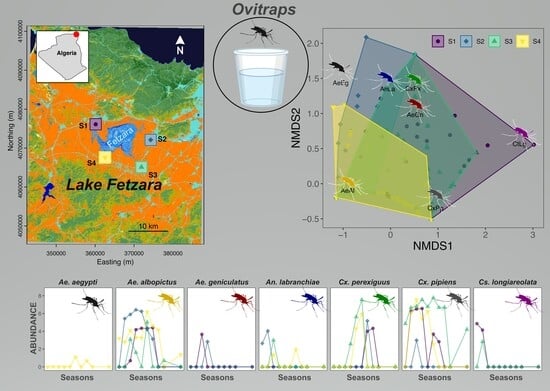
Spatial and Temporal Distribution of Mosquito Species (Culicidae) in a Ramsar Site, Fetzara Lake (Annaba, Algeria)
Simple Summary
Abstract
1. Introduction
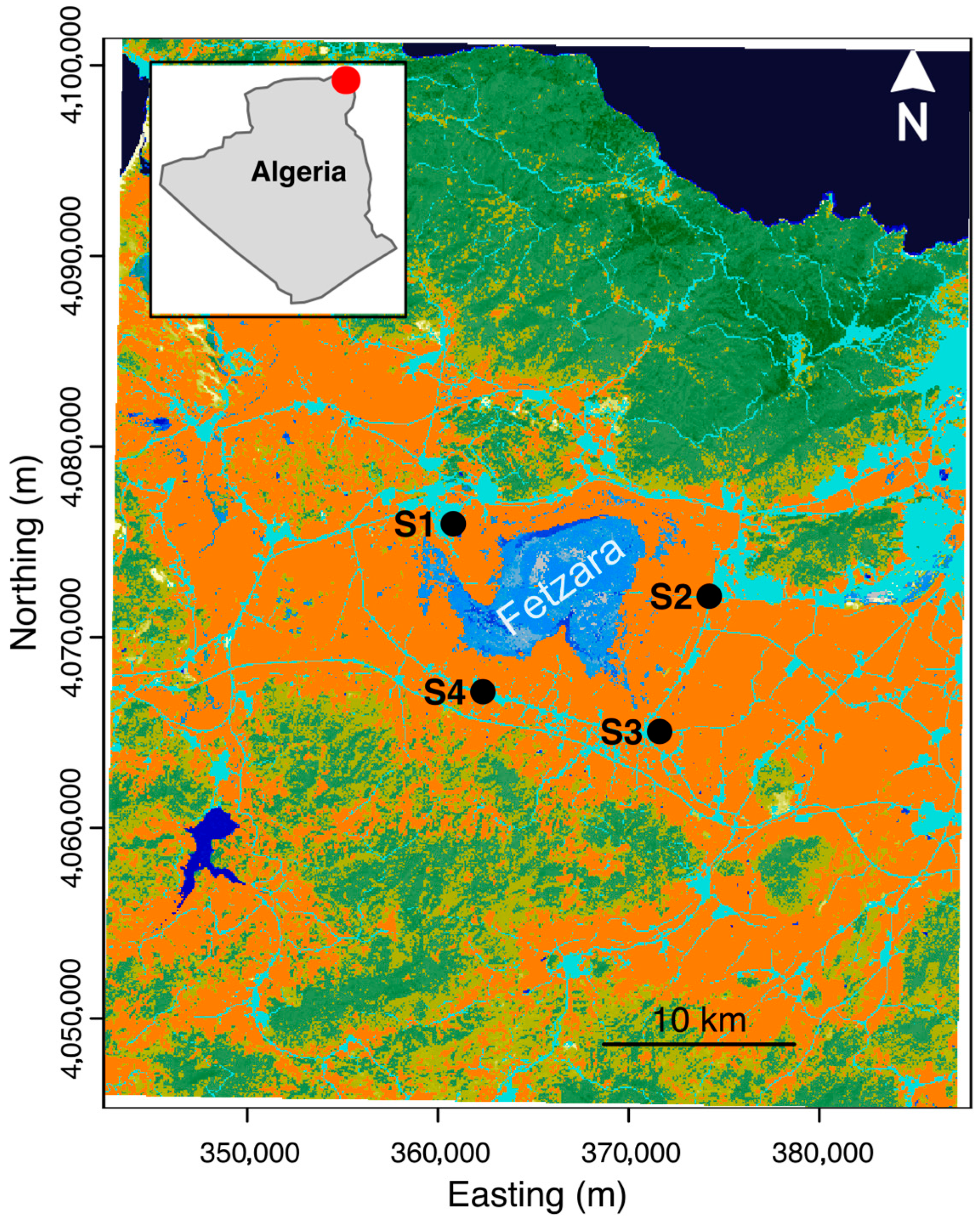
2. Materials and Methods
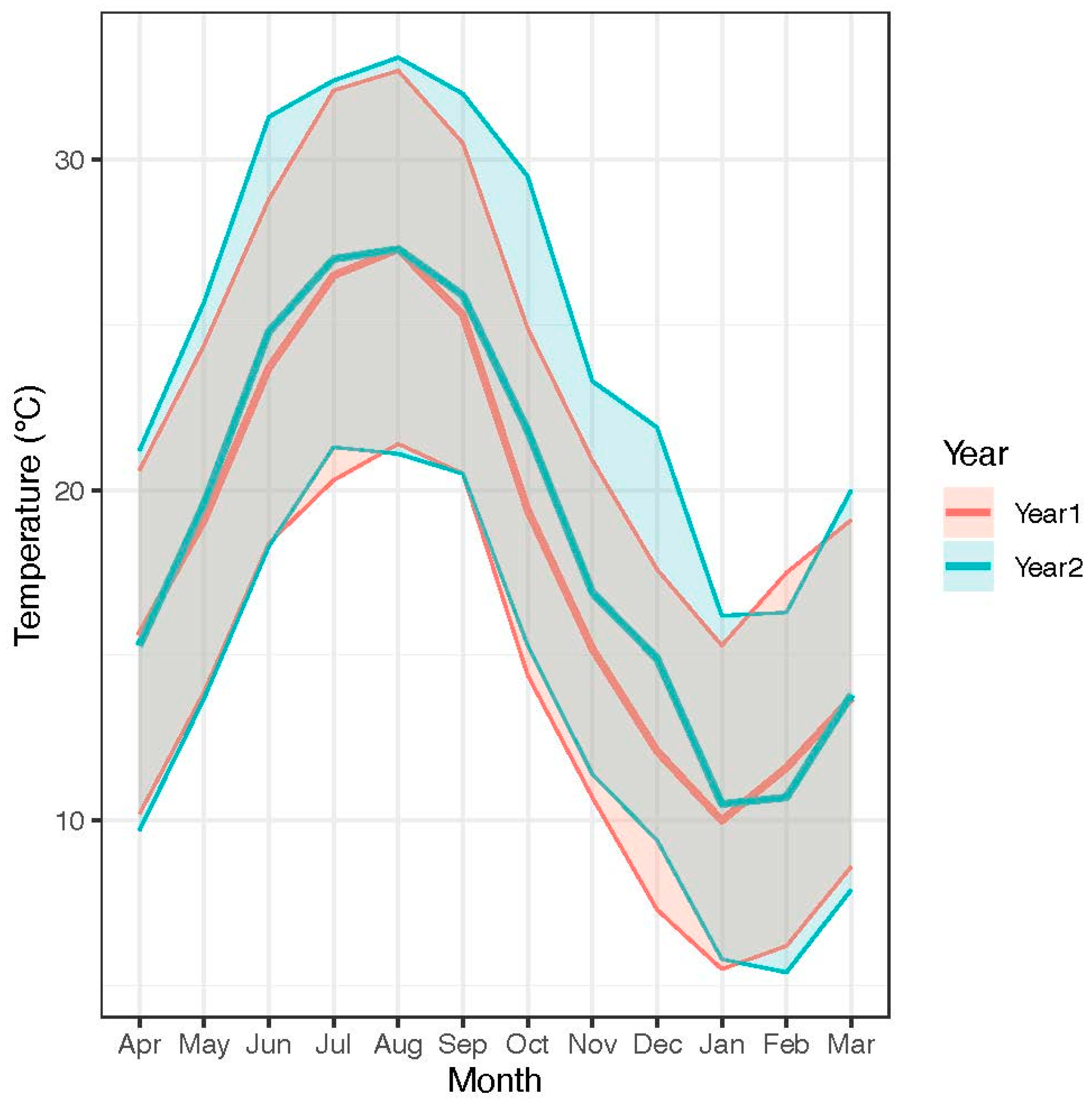
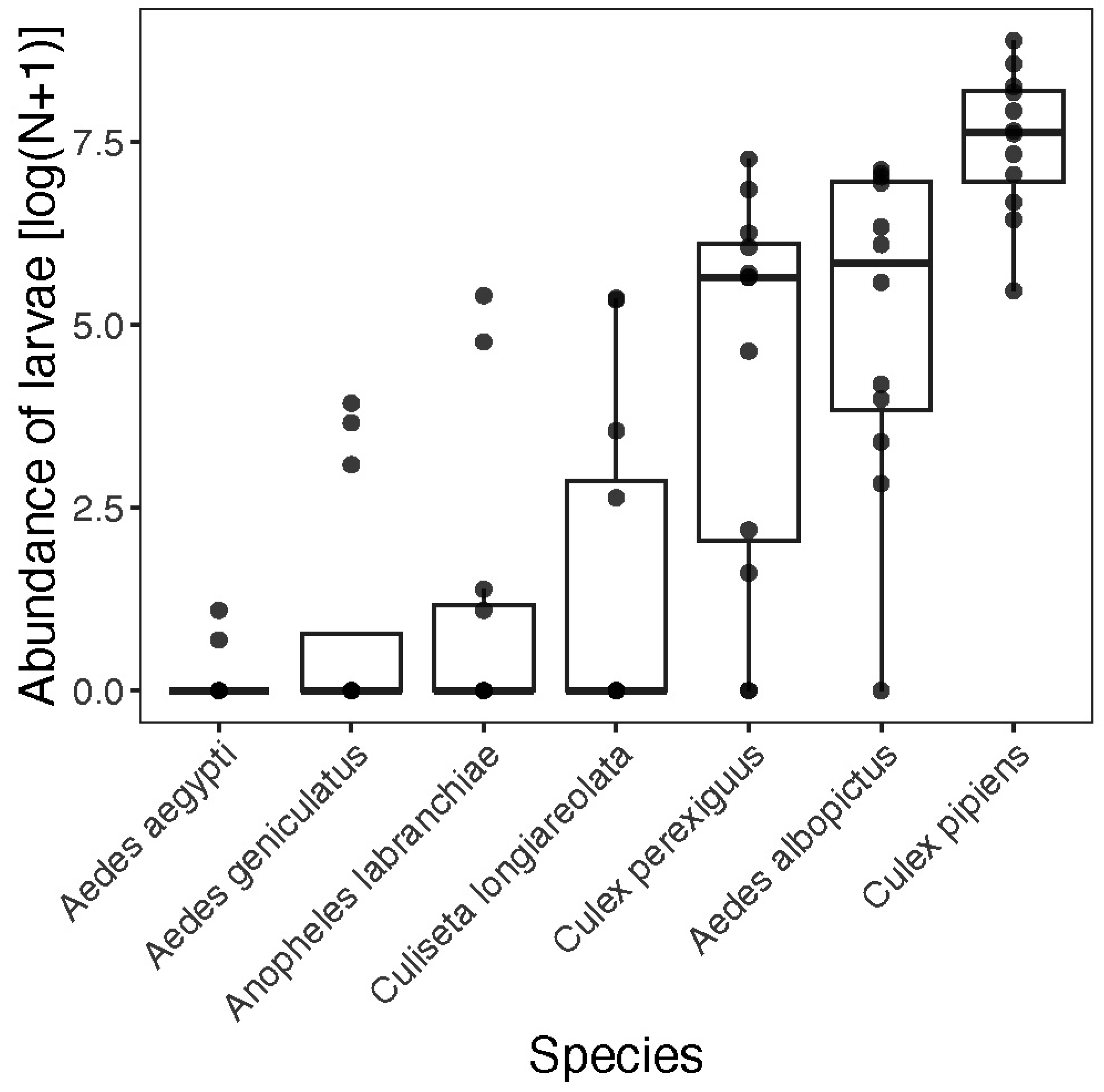
3. Results
4. Discussion
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
References
- Blaustein, L.; Chase, J.M. Interactions between mosquito larvae and species that share the same trophic level. Annu. Rev. Entomol. 2007, 52, 489–507. [Google Scholar] [CrossRef]
- Weterings, R.; Umponstira, C.; Buckley, H.L. Landscape variation influences trophic cascades in dengue vector food webs. Sci. Adv. 2018, 4, 9534–9542. [Google Scholar] [CrossRef]
- Dambach, P. The use of aquatic predators for larval control of mosquito disease vectors: Opportunities and limitations. Biol. Control 2020, 150, 104357. [Google Scholar] [CrossRef]
- Medlock, J.; Snow, K. Natural predators and parasites of British mosquitoes—A review. Eur. Mosq. Bull. 2008, 25, 1–11. [Google Scholar]
- Dale, P.E.R.; Knight, J.M. Wetlands and mosquitoes: A review. Wetl. Ecol. Manag. 2008, 16, 255–276. [Google Scholar] [CrossRef]
- Avramov, M.; Thaivalappil, A.; Ludwig, A.; Miner, L.; Cullingham, C.I.; Waddell, L.; Lapen, D.R. Relationships between water quality and mosquito presence and abundance: A systematic review and meta-analysis. J. Med. Entomol. 2023, 61, 1–33. [Google Scholar] [CrossRef]
- Dworrak, T.V.; Sauer, F.G.; Kiel, E. Wetland Conservation and Its Effects on Mosquito Populations. Wetlands 2022, 42, 96–109. [Google Scholar] [CrossRef]
- Khelifa, R.; Deacon, C.; Mahdjoub, H.; Suhling, F.; Simaika, J.P.; Samways, M.J. Dragonfly Conservation in the Increasingly Stressed African Mediterranean-Type Ecosystems. Front. Environ. Sci. 2021, 9, 301–316. [Google Scholar] [CrossRef]
- Khelifa, R.; Mahdjoub, H.; Samways, M.J. Combined climatic and anthropogenic stress threaten resilience of important wetland sites in an arid region. Sci. Total Environ. 2022, 806, 150806. [Google Scholar] [CrossRef] [PubMed]
- Nebbak, A.; Almeras, L.; Parola, P.; Bitam, I. Mosquito Vectors (Diptera: Culicidae) and Mosquito-Borne Diseases in North Africa. Insects 2022, 13, 962. [Google Scholar] [CrossRef]
