Report on Leg Sensilla of Notonectidae (Hemiptera, Heteroptera)
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Scanning Electron Microscope (SEM) Characterization
3. Results
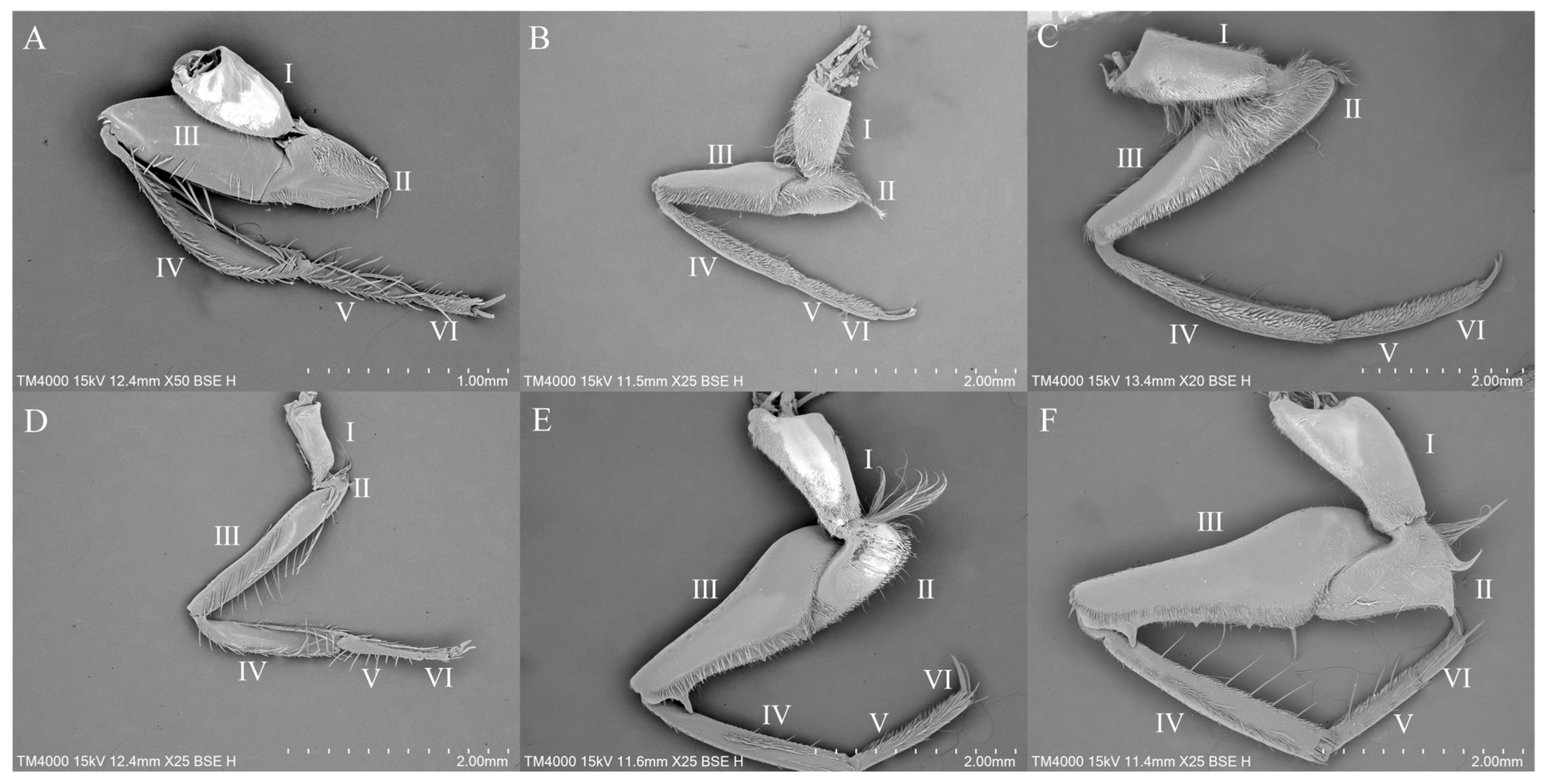
