Diversity, Distribution, and Host Blood Meals of Black Flies (Diptera: Simuliidae) in Laos
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Collection and Identification
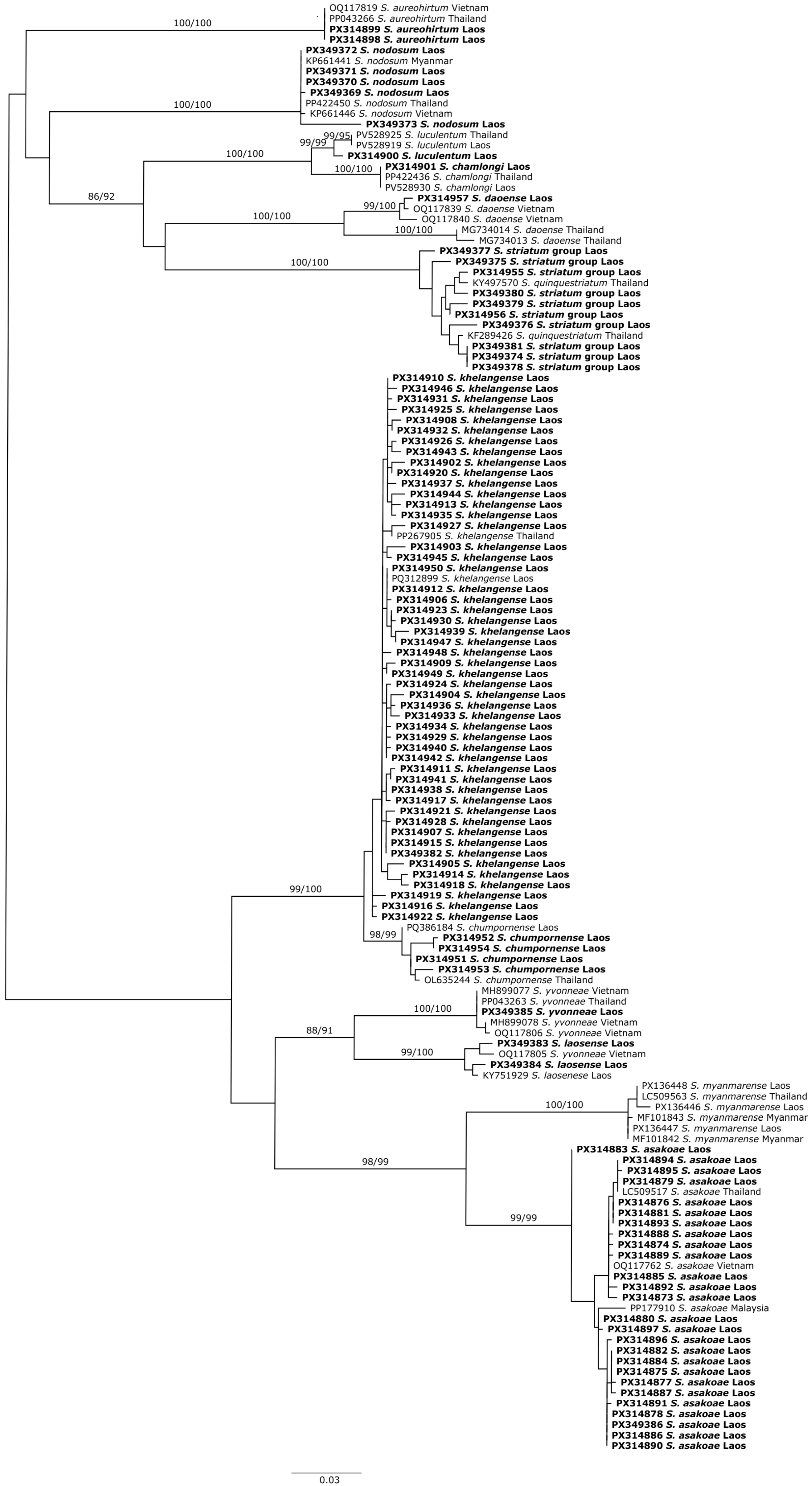
2.2. DNA Barcoding
2.3. Molecular Identification of Vertebrate Blood Meals
2.4. Data Analysis
3. Results
3.1. Adult Black Fly Diversity and Distribution
3.2. Blood Meal Identifications
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Adler, P.H.; McCreadie, J.W. Black flies (Simuliidae). In Medical and Veterinary Entomology, 3rd ed.; Mullen, G., Durden, L.A., Eds.; Elsevier: San Diego, CA, USA, 2019; pp. 237–259. [Google Scholar]
- WHO. Onchocerciasis. Available online: https://www.who.int/news-room/fact-sheets/detail/onchocerciasis (accessed on 21 August 2025).