- Rouibi, A.; Baaloudj, A.; Chahrour, F.; Kerfouf, A.; Rizi, H.; Berdja, R.; Belkhiria, W.; Chaib, S.; Gharbi, M. Characterization and diversity of macroinvertebrates of the Bouhamdane Stream (northeast of Algeria). Zool. Ecol. 2021, 31, 45–52. [Google Scholar] [CrossRef]
- Baaloudj, A.; Ouarab, S.; Kerfouf, A.; Bouriach, M.; Ali Hussein, A.; Hammana, C.; N’Diaye, D. Use of macro invertebrates to assess the quality of Seybouse River (North-East of Algeria). Ukr. J. Ecol. 2020, 10, 60–66. [Google Scholar] [CrossRef] [PubMed]
- Bekhouche, N.; Marniche, F.; Ouldjaoui, A.; Khammar, H.; Gherraf, N.; Ferroudja, M.O.; Mihoubi, L. Dipters from the sub-basin of Boumerzoug (Constantine–Algeria). Acta Sci. Nat. 2021, 8, 51–68. [Google Scholar] [CrossRef]
- Djamai, S.; Mimeche, F.; Bensaci, E.; Oliva-Paterna, F. Diversity of macro-invertebrates in Lake Tonga (northeast Algeria). Biharean Biol. 2019, 13, 8–11. [Google Scholar]
- Mellouk, K.; Aroua, N. Le lac Fetzara, une zone humide fragile, menacée par l’extension urbaine de la ville d’Annaba (littoral est algérien). Méditerranée 2015, 125, 133–140. [Google Scholar] [CrossRef]
- Ricci, G.; Faouzi, Z.; D’Ambrosio, E.; De Girolamo, A.; Parete, G.; Debieche, T.-H.; Gentile, F. Evaluating flow regime alterations due to point sources in intermittent rivers: A modelling approach. J. Agric. Eng. 2022, 53, 1333–1348. [Google Scholar]
- Ziouch, O.R.; Laskri, H.; Chenaker, H.; Ledjedel, N.E.; Daifallah, T.; Ounissi, M. Transport of nutrients from the Seybouse River to Annaba Bay (Algeria, SW Mediterranean). Mar. Pollut. Bull. 2020, 156, 111231–111245. [Google Scholar] [CrossRef]
- Shittu, R.A.; Thomas, S.M.; Roiz, D.; Ruiz, S.; Figuerola, J.; Beierkuhnlein, C. Modeling the effects of species associations and abiotic parameters on the abundance of mosquito species in a Mediterranean wetland. Wetl. Ecol. Manag. 2024, 32, 381–395. [Google Scholar] [CrossRef]
- Ruairuen, W.; Amnakmanee, K.; Primprao, O.; Boonrod, T. Effect of ecological factors and breeding habitat types on Culicine larvae occurrence and abundance in residential areas Southern Thailand. Acta Trop. 2022, 234, 106630. [Google Scholar] [CrossRef]
