Impact of Biogenic Structures of the Soil-Nesting Ants Lasius niger and Lasius flavus on the Soil Microarthropod Community in Urban Green Spaces
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Sites
2.2. Soil Microarthropod Sampling and Processing
2.3. Abiotic Factors
2.4. Data Analysis
3. Results
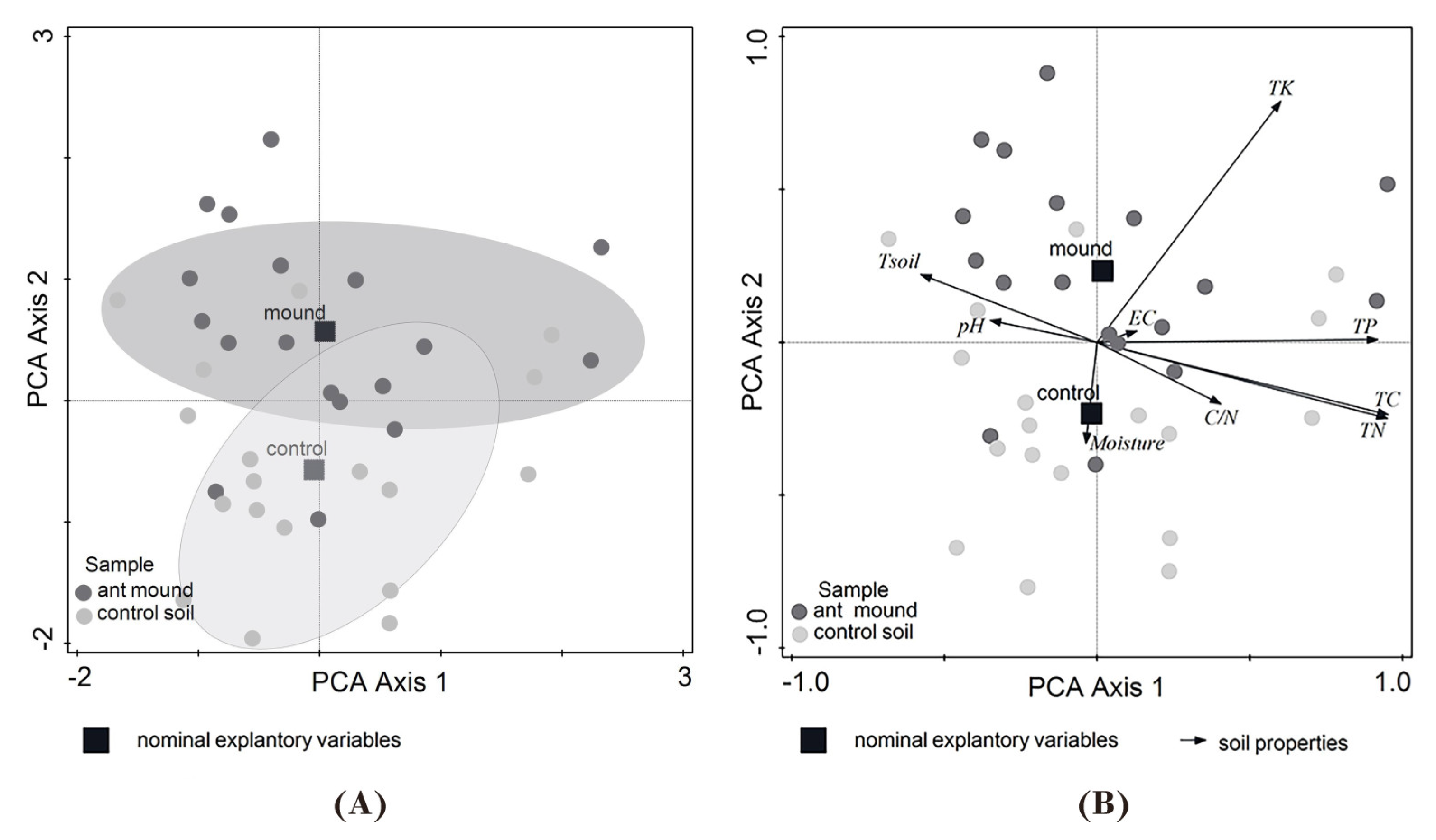
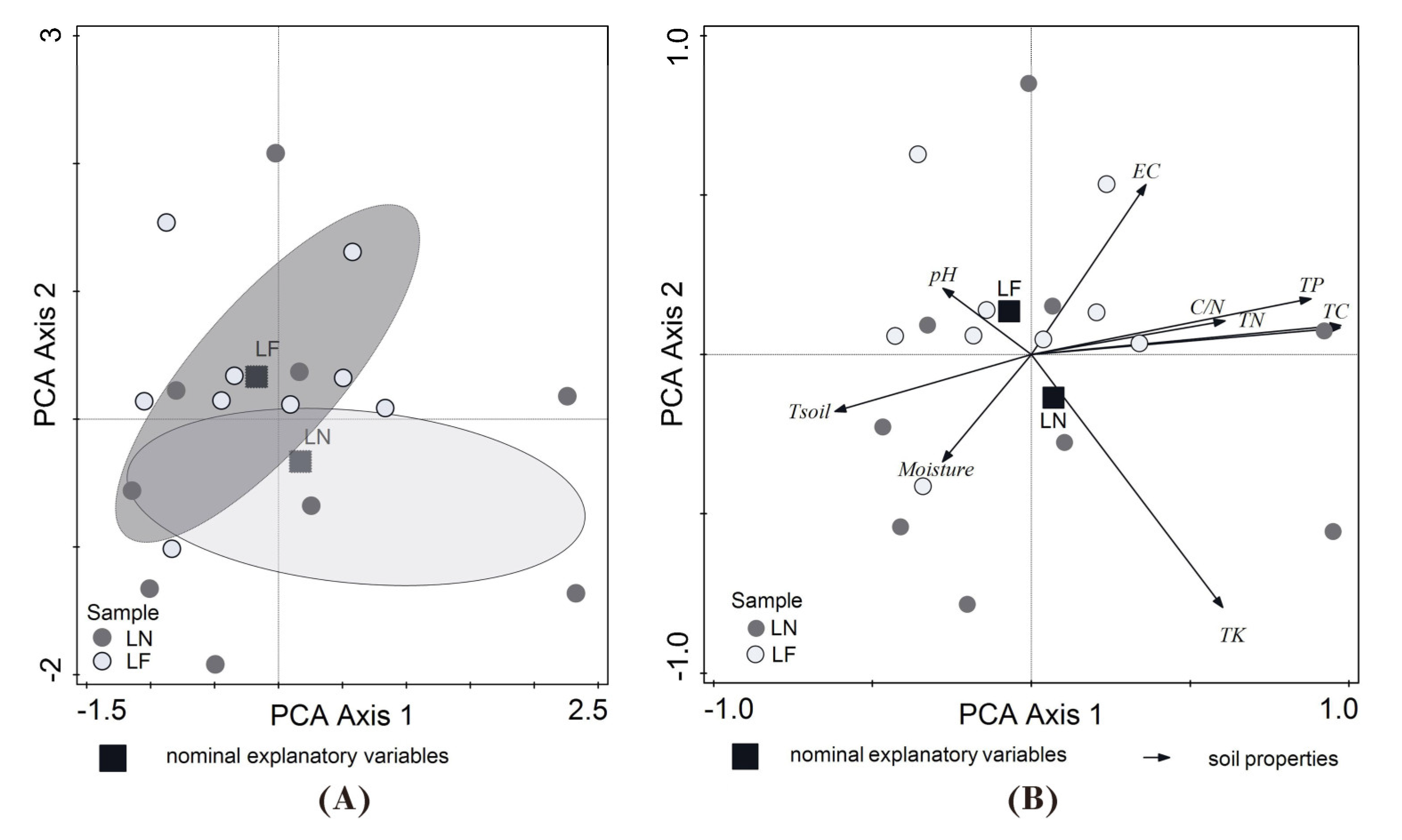
3.1. Soil Properties
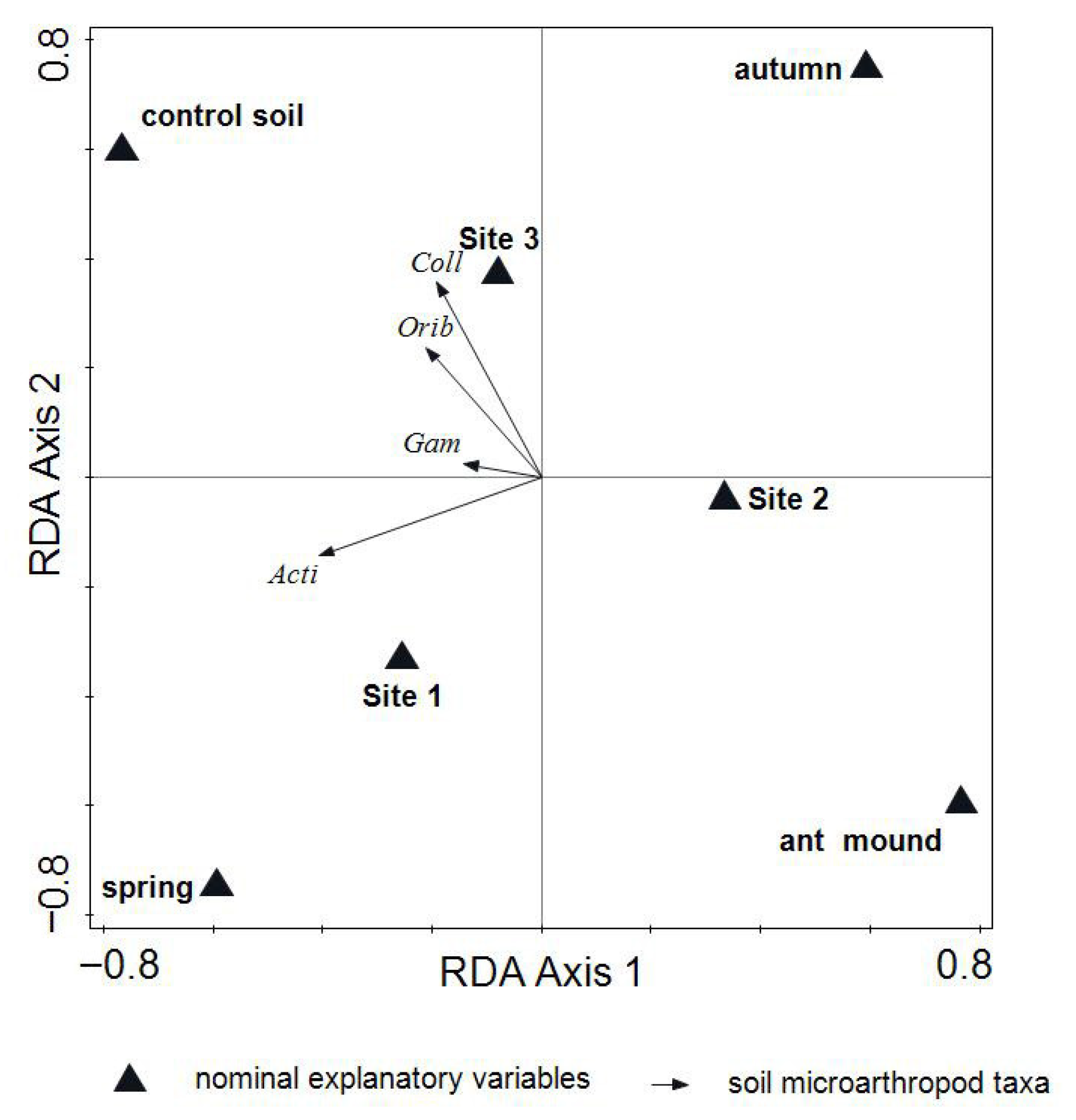
3.2. Soil Microarthropod Taxonomic Composition and Abundance Patterns
3.3. Diversity Pattern and Structure of Collembola Assemblages
3.4. Diversity Pattern and Structure of Mesostigmata Assemblages
4. Discussion
4.1. Effect of L. flavus and L. niger on Collembola Assemblage Composition, Abundance and Distribution Patterns
4.2. Effect of L. flavus and L. niger on Mesostigmata Assemblage Composition, Abundance and Distribution Patterns
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jones, C.G.; Lawton, J.H.; Shachak, M. Positive and negative effects of organisms as physical ecosystem engineers. Ecology 1997, 78, 1946–1957. [Google Scholar] [CrossRef]
- Lavelle, P. Faunal activities and soil processes: Adaptive strategies that determine ecosystem function. Adv. Ecol. Res. 1997, 21, 93–132. [Google Scholar]
- Taylor, A.R.; Lenoir, L.; Vegerfors, B.; Persson, T. Ant and earthworm bioturbation in cold-temperate ecosystems. Ecosystems 2019, 22, 981–994. [Google Scholar] [CrossRef]
- Jouquet, P.; Dauber, J.; Lagerlöf, J.; Lavelle, P.; Lepage, M. Soil invertebrates as ecosystem engineers: Intended and accidental effects on soil and feedback loops. Appl. Soil Ecol. 2006, 32, 153–164. [Google Scholar] [CrossRef]
- Coleman, D.C.; Callaham, M.A.; Crossley, D.A., Jr. Fundamentals of Soil Ecology, 3rd ed.; Academic Press: London, UK, 2018; pp. 1–361. [Google Scholar]
- Culliney, T. Role of Arthropods in maintaining soil fertility. Agriculture 2013, 3, 629–659. [Google Scholar] [CrossRef]
- Menta, C. Soil fauna diversity—Function, soil degradation, biological indices, soil restoration. In Biodiversity Conservation and Utilization in a Diverse World; Lameed, G.A., Ed.; IntechOpen: Rijeka, Croatia, 2012; pp. 59–94. [Google Scholar]
- Briones, M.J. Soil fauna and soil functions: A jigsaw puzzle. Front. Environ. Sci. 2014, 2, 7. [Google Scholar] [CrossRef]
