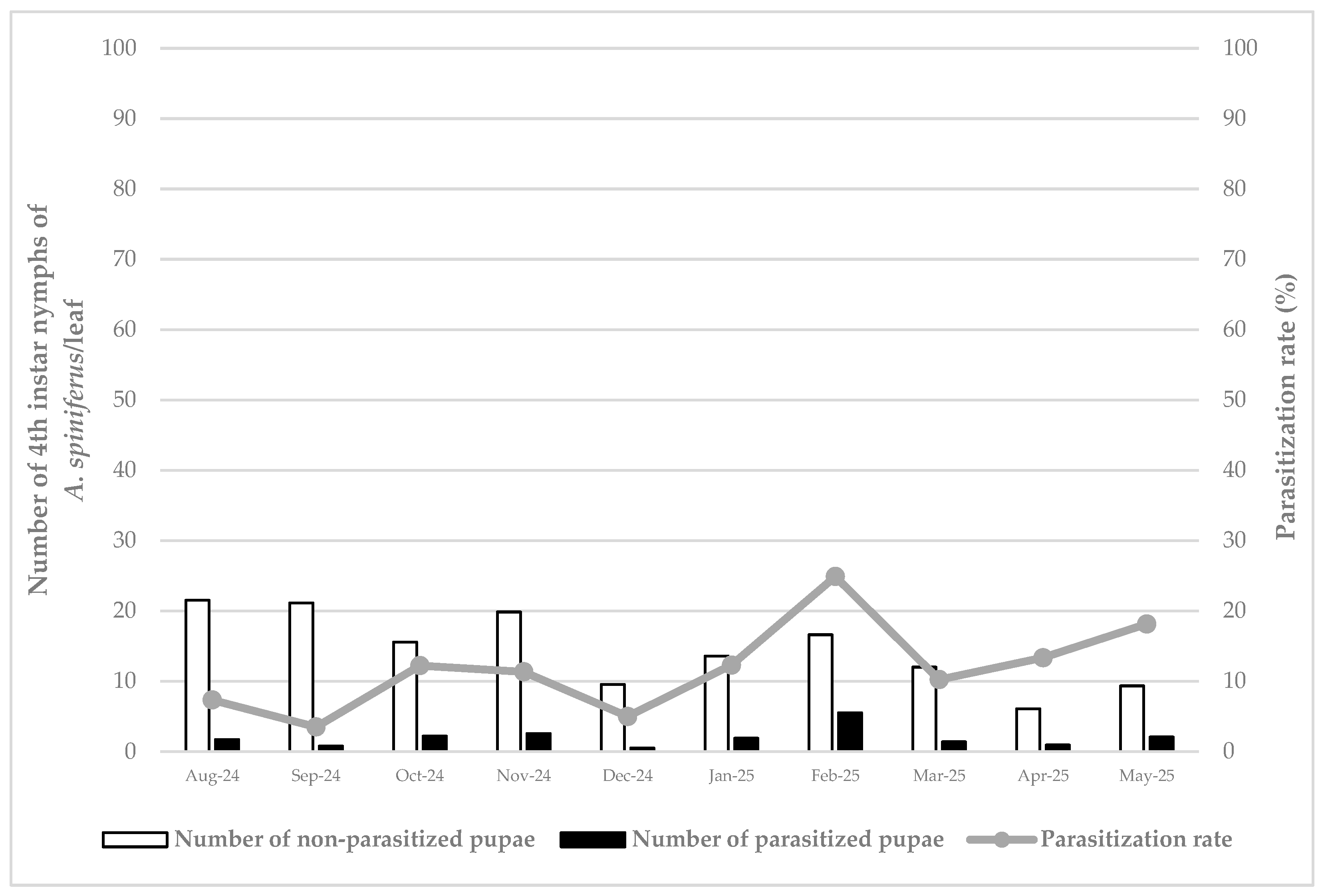
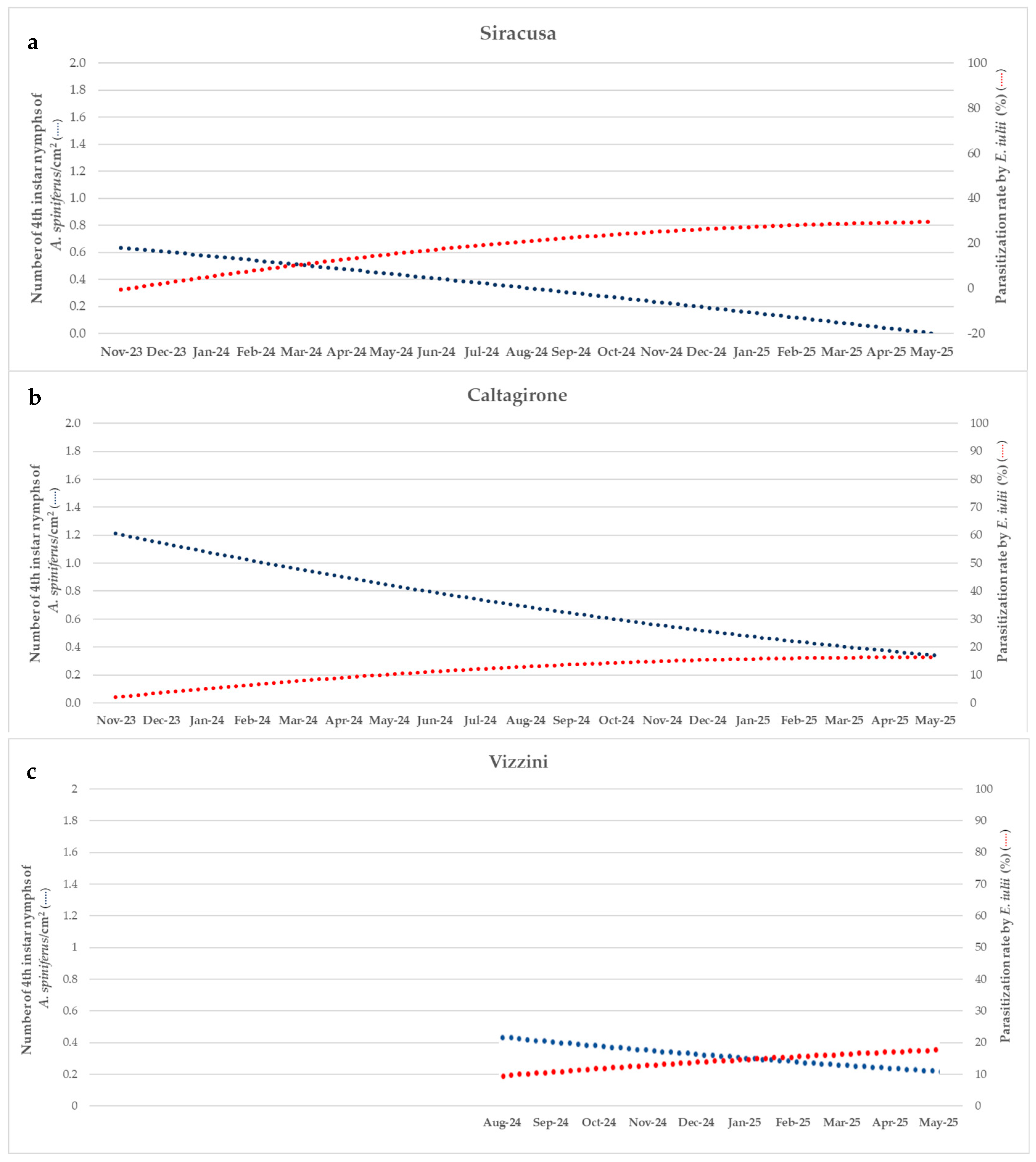
3. Results
3.1. Pest Abundance/Density
3.2. Parasitoid Parasitization Rate
3.3. Pest Density and Parasitization Rate Relationship
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Martin, J.H. An identification guide to common whitefly pest species of the world (Homoptera: Aleyrodidae). Int. J. Pest Manag. 1987, 33, 298–322. [Google Scholar] [CrossRef]
- Martin, J.H.; Mound, L.A. An annotated check list of the world’s whiteflies (Insecta: Hemiptera: Aleyrodidae). Zootaxa 2007, 1492, 1–84. [Google Scholar] [CrossRef]
- Saurabh, S.; Mishra, M.; Rai, P.; Pandey, R.; Singh, J.; Khare, A.; Jain, M.; Singh, P.K. Tiny flies: A mighty pest that threatens agricultural productivity—A case for next-generation control strategies of whiteflies. Insects 2011, 12, 585. [Google Scholar] [CrossRef]
- Navas-Castillo, J.; Fiallo-Olivé, E.; Sánchez-Campos, S. Emerging virus diseases transmitted by whiteflies. Annu. Rev. Phytopathol. 2011, 49, 219–248. [Google Scholar] [CrossRef]