- Smith, D.C.; Schafer, S.M.; Golding, N.; Nunn, M.A.; White, S.M.; Callaghan, A.; Purse, B.V. Vegetation structure drives mosquito community composition in UK’s largest managed lowland wetland. Parasites Vectors 2024, 17, 201. [Google Scholar] [CrossRef] [PubMed]
- Tarekegn, M.; Tekie, H.; Wolde-Hawariat, Y.; Dugassa, S. Habitat characteristics and spatial distribution of Anopheles mosquito larvae in malaria elimination settings in Dembiya District, Northwestern Ethiopia. Int. J. Trop. Insect Sci. 2022, 42, 2937–2947. [Google Scholar] [CrossRef]
- Hernandez-Triana, L.M.; Garza-Hernandez, J.A.; Ortega Morales, A.I.; Prosser, S.W.J.; Hebert, P.D.N.; Nikolova, N.I.; Barrero, E.; de Luna-Santillana, E.J.; Gonzalez-Alvarez, V.H.; Mendez-Lopez, R.; et al. An Integrated Molecular Approach to Untangling Host-Vector-Pathogen Interactions in Mosquitoes (Diptera: Culicidae) from Sylvan Communities in Mexico. Front. Vet. Sci. 2021, 7, 564791. [Google Scholar] [CrossRef]
- Mokany, A.; Shine, R. Competition between tadpoles and mosquito larvae. Oecologia 2003, 135, 615–620. [Google Scholar] [CrossRef]
- Noden, B.H.; O’Neal, P.A.; Fader, J.E.; Juliano, S.A. Impact of inter- and intra-specific competition among larvae on larval, adult, and life-table traits of Aedes aegypti and Aedes albopictus females. Ecol. Entomol. 2016, 41, 192–200. [Google Scholar] [CrossRef]
- Vieira, C.; Gyawali, N.; Onn, M.B.; Shivas, M.A.; Shearman, D.; Darbro, J.M.; Wallau, G.L.; van den Hurk, A.F.; Frentiu, F.D.; Skinner, E.B.; et al. Mosquito bloodmeals can be used to determine vertebrate diversity, host preference, and pathogen exposure in humans and wildlife. Sci. Rep. 2024, 14, 23203–23212. [Google Scholar] [CrossRef]
- Gleiser, R.M.; Schelotto, G.; Gorla, D.E. Spatial pattern of abundance of the mosquito, Ochlerotatus albifasciatus, in relation to habitat characteristics. Med. Vet. Entomol. 2002, 16, 364–371. [Google Scholar] [CrossRef] [PubMed]
- Golding, N.; Nunn, M.A.; Purse, B.V. Identifying biotic interactions which drive the spatial distribution of a mosquito community. Parasites Vectors 2015, 8, 367. [Google Scholar] [CrossRef] [PubMed]
- Ramsar Sites Information Service. Lac Fetzara. Available online: https://rsis.ramsar.org/ris/1299 (accessed on 15 July 2023).
