Culicidae Fauna (Diptera: Culicomorpha) of the Municipality of Mazagão, Amapá, in the Brazilian Amazon
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Aspects
2.2. Study Area
2.3. Entomological Collection
2.3.1. Protected Human Attraction (PHA) Method
2.3.2. Collection with CDC-Type Traps
2.3.3. Taxonomic Identification
2.4. Faunal Analysis
2.4.1. Species Dominance
2.4.2. Diversity Analysis
2.4.3. Species Overlap Across Different Periods
2.4.4. Environmental Variables
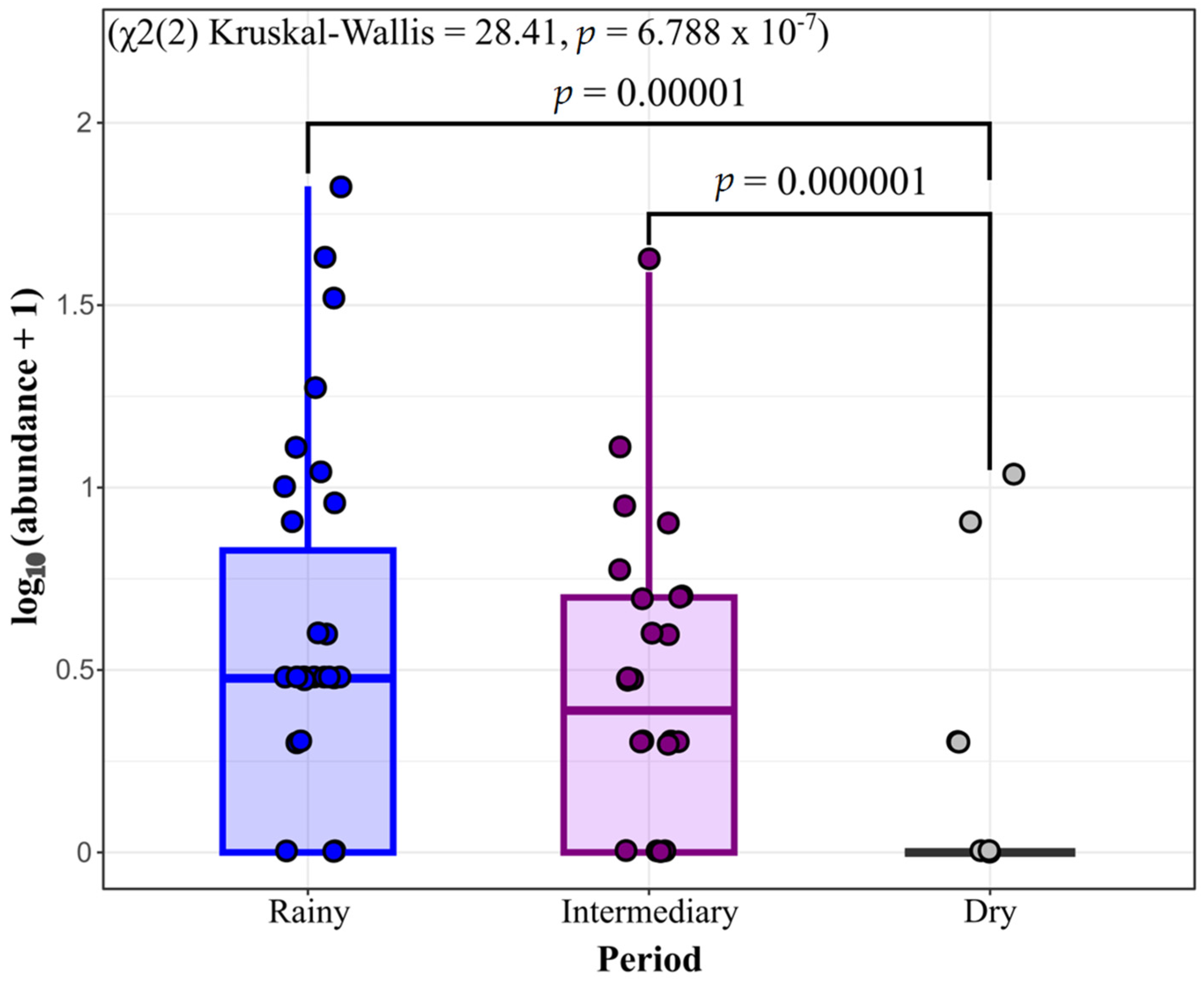
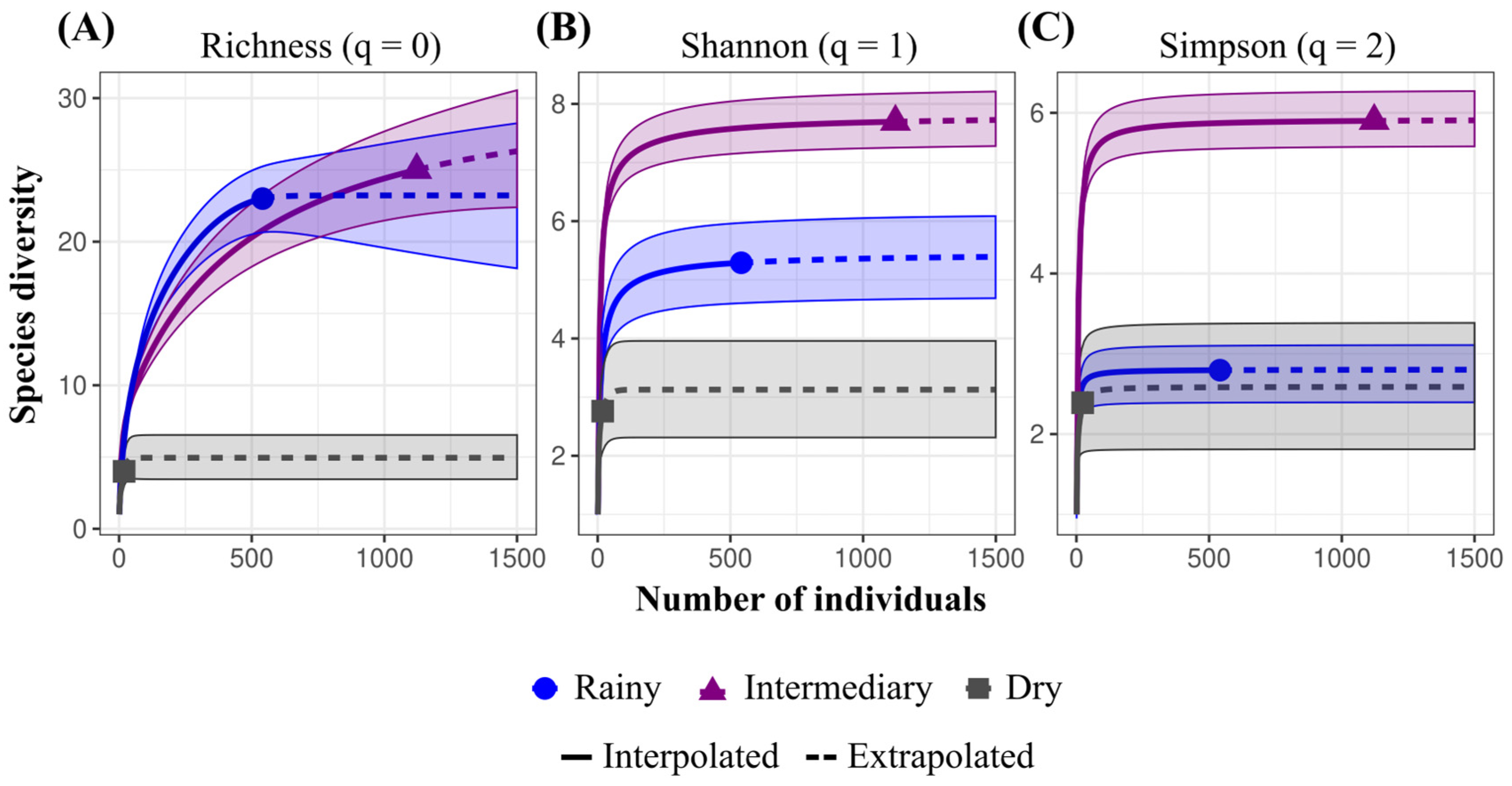
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Peleja, J.R.P.; Moura, J.M.S. Integrative Studies of the Amazon–EIA; Acquerello: São Paulo, Brazil, 2012; Volume 1. [Google Scholar]
- Wu, X.; Lu, Y.; Zhou, S.; Chen, L.; Xu, B. Impact of Climate Change on Human Infectious Diseases: Empirical Evidence and Human Adaptation. Environ. Int. 2016, 86, 14–23. [Google Scholar] [CrossRef]
- Reis, H.C.F.; Dias, D.D.; Nascimento, B.L.S.d.; Reis, L.A.M.; da Silva e Silva, L.H.; da Silva, F.S.; Vieira, D.B.R.; Brandão, R.C.F.; da Silva, E.V.P.; Nunes Neto, J.P. Culicidae Fauna (Diptera: Culicomorpha) of the Quilombola Community of Abacatal, Ananindeua, Pará, in the Brazilian Amazon. Insects 2025, 16, 397. [Google Scholar] [CrossRef]