- Takaoka, H.; Fukuda, M.; Otsuka, Y.; Aoki, C.; Uni, S.; Bain, O. Blackfly vectors of zoonotic onchocerciasis in Japan. Med. Vet. Entomol. 2012, 26, 372–378. [Google Scholar] [CrossRef]
- Valkiūnas, G. Avian Malaria Parasites and Other Haemosporidia; CRC: Boca Raton, FL, USA, 2005. [Google Scholar]
- Reisen, W.K. Landscape epidemiology of vector-borne diseases. Annu. Rev. Entomol. 2010, 55, 461–483. [Google Scholar] [CrossRef]
- Martínez-de la Puente, J.; Figuerola, J.; Soriguer, R. Fur or feather? Feeding preferences of species of Culicoides biting midges in Europe. Trends Parasitol. 2015, 31, 16–22. [Google Scholar] [CrossRef]
- Kitron, U. Landscape ecology and epidemiology of vector-borne diseases: Tools for spatial analysis. J. Med. Entomol. 1998, 35, 435–445. [Google Scholar] [CrossRef]
- Adler, P.H. World Blackflies (Diptera: Simuliidae): A Comprehensive Revision of the Taxonomic and Geographical Inventory. 2025. Available online: http://biomia.sites.clemson.edu/pdfs/blackflyinventory.pdf (accessed on 16 April 2025).
- Takaoka, H. The Black Flies of Subtropical and Tropical Asia: Taxonomy and Biology; Springer Nature: Singapore, 2024. [Google Scholar]
- Srisuka, W.; Sulin, C.; Aupalee, K.; Phankaen, T.; Taai, K.; Thongsahuan, S.; Saeung, A.; Takaoka, H. Community structure, biodiversity and spatiotemporal distribution of the black flies (Diptera: Simuliidae) using malaise traps on the highest mountain in Thailand. Insects 2021, 12, 504. [Google Scholar] [CrossRef]
- Gomontean, B.; Jumpato, W.; Wongpakam, K.; Tangkawanit, U.; Wannasingha, W.; Thanee, I.; Ya’cob, Z.; Pramual, P. Diversity, distribution and host blood meal analysis of adult black flies (Diptera: Simuliidae) from Thailand. Insects 2024, 15, 74. [Google Scholar] [CrossRef]
- Izwan-Anas, N.; Halim, M.R.A.; Low, V.L.; Adler, P.H.; Ya’cob, Z. Wild-caught adult black flies (Diptera: Simuliidae) from various ecological landscapes in Malaysia. Acta Trop. 2024, 259, 107374. [Google Scholar] [CrossRef]
- Mukherjee, A.; Kar, O.; Mukherjee, K.; Mukherjee, B.; Naskar, A.; Banerjee, D. Molecular identification of Onchocerciasis vectors (Diptera: Simuliidae) from the central Himalayan landscape of India: A DNA barcode approach. Vector Borne Zoonotic Dis. 2025, 25, 258–268. [Google Scholar] [CrossRef]