- Habes, S. Caractéristiques Chimiques d’un lac Appartenant aux Écosystèmes Humides du Nord de l’Algérie; Cas: Lac Fetzara Nord-Est Algérien. Ph.D. Thesis, University of Badji Mokhtar, Annaba, Algeria, 2013. [Google Scholar]
- Boumezbeur, A. Fiche Descriptive sur les Zones Humides Ramsar; Direction Generale Des Forets: Alger, Algeria, 2003; pp. 1–16. [Google Scholar]
- Djamai, R. Contribution à l’étude de la Salinité des Sols et des Eaux du Système Endoréique du lac Fetzara (NordEst Algérien). Approche Géochimique et Évolution Spatiotemporelle des Phénomènes. Ph.D. Thesis, Institut National Agronomique d’Alger, El-Harrach, Algeria, 2007. [Google Scholar]
- Coroian, M.; Silaghi, C.; Tews, B.A.; Baltag, E.S.; Marinov, M.; Alexe, V.; Kalmar, Z.; Cintia, H.; Lupse, M.S.; Mihalca, A.D. Serological Survey of Mosquito-Borne Arboviruses in Wild Birds from Important Migratory Hotspots in Romania. Pathogens 2022, 11, 1270. [Google Scholar] [CrossRef]
- Fekrache, F. Contribution A L’etude De L’origine De La Salinite Des Eaux Du Lac Fetzara-annaba. Ph.D. Thesis, Université Badji Mokhtar, Annaba, Algeria, 2015. [Google Scholar]
- Boulaksaa, K.; Laifa, A. Évaluation de la pollution azotée minérale des eaux superficielles de la zone humide Ramsar du lac Fetzara (Nord-Est algérien). Rev. Sci. L’eau 2020, 32, 409–419. [Google Scholar] [CrossRef]
- Zahi, F.; Djamai, R.; Drouiche, A.M.; Medjani, F. Contribution to study of soil salinity in the region of Fetzara Lake (Northeast of Algeria). J. Mater. Environ. Sci. 2011, 2, 439–444. [Google Scholar]
- Fay, R.W.; Eliason, D.A. A preferred oviposition site as a surveillance method for Aedes aegypti. Mosq. News 1966, 26, 531–535. [Google Scholar]
- Regis, L.; Monteiro, A.M.; Melo-Santos, M.A.; Silveira, J.C., Jr.; Furtado, A.F.; Acioli, R.V.; Santos, G.M.; Nakazawa, M.; Carvalho, M.S.; Ribeiro, P.J., Jr.; et al. Developing new approaches for detecting and preventing Aedes aegypti population outbreaks: Basis for surveillance, alert and control system. Mem. Inst. Oswaldo Cruz 2008, 103, 50–59. [Google Scholar] [CrossRef]
- Rouibi, A.; Adjami, Y.; Merabti, B.; Boumaza, M.; Khamsa, K.; Rouibi, A.; Ramdani, K.; Ouakid, M.L. Update on the distribution and the status the Asian tiger mosquito (Aedes albopictus) on the population of Culicidae in the North-east of Algeria. Ukr. J. Ecol. 2023, 13, 17–27. [Google Scholar]
- Marabuto, E.; Rebelo, M.T. The Asian tiger mosquito, Aedes (Stegomyia) albopictus (Skuse), a vector of dengue, chikungunya and zika viruses, reaches Portugal (Diptera: Culicidae). Zootaxa 2018, 4413, 197–200. [Google Scholar] [CrossRef]
- Schaffner, F.; Angel, G.; Geoffroy, B.; Hervy, J.-P.; Rhaiem, A.; Brunhes, J. The Mosquitoes of Europe. An Identification and Training Programme; IRD Editions & EID Méditerranée: Montpellier, France, 2001. [Google Scholar]
- Brunhes, J.; Rhaim, A.; Geoffroy, B.; Angel, G.; Hervy, J.-P. Les Moustiques de l’Afrique Méditerranéenne: Logiciel D’identification et D’enseignement; IRD and IPT, 1 CD-Rom collection didactique, Editions IRD; FRA: Paris, France; IRD: Tunis, Tunisia, 2000; p. 1 CD-ROM. [Google Scholar]
- Oksanen, J.; Simpson, G.L.; Blanchet, F.G.; Kindt, R.; Legendre, P.; Minchin, P.R.; O’Hara, R.B.; Solymos, P.; Stevens, M.H.H.; Szoecs, E.; et al. Vegan: Community Ecology Package. 2024. Available online: https://CRAN.R-project.org/package=vegan (accessed on 12 July 2024).
- R Core Development Team. R: A Language and Environment for Statistical Computing; Vienna, Austria. 2025. Available online: https://www.R-project.org/ (accessed on 1 March 2025).