- Lima-Camara, T.N. Emerging Arboviruses and Public Health Challenges in Brazil. Rev. Saude Publica 2016, 50, 36. [Google Scholar] [CrossRef]
- INPE Projeto PRODES: Monitoramento do Desmatamento da Floresta Amazônica Brasileira por Satélite. Available online: http://www.obt.inpe.br/OBT/assuntos/programas/amazonia/prodes (accessed on 25 August 2025).
- Ellwanger, J.H.; Kulmann-Leal, B.; Kaminski, V.L.; Valverde-Villegas, J.M.; da Veiga, A.B.G.; Spilki, F.R.; Fearnside, P.M.; Caesar, L.; Giatti, L.L.; Wallau, G.L.; et al. Beyond Diversity Loss and Climate Change: Impacts of Amazon Deforestation on Infectious Diseases and Public Health. An. Acad. Bras. Cienc. 2020, 92, e2019137. [Google Scholar] [CrossRef]
- Mohapatra, R.K.; Mishra, S.; Satapathy, P.; Kandi, V.; Tuglo, L.S. Surging Oropouche Virus (OROV) Cases in the Americas: A Public Health Challenge. New Microbes New Infect. 2024, 59, 101243. [Google Scholar] [CrossRef] [PubMed]
- Vasconcelos, P.F.C.; Travassos da Rosa, A.P.A.; Rodrigues, S.G.; Travassos da Rosa, E.S.; Dégallier, N.; Travassos da Rosa, J.F.S. Inadequate Management of Natural Ecosystem in the Brazilian Amazon Region Results in the Emergence and Reemergence of Arboviruses. Cad. Saude Publica 2001, 17, S155–S164. [Google Scholar] [CrossRef] [PubMed]
- Franco, O. The History of Yellow Fever in Brazil; Brazilian Ministry of Health: Rio de Janeiro, Brazil, 1969.