- Schmidt, S.; De Barro, P.; Jamieson, L. Parasitoids of the Australian citrus whitefly, Orchamoplatus citri (Takahashi) (Hemiptera: Aleyrodidae), with description of a new Eretmocerus species (Hymenoptera: Aphelinidae). Zootaxa 2011, 2873, 27–34. [Google Scholar] [CrossRef]
- Massimino Cocuzza, G.E.; Rapisarda, C. Integrated pest management in citrus. In Integrated Pest Management in Tropical Regions; Rapisarda, C., Massimino Cocuzza, G.E., Eds.; CAB International: Wallingford, UK, 2017; pp. 246–269. [Google Scholar] [CrossRef]
- Dubey, A.K.; Sundararaj, R. Whitefly species of the genus Aleurocanthus Quaintance & Baker (Hemiptera: Aleyrodidae) from India, with descriptions of six new species. Orient. Insects 2005, 39, 295–321. [Google Scholar] [CrossRef]
- Dubey, A.K.; Ko, C.-C. Sexual dimorphism among species of Aleurocanthus Quaintance & Baker (Hemiptera: Aleyrodidae) in Taiwan, with one new species and an identification key. Zootaxa 2012, 3177, 1–23. [Google Scholar] [CrossRef]
- Gillespie, P.S. A review of the whitefly genus Aleurocanthus Quaintance & Baker (Hemiptera: Aleyrodidae) in Australia. Zootaxa 2012, 3252, 1–42. [Google Scholar] [CrossRef]
- Andrianto, E.; Kasai, A. Taxonomic revision of tribe Aleurocanthini Takahashi, 1954 stat. rev. using consortium gene analysis (mito-nuclear-primary endosymbiont) with the first evidence for mitochondrial recombination in whitefly (Hemiptera: Aleyrodidae). Diversity 2023, 15, 80. [Google Scholar] [CrossRef]
- Bragard, C.; Dehnen-Schmutz, K.; Di Serio, F.; Gonthier, P.; Jacques, M.A.; Jaques Miret, J.A.; Fejer Justesen, A.; Magnusson, C.S.; Milonas, P.; Navas-Cortes, J.A.; et al. Pest categorisation of Aleurocanthus spp. EFSA J. 2018, 16, 5436. [Google Scholar] [CrossRef]
- EPPO Global Database. Aleurocanthus spiniferus (ALECSEN). Available online: https://gd.eppo.int/taxon/ALECSN (accessed on 9 June 2025).