- Merabti, B.; Boumaza, M.; Ouakid, M.L.; Carvajal, T.M.; Harbach, R.E. An updated checklist of the mosquitoes (Diptera: Culicidae) present in Algeria, with assessments of doubtful records and problematic species. Zootaxa 2021, 5027, 515–545. [Google Scholar] [CrossRef]
- Amara Korba, R.; Alayat, M.S.; Bouiba, L.; Boudrissa, A.; Bouslama, Z.; Boukraa, S.; Francis, F.; Failloux, A.B.; Boubidi, S.C. Ecological differentiation of members of the Culex pipiens complex, potential vectors of West Nile virus and Rift Valley fever virus in Algeria. Parasites Vectors 2016, 9, 455. [Google Scholar] [CrossRef]
- Aqeehal, H.; Shibani, N.; Annajar, B. Mosquito species composition at a selected area in eastern Tripoli, Libya. Int. J. Entomol. Res. 2019, 4, 122–125. [Google Scholar]
- Benallal, K.E.; Garni, R.; Bouiba, L.; Harrat, Z. First Detection of Aedes (Stegomyia) albopictus (Diptera: Culicidae) in Algiers, the Capital City of Algeria. J. Arthropod-Borne Dis. 2019, 13, 420–425. [Google Scholar] [CrossRef] [PubMed]
- Sergent, E.; Sergent, É. Observations sur les moustiques des environs d’Alger. J. Ann. Inst. Pasteur 1903, 17, 60–67. [Google Scholar]
- Senevet, G.; Andarelli, L. Mosquitoes of North Africa and the Mediterranean basin. 3. Aedes. I. Generalities. J. Arch. L’institut Pasteur d’Algerie 1963, 41, 115–141. [Google Scholar]
- Senevet, G.; Andarelli, L. The mosquitoes of North Africa and the Mediterranean. III. Aedes. Part 2. Sub-genus Ochlerotatus: Group H. Arch. Inst. Pasteur d’Algerie 1966, 44, 51–74. [Google Scholar]
- Foley, H. Les moustiques du Sahara algérien. Arch. l’Inst. Pasteur d’Algérie 1923, 1, 295–301. [Google Scholar]
- Ramsdale, C.D.; de Zulueta, J. Anophelism in the Algerian Sahara and some implications of the construction of a trans-Saharan highway. J. Trop. Med. Hyg. 1983, 86, 51–58. [Google Scholar] [PubMed]
- Berchi, S. Bioécologie de Culex pipiens L. (Diptera: Culicidae) Dans la Région de Constantine et Perspectives de Lutte. Ph.D. Thesis, University of Brothers Mentouri, Constantine, Algeria, 2000. [Google Scholar]
- Boudemagh, N.; Bendali-Saoudi, F.; Soltani, N. Inventory of Culicidae (Diptera: Nematocera) in the region of Collo (North-East Algeria). J. Ann. Biol. Res. 2013, 4, 94–99. [Google Scholar]
- Lafri, I.; Assia, B.; Ben-Mahdi, M. An inventory of mosquitoes (Diptera: Culicidae) in Algeria. Bull. Soc. Zool. Fr. 2014, 139, 257–263. [Google Scholar]
- Amara Korba, R.; Boukraa, S.; Saoucen, A.; Bendjeddou, M.; Francis, F.; Boubidi, S.; Bouslama, Z. Preliminary report of mosquitoes survey at Tonga Lake (North-East Algeria). Adv. Environ. Biol. 2015, 9, 288–294. [Google Scholar]
- Messai, N.; Aouati, A.; Berchi, S. Impact of the surface water physicochemical parameters on Culicidae (Diptera: Nematocera) of lakeside ecosystem “Sebkhet Ezzemoul”(Oum El Bouaghi-Algeria). J. Entomol. Zool. Stud. 2016, 3, 391–398. [Google Scholar]
- Dahchar, Z.; Oudainia, W.; Bendali-Saoudi, F.; Soltani, N. Inventory of Culicidae of the wetland (of the West region of Annaba). J. Entomol. Zool. Stud. 2017, 5, 430–436. [Google Scholar]