- Leão, R.N.Q.D.; Bichara, C.N.C.; Fraiha Neto, H.; Vasconcelos, P.F.D.C. Tropical Medicine and Infectious Diseases in the Amazon, 1st ed.; Samauma: Belém, Brazil, 2013; pp. 488–489. [Google Scholar]
- Paula, M.B.d.; Fernandes, A.; Medeiros-Sousa, A.R.; Ceretti-Júnior, W.; Christe, R.; Stroebel, R.C.; Pedrosa, L.; Almeida, R.M.M.d.S.; Carvalho, G.C.d.; Pereira, U.D.; et al. Mosquito (Diptera: Culicidae) Fauna in Parks in Greater São Paulo, Brazil. Biota Neotrop. 2015, 15, e20140026. [Google Scholar] [CrossRef]
- Forattini, O.P.; Gomes, A.C.; Galati, E.A.B.; Rabello, E.X.; Iversson, L.B. Estudos Ecológicos Sobre Mosquitos Culicidae No Sistema Serra Do Mar, Brasil: 1–Observações No Ambiente Extradomiciliar. Rev. Saude Publica 1978, 12, 297–325. [Google Scholar] [CrossRef] [PubMed]
- Catenacci, L.S.; Nunes-Neto, J.; Deem, S.L.; Palmer, J.L.; Travassos-da Rosa, E.S.; Tello, J.S. Diversity Patterns of Hematophagous Insects in Atlantic Forest Fragments and Human-Modified Areas of Southern Bahia, Brazil. J. Vector Ecol. 2018, 43, 293–304. [Google Scholar] [CrossRef]
- Carvalho, G.C.d.; Ceretti-Junior, W.; Barrio-Nuevo, K.M.; Wilk-da-Silva, R.; Christe, R.O.; Paula, M.B.d.; Vendrami, D.P.; Multini, L.C.; Evangelista, E.; Camargo, A.A.; et al. Composition and Diversity of Mosquitoes (Diptera: Culicidae) in Urban Parks in the South Region of the City of São Paulo, Brazil. Biota Neotrop. 2017, 17, e20160274. [Google Scholar] [CrossRef]
- Harbach, R. Mosquito Taxonomic Inventory. Valid Species List. Available online: https://mosquito-taxonomic-inventory.myspecies.info/valid-species-list (accessed on 5 August 2025).
- Guedes, M.P. Culicidae (Diptera) no Brasil: Relações entre diversidade, distribuição e enfermidades. Oecologia Aust. 2012, 16, 283–296. [Google Scholar] [CrossRef]
- Brazil, Ministério Da Saúde. DATASUS. Tabnet. Brasília, DF: Ministério Da Saúde. Available online: https://datasus.saude.gov.br/informacoes-de-saude-tabnet/ (accessed on 10 May 2025).
- Naveca, F.G.; Almeida, T.A.P.d.; Souza, V.; Nascimento, V.; Silva, D.; Nascimento, F.; Mejía, M.; Oliveira, Y.S.d.; Rocha, L.; Xavier, N.; et al. Human Outbreaks of a Novel Reassortant Oropouche Virus in the Brazilian Amazon Region. Nat. Med. 2024, 30, 3509–3521. [Google Scholar] [CrossRef]
- Brazil, Gerenciador de Ambiente Laboratorial (GAL)—Secretaria de Vigilância Em Saúde e Ambiente. Available online: http://gal.datasus.gov.br (accessed on 3 September 2025).