- Mound, L.A.; Halsey, S.H. Whitefly of the World; British Museum (Natural History) and John Wiley & Sons: Chichester, UK, 1978; 340p. [Google Scholar] [CrossRef]
- Kapantaidaki, D.E.; Antonatos, S.; Kontodimas, D.; Milonas, P.; Papachristos, D.P. Presence of the invasive whitefly Aleurocanthus spiniferus (Hemiptera: Aleyrodidae) in Greece. EPPO Bull. 2019, 49, 127–131. [Google Scholar] [CrossRef]
- Nugnes, F.; Laudonia, S.; Jesu, G.; Jansen, M.G.M.; Bernardo, U.; Porcelli, F. Aleurocanthus spiniferus (Hemiptera: Aleyrodidae) in some European countries: Diffusion, hosts, molecular characterization, and natural enemies. Insects 2020, 11, 42. [Google Scholar] [CrossRef]
- Rapisarda, C.; Longo, S. First report from Sicily (Italy) of the orange spiny whitefly, Aleurocanthus spiniferus (Quaintance) (Hemiptera: Aleyrodidae), and its potential risk for the Italian citrus industry. EPPO Bull. 2021, 51, 329–332. [Google Scholar] [CrossRef]
- Porcelli, F. First record of Aleurocanthus spiniferus (Homoptera: Aleyrodidae) in Apulia, Southern Italy. EPPO Bull. 2008, 38, 516–518. [Google Scholar] [CrossRef]
- Radonjić, S.; Hrnčić, C.; Malumphy, C. First record of Aleurocanthus spiniferus (Quaintance) (Hemiptera: Aleyrodidae) in Montenegro. Redia 2014, 77, 141–145. [Google Scholar]
- Šimala, M.; Masten Milek, T.; Pintar, M. The whitefly species (Hemiptera: Aleyrodidae) with dark puparium and pupal case recorded in Croatia. Nat. Croat. 2015, 24, 111–125. [Google Scholar] [CrossRef]
- Streito, J.C.; Mendes, E.; Sanquer, E.; Strugarek, M.; Ouvrad, D.; Robin-Havret, V.; Poncet, L.; Lannou, C.; Rossi, J.P. Incursion preparedness, citizen science and early detection of invasive insects: The case of Aleurocanthus spiniferus (Hemiptera: Aleyrodidae) in France. Insects 2023, 14, 916. [Google Scholar] [CrossRef] [PubMed]
- Cioffi, M.; Cornara, D.; Corrado, I.; Jansen, M.G.M.; Porcelli, F. The status of Aleurocanthus spiniferus from its unwanted introduction in Italy to date. Bull. Insectol. 2013, 66, 273–281. [Google Scholar]
- Bariselli, M.; Bortolotti, P.P.; Nannini, R. Scheda 27—Aleurocanthus spiniferus. In Schede Tecniche Per Il Riconoscimento Degli Organismi Nocivi Da Quarantena (Direttiva 2000/89/CE); Regione Emilia-Romagna, Assessorato Agricoltura, Caccia e Pesca, Servizio Fitosanitario Emilia-Romagna: Bologna, Italy, 2019; 4p. [Google Scholar]
- Melone, G.; Ascolese, R.; Nugnes, F.; Porcelli, F.; Rapisarda, C.; Farina, A.; Picciotti, U.; Garganese, F.; Laudonia, S. An Eretmocerus species, parasitoid of Aleurocanthus spiniferus, was found in Europe: The secret savior of threatened plants. Sustainability 2024, 16, 2970. [Google Scholar] [CrossRef]
- Mendel, Z.; Avtabi, A.; Massimino Cocuzza, G.E. Citron arthropod pests in the Mediterranean, their origin and notes on their biology and management. In The Citron Compendium; Goldschmidt, E.E., Bar-Joseph, M., Eds.; Springer: Cham, Switzerland, 2023; pp. 131–168. [Google Scholar] [CrossRef]
- Singla, A.; Barmota, H.; Sahoo, S.K.; Kang, B.K. Influence of neonicotinoids on pollinators: A review. J. Apic. Res. 2020, 60, 19–32. [Google Scholar] [CrossRef]
- Mokrane, S.; Cavallo, G.; Tortorici, F.; Romero, E.; Fereres, A.; Djelouha, K.; Verrastro, V.; Cornara, D. Behavioral effects induced by organic insecticides can be exploited for a sustainable control of the orange spiny whitefly Aleurocanthus spiniferus. Sci. Rep. 2020, 10, 15746. [Google Scholar] [CrossRef] [PubMed]
- Rajna, S.; Praveen, K.V.; Laneesha, M.; Kelageri, S.S. Recent trends in insecticide resistance research on whiteflies (Hemiptera: Aleyrodidae): A bibliometric profile. Curr. Sci. 2021, 120, 1433–1440. [Google Scholar] [CrossRef]
- Evans, G. The Whiteflies (Hemiptera: Aleyrodidae) of the World and Their Host Plants and Natural Enemies; USDA/APHIS: Washington, DC, USA, 2007; 708p, Available online: https://keys.lucidcentral.org/keys/v3/whitefly/ (accessed on 23 September 2025).