- Houmani, M.; Bendali-Saoudi, F.; Soltani, N. Inventory of Culicidae in the region of El Taref (North-east Algeria). J. Entomol. Zool. Stud. 2017, 5, 263–267. [Google Scholar]
- Serradj, N.; Bendali-Saoudi, F.; Soltani, N. Inventory of the invertebrate fauna at the level of the Lake of Birds (North-east-Algeria). J. Entomol. Zool. Stud. 2018, 6, 98–106. [Google Scholar]
- Arroussi, D.E.R.; Bouaziz, A.; Boudjelida, H. Mosquito survey reveals the first record of Aedes (Diptera: Culicidae) species in urban area, Annaba district, Northeastern Algeria. Pol. J. Entomol. 2021, 90, 14–26. [Google Scholar] [CrossRef]
- Halimi, I.; Lebbal, S.; Mari, R.B.; Ghorab, A.; Saidi, F. Biodiversity of Culicidae (Insecta: Diptera) in the Region of Khenchela (Northeast Algeria). J. Bioresour. Manag. 2022, 9, 8. [Google Scholar]
- Boulares, M.; Rehimi, N.; Houhamdi, I.; Baaloudj, A.; Soltani, N.; Houhamdi, M. Systematic and ecological study of mosquitoes (Diptera: Culicidae) at lake Fetzara (Annaba, Northeast Algeria). Ukr. J. Ecol. 2023, 13, 1–7. [Google Scholar]
- Rouibi, A.; Rouibi, A.; Rouibi, A. The First Culicidae Inventory in the Region of Guelma (Northeast Algeria). Transylv. Rev. Syst. Ecol. Res. 2024, 26, 75–86. [Google Scholar] [CrossRef]
- Carrieri, M.; Bacchi, M.; Bellini, R.; Maini, S. On the Competition Occurring Between Aedes albopictus and Culex pipiens (Diptera: Culicidae) in Italy. Environ. Entomol. 2003, 32, 1313–1321. [Google Scholar] [CrossRef]
- Brunhes, J.; Hassaïne, K.; Rhaiem, A.; Hervy, J.-P. Les Culicides de l’Afrique méditerranéenne: Espèces présentes et répartition (Diptera, Nematocera). Bull. Société Entomol. Fr. 2000, 105, 195–204. [Google Scholar] [CrossRef]
- Dalla Pozza, G.; Majori, G. First record of Aedes albopictus establishment in Italy. J. Am. Mosq. Control Assoc. 1992, 8, 318–320. [Google Scholar] [PubMed]
- Adhami, J.; Reiter, P. Introduction and establishment of Aedes (Stegomyia) albopictus skuse (Diptera: Culicidae) in Albania. J. Am. Mosq. Control Assoc. 1998, 14, 340–343. [Google Scholar]
- Izri, A.; Bitam, I.; Charrel, R.N. First entomological documentation of Aedes (Stegomyia) albopictus (Skuse, 1894) in Algeria. Clin. Microbiol. Infect. 2011, 17, 1116–1118. [Google Scholar] [CrossRef]
- Hamaidia, K.; Soltani, N. New report of Aedes albopictus in Souk Ahras, Northeast Algeria. Biodivers. J. Biol. Divers. 2021, 22, 2901–2906. [Google Scholar] [CrossRef]
- Senevet, G.; Prunelle, M. Larves de Culicides recueillies en Algérie et en Tunisie par M.H.Gauthier. Bull. Société d’Histoire Nat. d’Afrique Nord 1928, 19, 94–99. [Google Scholar]
- Bacqué, B.; Keiffer, J. Existence de Stegomyia fasciata au Sahara, à 500 kilomètres de la côte méditerranéenne. Arch. l’Inst. Pasteur d’Algérie 1923, 1, 169–171. [Google Scholar]
- Senevet, G.; Andarelli, L. Observations on Geographical Distribution of Subspecies, Varieties and Forms of Aedes aegypti. Arch. l’Inst. Pasteur d’Algérie 1961, 39, 100–103. [Google Scholar]