- Pramual, P.; Tangkawanit, U.; Kunprom, C.; Vaisusuk, K.; Chatan, W.; Wongpakam, K.; Thongboonma, S. Seasonal population dynamics and a role as natural vector of Leucocytozoon of black fly, Simulium chumpornense Takaoka & Kuvangkadilok. Acta Trop. 2020, 211, 105617. [Google Scholar] [CrossRef]
- Pramual, P. Black fly diversity and impacts on human welfare in Southeast Asia. In Biodiversity of Southeast Asian Parasites and Vectors Causing Human Disease; Petney, T.N., Saijuntha, W., Mehlhorn, H., Eds.; Parasitology Research Monographs; Springer: Cham, Switzerland, 2021; Volume 14, pp. 143–164. [Google Scholar]
- Adler, P.H.; Gomontean, B.; Jumpato, W.; Mintara, R.; Namtaku, S.; Thanee, I.; Wannasingha, W.; Wongpakam, K.; Jaroenchaiwattanachote, C.; Inkhavilay, K.; et al. An integrated analysis of mammalophilic blackflies in the Simulium variegatum group (Diptera: Simuliidae) in Laos. Med. Vet. Entomol. 2025. [Google Scholar] [CrossRef]
- Jumpato, W.; Tangkawanit, U.; Wongpakam, K.; Pramual, P. Molecular detection of Leucocytozoon (Apicomplexa: Haemosporida) in black flies (Diptera: Simuliidae) from Thailand. Acta Trop. 2019, 190, 228–234. [Google Scholar] [CrossRef]
- Jumpato, W.; Wannasingha, W.; Jaroenchaiwattanachote, C.; Mintara, R.; Wongpakam, K.; Adler, P.H.; Pramual, P. Diversity and prevalence of Leucocytozoon in black flies (Diptera: Simuliidae) of Thailand. Parasit. Vectors 2024, 17, 475. [Google Scholar] [CrossRef]
- Gomontean, B.; Jumpato, W.; Namtaku, S.; Wannasingha, W.; Wongpakam, K.; Thanee, I.; Inkhavilay, K.; Malavong, B.; Pramual, P. Black fly diversity and molecular detection of blood parasites in Simulium khelangense (Diptera, Simuliidae) from Laos. J. Med. Entomol. 2025, 62, 409–415. [Google Scholar] [CrossRef]
- Pramual, P.; Nanork, P. Phylogenetic analysis based on multiple gene sequences revealing cryptic biodiversity in Simulium multistriatum group (Diptera: Simuliidae) in Thailand. Entomol. Sci. 2012, 15, 202–213. [Google Scholar] [CrossRef]
- Takaoka, H.; Srisuka, W.; Saeung, A.; Maleewong, W.; Low, V.L. A new black fly species of Simulium (Gomphostilbia) (Diptera: Simuliidae) from Laos. J. Med. Entomol. 2017, 54, 1543–1551. [Google Scholar] [CrossRef]
- Thanee, I.; Gomontean, B.; Jumpato, W.; Namtaku, S.; Wongpakam, K.; Wannasingha, W.; Inkhavilay, K.; Malavong, B.; Pramual, P. Genetic characterization and breeding habitats of black fly (Diptera, Simuliidae) vector species in Laos. Diversity 2014, 16, 653. [Google Scholar] [CrossRef]