- Nunes, M.R.T.; Faria, N.R.; de Vasconcelos, J.M.; Golding, N.; Kraemer, M.U.G.; de Oliveira, L.F.; da Silva Azevedo, R.d.S.; da Silva, D.E.A.; da Silva, E.V.P.; da Silva, S.P.; et al. Emergence and Potential for Spread of Chikungunya Virus in Brazil. BMC Med. 2015, 13, 102. [Google Scholar] [CrossRef] [PubMed]
- Souto, R.N.P.; Saraiva, J.F.; Ferreira, R.M.; Barbosa, L.M. Culicideos (DIPTERA: CULICIDAE) Da Coleção Entomológica Do Instituto de Pesquisas Cientificas e Tecnológicas Do Amapá. Biota Amaz. 2011, 1, 60–65. [Google Scholar] [CrossRef]
- Vidal, L. Mazagão a Cidade que Atravessou o Atlântico: Do Marrocos à Amazônia (1769–1783); Martins Fontes: São Paulo, Brazil, 2008; 294p. [Google Scholar]
- Brazil, Ministry of Health, Health Surveillance Secretariat, Department of Communicable Disease Surveillance. Guide to Epizootics Surveillance in Non-Human Primates and Entomology Applied to the Yellow Fever Surveillance, 2nd ed.; Ministry of Health: Brasília, Brazil, 2017. Available online: https://www.gov.br/saude/pt-br/assuntos/saude-de-a-a-z/f/febreamarela/publicacoes/guia_vigilancia_epizootias_primatas_entomologia.pdf/view (accessed on 18 July 2025). (In Portuguese)
- Sudia, W.; Chamberlain, R. Battery-Operated Light Trap, an Improved Model. J. Am. Mosq. Control Assoc. 1988, 4, 536–538. [Google Scholar] [PubMed]
- Consoli, R.A.G.B.; Oliveira, R.L. Principais Mosquitos de Importância Sanitária No Brasil, 1st ed.; Fiocruz: Rio de Janeiro, Brazil, 1994. [Google Scholar]
- Galindo, P.; Blanton, F.S.; Peyton, E.L. A revision of the Uranotaenia of Panama with notes on other American species of the genus (Diptera, Culicidae). Ann. Entomol. Soc. Am. 1954, 47, 107–177. [Google Scholar] [CrossRef]
- Sallum, M.A.M.; Forattini, O.P. Revision of the Spissipes Section of Culex (Melanoconion) (Diptera: Culicidae). J. Am. Mosq. Control Assoc. 1996, 12, 517–600. [Google Scholar]
- Rodriguez de Sá, I.L.; Hutchings, R.S.G.; Hutchings, R.W.; Mureb Sallum, M.A. Revision of the Atratus Group of Culex (Melanoconion) (Diptera: Culicidae). Parasites Vectors 2020, 13, 269. [Google Scholar] [CrossRef]
- Lane, J. Neotropical Culicidae; USP: São Paulo, Brazil, 1953; Volume II. [Google Scholar]
- Forattini, O.P. Culicidologia Médica, Identificação, Biologia, Epidemiologia; edUSP: São Paulo, Brazil, 2002; Volume 2. [Google Scholar]
- Reinert, J.F. List of Abbreviations for Currently Valid Generic-Level Taxain Family Culicidae (Diptera). Eur. Mosq. Bull. 2009, 27, 68–76. [Google Scholar]
- Friebe, B. Zur Biologie eines Buchenwaldbodens. 3. Die Kaferfauna. Beitrage Naturkd Forsch Sudwestdtsch Beiheft 1983, 41, 45–80. [Google Scholar]
- Hill, M.O. Diversity and Evenness: A Unifying Notation and Its Consequences. Ecology 1973, 54, 427–432. [Google Scholar] [CrossRef]
- Li, D. HillR: Taxonomic, Functional, and Phylogenetic Diversity and Similarity through Hill Numbers. J. Open Source Softw. 2018, 3, 1041. [Google Scholar] [CrossRef]
- Hsieh, T.C.; Ma, K.H.; Chao, A. INEXT: An R Package for Rarefaction and Extrapolation of Species Diversity (Hill Numbers). Methods Ecol. Evol. 2016, 7, 1451–1456. [Google Scholar] [CrossRef]
- Chao, A.; Gotelli, N.J.; Hsieh, T.C.; Sander, E.L.; Ma, K.H.; Colwell, R.K.; Ellison, A.M. Rarefaction and Extrapolation with Hill Numbers: A Framework for Sampling and Estimation in Species Diversity Studies. Ecol. Monogr. 2014, 84, 45–67. [Google Scholar] [CrossRef]
- Chao, A.; Colwell, R.K.; Lin, C.-W.; Gotelli, N.J. Sufficient Sampling for Asymptotic Minimum Species Richness Estimators. Ecology 2009, 90, 1125–1133. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.-H.; Yu, G.; Cai, P. GgVennDiagram: An Intuitive, Easy-to-Use, and Highly Customizable R Package to Generate Venn Diagram. Front. Genet. 2021, 12, 706907. [Google Scholar] [CrossRef] [PubMed]
- Medeiros, D.B.A.; Vasconcelos, P.F.C. Is the Brazilian Diverse Environment Is a Crib for the Emergence and Maintenance of Exotic Arboviruses? An. Acad. Bras. Cienc. 2019, 91 (Suppl. 3), e20190407. [Google Scholar] [CrossRef]
- Silva, C.B. Diversidade de Mosquitos (Diptera: Culicidae) na Reserva Biológica do Lago Piratuba, Amapá, Amazônia Oriental, Brasil. Master’s Thesis, Universidade Federal do Amapá, Macapá, Brazil, 2018. [Google Scholar]
- Souto, R.N.P. Inventário Da Fauna Culicidiana (Diptera:Culicidae) Nas Ressacas Do Curralinho e Da Lagoa Dos Índios. In Diagnóstico das Ressacas do Estado do Amapá: Bacias do Igarapé da Fortaleza e Rio Curiaú; CPAQ/IEPA e DGEO/SEMA: Macapá, Brazil, 2003; Volume 1, pp. 63–72. [Google Scholar]
- Cerqueira, N.L. Distribuição Geográfica Dos Mosquitos da Amazônia. Rev. Bras. Entomol. 1961, 10, 111–168. [Google Scholar]