- Holstein, M. Dynamics of Aedes aegypti Distribution, Density and Seasonal Prevalence in the Mediterranean Area. Bull. World Health Organ. 1967, 36, 541–543. [Google Scholar]
- Senevet, G.; Andarelli, L. Le genre Aedes en Afrique du Nord. Arch. l’Inst. Pasteur Alger 1954, 41, 115–141. [Google Scholar]
- Hongo, H.; Masikini, M. Impact of immigrant pastoral herds to fringing wetlands of lake Victoria in Magu district Mwanza region, Tanzania. Phys. Chem. Earth Parts A/B/C 2003, 28, 1001–1007. [Google Scholar] [CrossRef]
- Mironga, J. Effect of farming practices on wetlands of Kisii District, Kenya. Appl. Ecol. Environ. Res. 2005, 3, 81–91. [Google Scholar] [CrossRef]
- Becker, N.; Hoffmann, D. First record of Culiseta longiareolata (Macquart) for Germany. Eur. Mosq. Bull. 2011, 29, 143–150. [Google Scholar]
- Roiz, D.; Ruiz, S.; Soriguer, R.; Figuerola, J. Landscape Effects on the Presence, Abundance and Diversity of Mosquitoes in Mediterranean Wetlands. PLoS ONE 2015, 10, e0128112. [Google Scholar] [CrossRef] [PubMed]
- Spanoudis, C.G.; Pappas, C.S.; Savopoulou-Soultani, M.; Andreadis, S.S. Composition, seasonal abundance, and public health importance of mosquito species in the regional unit of Thessaloniki, Northern Greece. Parasitol. Res. 2021, 120, 3083–3090. [Google Scholar] [CrossRef]
- Mayi, M.P.A.; Bamou, R.; Djiappi-Tchamen, B.; Fontaine, A.; Jeffries, C.L.; Walker, T.; Antonio-Nkondjio, C.; Cornel, A.J.; Tchuinkam, T. Habitat and Seasonality Affect Mosquito Community Composition in the West Region of Cameroon. Insects 2020, 11, 312. [Google Scholar] [CrossRef]
- Merabti, B.; Boumaza, M.; Lebbouz, I.; Ouakid, M.L. First record of the avian malaria vector Cs. longiareolata (Diptera: Culicidae) for the Southeast of Algeria. J. Appl. Biosci. 2020, 154, 15842–15861. [Google Scholar]
- Baril, C.; Pilling, B.G.; Mikkelsen, M.J.; Sparrow, J.M.; Duncan, C.A.M.; Koloski, C.W.; LaZerte, S.E.; Cassone, B.J. The influence of weather on the population dynamics of common mosquito vector species in the Canadian Prairies. Parasites Vectors 2023, 16, 153. [Google Scholar] [CrossRef]
- Schäfer, M.L.; Lundström, J.O.; Petersson, E. Comparison of mosquito (Diptera: Culicidae) populations by wetland type and year in the lower River Dalälven region, Central Sweden. J. Vector Ecol. 2008, 33, 150–157. [Google Scholar] [CrossRef] [PubMed]
- Ciota, A.T.; Matacchiero, A.C.; Kilpatrick, A.M.; Kramer, L.D. The effect of temperature on life history traits of Culex mosquitoes. J. Med. Entomol. 2014, 51, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Agyekum, T.P.; Arko-Mensah, J.; Botwe, P.K.; Hogarh, J.N.; Issah, I.; Dwomoh, D.; Billah, M.K.; Dadzie, S.K.; Robins, T.G.; Fobil, J.N. Effects of elevated temperatures on the development of immature stages of Anopheles gambiae (s.l.) mosquitoes. Trop. Med. Int. Health 2022, 27, 338–346. [Google Scholar] [CrossRef]
- Kirik, H.; Burtin, V.; Tummeleht, L.; Kurina, O. Friends in All the Green Spaces: Weather Dependent Changes in Urban Mosquito (Diptera: Culicidae) Abundance and Diversity. Insects 2021, 12, 352. [Google Scholar] [CrossRef]