- Adler, P.H.; Vlasov, S.; Huang, Y.T.; Hadi, U.K.; Inkhavilay, K.; Malavong, B.; Topolenko, V.; Gomontean, B.; Jumpato, W.; Mintara, R.; et al. Rare chromosomal uniformity in black flies of the Simulium striatum species group (Diptera: Simuliidae). Insects 2025, 16, 511. [Google Scholar] [CrossRef]
- Adler, P.H.; Wannasingha, W.; Gomontean, B.; Jumpato, W.; Mintara, R.; Namtaku, S.; Thanee, I.; Wongpakam, K.; Jaroenchaiwattanachote, C.; Inkhavilay, K.; et al. Laos, a New Frontier for Investigating Black Flies (Diptera: Simuliidae). J. Med. Entomol. 2025. submitted. [Google Scholar]
- Takaoka, H.; Choochote, W.; Aoki, C.; Fukuda, M.; Bain, O. Black flies (Diptera: Simuliidae) attracted to humans and water buffalos and natural infections with filarial larvae, probably Onchocerca sp., in northern Thailand. Parasite 2003, 10, 3–8. [Google Scholar] [CrossRef][Green Version]
- Fukuda, M.; Choochote, W.; Bain, O.; Aoki, C.; Takaoka, H. Natural infections with filarial larvae in two species of black flies (Diptera: Simuliidae) in northern Thailand. Jpn. J. Trop. Med. Hyg. 2003, 31, 99–102. [Google Scholar] [CrossRef][Green Version]
- Takaoka, H.; Srisuka, W.; Saeung, A. Checklist and keys for the black flies (Diptera: Simuliidae) in Thailand. Med. Entomol. Zool. 2019, 70, 53–77. [Google Scholar] [CrossRef]
- Takaoka, H.; Sofian-Azirun, M.; Ya’Cob, Z.; Chen, C.D.; Lau, K.W.; Low, V.L.; Da Pham, X.; Adler, P.H. The black flies (Diptera: Simuliidae) of Vietnam. Zootaxa 2017, 4261, 1–165. [Google Scholar] [CrossRef]
- Folmer, O.; Black, M.; Hoeh, W.; Lutz, R.; Vrijenhoek, R. DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Mol. Mar. Biol. Biotechnol. 1994, 3, 294–299. [Google Scholar]
- Tangkawanit, U.; Wongpakam, K.; Pramual, P. A new black fly (Diptera: Simuliidae) species of the subgenus Asiosimulium Takaoka Choochote from Thailand. Zootaxa 2018, 4388, 111–122. [Google Scholar] [CrossRef]
- Kocher, T.D.; Thomas, W.K.; Meyer, A.; Edwards, S.V.; Pääbo, S.; Villablanca, F.X.; Wilson, A.C. Dynamics of mitochondrial DNA evolution in animals: Amplification and sequencing with conserved primers. Proc. Nat. Acad. Sci. USA 1989, 86, 6196–6200. [Google Scholar] [CrossRef]
- Malmqvist, B.; Strasevicius, D.; Hellgren, O.; Adler, P.H.; Bensch, S. Vertebrate host specificity of wild–caught blackflies revealed by mitochondrial DNA in blood. Proc. R. Soc. Lond. B 2004, 271 (Suppl. 4), S152–S155. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef]