- Niu, J.Z.; Hull-Sanders, H.; Zhang, Y.X.; Lin, J.Z.; Dou, W.; Wang, J.J. Biological control of arthropod pests in citrus orchards in China. Biol. Control 2014, 68, 15–22. [Google Scholar] [CrossRef]
- Noyes, J.S. Universal Chalcidoidea Database; World Wide Web Electronic Publication: London, UK, 2019; Available online: https://ucd.chalcid.org/#/ (accessed on 23 September 2025).
- Van den Berg, M.A.; De Beer, M.S. Natural enemies of the spiny blackfly, Aleurocanthus spiniferus (Hemiptera: Aleyrodidae), in Mpumalanga, South Africa. In Proceedings of the International Society of Citriculture, Sun City Resort, South Africa, 12–17 May 1996; Volume 1, pp. 667–669. [Google Scholar]
- Van den Berg, M.A.; Hoppner, G.; Greenland, J. An economic study of the biological control of the spiny blackfly, Aleurocanthus spiniferus (Hemiptera: Aleyrodidae), in a citrus orchard in Swaziland. Biocontrol Sci. Technol. 2000, 10, 27–32. [Google Scholar] [CrossRef]
- Muniappan, R.; Purea, M.; Sengebau, F.; Reddy, G.V.P. Orange spiny whitefly, Aleurocanthus spiniferus (Quaintance) (Homoptera: Aleyrodidae), and its parasitoids in the Republic of Palau. Proc. Hawaiian Entomol. Soc. 2006, 38, 21–25. [Google Scholar]
- Nguyen, R.; Fasulo, T.R. A Citrus Blackfly Parasitoid, Encarsia perplexa Huang & Polaszek (Insecta: Hymenoptera: Aphelinidae); University of Florida IFAS Extension: Gainesville, FL, USA, 2010; 3p, Available online: https://edis.ifas.ufl.edu/publication/IN510 (accessed on 23 September 2025).
- Gyeltshen, J.; Hodges, A.; Hodges, G.S. Orange Spiny Whitefly, Aleurocanthus spiniferus (Quaintance) (Insecta: Hemiptera: Aleyrodidae); University of Florida IFAS Extension: Gainesville, FL, USA, 2005; Publication #EENY341. [Google Scholar] [CrossRef]
- Massimino Cocuzza, G.E.; Jovičić, I.; Frisenna, F.; Tumminelli, R.; Siscaro, G. Discovery of Serangium montazerii Fürsch (Coleoptera: Coccinellidae) as a predator of Aleurocanthus spiniferus (Quaintance) (Hemiptera: Aleyrodidae) in Italy. EPPO Bull. 2023, 52, 376–386. [Google Scholar] [CrossRef]
- Laudonia, S.; Melone, G.; Ascolese, R.; Nugnes, F. Eretmocerus iulii Laudonia et Melone sp. n.: Parasitoid associated with Aleurocanthus spiniferus. Bull. Insectol. 2024, 77, 263–269. [Google Scholar]
- Costi, E.; Giannetti, D.; Cesari, M.; Rapisarda, C.; Polaszek, A.; Kresslein, R.L.; Maistrello, L. A European début: The Asian parasitoid Encarsia nipponica targets the invasive Aleurocanthus spiniferus in Northern Italy. Preprints 2025. [Google Scholar] [CrossRef]
- Melone, G.; Andretta, L.; Pica, F.; Donnarumma, F.P.; Ascolese, R.; Nugnes, F.; Laudonia, S. First detection of Encarsia smithi in Italy and co-occurrence with Eretmocerus iulii: A case of unintentional introductions and new associations with the invasive species Aleurocanthus spiniferus. Insects 2025, 16, 891. [Google Scholar] [CrossRef] [PubMed]
- Noyes, J.S. Collecting and preserving chalcid wasps (Hymenoptera: Chalcidoidea). J. Nat. Hist. 1982, 16, 315–334. [Google Scholar] [CrossRef]
- Silvestri, F. Contribuzione alla conoscenza degli Aleurodidae (Insecta: Hemiptera) viventi su Citrus in Estremo Oriente e dei loro parassiti. Boll. Lab. Zool. Gen. Agrar. Portici 1928, 21, 1–60. [Google Scholar]