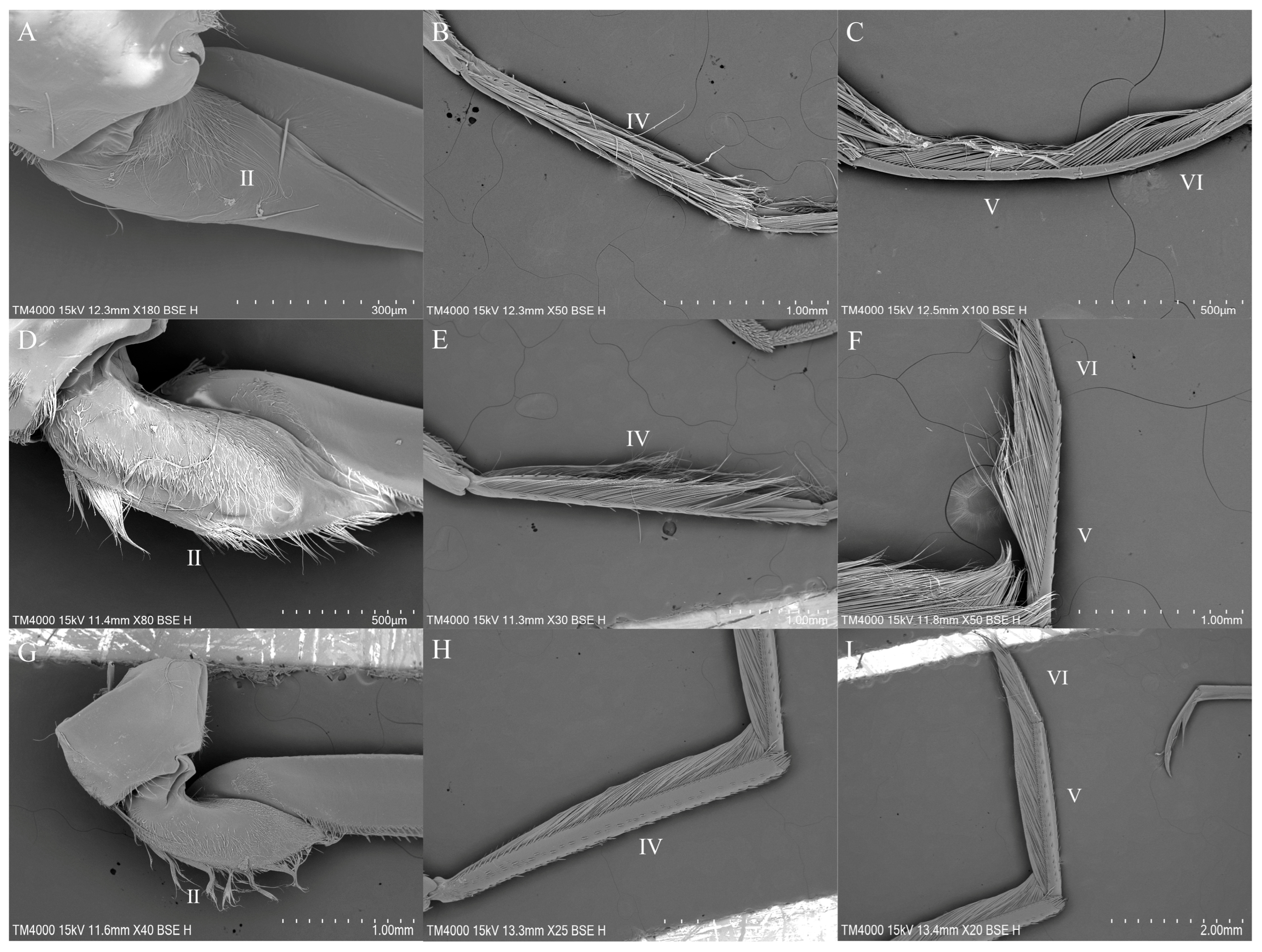
3.1. Gross Morphology of the Legs
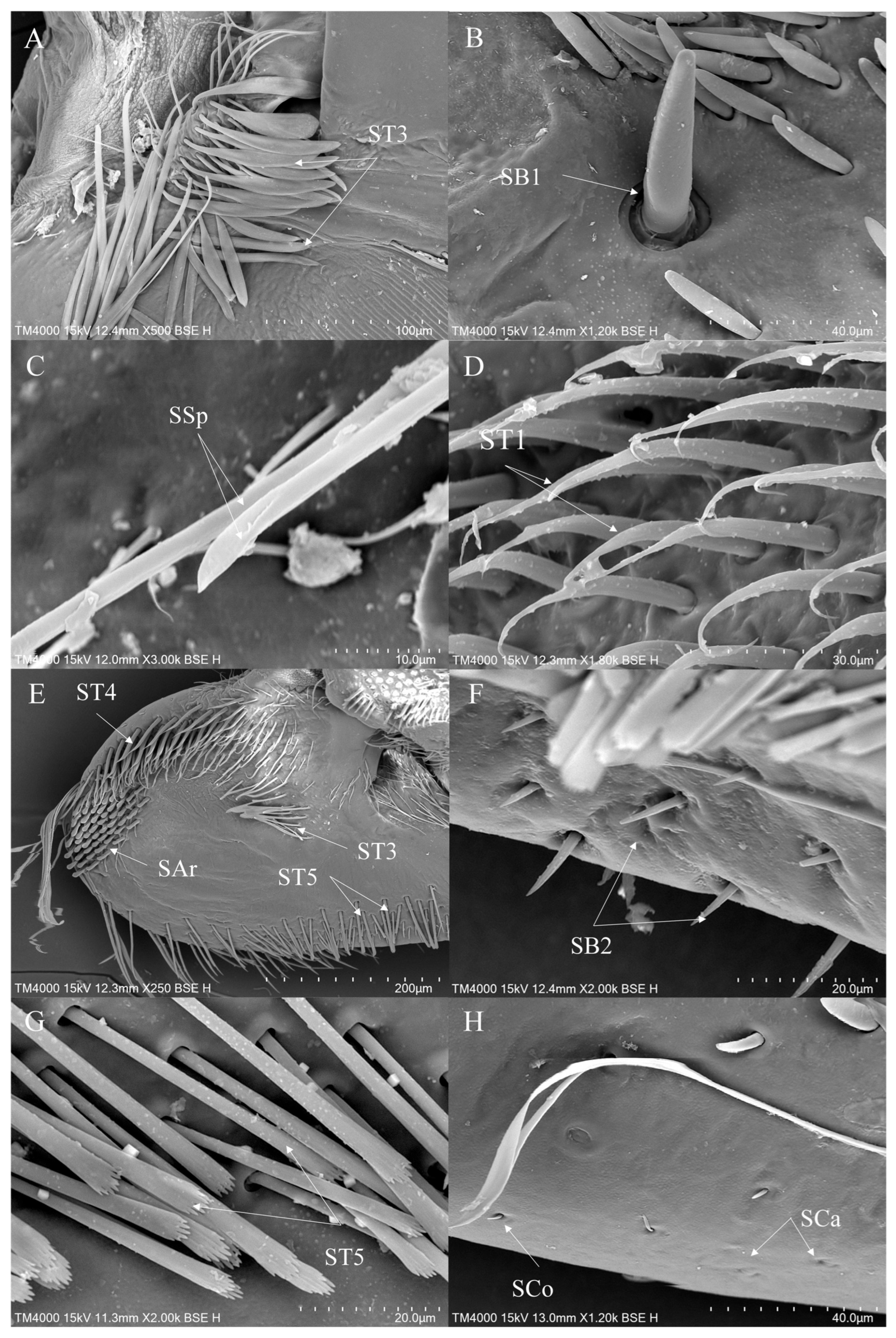
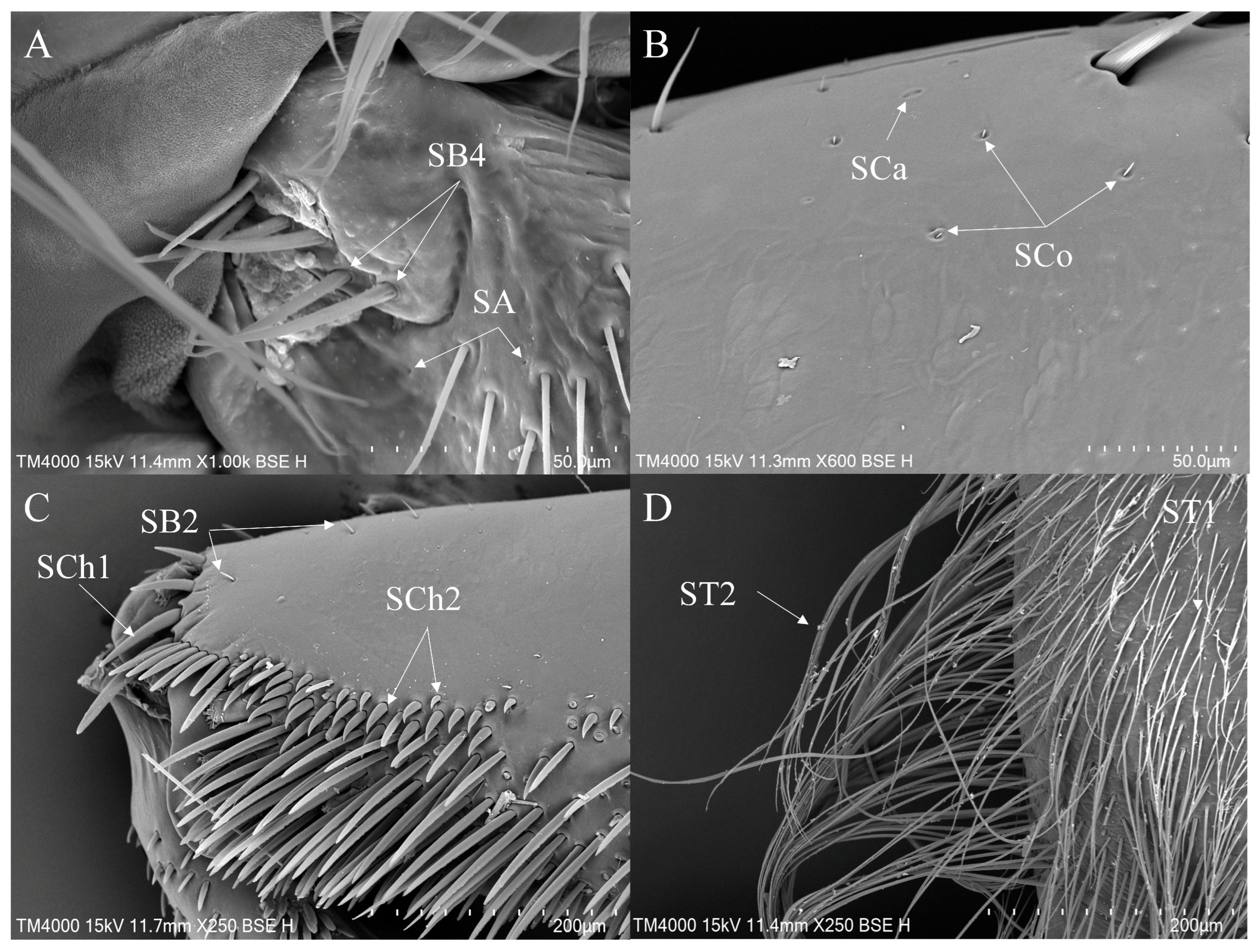
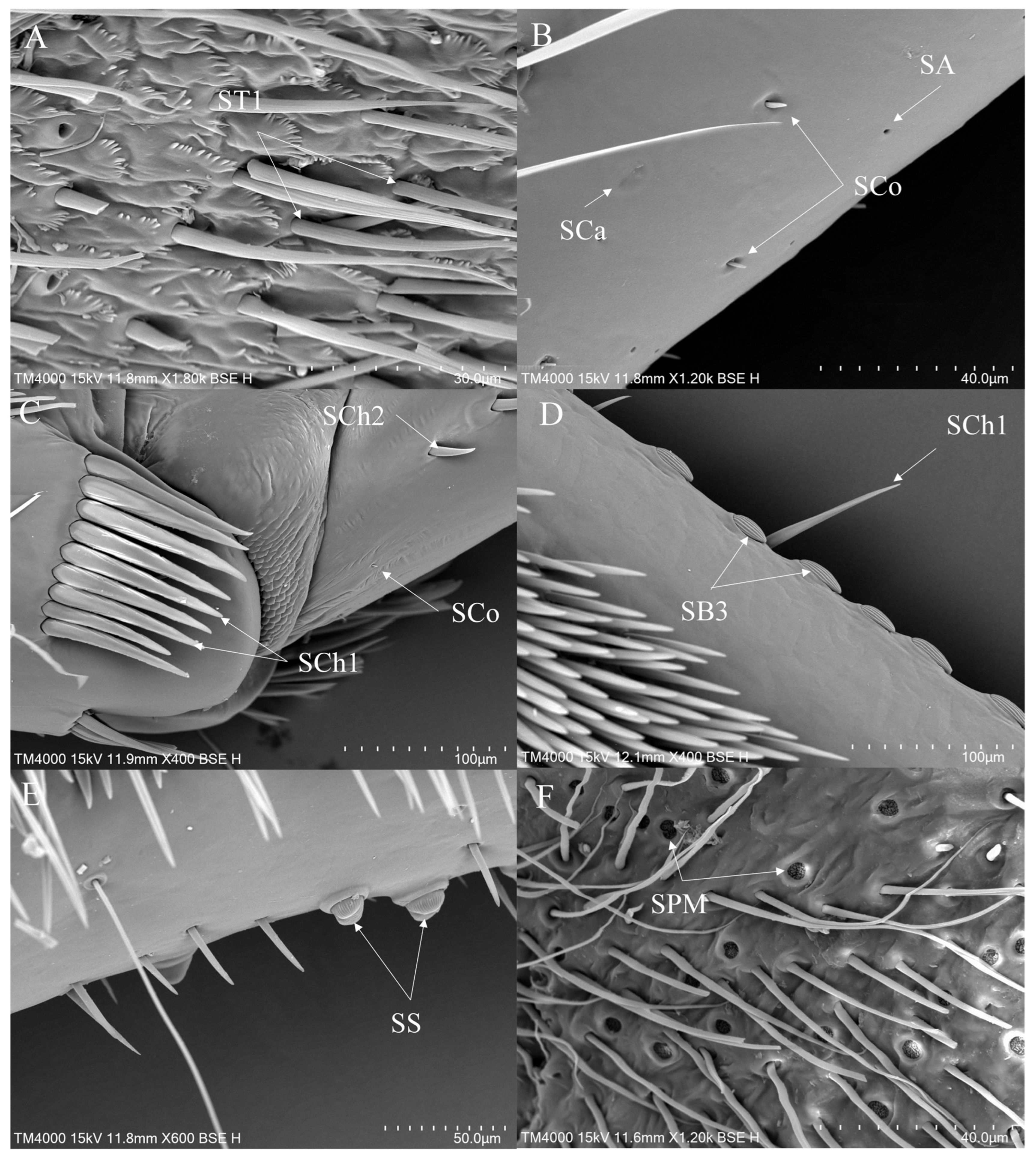
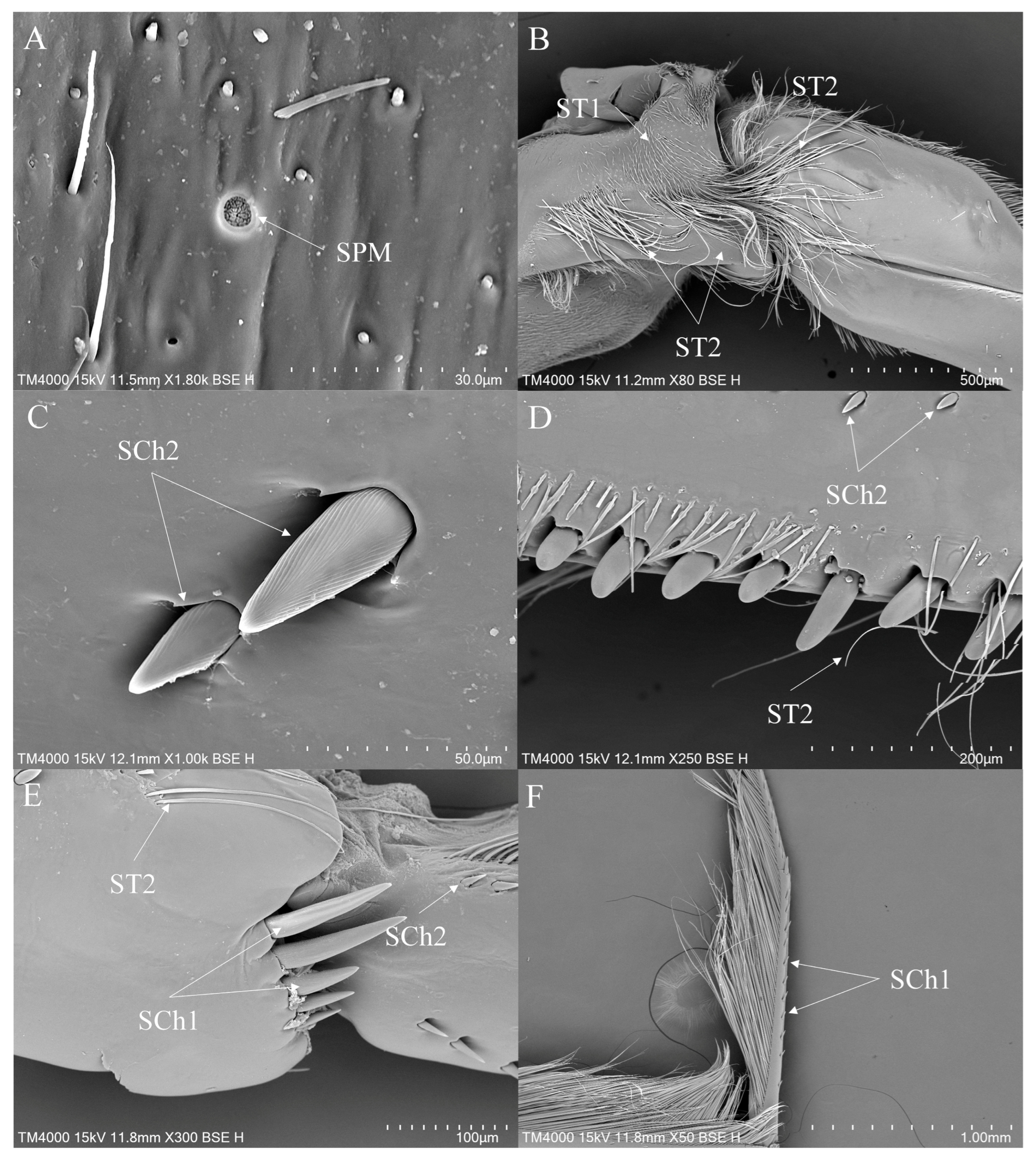
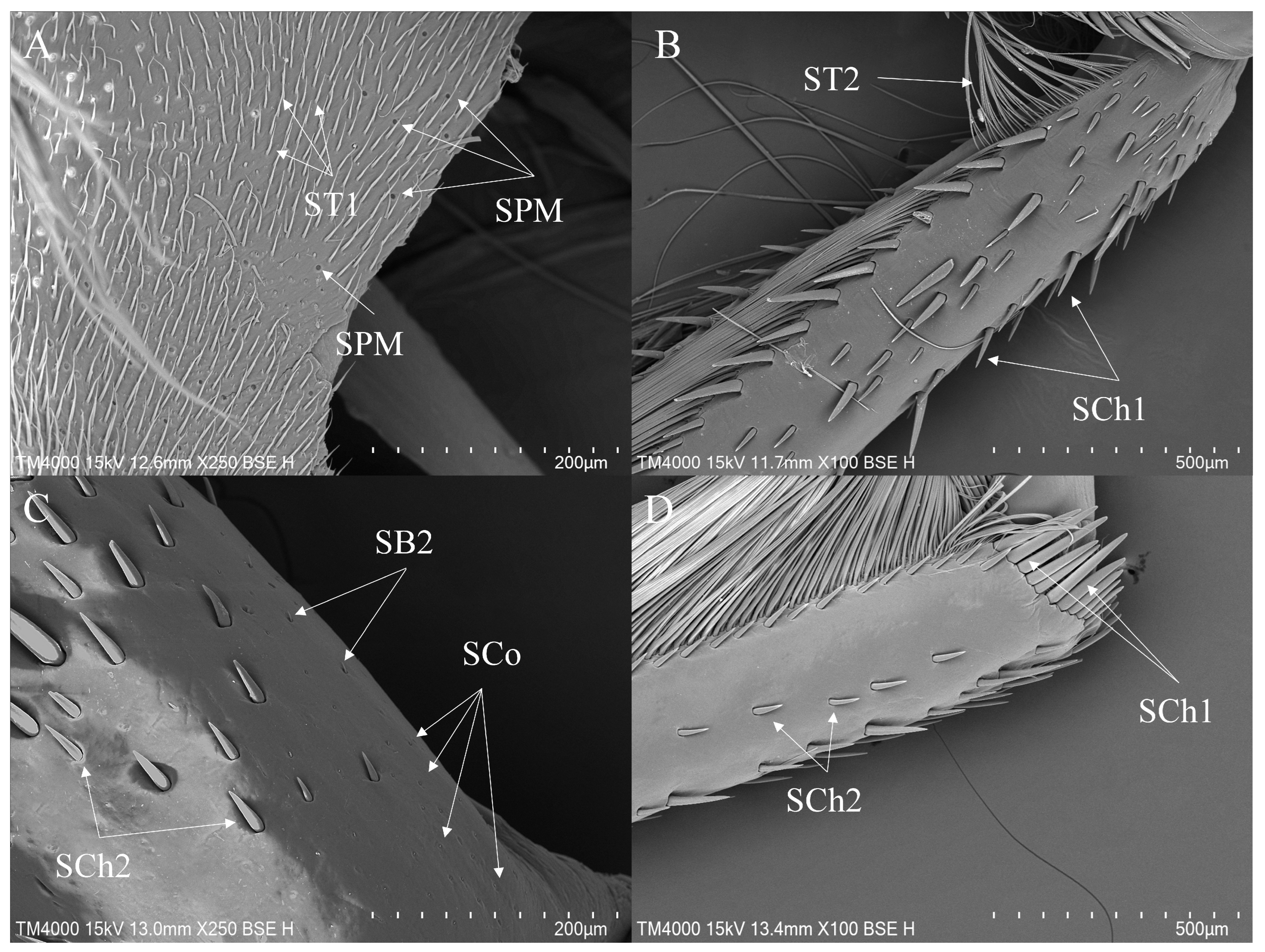