- Putt, Q.Y.; Ya’cob, Z.; Adler, P.H.; Chen, C.D.; Hew, Y.X.; Izwan-Anas, N.; Lau, K.W.; Sofian-Azirun, M.; Pham, X.D.; Takaoka, H.; et al. From bites to barcodes: Uncovering the hidden diversity of black flies (Diptera: Simuliidae) in Vietnam. Parasit. Vectors 2023, 16, 266. [Google Scholar] [CrossRef]
- Thanee, I.; Jumpato, W.; Jaroenchaiwattanachote, C.; Gomontean, B.; Wannasingha, W.; Namtaku, S.; Adler, P.H.; Pramual, P. Discovery of the larvae and pupae of the black fly Simulium (Gomphostilbia) khelangense and breeding habitats of potential pest species of the S.(G.) chumpornense subgroup (Simuliidae). Insects 2024, 15, 346. [Google Scholar] [CrossRef]
- Low, V.L.; Adler, P.H.; Sofian-Azirun, M.; Srisuka, W.; Saeung, A.; Huang, Y.T.; Hadi, U.K.; Da Pham, X.; Takaoka, H. Tests of conspecificity for allopatric vectors: Simulium nodosum and Simulium shirakii (Diptera: Simuliidae) in Asia. Parasit. Vectors 2015, 8, 297. [Google Scholar] [CrossRef]
- Adler, P.H.; Currie, D.C.; Wood, D.M. The Black Flies (Simuliidae) of North America; Cornell University Press: Ithaca, NY, USA, 2004. [Google Scholar]
- Pramual, P.; Thaijarern, J.; Tangkawanit, U.; Wongpakam, K. Molecular identification of blood meal sources in black flies (Diptera: Simuliidae) suspected as Leucocytozoon vectors. Acta Trop. 2020, 205, 105383. [Google Scholar] [CrossRef] [PubMed]
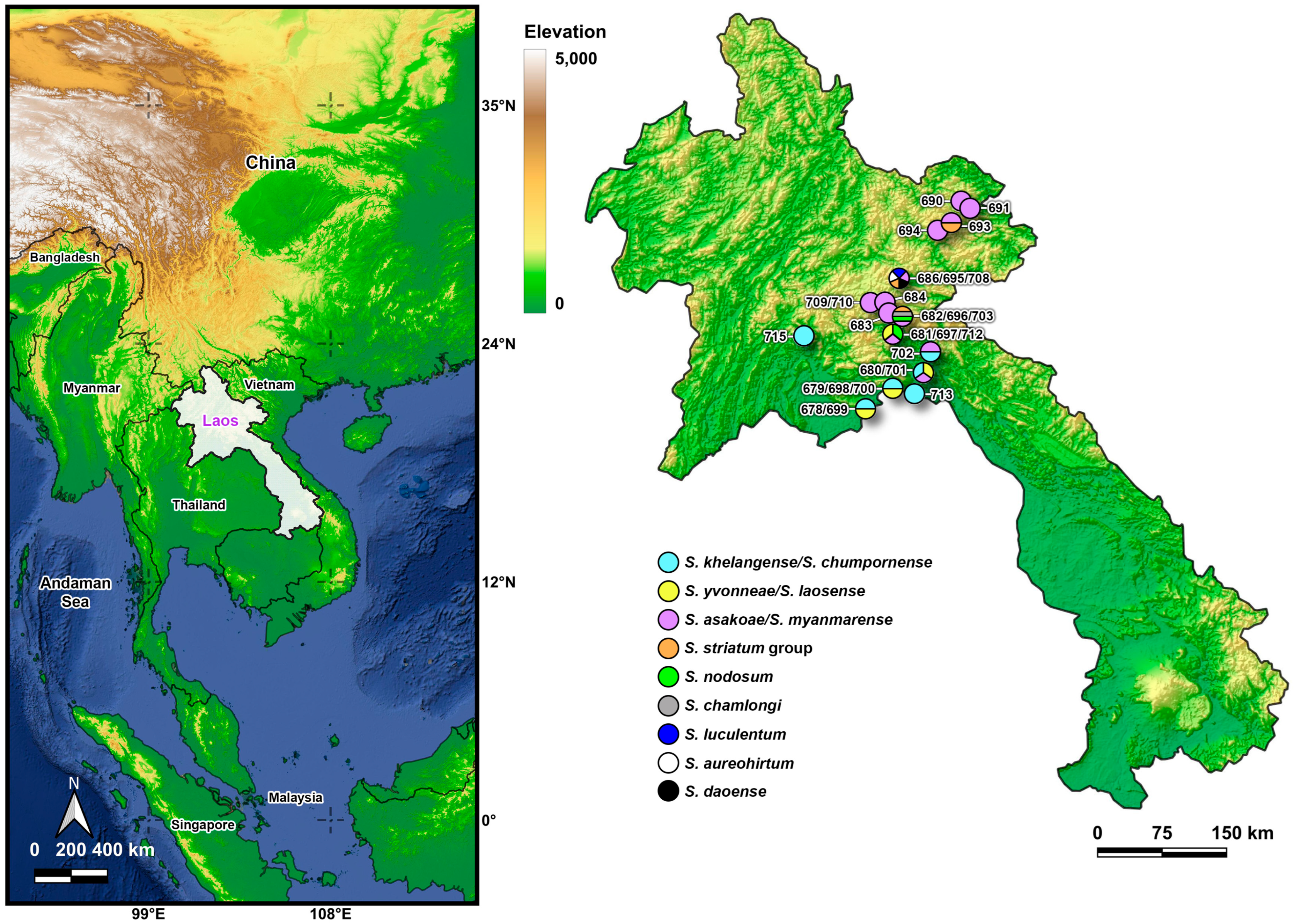
| Location (Code) | Coordinates | Elevation (m) | Date | Species | Males | Females | Blood-Fed Females | Total |
|---|---|---|---|---|---|---|---|---|
| Muang Thaphabat, Bolikhamxai Province (678) | 18.231388 N 103.115357 E | 172 | 4 January 2025 | S. khelangense/ S. chumpornense | 3 | 86 | - | 89 |
| S. yvonneae/ S. laosense | - | 14 | - | 14 | ||||
| 699 | 7 February 2025 | S. khelangense/ S. chumpornense | 4 | 503 | 5 | 512 | ||
| Ban Nong Keun, Bolikhamxai Province (679) | 18.452807 N 103.406923 E | 162 | 4 January 2025 | S. khelangense/ S. chumpornense | 8 | 312 | 7 | 327 |
| S. yvonneae/ S. laosense | - | 20 | - | 20 | ||||
| 698 | 9 January 2025 | S. khelangense/ S. chumpornense | - | 182 | 6 | 188 | ||
| S. yvonneae/ S. laosense | - | 3 | - | 3 | ||||
| 700 | 7 February 2025 | S. khelangense/ S. chumpornense | 27 | 494 | 4 | 525 | ||
| S. yvonneae/ S. laosense | - | 35 | - | 35 | ||||
| Borikham, Bolikhamxai Province (680) | 18.620325 N 103.737773 E | 174 | 5 January 2025 | S. asakoae/ S. myanmarense | - | 58 | 1 | 59 |