- Coleman, D.C.; Wall, D.H. Soil Fauna: Occurrence, biodiversity and role in ecosystem function. In Soil Microbiology, Ecology and Biochemistry, 4th ed.; Paul, E.A., Ed.; Elsevier: Amsterdam, The Netherlands, 2024; pp. 111–149. [Google Scholar]
- Lavelle, P.; Decäens, T.; Aubert, M.; Barot, S.; Blouin, M.; Bureau, F.; Margerie, P.; Mora, P.; Rossi, J.-P. Soil invertebrates and ecosystem services. Eur. J. Soil Biol. 2006, 42, 3–15. [Google Scholar] [CrossRef]
- Oliveira, L.R.D.; Frazão, L.A.; Silva, S.C.O.; Araújo, W.S.; Nunes, Y.R.F.; Fernandes, V.M.; Veloso, M.D.M. Nutrient cycling and soil quality in threatened veredas in two protected areas of the Brazilian Cerrado. Nat. Conserv. Res. 2024, 9, 34–46. [Google Scholar] [CrossRef]
- Potapov, M.; Beaulieu, F.; Birkhofer, K.; Bluhm, S.L.; Degtyarev, M.I.; Devetter, M.; Goncharov, A.A.; Gongalsky, K.B.; Klarner, B.; Korobushkin, D.I.; et al. Feeding habits and multifunctional classification of soil associated consumers from protists to vertebrates. Biol. Rev. 2022, 97, 1057–1117. [Google Scholar] [CrossRef]
- Klimek, B.; Poliwka-Modliborek, H.; Grześ, I.M. Ant nests as a microbial hot spots in a long-term heavy metal-contaminated soils. Environ. Sci. Pollut. Res. 2022, 29, 10848–10857. [Google Scholar] [CrossRef]
- Vilkova, V.V.; Kazeev, K.S.; Nizhelskiy, M.S.; Kolesnikov, S.I.; Kozun, Y.S. Changes in soil properties of xerophytic forests in Southern Russia after anthropogenic impact. Nat. Conserv. Res. 2024, 9, 61–72. [Google Scholar] [CrossRef]
- Bardgett, R.; van der Putten, W. Belowground biodiversity and ecosystem functioning. Nature 2014, 515, 505–511. [Google Scholar] [CrossRef]
- Wills, B.D.; Landis, D.A. The role of ants in north temperate grasslands: A review. Oecologia 2018, 186, 323–338. [Google Scholar] [CrossRef]
- Winsome, T. Fauna. In Encyclopedia of Soils in the Environment; Hillel, D., Ed.; Elsevier: Oxford, UK, 2005; pp. 539–548. [Google Scholar]
- Jónsson, J.Ö.G.; Davidsdóttir, B. Classification and valuation of soil ecosystem services. Agric. Syst. 2016, 145, 866–877. [Google Scholar] [CrossRef]
- Farji-Brener, A.G.; Werenkraut, V. The effects of ant nests on soil fertility and plant performance: A meta-analysis. J. Anim. Ecol. 2017, 86, 866–877. [Google Scholar] [CrossRef]
- Turner, J.S. The Soul of the Superorganism. In The Extended Organism: The Physiology of Animal-Built Structures; Harvard University Press: Cambridge, MA, USA; London, UK, 2000; pp. 179–200. [Google Scholar]