3.2. Morphology and Types of Leg Sensilla
- 1.
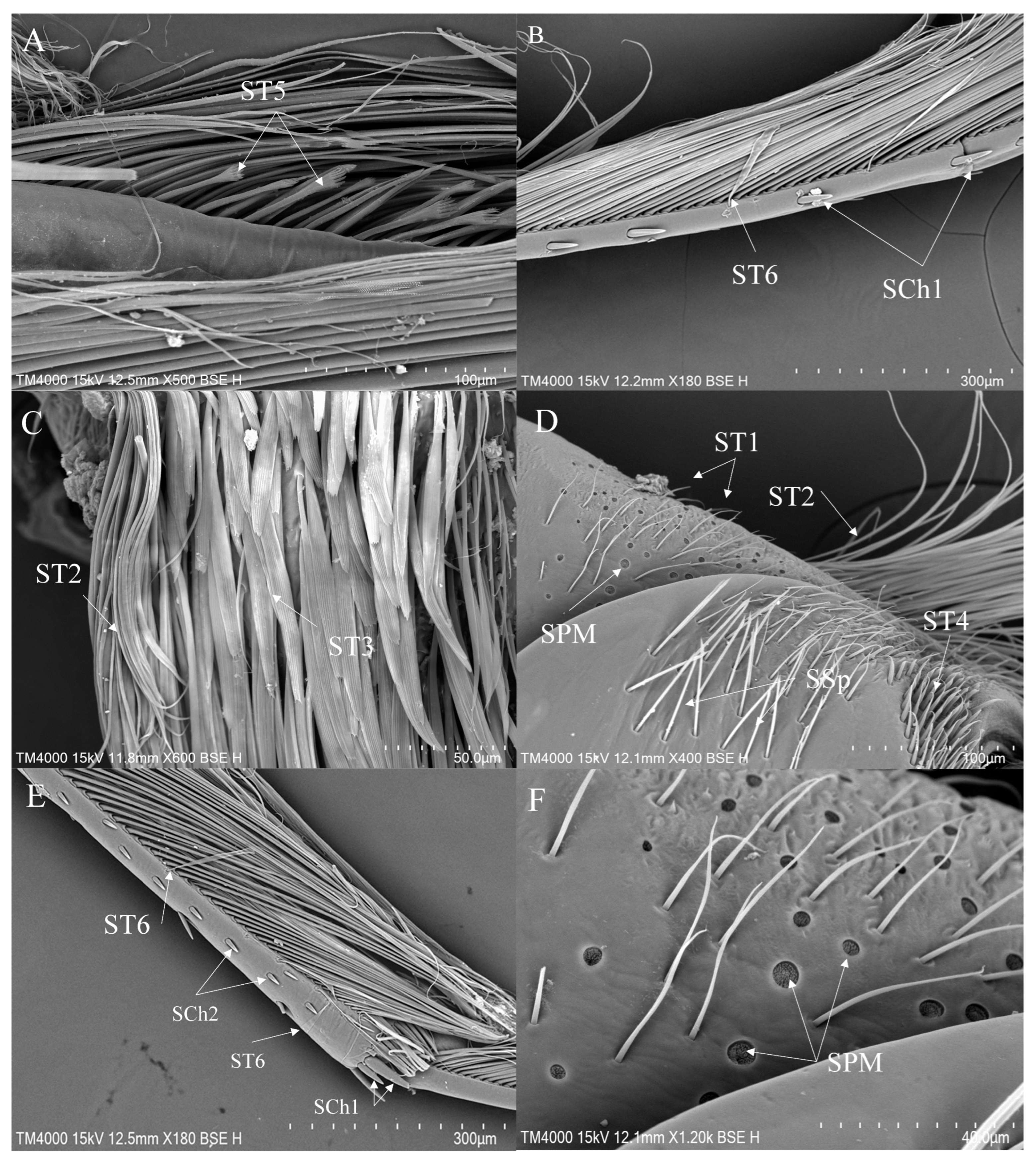
- Sensilla trichodea (ST)—Long, thin, hairlike sensilla, with smooth or ribbed surfaces. They have a flexible socket (a thin membrane connects the cuticle of the leg with the cuticle of the sensillum, making it movable at the base), which gives them a putative mechanoreceptive function. The shape of this sensillum varies from tapered at the top to flattened at the top. The tip is either straight or bent. This type of sensilla appears in groups, covering large areas of the surface. The other function performed by sensilla trichodea is gustation. In this case, the sensillum occurs with a single pore on the tip.