| S. khelangense/ S. chumpornense | - | 3 | - | 3 | ||||
| S. yvonneae/ S. laosense | - | 1 | - | 1 | ||||
| 701 | 8 February 2025 | S. khelangense/ S. chumpornense | - | 144 | 4 | 148 | ||
| S. asakoae/ S. myanmarense | - | 87 | 3 | 90 | ||||
| S. ynonneae/ S. laosense | - | 3 | - | 3 | ||||
| Thathom, Xaisomboun Province (681) | 19.037533 N 103.407373 E | 321 | 5 January 2025 | S. asakoae/ S. myanmarense | - | 51 | 1 | 52 |
| 697 | 8 January 2025 | S. asakoae/ S. myanmarense | - | 357 | 4 | 361 | ||
| S. yvonneae/ S. laosense | - | 1 | - | 1 | ||||
| 712 | 10 February 2025 | S. asakoae/ S. myanmarense | 4 | 247 | 1 | 252 | ||
| S. nodosum | - | 15 | - | 15 | ||||
| Muang Khoune (1), Xiang Khouang Province (682) | 19.228120 N 103.365560 E | 995 | 5 January 2025 | S. asakoae/ S. myanmarense | 26 | 148 | 8 | 182 |
| S. striatum group | - | 9 | - | 9 | ||||
| 696 | 8 January 2025 | S. asakoae/ S. myanmarense | 4 | 168 | 4 | 176 | ||
| S.chamlongi | - | - | 1 | 1 | ||||
| S. striatum group | - | 2 | - | 2 | ||||
| S. nodosum | - | 2 | - | 2 | ||||
| 703 | 8 February 2025 | S. asakoae/ S. myanmarense | - | 57 | 1 | 58 | ||
| S. striatum group | - | 2 | - | 2 | ||||
| Muang Khoune (2), Xiang Khouang Province (683) | 19.263842 N 103.361737 E | 1045 | 5 January 2025 | S. asakoae/ S. myanmarense | - | 35 | - | 35 |
| Muang Khoune (3), Xiang Khouang Province (684) | 19.374480 N 103.246903 E | 1146 | 5 January 2025 | S. asakoae/ S. myanmarense | - | 8 | - | 8 |
| Muang Kham (1), Xiang Khouang Province (686) | 19.637827 N 103.473968 E | 756 | 6 January 2025 | S. asakoae/ S. myanmarense | - | 26 | 3 | 29 |
| S.luculentum | - | - | 1 | 1 | ||||
| S. striatum group | 4 | 9 | - | 13 | ||||
| 695 | 8 January 2025 | S. asakoae/ S. myanmarense | - | 37 | 2 | 39 | ||
| S. striatum group | - | 1 | 3 | 4 | ||||
| S.daoense | - | - | 1 | 1 | ||||
| 708 | 9 February 2025 | S.aureohirtum | 7 | 6 | 2 | 15 | ||
| Vieng Xai, Huaphan Province (690) | 20.470123 N 104.143545 E | 848 | 7 January 2025 | S. asakoae/ S. myanmarense | - | 6 | - | 6 |
| Ban Mueang Nga, Vieng Xai, Huaphan Province (691) | 20.431587 N 104.178603 E | 835 | 7 January 2025 | S. asakoae/ S. myanmarense | - | 93 | 5 | 98 |
| Tad Seleuy waterfall, Sam Neua, Huaphan Province (693) | 20.231111 N 104.005194 E | 1219 | 7 January 2025 | S. asakoae/ S. myanmarense | - | 293 | - | 293 |
| S. striatum group | - | 1 | - | 1 | ||||