- Odling-Smee, F.J.; Laland, K.N.; Feldman, M.W. Niche Construction: The Neglected Process in Evolution; Princeton University Press: Princeton, NJ, USA, 2003; pp. 1–468. [Google Scholar]
- Lavelle, P. Functional domains in soils. Ecol. Res. 2002, 17, 441–450. [Google Scholar] [CrossRef]
- Holec, M.; Frouz, J. The effect of two ant species Lasius niger and Lasius flavus on soil properties in two contrasting habitats. Eur. J. Soil Biol. 2006, 42, 213–217. [Google Scholar] [CrossRef]
- Frouz, J.; Jilková, V. The effect of ants on soil properties and processes (Hymenoptera: Formicidae). Myrmecol. News 2008, 11, 191–199. [Google Scholar]
- Cammeraat, E.L.H.; Risch, A.C. The impact of ants on mineral soil properties and processes at different spatial scales. J. Appl. Entomol. 2008, 132, 285–294. [Google Scholar] [CrossRef]
- Haitao, W.; Donghui, W.; Xianguo, L.; Xiaomin, Y. Spatial distribution of ant mounds and effects on soil physical properties in wetlands of the Sanjiang plain, China. Acta Ecol. Sin. 2010, 30, 270–275. [Google Scholar]
- Bierbaß, P.; Gutknecht, J.L.M.; Michalzik, B. Nest-mounds of the yellow meadow ant (Lasius flavus) at the “Alter Gleisberg”, Central Germany: Hot or cold spots in nutrient cycling? Soil Biol. Biochem. 2015, 80, 209–217. [Google Scholar] [CrossRef]
- De Almeida, T.; Mesiéard, F.; Santonja, M.; Gros, R.; Dutoit, T.; Blight, O. Above- and below-ground effects of an ecosystem engineer ant in Mediterranean dry grasslands. Proc. R. Soc. B 2020, 287, 20201840. [Google Scholar] [CrossRef]
- Folgarait, P.F. Ant biodiversity and its relationship to ecosystem functioning: A review. Biodivers. Conserv. 1998, 7, 1221–1244. [Google Scholar] [CrossRef]
- Danoff-Burg, J.A. Myrmecophiles. In Encyclopedia of Entomology; Capinera, J.L., Ed.; Springer: Dordrecht, The Netherlands, 2008. [Google Scholar] [CrossRef]
- Dauber, J.; Wolters, V. Microbial activity and functional diversity in the mounds of three different ant species. Soil Biol. Biochem. 2000, 32, 93–99. [Google Scholar] [CrossRef]
- Dauber, J.; Niechoj, R.; Baltruschat, H.; Wolters, V. Soil engineering ants increase grass root arbuscular mycorrhizal colonization. Biol. Fertil. Soils 2008, 44, 791–796. [Google Scholar] [CrossRef]
- Boots, B.; Clipson, N. Linking ecosystem modification by the yellow meadow ant (Lasius flavus) to microbial assemblages in different soil environments. Eur. J. Soil Biol. 2013, 55, 100–106. [Google Scholar] [CrossRef]