- Dias, D.D. Fauna de Culicidae (Diptera: Culicomorpha) de Um Fragmento Florestal No Município de Belém, Pará, Brasil. Master’s Thesis, State University of Pará, Belém, Brazil, 2024. [Google Scholar]
- Ewing, D.A.; Cobbold, C.A.; Purse, B.V.; Nunn, M.A.; White, S.M. Modelling the effect of temperature on the seasonal population dynamics of temperate mosquitoes. J. Theor. Biol. 2016, 400, 65–79. [Google Scholar] [CrossRef]
- Fé, N.F.; Barbosa, M.d.G.V.; Fé, F.A.A.; Guerra, M.V.d.F.; Alecrim, W.D. Fauna de Culicidae Em Municípios da Zona Rural Do Estado Do Amazonas, Com Incidência de Febre Amarela. Rev. Soc. Bras. Med. Trop. 2003, 36, 343–348. [Google Scholar] [CrossRef]
- Almeida, J.F.; Belchior, H.C.M.; Batista, F.A.J.C.; Guimarães, R.C.d.S.; Maitra, A.; Ríos Velásquez, C.M.; Izzo, T.J.; Pessoa, F.A.C. Change in the Faunal Composition of Mosquitoes (Diptera: Culicidae) along a Heterogeneous Landscape Gradient in the Brazilian Amazon. PLoS ONE 2023, 18, e0288646. [Google Scholar] [CrossRef]
- Hendy, A.; Valério, D.; Fé, N.F.; Hernandez-Acosta, E.; Mendonça, C.; Andrade, E.; Pedrosa, I.; Costa, E.R.; Júnior, J.T.A.; Assunção, F.P.; et al. Microclimate and the Vertical Stratification of Potential Bridge Vectors of Mosquito-borne Viruses Captured by Nets and Ovitraps in a Central Amazonian Forest Bordering Manaus, Brazil. Sci. Rep. 2021, 11, 21129. [Google Scholar] [CrossRef] [PubMed]
- Hutchings, R.S.G.; Honegger, R.W.H.; Sallum, M.A.M. Culicidae (Diptera: Culicomorpha) from the central Brazilian Amazon: Nhamundá and Abacaxis rivers. Zoologia 2013, 30, 1–14. [Google Scholar] [CrossRef]
- Hutchings, R.S.G.; Hutchings, R.W.; Menezes, I.S.; Motta, M.D.A.; Sallum, M.A.M. Mosquitoes (Diptera: Culicidae) from the northwestern Brazilian Amazon: Padauari river. J. Med. Entomol. 2016, 53, 1330–1347. [Google Scholar] [CrossRef]
- Confalonieri, U.; Neto, C. Análises da diversidade e similaridade entre uma população de mosquitos (Diptera: Culicidae) de Caxiuanã, Pará-Brasil. In Proceedings of the Anais do VIII Congresso de Ecologia do Brasil, Caxambu, Brazil, 23–28 September 2007. [Google Scholar]
- Farias, M.A. Avaliação Entomofaunística e Pesquisa de Arbovirus na Área de Proteção Ambiental Ilha do Combu, Belém, Pará. Master’s Thesis, Evandro Chagas Institute, Ananindeua, Brazil, 2019. [Google Scholar]
- Mucci, L.F.; Bergo, E.S.; Deus, J.T.D.; Reginato, S.L.; Pereira, M.; Camargo-Neves, V.L.F.D. Evaluation of methods for collecting mosquitoes (Culicidae: Diptera) in canopy and ground Strata in the Brazilian Savanna. Trop. Med. Infect. Dis. 2022, 7, 446. [Google Scholar] [CrossRef]
- Deus, J.T.D.; Mucci, L.F.; Lucheta Reginatto, S.; Pereira, M.; Bergo, E.S.; de Camargo-Neves, V.L.F. Evaluation of methods to collect diurnal Culicidae (Diptera) at canopy and ground strata, in the atlantic forest biome. Insects 2022, 13, 202. [Google Scholar] [CrossRef] [PubMed]
- Service, M.W. Mosquito Ecology: Field Sampling Methods; Springer: Berlin/Heidelberg, Germany; Dordrecht, The Netherlands, 1993. [Google Scholar]
- Silver, J.B. Mosquito Ecology: Field Sampling Methods, 3rd ed.; Springer: Dordrecht, The Netherlands, 2008. [Google Scholar]
- Brilhante, A.F.; Fernandes, A.; Souza, J.F.; Paula, M.B.D.; Melchior, L.A.; Cardoso, C.O.; Galati, E.A.B.; Marelli, M.T.; Lima-Camara, T.N. Entomological survey of the mosquitoes in an area of ecological tourism in the Brazilian Amazon Basin. J. Am. Mosq. Control Assoc. 2018, 34, 42–46. [Google Scholar] [CrossRef] [PubMed]
- Hawkes, F.M.; Hopkins, R.J. The Mosquito. In Mosquitopia; Routledge: London, UK, 2021; pp. 16–31. [Google Scholar]
- Freire, R.C.d.M.; Barbosa, T.d.M.; Jales, J.T.; Ximenes, M.d.F.F.d.M.; La Corte, R.; Gama, R.A. Ecological Aspects of Mosquitoes (Diptera: Culicidae) in a Fragment of Seasonal Dry Tropical Forest (Caatinga) in Brazil. J. Arid. Environ. 2021, 190, 104528. [Google Scholar] [CrossRef]
- Velásquez, G.; Ulloa, A.; Montañez, H.; Guimarães, A.E.; Maldonado, A.J.; Bastardo, J.W. Evidence of the Presence of West Nile Virus in Mosquito Pools in North Eastern Region of Venezuela. Glob. Adv. Res. J. Med. Med. Sci. (GARJMMS) 2013, 2, 20–25. [Google Scholar]
- Aitken, T.H.G.; Spence, L.; Manuel, R. Virus Transmission Studies with Trinidadian Mosquitoes Part Iv. Kairi Virus1. J. Med. Entomol. 1964, 1, 50–52. [Google Scholar] [CrossRef]
- Spence, L.; Anderson, C.R.; Aitken, T.H.G.; Downs, W.G. Bimiti Virus, a New Agent Isolated from Trinidadian Mosquitoes. Am. J. Trop. Med. Hyg. 1962, 11, 414–417. [Google Scholar] [CrossRef]
- Panday, R.S. Guamá-Group Virus Activity in Surinam. Trop. Geogr. Med. 1981, 33, 123–127. [Google Scholar]
- Segura, M.; De Oliveira, D.M.; Castro, F. Atlas of Culicidae in the Brazilian Amazon: Specific Characteristics of Hematophagous Insects of the Culicidae Family; Instituto Evandro Chagas: Belém, Brazil, 2007.