- a.
- ST1—Long, thin, hairlike sensilla with a ribbed surface, without pores—they perform a mechanoreceptive function.
- b.
- ST2—Long, thin, hairlike sensilla with a smooth surface, without pores—they perform a mechanoreceptive function.
- c.
- ST3—Long, flattened sensilla. Their bases are cylindrical and gradually flatten toward the tips. Their surfaces have more or less distinct stripes with no pores present—they perform a mechanoreceptive function.
- d.
- ST4—Long, flattened, sometimes curved inwards sensilla. The surface has shallow stripes, without pores—they perform a mechanoreceptive function.
- e.
- ST5—Long ribbed sensilla, flattening and widening along the length, with a ribbed frayed end resembling a brush, without pores—they perform a mechanoreceptive function.
- f.
- ST6—Their bases are flattened and gradually widen toward the obtusely rounded tips. These types of sensilla also perform a mechanoreceptive function.
- 2.
- Sensilla chaetica (SCh)—Thick sensilla with pronounced ribs on the surface. The length varies. The tip is either pointed or rounded. It has a flexible socket, like sensilla trichodea, but it is easily distinguished from this other type because it is visibly thicker and more rigid. This type is also described in the literature as mechanoreceptive sensilla.
- a.
- SCh1—These sensilla are thick, rigid, and marked with distinct stripes. They taper from a thicker base to a sharply pointed or slightly rounded tip.
- b.
- SCh2—These sensilla are thick and rigid, with pronounced ribs on the surface. The sensilla are short and slightly bent.
- 3.
- Sensilla campaniformia (SCa)—Oval or elongated disks lying flat on the surface, usually with a visible pore in the middle. This type also has a flexible socket. These sensilla belong to mechanoreceptors and are described as pressure sensilla.
- 4.
- Sensilla basiconica (SB)—Conical structure, usually smaller than the sensilla trichodea, with porous or non-porous surfaces and a flexible or inflexible socket.
- a.
- SB1—These sensilla are relatively large, and the socket is flexible. The surface is smooth, without any streaks, and the tips are obtusely rounded. They are usually distributed on the tibia.
- b.
- SB2—The sockets of these sensilla are flexible. The sensilla are relatively small and taper gradually from their bases to the tips. Their surfaces have striations. They are the smallest type among the sensilla basiconica.
- c.
- SB3—These sensilla have stripes on their surfaces and sharp ends, are short in size, and usually cling closely to the surface of the leg.
- d.
- SB4—These are conical structures that are either straight or branched at the base, gradually converging towards the tip. They have a smooth surface and a flexible socket.
- 5.
- Sensilla coeloconica (SCo)—Small cone-like with an inflexible socket and smooth surfaces. Individually distributed or concentrated in smooth areas of the legs, they are considered to have a thermo-hygroreceptive function.
- 6.
- Sensilla ampullacea (SA)—These sensilla have thermo-hygroreceptive functions. Their sockets are inflexible and hidden in a deep cavity. Only a circular opening can be seen on the surface.
- 7.
- Sensilla styloconica (SS)—Pegs arise from a bulge of cuticle, each peg with a flexible socket and a ribbed surface. These sensilla are believed to perform mechanoreceptive or gustatory functions.
- 8.
- Sensilla placodea multilobated (SPM)—Round cavities with small, fingerlike protuberances. As they were observed before on the antennae of the studied species, the name was given according to these other studies. The probable function is olfaction; however, olfactory structures are not specific to leg sensilla. Therefore, they might play another role.
- 9.
- Sensilla arch-shaped (SAr)—Thick, perpendicular to the surface of the legs, with obtusely rounded tips and striated surfaces.
- 10.
- Sensilla spoon-shaped (SSp)—Slender, cylindrical base adorned with stripes. The end expands into a spoon-like shape.
3.3. Sensilla Observed Among Representatives of the Studied Genera
3.3.1. Sensilla on the Legs of Anisops
3.3.2. Sensilla on the Legs of Enithares
3.3.3. Sensilla on the Legs of Notonecta
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dalal, A.; Cuthbert, R.N.; Dick, J.T.; Gupta, S. Water depth-dependent notonectid predatory impacts across larval mosquito ontogeny. Pest Manag. Sci. 2019, 75, 2610–2617. [Google Scholar] [CrossRef] [PubMed]
- Dambach, P. The use of aquatic predators for larval control of mosquito disease vectors: Opportunities and limitations. Biol. Control 2020, 150, 104357. [Google Scholar] [CrossRef]
- Arnér, M.; Koivisto, S.; Norberg, J.; Kautsky, N. Trophic interactions in rockpool food webs: Regulation of zooplankton and phytoplankton by Notonecta and Daphnia. Freshw. Biol. 1998, 39, 79–90. [Google Scholar] [CrossRef]
- Bakonyi, G.; Vásárhelyi, T.; Szabó, B. Pollution impacts on water bugs (Nepomorpha, Gerromorpha): State of the art and their biomonitoring potential. Environ. Monit. Assess. 2022, 194, 301. [Google Scholar] [CrossRef] [PubMed]
- Novais, A.M.; Dias, G.; Lino-Neto, J. Testicular, spermatogenesis and sperm morphology in Martarega bentoi (Heteroptera: Notonectidae). Arthropod Struct. Dev. 2017, 46, 635–643. [Google Scholar] [CrossRef]