- Dauber, J.; Rommeler, A.; Wolters, V. The ant Lasius flavus alters the viable seed bank in pastures. Eur. J. Soil Biol. 2006, 42, 157–163. [Google Scholar] [CrossRef]
- O’Grady, A.; Breen, J.; Harrington, T.J.; Courtney, R. The seed bank in soil from the nests of grassland ants in a unique limestone grassland community in Ireland. Ecol. Eng. 2013, 61, 58–64. [Google Scholar] [CrossRef]
- Laakso, J.; Setälä, H. Composition and trophic structure of detrital food web in ant nest mounds of Formica aquilonia and in the surrounding forest soil. Oikos 1998, 81, 266–278. [Google Scholar] [CrossRef]
- Sleptzova, E.V.; Reznikova, Z.I. Formation of springtail (Collembola) communities during colonization of ant-hills. Entomol. Rev. 2006, 86, 373–382. [Google Scholar] [CrossRef]
- Elo, R.A.; Penttinen, R.; Sorvari, J. Distribution of Oribatid mite is moisture related within red wood ant Formica polyctena nest mounds. Appl. Soil Ecol. 2018, 124, 203–210. [Google Scholar] [CrossRef]
- Çakur, M.; Akburak, S.; Sargıncı, M. The negative effect of wood ants (Formica rufa) on microarthropod density and soil biological quality in a semi-arid pine forest. Pedobiologia 2019, 77, 150593. [Google Scholar] [CrossRef]
- Kamczyc, J.; Gwiazdowicz, D.J. The diversity of soil mites (Acari: Mesostigmata) in yellow ant (Lasius flavus) nests along a gradient of land use. Biologia 2013, 68, 314–318. [Google Scholar] [CrossRef]
- Arroyo, J.; O’Grady, A.; Vance, H.; Bolger, T. The mite (Acari: Oribatida, Mesostigmata) assemblages associated with Lasius flavus (Hymenoptera: Formicidae) nests and surrounding soil in an Irish grassland. Biol. Environ. Proc. R. Ir. Acad. 2015, 115B, 17–28. [Google Scholar] [CrossRef]
- Scharenbroch, B.C.; Lloyd, J.E.; Johnson-Maynard, J.L. Distinguishing urban soils with physical, chemical, and biological properties. Pedobiologia 2005, 49, 283–296. [Google Scholar] [CrossRef]
- Pickett, S.T.A.; Cardenasso, M.L. Altered resources, disturbance, and heterogeneity: A framework for comparing urban and non-urban soils. Urban Ecosyst. 2009, 12, 23–44. [Google Scholar] [CrossRef]
- Pickett, S.T.A.; Cadenasso, M.L.; Grove, J.M.; Boone, C.G.; Groffman, P.M.; Irwin, E.; Kaushal, S.S.; Marshall, V.; McGrath, B.P.; Nilon, C.H.; et al. Urban ecological systems: Scientific foundations and a decade of progress. J. Environ. Manag. 2011, 92, 331–362. [Google Scholar] [CrossRef]
- Sudnik-Wójcikowska, B.; Galera, H. Warsaw. In Plants and Habitats of European Cities; Kelcey, J.G., Müller, N., Eds.; Springer: New York, NY, USA, 2011; pp. 499–545. [Google Scholar]