| Ban Nator, Houamuang, Huaphan Province (694) | 20.158222 N 103.888923 E | 1199 | 7 January 2025 | S. asakoae/ S. myanmarense | - | 8 | 1 | 9 |
| Ban Thasi, Muaung Thathom, Nammang River, Xaisomboun Province, (702) | 18.847473 N 103.811762 E | 209 | 8 February 2025 | S. asakoae/ S. myanmarense | - | 1 | 0 | 1 |
| S.khelangense | - | 1 | 0 | 1 | ||||
| Ban Kang Yao, Muang Phonsavan, Xiangkhoang Province (709–710) | 19.376930 N 103.168240 E | 1119 | 9 February 2025 | S. asakoae/ S. myanmarense | - | 2 | 0 | 2 |
| Sokbounma hotel, Muang Pakxane, Borikhamxai Province (713) | 18.392865 N 103.641953 E | 159 | 11 February 2025 | S. khelangense/ S. chumpornense | - | 337 | - | 337 |
| Muang Vangvieng, Nam Xong River, Vientiane Province (715) | 19.018830 N 102.446027 E | 250 | 21 March 2025 | S. khelangense/ S. chumpornense | - | 1 | - | 1 |
| Ban Pha Hom, Muang Vangvieng, Vientiane Province (716) | 19.125217 N 102.344787 E | 478 | 21 March 2025 | S. striatum group | - | 2 | - | 2 |
| Ban Nong, Muang Kasi, Vientiane Province (717) | 19.125457 N 102.249120 E | 456 | 21 March 2025 | S. khelangense/ S. chumpornense | - | 3 | - | 3 |
| S. asakoae/ S. myanmarense | - | 38 | - | 38 | ||||
| S. striatum group | - | 2 | - | 2 | ||||
| Ban Lao Kham (1), Muang Feuang, Vientiane Province (726) | 18.652842 N 102.110338 E | 227 | 22 March 2025 | S. khelangense/ S. chumpornense | 2 | 268 | 2 | 272 |
| Ban Na Keo, Muang Thoulakhom, Vientiane Province (728) | 18.341101 N 102.643600 E | 176 | 23 March 2025 | S. khelangense/ S. chumpornense | - | 294 | 24 | 318 |
| Subgenus Species | Males | Females | Blood-Fed Females | Total | % OC | % OL | Elevation Range (m) |
|---|---|---|---|---|---|---|---|
| subgenus Gomphostilbia | |||||||
| S. asakoae/ S. myanmarense | 34 | 1720 | 34 | 1788 | 63.3 | 65.0 | 174–1219 |
| S. khelangense/ S. chumpornense | 44 | 2628 | 52 | 2724 | 43.3 | 45.0 | 159–456 |
| S. yvonneae/S. laosense | 0 | 77 | 0 | 77 | 23.3 | 20.0 | 162–321 |
| subgenus Nevermannia | |||||||
| S.aureohirtum | 7 | 6 | 2 | 15 | 3.3 | 5.0 | 756 |
| subgenus Simulium | |||||||
| S.chamlongi | 0 | 0 | 1 | 1 | 3.3 | 5.0 | 995 |
| S.daoense | 0 | 0 | 1 | 1 | 3.3 | 5.0 | 756 |
| S.luculentum | 0 | 0 | 1 | 1 | 3.3 | 5.0 | 756 |
| S. nodosum | 0 | 17 | 0 | 17 | 6.7 | 10.0 | 321–995 |
| S. striatum group | 4 | 28 | 3 | 35 | 23.3 | 25.0 | 456–1219 |
| Total | 89 | 4476 | 93 | 4658 |
| Subgenus/Species | Blood Hosts | ||