- Pinheiro, F.P.; Travassos da Rosa, A.P.A.; Travassos da Rosa, J.F.S.; Ishak, R.; Freitas, R.B.; Gomes, M.L.C.; LeDuc, J.W.; Oliva, O.F.P. Oropouche Virus: A Review of Clinical, Epidemiological, and Ecological Findings. Am. J. Trop. Med. Hyg. 1981, 30, 149–160. [Google Scholar] [CrossRef]
- Sakkas, H.; Bozidis, P.; Franks, A.; Papadopoulou, C. Oropouche Fever: A Review. Viruses 2018, 10, 175. [Google Scholar] [CrossRef]
- Dantur Juri, M.J.; Stein, M.; Rossi, G.C.; Navarro, J.C.; Zaidenberg, M.; Mureb Sallum, M.A. New Records of Mosquitoes from Northwestern Argentina. J. Am. Mosq. Control Assoc. 2012, 28, 111–113. [Google Scholar] [CrossRef]
- Moutailler, S.; Yousfi, L.; Mousson, L.; Devillers, E.; Vazeille, M.; Vega-Rúa, A.; Perrin, Y.; Jourdain, F.; Chandre, F.; Cannet, A.; et al. A New High-Throughput Tool to Screen Mosquito-Borne Viruses in Zika Virus Endemic/Epidemic Areas. Viruses 2019, 11, 904. [Google Scholar] [CrossRef]
- Robin, Y.; Lhuillier, M.; Girault, G.; Pajot, F.X.; Dégallier, N. Dengue Viruses and Other Arboviruses in French Guiana. In Proceedings of the International Symposium on Tropical Arboviruses and Haemorrhagic Fevers, Belem, Brazil, 14–18 April 1980; pp. 391–396. [Google Scholar]
- Rojas-Araya, D.; Mathias, D.; Burkett-Cadena, N. An Mosquito Mansonia Titillans (Walker) (Insecta: Diptera: Culicidae: Culicinae: Mansoniini). EDIS 2021, 2021, 6. [Google Scholar] [CrossRef]
- Ganjian, N.; Riviere-Cinnamond, A. Mayaro Virus in Latin America and the Caribbean. Rev. Panam. Salud Pública 2020, 44, e14. [Google Scholar] [CrossRef] [PubMed]
- Turell, M.J.; Jones, J.W.; Sardelis, M.R.; Dohm, D.J.; Coleman, R.E.; Watts, D.M.; Fernandez, R.; Calampa, C.; Klein, T.A. Vector Competence of Peruvian Mosquitoes (Diptera: Culicidae) for Epizootic and Enzootic Strains of Venezuelan Equine Encephalomyelitis Virus. J. Med. Entomol. 2000, 37, 835–839. [Google Scholar] [CrossRef]
- Méndez, W.; Liria, J.; Navarro, J.-C.; García, C.Z.; Freier, J.E.; Salas, R.; Weaver, S.C.; Barrera, R. Spatial Dispersion of Adult Mosquitoes (Diptera: Culicidae) in a Sylvatic Focus of Venezuelan Equine Encephalitis Virus. J. Med. Entomol. 2001, 38, 813–821. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Beranek, M.D.; Gallardo, R.; Almirón, W.R.; Contigiani, M.S. First Detection of Mansonia Titillans (Diptera: Culicidae) Infected with St. Louis Encephalitis Virus (Flaviviridae: Flavivirus) and Bunyamwera Serogroup (Peribunyaviridae: Orthobunyavirus) in Argentina. J. Vector Ecol. 2018, 43, 340–343. [Google Scholar] [CrossRef]
- Reis, L.A.M.; Pampolha, A.B.O.; Nascimento, B.L.S.d.; Dias, D.D.; Araújo, P.A.d.S.; Silva, F.S.d.; Silva, L.H.d.S.e.; Reis, H.C.F.; Silva, E.V.P.d.; Nunes Neto, J.P. Genus Culex Linnaeus, 1758 (Diptera: Culicidae) as an Important Potential Arbovirus Vector in Brazil: An Integrative Review. Life 2023, 13, 2179. [Google Scholar] [CrossRef]
- Lord, R.D. Encefalitis Equina Venezolana Su Historia y Distribucion Geografica. Boletín Oficina Sanit. Panam (OSP) 1973, 75, 530–541. [Google Scholar]
- Culex Quinquefasciatus Surveillance Guide. Available online: https://www.gov.br/saude/pt-br/centrais-de-conteudo/publicacoes/svsa/culex-quinquefasciatus/guia_vigilancia_culex_quinquefasciatus.pdf/view (accessed on 14 August 2025).