- Ślipiński, P.; Żmihorski, M.; Czechowski, W. Species diversity and nestedness of ant assemblages in an urban environment. Eur. J. Entomol. 2012, 109, 197–206. [Google Scholar] [CrossRef]
- Czechowski, W.; Radchenko, A.; Czechowska, W.; Vepsäläinen, K. The Ants (Hymenoptera: Formicidae) of Poland with Reference to the Myrmecofauna of Europe (Fauna Poloniae n.s.4); Natura Optima Dux Foundation: Warsaw, Poland, 2012; pp. 1–496. [Google Scholar]
- Trigos-Peral, G.; Rutkowski, T.; Witek, M.; Ślipiński, P.; Babik, H.; Czechowski, W. Three categories of urban green areas and the effect of their different management on the communities of ants, spiders and harvestmen. Urban Ecosyst. 2020, 23, 803–818. [Google Scholar] [CrossRef]
- IUSS Working Group WRB. World Reference Base for Soil Resources a Framework for International Classification and Communication; World Soil Resource Reports; FAO: Rome, Italy, 2006; Volume 103, pp. 1–116. [Google Scholar]
- Fjellberg, A. The Collembola of Fennoscandia and Denmark. Part I: Poduromorpha. Entomol. Scand. 1998, 35, 1–183. [Google Scholar]
- Fjellberg, A. The Collembola of Fennoscandia and Denmark. Part II: Entomobryomorpha and Symphypleona. Entomol. Scand. 2007, 42, 1–264. [Google Scholar]
- Pomorski, R.J. Onychiurinae of Poland (Collembola: Onychiuridae); Wrocław University/Polish Taxonomical Society: Wrocław, Poland, 1998; pp. 1–201. ISBN 83-909804-1-X. [Google Scholar]
- Bretfeld, G. Symphypleona. Synopses on Palaearctic Collembola. Abh. Ber. Nat.kd. mus. Görlitz 1999, 71, 1–318. [Google Scholar]
- Potapov, M. Isotomidae. Synopses on Palaearctic Collembola. Abh. Ber. Nat.kd. mus. Görlitz 2001, 73, 1–603. [Google Scholar]
- Thibaud, J.-M.; Schulz, H.-J.; da Gama Assalino, M.M. Hypogastruridae. In Synopses on Palaearctic Collembola, Vol. 4: Hypogastruridae; Dunger, W., Ed.; State Museum of the Natural History Museum of Görlitz: Görlitz, Germany, 2004; pp. 1–287. [Google Scholar]
- Dunger, W.; Schlitt, B. Tulbergiidae. Synopses on Palaearctic Collembola. Soil Org. 2011, 83, 1–168. [Google Scholar]
- Jordana, R. Capbryinae and Entomobryini. Synopses on Palaearctic Collembola. Soil Org. 2012, 84, 1–390. [Google Scholar]
- Bellinger, P.F.; Christiansen, K.A.; Janssens, F. Checklist of the Collembola of the World; 1996–2020. Available online: http://www.collembola.org/ (accessed on 29 May 2020).