- Polhemus, J.T.; Polhemus, D.A. Global diversity of true bugs (Heteroptera; Insecta) in freshwater. Hydrobiologia 2008, 595, 379–391. [Google Scholar] [CrossRef]
- Jordão, R.; Barbosa, J.F.; Moreira, F.F.F. Survey of the Notonectidae (Insecta, Hemiptera, Heteroptera, Nepomorpha) from northeastern Brazil. ZooKeys 2025, 1233, 245–288. [Google Scholar] [CrossRef]
- Xie, T.Y.; He, F.X.; Liu, G.Q. Checklist and distribution of Nepomorpha (Hemiptera: Heteroptera) from China. Zootaxa 2024, 5410, 325–375. [Google Scholar] [CrossRef]
- Ali, S.A.I.; Diakite, M.M.; Ali, S.; Wang, M.-Q. Effects of the antennal sensilla distribution pattern on the behavioral responses of Tribolium castaneum (Coleoptera: Tenebrionidae). Fla. Entomol. 2016, 99, 52–59. [Google Scholar] [CrossRef]
- Wisenden, B.D. Olfactory assessment of predation risk in the aquatic environment. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2000, 355, 1205–1208. [Google Scholar] [CrossRef]
- Brönmark, C.; Hansson, L. Chemical communication in aquatic systems: An introduction. Oikos 2000, 88, 103–109. [Google Scholar] [CrossRef]
- Nowińska, A. Report on the types and distribution of antennal sensilla in Lygaeidae (Heteroptera: Lygaeoidea) and their putative functions. Insects 2025, 16, 44. [Google Scholar] [CrossRef]
- Crespo, J.G. A review of chemosensation and related behavior in aquatic insects. J. Insect Sci. 2011, 11, 62. [Google Scholar] [CrossRef] [PubMed]
- Zacharuk, R.Y. Ultrastructure and function of insect chemosensilla. Annu. Rev. Entomol. 1980, 25, 27–47. [Google Scholar] [CrossRef]
- Nowińska, A.; Brożek, J. Insect evolution toward aquatic habitats; reassessment of antennal sensilla in the water bug families Ochteridae, Gelastocoridae and Aphelocheiridae (Hemiptera: Heteroptera: Nepomorpha). Contrib. Zool. 2020, 89, 412–433. [Google Scholar] [CrossRef]
- Nowińska, A.; Franielczyk-Pietyra, B.; Polhemus, D.A. The leg sensilla of insects from different habitats-comparison of strictly aquatic and riparian bugs (Corixidae, Ochteridae, Gelastocoridae: Nepomorpha: Insecta: Heteroptera). Insects 2023, 14, 441. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, A.; Parveen, S.; Brożek, J.; Dey, D. Antennal sensilla of phytophagous and predatory pentatomids (Hemiptera: Pentatomidae): A comparative study of four genera. Zool. Anz. J. Comp. Zool. 2016, 261, 48–55. [Google Scholar] [CrossRef]
- Li, Z.B.; Zhang, Y.Y.; An, X.K.; Wang, Q.; Khashaveh, A.; Gu, S.H.; Liu, S.; Zhang, Y.J. Identification of leg chemosensory genes and sensilla in the Apolygus lucorum. Front. Physiol. 2020, 11, 276. [Google Scholar] [CrossRef]
- Ling, F.; Dahanukar, A.; Weiss, L.A.; Kwon, J.Y.; Carlson, J.R. The molecular and cellular basis of taste coding in the legs of Drosophila. J. Neurosci. 2014, 34, 7148–7164. [Google Scholar] [CrossRef]
- Koh, T.-W.; He, Z.; Gorur-Shandilya, S.; Menuz, K.; Larter, N.K.; Stewart, S.; Carlson, J.R. The Drosophila IR20a clade of ionotropic receptors are candidate taste and pheromone receptors. Neuron 2014, 83, 850–865. [Google Scholar] [CrossRef]
- Zhang, Y.F.; Van Loon, J.J.A.; Wang, C.-Z. Tarsal taste neuron activity and proboscis extension reflex in response to sugars and amino acids in Helicoverpa armigera (Hübner). J. Exp. Biol. 2010, 213, 2889–2895. [Google Scholar] [CrossRef] [PubMed]
- Yosano, S.; Kutsuwada, Y.; Akatsu, M.; Masuta, S.; Kakazu, R.; Masuoka, N.; Matsuda, K.; Hori, M. Taste recognition through tarsal gustatory sensilla potentially important for host selection in leaf beetles (Coleoptera: Chrysomelidae). Sci. Rep. 2020, 10, 4931. [Google Scholar] [CrossRef]
- Nowińska, A.; Brożek, J. Morphological study of the antennal sensilla in Gerromorpha (Insecta: Hemiptera: Heteroptera). Zoomorphology 2017, 136, 327–347. [Google Scholar] [CrossRef]
- Nowińska, A.; Brożek, J. The variability of antennal sensilla in Naucoridae (Heteroptera: Nepomorpha). Sci. Rep. 2021, 11, 19651. [Google Scholar] [CrossRef] [PubMed]
- Nowińska, A.; Brożek, J. Antennal sensory structures in water bugs of Nepoidea (Insecta: Hemiptera: Nepomorpha), their morphology and function. Zoomorphology 2019, 138, 307–319. [Google Scholar] [CrossRef]
- Nowińska, A.; Brożek, J. Morphology of the antennal sensilla of Notonectoidea and comparison of evolutionary changes in sensilla types and distribution in infraorder Nepomorpha (Insecta: Heteroptera). Insects 2021, 12, 1121. [Google Scholar] [CrossRef]
- Nowińska, A.; Chen, P.; Brożek, J. Comparative study of antennal sensilla of Corixidae and Micronectidae (Hemiptera: Heteroptera: Nepomorpha: Corixoidea). Insects 2020, 11, 734. [Google Scholar] [CrossRef]
- Brożek, J. Comparative analysis and systematic mapping of the labial sensilla in the Nepomorpha (Heteroptera: Insecta). Sci. World J. 2013, 2013, 1–44. [Google Scholar] [CrossRef]
- Gergs, A.; Hoeltzenbein, N.I.; Ratte, H.T. Diurnal and nocturnal functional response of juvenile Notonecta maculata considered as a consequence of shifting predation behaviour. Behav. Processes 2010, 85, 151–156. [Google Scholar] [CrossRef]