- Koenraadt, C.J.M.; Harrington, L.C. Flushing Effect of Rain on Container-Inhabiting Mosquitoes Aedes aegypti and Culex pipiens (Diptera: Culicidae). J. Med. Entomol. 2008, 45, 28–35. [Google Scholar] [CrossRef]
- Valdez, L.D.; Sibona, G.J.; Diaz, L.A.; Contigiani, M.S.; Condat, C.A. Effects of rainfall on Culex mosquito population dynamics. J. Theor. Biol. 2017, 421, 28–38. [Google Scholar] [CrossRef] [PubMed]
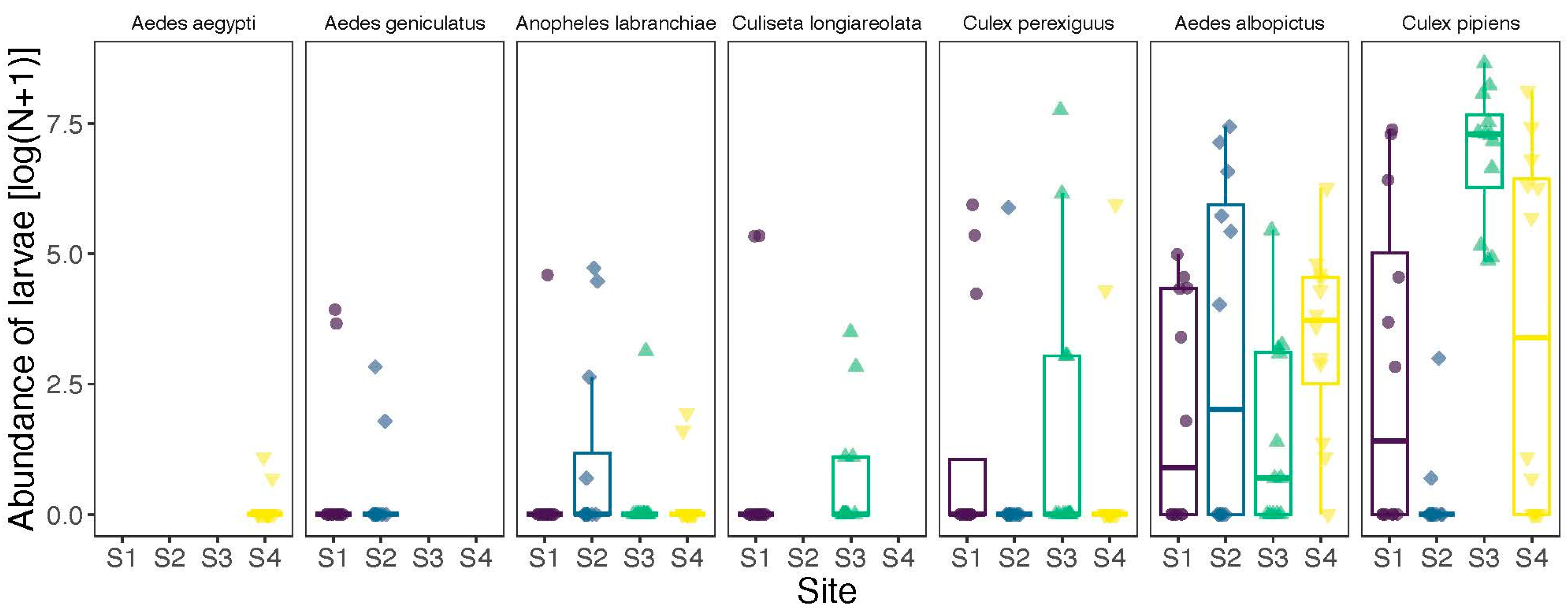
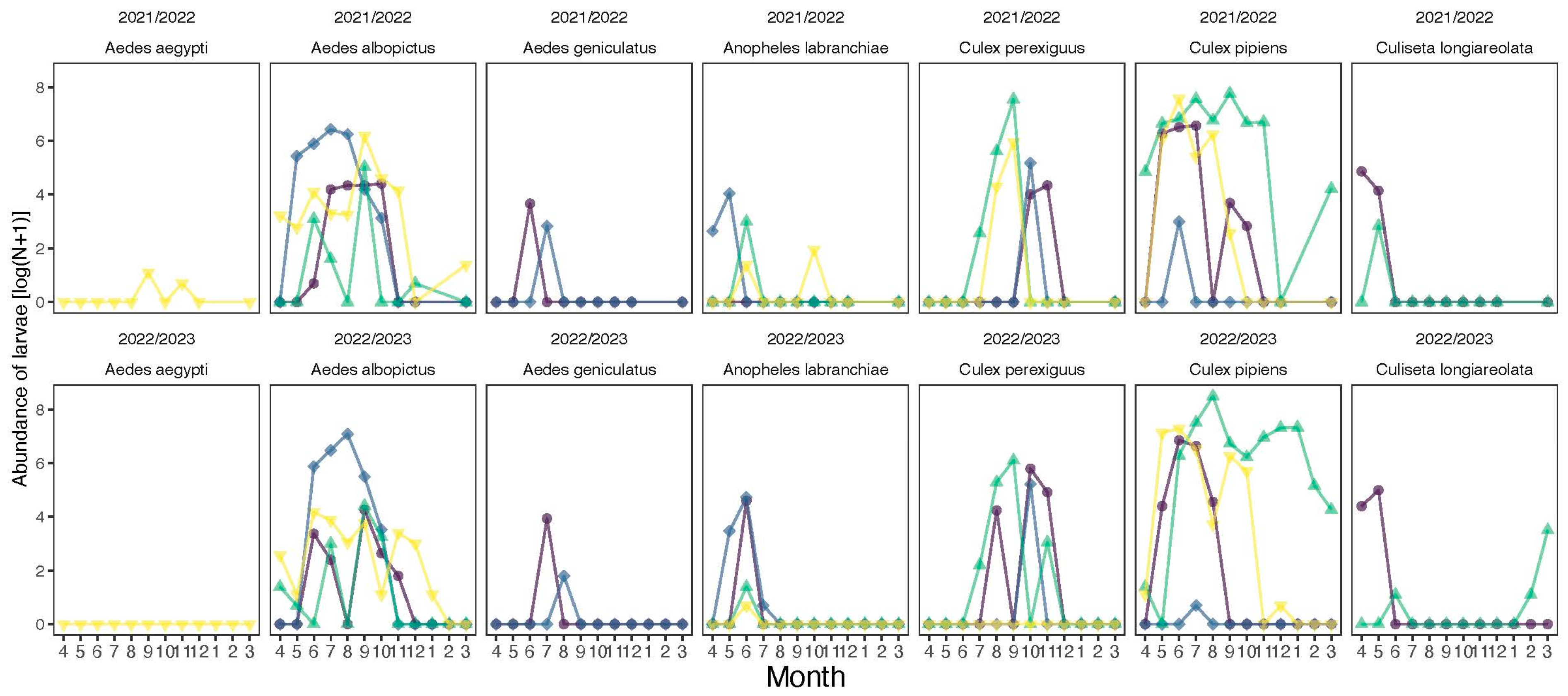
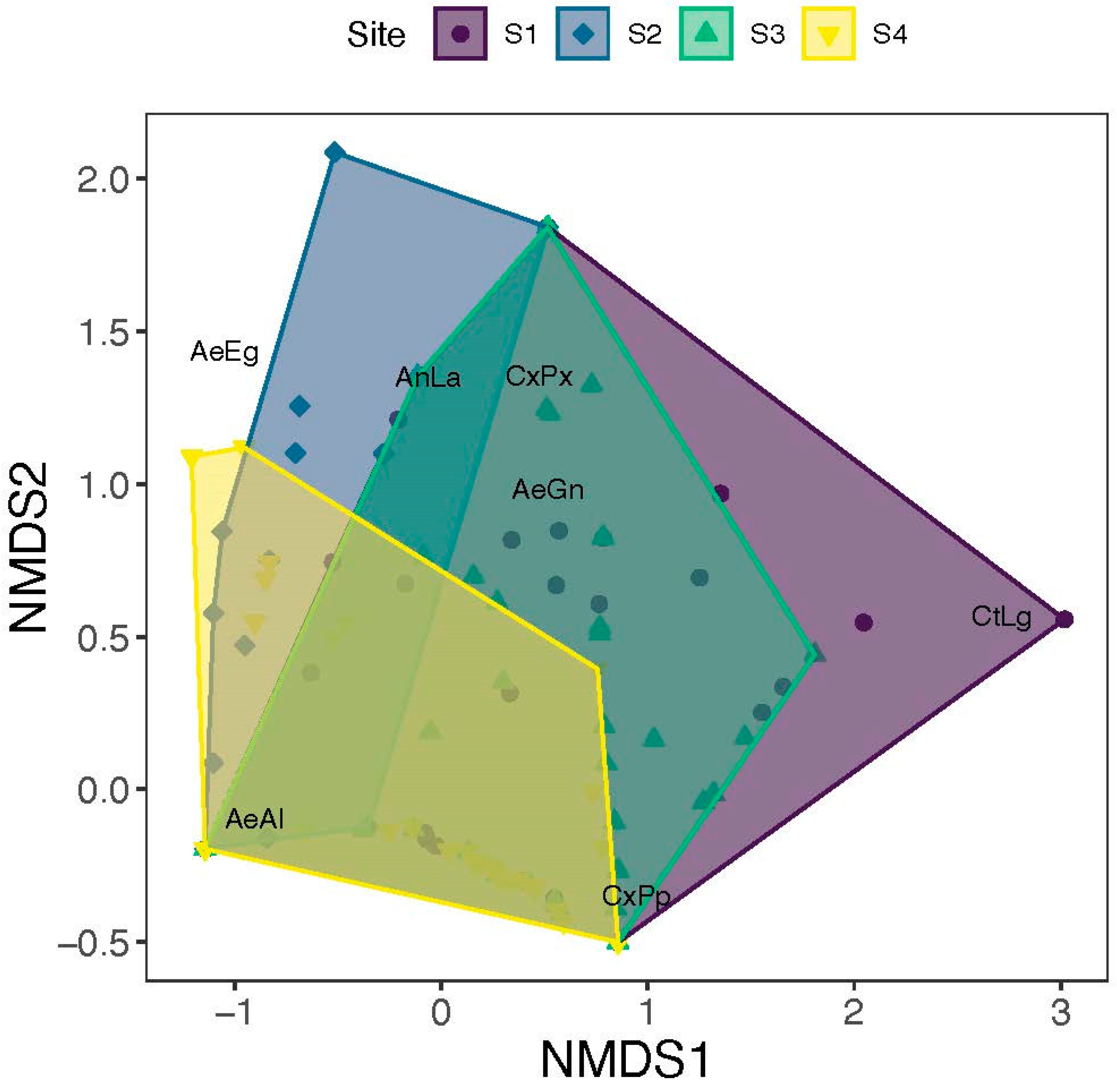

| Species | Code | Region | Site | Mean | SD | Min | Max | N |
|---|---|---|---|---|---|---|---|---|
| Aedes aegypti | AeEg | SW | S4 | 0.02 | 0.19 | 0 | 2 | 143 |
| Aedes albopictus | AeAl | NE | S2 | 29.90 | 70.00 | 0 | 606 | 143 |
| Aedes albopictus | AeAl | NW | S1 | 2.97 | 7.64 | 0 | 49 | 143 |
| Aedes albopictus | AeAl | SE | S3 | 2.15 | 13.70 | 0 | 152 | 143 |
| Aedes albopictus | AeAl | SW | S4 | 7.25 | 21.40 | 0 | 219 | 143 |
| Aedes geniculatus | AeGn | NE | S2 | 0.15 | 1.40 | 0 | 16 | 143 |
| Aedes geniculatus | AeGn | NW | S1 | 0.62 | 4.66 | 0 | 49 | 143 |
| Anopheles labranchiae | AnLa | NE | S2 | 1.49 | 7.20 | 0 | 58 | 143 |
| Anopheles labranchiae | AnLa | NW | S1 | 0.69 | 4.95 | 0 | 52 | 143 |
| Anopheles labranchiae | AnLa | SE | S3 | 0.15 | 1.20 | 0 | 13 | 143 |
| Anopheles labranchiae | AnLa | SW | S4 | 0.07 | 0.41 | 0 | 3 | 143 |
| Culex perexiguus | CxPx | NE | S2 | 2.51 | 18.60 | 0 | 183 | 143 |
| Culex perexiguus | CxPx | NW | S1 | 4.61 | 28.70 | 0 | 298 | 143 |
| Culex perexiguus | CxPx | SE | S3 | 19.90 | 120.00 | 0 | 1252 | 143 |
| Culex perexiguus | CxPx | SW | S4 | 3.20 | 22.30 | 0 | 236 | 143 |
| Culex pipiens | CxPp | NE | S2 | 0.14 | 1.59 | 0 | 19 | 143 |
| Culex pipiens | CxPp | NW | S1 | 26.90 | 67.00 | 0 | 455 | 143 |
| Culex pipiens | CxPp | SE | S3 | 150.00 | 252.00 | 0 | 1604 | 143 |
| Culex pipiens | CxPp | SW | S4 | 51.40 | 138.00 | 0 | 948 | 143 |
| Culiseta longiareolata | CtLg | NW | S1 | 2.92 | 11.40 | 0 | 76 | 143 |
| Culiseta longiareolata | CtLg | SE | S3 | 0.36 | 2.86 | 0 | 32 | 143 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rouibi, A.; Rouibi, A.; Khelifa, R. Spatial and Temporal Distribution of Mosquito Species (Culicidae) in a Ramsar Site, Fetzara Lake (Annaba, Algeria). Insects 2025, 16, 1057. https://doi.org/10.3390/insects16101057
Rouibi A, Rouibi A, Khelifa R. Spatial and Temporal Distribution of Mosquito Species (Culicidae) in a Ramsar Site, Fetzara Lake (Annaba, Algeria). Insects. 2025; 16(10):1057. https://doi.org/10.3390/insects16101057
Chicago/Turabian StyleRouibi, Amna, Abdelhakim Rouibi, and Rassim Khelifa. 2025. "Spatial and Temporal Distribution of Mosquito Species (Culicidae) in a Ramsar Site, Fetzara Lake (Annaba, Algeria)" Insects 16, no. 10: 1057. https://doi.org/10.3390/insects16101057
APA StyleRouibi, A., Rouibi, A., & Khelifa, R. (2025). Spatial and Temporal Distribution of Mosquito Species (Culicidae) in a Ramsar Site, Fetzara Lake (Annaba, Algeria). Insects, 16(10), 1057. https://doi.org/10.3390/insects16101057