- Karg, W. Raubmilben. Acari (Acarina), Miben Parasitiformes (Anactinochaeta), Cohors Gamasina Leach. Die Tierwelt Dtschl. 1993, 59, 1–523. [Google Scholar]
- Mašán, P. Mites of the Cohort Uropodina (Acarina, Mesostigmata) in Slovakia. Annot. Zool. Bot. 2001, 223, 1–320. [Google Scholar]
- Gwiazdowicz, D.J. Ascid Mites (Acari, Mesostigmata) from Selected Forest Ecosystems and Microhabitats in Poland; Wydawnictwo Akademii Rolniczej: Poznań, Poland, 2007; pp. 1–248. [Google Scholar]
- Ostrowska, A.; Gawliński, S.; Szczubiałka, Z. Metody Analizy i Oceny Właściwości Gleb i Roślin. Katalog; Instytut Ochrony Środowiska: Warszawa, Poland, 1991; pp. 1–350. (In Polish) [Google Scholar]
- Lepš, J.; Šmilauer, P. Multivariate Analysis of Ecological Data Using CANOCO; Cambridge University Press: Cambridge, UK, 2003; pp. 1–376. [Google Scholar]
- Borcard, D.; Legendre, P.; Drapeau, P. Partialling out the spatial component of ecological variation. Ecology 1992, 73, 1045–1055. [Google Scholar] [CrossRef]
- Velazco, V.N.; Saravia, L.A.; Coviella, C.E.; Falco, L.B. Trophic resources of the edaphic microarthropods: A worldwide review of the empirical evidence. Heliyon 2023, 9, e20439. [Google Scholar] [CrossRef]
- Sankovitz, M.; Purcell, J. Ant nests differentially affect soil chemistry across elevational gradients. Insectes Soc. 2022, 69, 293–298. [Google Scholar] [CrossRef]
- Zhou, W.; Zhan, P.; Zeng, M.; Chen, T.; Ahang, X.; Yang, G.; Guo, Y. Effects of ant bioturbation and foraging activities on soil mechanical properties and stability. Glob. Ecol. Conserv. 2023, 46, e02575. [Google Scholar] [CrossRef]
- Ehrle, A.; Kolle, O.; Tischer, A.; Trumbore, S.E.; Michalzik, B. Effects of mound building Lasius flavus on organic carbon and nutrient fluxes in soils of temperate grassland ecosystems. Pedobiologia 2021, 84, 150701. [Google Scholar] [CrossRef]
- Nkem, J.N.; Lobry de Bruyn, L.A.; Grant, C.D.; Hulugalle, N.R. The impact of ant bioturbation and foraging activities on surrounding soil properties. Pedobiologia 2000, 44, 609–621. [Google Scholar] [CrossRef]
- Kardol, P.; Reynolds, W.N.; Norby, R.J.; Classon, A.T. Climate change effects on soil microarthropod abundance and community structure. Appl. Soil Ecol. 2011, 47, 37–44. [Google Scholar] [CrossRef]
- Xu, G.-L.; Kuster, T.M.; Günthardt-Goerg, M.S.; Dobbertin, M.; Li, M.-H. Seasonal exposure to drought and air warming affects soil Collembola and mites. PLoS ONE 2012, 7, e43102. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Su, F.; Han, H.; Du, Y.; Yu, C.; Wan, S. Responses of soil microarthropods to warming and increased precipitation in a semiarid temperate steppe. Appl. Soil Ecol. 2014, 84, 200–207. [Google Scholar] [CrossRef]
- Liu, J.-L.; Li, F.-R.; Liu, L.-L.; Yang, K. Responses of different Collembola and mite taxa to experimental rain pulses in an arid ecosystem. Catena 2017, 155, 53–61. [Google Scholar] [CrossRef]
- Guo, Z.; Chang, H.; Liu, R. Response of soil microarthropod community to seasonal changes in Urat Desert Steppe, Inner Mongolia. Front. Environ. Sci. 2022, 10, 893913. [Google Scholar] [CrossRef]
- Flórián, N.; Ladányi, M.; Ittzés, A.; Kröel-Dulay, G.; Ónodi, G.; Mucsi, M.; Szili-Kovács, T.; Gergócs, V.; Dányi, L.; Dombos, M. Effects of single and repeated drought on soil microarthropods in a semi-arid ecosystem depend more on timing and duration than drought severity. PLoS ONE 2019, 14, e0219975. [Google Scholar] [CrossRef]