- McIver, S.B. Structure of cuticular mechanoreceptors of arthropods. Annu. Rev. Entomol. 1975, 20, 381–397. [Google Scholar] [CrossRef]
- Ryan, M.F. The chemoreceptive organs: Structural aspects. In Insect Chemoreception; Kluwer Academic Publishers: Dordrecht, The Netherland, 2002; pp. 113–139. ISBN 978-1-4020-0270-0. [Google Scholar]
- Lang, H.H. Surface wave discrimination between prey and nonprey by the back swimmer Notonecta glauca L. (Hemiptera, Heteroptera). Behav. Ecol. Sociobiol. 1980, 6, 233–246. [Google Scholar] [CrossRef]
- Chen, P.P.; Nieser, N.; Zettel, H. The Aquatic and Semi-Aquatic Bugs (Heteroptera: Nepomorpha & Gerromorpha) of Malesia; BRILL: South Holland, The Netherlands, 2005; ISBN 978-90-474-1680-7. [Google Scholar]
- Xie, T.Y.; Ma, S.Y.; He, F.X. Report on antennal sensilla of Aphelocheirus ellipsoideus (Hemiptera, Heteroptera, Aphelocheiridae). Arch. Insect Biochem. Physiol. 2022, 111, e21917. [Google Scholar] [CrossRef]
- Brożek, J. Phylogenetic signals from Nepomorpha (Insecta: Hemiptera: Heteroptera) mouthparts: Stylets bundle, sense organs, and labial segments. Sci. World J. 2014, 2014, 237854. [Google Scholar] [CrossRef]
- Garza, C.; Ramos, D.; Cook, J.L. Comparative morphology of antennae in the family Pleidae (Hemiptera: Heteroptera). Zoomorphology 2021, 140, 243–256. [Google Scholar] [CrossRef]
- Saltin, B.D.; Goldsmith, C.; Haustein, M.; Büschges, A.; Szczecinski, N.S.; Blanke, A. A parametric finite element model of leg campaniform sensilla in drosophila to study campaniform sensilla location and arrangement. J. R. Soc. Interface 2025, 22, 20240559. [Google Scholar] [CrossRef]
- Castro-Huertas, V.; Forero, D.; Grazia, J. Comparative morphology of the raptorial leg in thread-legged bugs of the tribe Metapterini Stål, 1859 (Hemiptera, Heteroptera, Reduviidae, Emesinae). Zoomorphology 2019, 138, 97–116. [Google Scholar] [CrossRef]
- Wang, Y.; Brożek, J.; Dai, W. Morphological disparity of the mouthparts in polyphagous species of Largidae (Heteroptera: Pentatomomorpha: Pyrrhocoroidea) reveals feeding specialization. Insects 2020, 11, 145. [Google Scholar] [CrossRef] [PubMed]
- Merritt, D.J.; Murphey, R.K. Projections of leg proprioceptors within the CNS of the fly Phormia in relation to the generalized insect ganglion. J. Comp. Neurol. 1992, 322, 16–34. [Google Scholar] [CrossRef] [PubMed]
- Altner, H.; Prillinger, L. Ultrastructure of Invertebrate Chemo-, Thermo-, and Hygroreceptors and Its Functional Significance. In International Review of Cytology; Bourne, G.H., Danielli, J.F., Eds.; Academic Press: Cambridge, MA, USA, 1980; Volume 67, pp. 69–139. [Google Scholar]
- Ye, Z.; Damgaard, J.; Yang, H.H.; Hebsgaard, M.B.; Weir, T.; Bu, W.J. Phylogeny and diversification of the true water bugs (Insecta: Hemiptera: Heteroptera: Nepomorpha). Cladistics 2020, 36, 72–87. [Google Scholar] [CrossRef]



| Family | Genus | Species | Number of Studied Specimens |
|---|---|---|---|
| Notonectidae | Anisops | Anisops bouvieri Kirkaldy, 1904 | 2 |
| Anisops elstoni Brooks, 1951 | 2 | ||
| Anisops exiguus Horváth, 1919 | 2 | ||
| Anisops kuroiwae Matsumura, 1915 | 3 | ||
| Anisops ogasawarensis Matsumura, 1915 | 2 | ||
| Anisops stali Kirkaldy, 1904 | 2 | ||
| Enithares | Enithares biimpressa (Uhler, 1860) | 3 | |
| Enithares ciliata (Fabricius, 1798) | 2 | ||
| Enithares sinica (Stål, 1854) | 2 | ||
| Enithares tibialis Liu et Zhang, 1991 | 2 | ||
| Notonecta | Notonecta amplifica Kiritshenko, 1930 | 2 | |
| Notonecta chinensis Fallou, 1887 | 2 | ||
| Notonecta kiangsis Kirkaldy, 1897 | 2 | ||
| Notonecta montandoni Kirkaldy, 1897 | 2 | ||
| Notonecta reuteri reuteri Hungerford, 1928 | 2 | ||
| Notonecta triguttata Motschulsky, 1861 | 3 | ||
| Notonecta violacea Kirkaldy, 1897 | 2 |
| Sensillum Type | Anisops | Enithares | Notonecta | ||||||
|---|---|---|---|---|---|---|---|---|---|
| FL | ML | HL | FL | ML | HL | FL | ML | HL | |
| ST1 | + | + | + | + | + | + | + | + | + |
| ST2 | + | + | + | + | + | + | + | + | + |
| ST3 | + | + | + | ||||||
| ST4 | + | + | |||||||
| ST5 | + | + | + | ||||||
| ST6 | + | + | + | ||||||
| SB1 | + | ||||||||
| SB2 | + | + | + | + | + | ||||
| SB3 | + | + | |||||||
| SB4 | + | + | |||||||
| SCh1 | + | + | + | + | + | + | + | + | + |
| SCh2 | + | + | + | + | + | + | + | + | |
| SS | + | + | |||||||
| SA | + | + | + | + | + | ||||
| SCa | + | + | + | + | + | + | + | + | |
| SCo | + | + | + | + | + | + | + | ||
| SPM | + | + | + | + | + | + | |||
| SAr | + | ||||||||
| SSp | + | + | + | ||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fan, M.-Y.; Xie, T.-Y. Report on Leg Sensilla of Notonectidae (Hemiptera, Heteroptera). Insects 2025, 16, 1048. https://doi.org/10.3390/insects16101048
Fan M-Y, Xie T-Y. Report on Leg Sensilla of Notonectidae (Hemiptera, Heteroptera). Insects. 2025; 16(10):1048. https://doi.org/10.3390/insects16101048
Chicago/Turabian StyleFan, Meng-Yao, and Tong-Yin Xie. 2025. "Report on Leg Sensilla of Notonectidae (Hemiptera, Heteroptera)" Insects 16, no. 10: 1048. https://doi.org/10.3390/insects16101048
APA StyleFan, M.-Y., & Xie, T.-Y. (2025). Report on Leg Sensilla of Notonectidae (Hemiptera, Heteroptera). Insects, 16(10), 1048. https://doi.org/10.3390/insects16101048