|---|---|---|---|
| Morphological identification (n) | Molecular (total blood engorged/successful identification) | Host species | n |
| subgenus Gomphostilbia | |||
| S. asakoae (30) | S. asakoae (27/14) | Chicken (Gallus gallus) | 11 |
| Turkey (Meleagris gallopavo) | 2 | ||
| Human (Homo sapiens) | 1 | ||
| S. myanmarense (3/2) | Chicken (Gallus gallus) | 2 | |
| S. khelangense (52) | S. khelangense (48/47) | Chicken (Gallus gallus) | 42 |
| Human (Homo sapiens) | 1 | ||
| S. chumpornense (4/1) | Turkey (Meleagris gallopavo) | 1 | |
| subgenus Nevermannia | |||
| S. aureohirtum (2) | S. aureohirtum (2/1) | Chicken (Gallus gallus) | 1 |
| subgenus Simulium | |||
| S. luculentum (1) | S. luculentum (1) | Water buffalo (Bubalus bubalis) | 1 |
| S. chamlongi (1) | S. chamlongi (1) | Human (Homo sapiens) | 1 |
| S. striatum group (2) | S. striatum group (2) | Water buffalo (Bubalus bubalis) | 1 |
| Chicken (Gallus gallus) | 1 | ||
| S. daoense (1) | S. daoense (1) | Water buffalo (Bubalus bubalis) | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Namtaku, S.; Wannasingha, W.; Jumpato, W.; Inkhavilay, K.; Gomontean, B.; Wongpakam, K.; Jaroenchaiwattanachote, C.; Thanee, I.; Mintara, R.; Adler, P.H.; et al. Diversity, Distribution, and Host Blood Meals of Black Flies (Diptera: Simuliidae) in Laos. Insects 2025, 16, 1053. https://doi.org/10.3390/insects16101053
Namtaku S, Wannasingha W, Jumpato W, Inkhavilay K, Gomontean B, Wongpakam K, Jaroenchaiwattanachote C, Thanee I, Mintara R, Adler PH, et al. Diversity, Distribution, and Host Blood Meals of Black Flies (Diptera: Simuliidae) in Laos. Insects. 2025; 16(10):1053. https://doi.org/10.3390/insects16101053
Chicago/Turabian StyleNamtaku, San, Wannachai Wannasingha, Waraporn Jumpato, Khamla Inkhavilay, Bhuvadol Gomontean, Komgrit Wongpakam, Chavanut Jaroenchaiwattanachote, Isara Thanee, Ronnalit Mintara, Peter H. Adler, and et al. 2025. "Diversity, Distribution, and Host Blood Meals of Black Flies (Diptera: Simuliidae) in Laos" Insects 16, no. 10: 1053. https://doi.org/10.3390/insects16101053
APA StyleNamtaku, S., Wannasingha, W., Jumpato, W., Inkhavilay, K., Gomontean, B., Wongpakam, K., Jaroenchaiwattanachote, C., Thanee, I., Mintara, R., Adler, P. H., & Pramual, P. (2025). Diversity, Distribution, and Host Blood Meals of Black Flies (Diptera: Simuliidae) in Laos. Insects, 16(10), 1053. https://doi.org/10.3390/insects16101053