- Karabatsos, N. International Catalogue of Arboviruses: Including Certain Other Viruses of Vertebrates, 3rd ed.; American Society of Tropical Medicine and Hygiene: San Antonio, TX, USA, 1985. [Google Scholar]
- Araújo, P.A.; Freitas, M.O.; Chiang, J.O.; Silva, F.A.; Chagas, L.L.; Casseb, S.M.; Silva, S.P.; Nunes-Neto, J.P.; Rosa-Júnior, J.W.; Nascimento, B.S.; et al. Investigation about the Occurrence of Transmission Cycles of Arbovirus in the Tropical Forest, Amazon Region. Viruses 2019, 11, 774. [Google Scholar] [CrossRef]
- Hahn, C.E. A New Virus Causing Encephalitis in Man. Am. J. Trop. Med. Hyg. 1934, 14, 377–382. [Google Scholar]
- INPE Monitoramento dos Focos Ativos por Estado—Programa Queimadas. Available online: https://terrabrasilis.dpi.inpe.br/queimadas/situacao-atual/estatisticas/estatisticas_estados/ (accessed on 10 September 2025).
- Tavares, J.P.N. Características da climatologia de Macapá-AP. Caminhos Geogr. 2014, 15, 138–151. [Google Scholar] [CrossRef]
- Marchi, M.J.; Müller, G.A.; Marcondes, C.B. Mosquitos (Diptera: Culicidae) de Uma Futura Unidade de Conservação Em Área de Mata Atlântica No Sul Do Brasil. EntomoBrasilis 2010, 3, 34–37. [Google Scholar] [CrossRef][Green Version]
- Guedes, M.L.P. Análise de Fatores Regulatórios na Variação Interpopulacional e na Composição das Comunidades de Culicidae (Diptera). Ph.D. Thesis, Federal University of Paraná, Curitiba, Brazil, 2014. [Google Scholar][Green Version]
- Vasconcelos, P.F.d.C. Febre Amarela. Rev. Soc. Bras. Med. Trop. 2003, 36, 275–293. [Google Scholar] [CrossRef]
- de Oliveira, C.H.; Andrade, M.S.; Campos, F.S.; da Cardoso, J.C.; Gonçalves-dos-Santos, M.E.; Oliveira, R.S.; Aquino-Teixeira, S.M.; Campos, A.A.; Almeida, M.A.; Simonini-Teixeira, D.; et al. Yellow Fever Virus Maintained by Sabethes Mosquitoes during the Dry Season in Cerrado, a Semiarid Region of Brazil, in 2021. Viruses 2023, 15, 757. [Google Scholar] [CrossRef]
- Abreu, F.V.S.d.; Ribeiro, I.P.; Ferreira-de-Brito, A.; Santos, A.A.C.d.; Miranda, R.M.d.; Bonelly, I.d.S.; Neves, M.S.A.S.; Bersot, M.I.; Santos, T.P.d.; Gomes, M.Q.; et al. Haemagogus Leucocelaenus and Haemagogus Janthinomys Are the Primary Vectors in the Major Yellow Fever Outbreak in Brazil, 2016–2018. Emerg. Microbes. Infect. 2019, 8, 218–231. [Google Scholar] [CrossRef] [PubMed]
- Sanders, E.J.; Rwaguma, E.B.; Kawamata, J.; Kiwanuka, N.; Lutwama, J.J.; Ssengooba, F.P.; Lamunu, M.; Najjemba, R.; Were, W.A.; Bagambisa, G.; et al. O’nyong-nyong Fever in South-Central Uganda, 1996–1997: Description of the Epidemic and Results of a Household-Based Seroprevalence Survey. J. Infect. Dis. 1999, 180, 1436–1443. [Google Scholar] [CrossRef]
- Deane, L.; Causey, O.; Deane, M. Notas sobre a distribuição e biologia dos anofelinos das regiões Nordestina e Amazônica do Brasil. Rev. Serviço Espec. 1948, 1, 827–965. [Google Scholar]
- Lorosa, E.S.; Faria, M.S.; De Oliveira, L.C.M.; Alencar, J.; Marcondes, C.B. Blood meal identification of selected mosquitoes in Rio De Janeiro, Brazil. J. Am. Mosq. Control Assoc. 2010, 26, 18–23. [Google Scholar] [CrossRef]
- Barbosa, L.M.C.; Scarpassa, V.M. Blood-Feeding Behavior of Anopheles Species (Diptera: Culicidae) in the District of Ilha de Santana, State of Amapá, Eastern Brazilian Amazon. Rev. Bras. Entomol. 2021, 65, e20200048. [Google Scholar] [CrossRef]
- Tadei, W.P.; Dutary-Thatcher, B. Malaria vectors in the Brazilian Amazon: Anopheles of the subgenus Nyssorhynchus. Rev. Inst. Med. Trop. 2000, 42, 94–97. [Google Scholar] [CrossRef] [PubMed]
- Rosero, D.A.; Naranjo-Diaz, N.; Alvarez, N.; Cienfuegos, A.V.; Torres, C.; Luckhart, S.; Correa, M.M. Colombian Anopheles triannulatus (Diptera: Culicidae) naturally infected with Plasmodium spp. ISRN Parasitol. 2013, 2013, 927453. [Google Scholar] [CrossRef]