- Compere, H. Notes on the classification of the Aphelinidae with descriptions of new species. Univ. Calif. Publ. Entomol. 1936, 6, 277–322. [Google Scholar]
- Gerling, D. Comments on Eretmocerus serius Silvestri (Hymenoptera: Aphelinidae) with a description of two new species. Entomophaga 1969, 14, 213–222. [Google Scholar]
- Hayat, M. Aphelinidae of India (Hymenoptera: Chalcidoidea): A Taxonomic Revision; Memoirs of Entomology International: Gainesville, FL, USA, 1998; Volume 13, viii + 416p. [Google Scholar]
- Kuwana, I.; Ishii, T. On Prospaltella smithi Silv., and Cryptognatha sp., the enemies of Aleurocanthus spiniferus Quaintance, imported from Canton, China. Rev. Appl. Entomol. 1927, 15, 463. [Google Scholar]
- Peterson, G.D. Biological control of the orange spiny whitefly. J. Econ. Entomol. 1955, 48, 681–683. [Google Scholar] [CrossRef]
- Lin, H.C.; Wei, H.L.; Tao, C.C. Integrated control of citrus spiny white fly, Aleurocanthus spiniferus Quaintance & Baker (Homoptera: Aleyrodidae). J. Agric. Res. China 1975, 24, 55–61. [Google Scholar]
- Clausen, C.P. Introduced Parasites and Predators of Arthropod Pests and Weeds: A World Review; Agriculture Handbook No. 480; USDA: Washington, DC, USA, 1978; 545p. [Google Scholar]
- CABI. Aleurocanthus spiniferus (orange spiny whitefly). CABI Compendium 2021, 4136. [Google Scholar] [CrossRef]
- Giakoumaki, M.-V.; Milonas, P.; Antonatos, S.; Evangelou, V.; Partsinevelos, G.; Papachristos, D.; Ramadan, M.M. A survey in Hawaii for parasitoids of citrus whiteflies (Hemiptera: Aleyrodidae), for introduction into Greece. Insects 2023, 14, 858. [Google Scholar] [CrossRef]
- Van den Berg, M.A.; Greenland, J. Classical biological control of Aleurocanthus spiniferus (Hemiptera: Aleyrodidae) on citrus in Southern Africa. Entomophaga 1997, 42, 459–465. [Google Scholar] [CrossRef]

| Locality | Altitude | Coordinates | Host Plants | Starting Month |
|---|---|---|---|---|
| Siracusa (SR) | 17 m a.s.l. | 37.075136, 15.278588 | Citrus x aurantium L. Citrus limon (L.) | November 2023 |
| Caltagirone (CT) | 608 m a.s.l. | 37.237851, 14.513141 | Citrus x aurantium L. | November 2023 |
| Vizzini (CT) | 586 m a.s.l. | 37.164975, 14.752564 | Citrus x aurantium L. Citrus limon (L.) Citrus reticulata (L.) | August 2024 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Farina, A.; Rapisarda, C. Parasitization Activity by Eretmocerus iulii over the Orange Spiny Whitefly, Aleurocanthus spiniferus, in Sicily. Insects 2025, 16, 1074. https://doi.org/10.3390/insects16101074
Farina A, Rapisarda C. Parasitization Activity by Eretmocerus iulii over the Orange Spiny Whitefly, Aleurocanthus spiniferus, in Sicily. Insects. 2025; 16(10):1074. https://doi.org/10.3390/insects16101074
Chicago/Turabian StyleFarina, Alessia, and Carmelo Rapisarda. 2025. "Parasitization Activity by Eretmocerus iulii over the Orange Spiny Whitefly, Aleurocanthus spiniferus, in Sicily" Insects 16, no. 10: 1074. https://doi.org/10.3390/insects16101074
APA StyleFarina, A., & Rapisarda, C. (2025). Parasitization Activity by Eretmocerus iulii over the Orange Spiny Whitefly, Aleurocanthus spiniferus, in Sicily. Insects, 16(10), 1074. https://doi.org/10.3390/insects16101074