- Cole, L.; Buckland, S.M.; Bardgett, R.D. Relating microarthropod community structure and diversity to soil fertility manipulations in temperate grasslands. Soil Biol. Biochem. 2005, 37, 1707–1717. [Google Scholar] [CrossRef]
- Erktan, A.; Or, D.; Scheu, S. The physical structure of soil: Determinant and consequence of trophic interactions. Soil Biol. Biochem. 2020, 148, 107876. [Google Scholar] [CrossRef]
- Potapov, M. Multifunctionality of belowground food webs: Resource, size and spatial energy channels. Biol. Rev. 2022, 97, 1691–1711. [Google Scholar] [CrossRef]
- Jílková, V.; Šebek, O.; Frouz, J. Mechanisms of pH change in wood ant (Formica polyctena) nests. Pedobiologia 2012, 55, 247–251. [Google Scholar] [CrossRef]
- Castaño-Meneses, G.; Palacios-Vargas, J.G.; Delabie, J.H.C.; Zeppelini, D.; Mariano, C.S.F. Springtails (Collembola) associated with nests of fungus-growing ants (Formicidae: Myrmicinae: Attini) in southern Bahia, Brazil. Fla. Entomol. 2017, 100, 740–742. [Google Scholar] [CrossRef]
- Gwiazdowicz, D.J. Mesostigmatid mites (Acari) associated in nests of Formicidae in Poland. In Selected Problems of Acarological Research in Forests; Gwiazdowicz, D.J., Ed.; Wydawnictwo Uniwersytetu Przyrodniczego: Poznań, Poland, 2013; pp. 97–112. [Google Scholar]
- Wiśniewski, J. Ergänzung der Deutonymphenbeschreibung von Uropoda (Phaulodinychus) spinosula (Kneissl 1916) (Uropodini, Uropodinae). Acarologie 1980, 27, 15. [Google Scholar]
- Wiśniewski, J. Für die Fauna Polens neue Uropodina (Acari: Parasitiformes). Teil II. Fragm. Faun. 1982, 27, 143–147. [Google Scholar] [CrossRef]
- Wiśniewski, J. (Forest Research Institute, Warsaw, Poland). Studies on a Biological Complex of Factors Regulating Forest Ant Populations; Final Report FG-PO-366; Unpublished Work. 1983. [Google Scholar]
- Magura, T.; Lövei, G.L. Consequences of urban living: Urbanization and ground beetles. Curr. Landsc. Ecol. Rep. 2021, 6, 9–21. [Google Scholar] [CrossRef]
- Gwiazdowicz, D.J. Roztocze (Acari, Gamasida) występujące w mrowiskach Formica polyctena Förster na terenie Białowieskiego Parku Narodowego. Parki Nar. Rez. Przyr. 2001, 20, 97–101. (In Polish) [Google Scholar]

| Soil Properties | LN Mound | LF Mound | LN + LF | Control (C) | p-Value a | p-Value b | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | |||
| pHH20 | 6.86 | 0.45 | 6.87 | 0.38 | 6.87 | 0.40 | 6.77 | 0.49 | 0.863 | 0.658 |
| TK (g kg−1) | 1.45 | 0.49 | 1.16 | 0.19 | 1.31 | 0.39 | 0.99 | 0.32 | 0.222 | 0.006 |
| TP (g kg−1) | 0.84 | 0.37 | 0.82 | 0.22 | 0.83 | 0.30 | 0.79 | 0.32 | 0.796 | 0.517 |
| TN (g kg−1) | 6.21 | 2.67 | 5.37 | 1.38 | 5.79 | 2.10 | 6.20 | 2.03 | 0.863 | 0.420 |
| TC (g kg−1) | 72.27 | 34.94 | 58.96 | 14.71 | 65.61 | 26.89 | 70.15 | 23.31 | 0.863 | 0.420 |
| C/N ratio | 11.44 | 0.75 | 11.00 | 0.30 | 11.22 | 0.60 | 11.30 | 0.35 | 0.258 | 0.137 |
| Moisture (%) | 12.20 | 5.05 | 14.16 | 4.61 | 13.18 | 4.86 | 15.91 | 5.36 | 0.252 | 0.040 |
| Tsoil (°C) | 25.90 | 4.79 | 27.72 | 4.56 | 26.81 | 4.70 | 26.79 | 4.65 | 0.161 | 0.978 |
| EC (µS cm−1) | 0.03 | 0.04 | 0.03 | 0.02 | 0.03 | 0.03 | 0.02 | 0.02 | 0.888 | 0.827 |
| Microarthropod Taxon | LN Mound | LF Mound | LN + LF | Control (C) | p-Value a | p-Value b | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | |||
| Collembola | 4444.44 | 3289.42 | 4361.11 | 5315.92 | 4402.79 | 4356.96 | 9638.89 | 8072.54 | 0.365 | <0.001 |
| Mesostigmata | 4138.89 | 4186.13 | 1583.33 | 2088.13 | 2861.11 | 3508.38 | 2972.22 | 3200.32 | 0.076 | 0.505 |
| Oribatida | 10,111.11 | 11,245.33 | 3638.89 | 3109.78 | 6875.00 | 8768.76 | 18,013.89 | 21,163.14 | 0.062 | <0.001 |
| Actinedida | 2527.78 | 4974.86 | 1222.22 | 2157.22 | 1875.00 | 3836.62 | 1888.89 | 1848.21 | 0.584 | 0.086 |
| Model Fraction | Explained Variation (%) | Contribution to the Total Variation (%) | DF | Mean Square | Pseudo-F | p-Value |
|---|---|---|---|---|---|---|
| Soil microhabitat M (ant mound + control soil) | 68.5 | 5.2 | 1 | 0.063 | 4.9 | 0.040 |
| Site Sl | −19.2 | −1.5 | 2 | 0.006 | 0.5 | 0.884 |
| Season S | 55.4 | 4.2 | 1 | 0.054 | 4.1 | 0.004 |
| Overlap of M + Sl | −1.7 | −0.1 | ||||
| Overlap of M + S | −1.3 | −0.1 | ||||
| Overlap of Sl + S | −1.8 | −0.1 | ||||
| Joint overlap of M + Sl + S | <0.1 | <0.1 | ||||
| Total explained | 100 | 7.6 | 4 | 0.032 | ||
| All variation | 1 | 100 | 71 |
| Variable | Contribution (%) | Pseudo-F | p-Value | p-Value (adj) |
|---|---|---|---|---|
| Soil microhabitat: ant mound | 49.2 | 4.7 | 0.008 | 0.056 |
| Soil microhabitat: control | 49.2 | 4.7 | 0.002 | 0.014 |
| Season: spring | 41.7 | 4.2 | 0.008 | 0.056 |
| Season: autumn | 41.7 | 4.2 | 0.007 | 0.028 |
| Site: site 2 | 4.9 | 0.5 | 0.716 | 0.784 |
| Site: site 1 | 4.2 | 0.4 | 0.780 | 0.806 |
| Model Fraction | Explained Variation (%) | Contribution to the Total Variation (%) | DF | Mean Square | Pseudo-F | p-Value |
|---|---|---|---|---|---|---|
| Ant mound AM | 20.2 | 0.7 | 1 | 0.281 | 1.2 | 0.200 |
| Site Sl | 15.9 | 0.6 | 2 | 0.255 | 1.1 | 0.334 |
| Season S | 86.6 | 3.1 | 1 | 0.430 | 1.8 | 0.006 |
| Overlap of AM + Sl | −8.4 | −0.3 | ||||
| Overlap of AM + S | −8.4 | −0.3 | ||||
| Overlap of Sl + S | −11.5 | −0.4 | ||||
| Joint overlap of AM + Sl + S | 5.7 | 0.2 | ||||
| Total explained | 100 | 3.6 | 4 | 0.299 |
| Model Fraction | Explained Variation (%) | Contribution to the Total Variation (%) | DF | Mean Square | Pseudo-F | p-Value |
|---|---|---|---|---|---|---|
| Ant mound AM | 20.6 | 0.1 | 1 | 0.365 | 1.0 | 0.428 |
| Site Sl | 138.8 | 0.8 | 1 | 0.318 | 1.1 | 0.282 |
| Season S | −78.9 | −0.5 | 2 | 0.390 | 0.9 | 0.688 |
| Overlap of AM + Sl | −2.8 | −0.0 | ||||
| Overlap of AM + S | 6.7 | <0.1 | ||||
| Overlap of Sl + S | 11.6 | <0.1 | ||||
| Joint overlap of AM + Sl + S | 4.1 | <0.1 | ||||
| Total explained | 100 | 4 | 0.369 | |||
| All variation | 100 | 26 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sterzyńska, M.; Gwiazdowicz, D.J.; Nicia, P.; Zadrożny, P.; Trigos-Peral, G.; Negm, M.W. Impact of Biogenic Structures of the Soil-Nesting Ants Lasius niger and Lasius flavus on the Soil Microarthropod Community in Urban Green Spaces. Insects 2025, 16, 1058. https://doi.org/10.3390/insects16101058
Sterzyńska M, Gwiazdowicz DJ, Nicia P, Zadrożny P, Trigos-Peral G, Negm MW. Impact of Biogenic Structures of the Soil-Nesting Ants Lasius niger and Lasius flavus on the Soil Microarthropod Community in Urban Green Spaces. Insects. 2025; 16(10):1058. https://doi.org/10.3390/insects16101058
Chicago/Turabian StyleSterzyńska, Maria, Dariusz J. Gwiazdowicz, Paweł Nicia, Paweł Zadrożny, Gema Trigos-Peral, and Mohamed W. Negm. 2025. "Impact of Biogenic Structures of the Soil-Nesting Ants Lasius niger and Lasius flavus on the Soil Microarthropod Community in Urban Green Spaces" Insects 16, no. 10: 1058. https://doi.org/10.3390/insects16101058
APA StyleSterzyńska, M., Gwiazdowicz, D. J., Nicia, P., Zadrożny, P., Trigos-Peral, G., & Negm, M. W. (2025). Impact of Biogenic Structures of the Soil-Nesting Ants Lasius niger and Lasius flavus on the Soil Microarthropod Community in Urban Green Spaces. Insects, 16(10), 1058. https://doi.org/10.3390/insects16101058