- Galeno, E.O. Aspectos Ecológicos e Paridade de Anopheles spp. MEIGEN, 1818 (Diptera: Culicidae) em Cinco Comunidades do Município de Mazagão, Amapá, Amazônia Oriental. Master’s Thesis, Federal University of Amapá, Macapá, Brazil, 2018. [Google Scholar]
- Barbosa, L.M.C.; Souto, R.N.P.; Ferreira, R.M.A. Behavioral patterns, parity rate and natural infection analysis in anopheline species involved in the transmission of malaria in the northeastern Brazilian Amazon region. Acta Trop. 2016, 164, 216–225. [Google Scholar] [CrossRef] [PubMed]
- Póvoa, M.; Wirtz, R.; Lacerda, R.; Miles, M.; Warhurst, D. Malaria vectors in themunicipality of Serra do Navio, State of Amapa, Amazon Region, Brazil. Memórias Inst. Oswaldo Cruz 2001, 96, 179–184. [Google Scholar] [CrossRef]
- da Costa Fonseca, E.; David, E.D.S.; Komninakis, E.; Souto, R.N. Diversidade, distribuição e aspectos ecológicos das espécies de Anopheles Vetores de Malária, em comunidades agroextrativistas do município de Mazagão, Amapá, Brasil. Cuad. Educ. Desarro. 2023, 15, 15047–15063. [Google Scholar] [CrossRef]
- McKeon, S.N.; Schlichting, C.D.; Povoa, M.M.; Conn, J.E. Ecological suitability and spatial distribution of five Anopheles species in Amazonian Brazil. Am. J. Trop. Med. Hyg. 2013, 88, 1079–1086. [Google Scholar] [CrossRef][Green Version]
- Reis, I.C.; Codeço, C.T.; Degener, C.M.; Keppeler, E.C.; Muniz, M.M.; Silva de Oliveira, F.G.; Cortes, J.J.C.; de Freitas Monteiro, A.; Albano de Souza, C.A.; Morone Rodriguez, F.C. Contribution of fish farming ponds to the production of immature Anopheles spp. in a malaria-endemic Amazonian town. Malar. J. 2015, 14, 452. [Google Scholar] [CrossRef] [PubMed]



| Species | Rainy | Intermediary | Dry | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PHA | CDC | PHA | CDC | PHA | CDC | |||||||||
| Gr | Ca | Gr | Ca | Gr | Ca | Gr | Ca | Gr | Ca | Gr | Ca | Total | Fre. (%) | |
| Ad. (Ady.) squamipennis | 0 | 0 | 28 | 4 | 0 | 0 | 5 | 3 | 0 | 0 | 0 | 0 | 40 | 1.14 |
| Ae. (Och.) serratus | 5 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 7 | 0.20 |
| An. (Ano.) peryassui | 0 | 0 | 0 | 0 | 0 | 0 | 33 | 5 | 0 | 0 | 0 | 0 | 38 | 1.09 |
| An. (Ano.) spp. | 0 | 0 | 0 | 0 | 0 | 0 | 39 | 7 | 0 | 0 | 0 | 0 | 46 | 1.31 |
| An. (Nys.) aquasalis | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0.03 |
| An. (Nys.) triannulatus | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0.03 |
| An. (Nys.) spp. | 7 | 0 | 0 | 0 | 8 | 1 | 39 | 46 | 0 | 0 | 0 | 0 | 101 | 2.89 |
| Anopheles spp. | 0 | 0 | 0 | 0 | 0 | 0 | 17 | 0 | 0 | 0 | 0 | 0 | 17 | 0.49 |
| Cq. (Rhy.) albicosta | 2 | 1 | 9 | 6 | 2 | 3 | 130 | 55 | 0 | 0 | 0 | 0 | 208 | 5.94 |
| Cq. (Rhy.) arribalzagae | 1 | 0 | 0 | 0 | 1 | 0 | 2 | 1 | 9 | 1 | 0 | 0 | 15 | 0.43 |
| Cq. (Rhy.) juxtamansonia | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 2 | 0.06 |
| Cq. (Rhy.) nigricans | 0 | 0 | 2 | 1 | 2 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 7 | 0.20 |
| Cq. (Rhy.) spp. | 20 | 0 | 27 | 40 | 89 | 21 | 327 | 90 | 0 | 0 | 0 | 2 | 616 | 17.60 |
| Cq. (Rhy.) venezuelensis | 15 | 3 | 143 | 150 | 10 | 0 | 195 | 110 | 0 | 0 | 7 | 0 | 633 | 18.09 |
| Cx. (Cux.) spp. | 5 | 0 | 22 | 6 | 1 | 0 | 13 | 5 | 0 | 0 | 4 | 0 | 56 | 1.60 |
| Cx. (Mel.) ensiformis | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0.03 |
| Cx. (Mel.) group atratus | 0 | 0 | 27 | 15 | 0 | 0 | 79 | 36 | 0 | 0 | 0 | 0 | 157 | 4.49 |
| Cx. (Mel.) pedroi | 0 | 0 | 8 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 9 | 0.26 |
| Cx. (Mel.) portesi | 0 | 1 | 0 | 9 | 0 | 0 | 110 | 26 | 0 | 0 | 0 | 0 | 146 | 4.17 |
| Cx. (Mel.) saetilatus | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 2 | 0.06 |
| Cx. (Mel.) spp. | 0 | 0 | 13 | 34 | 4 | 0 | 212 | 67 | 0 | 0 | 60 | 35 | 425 | 12.14 |
| Cx. (Mel.) spissipes | 6 | 0 | 37 | 23 | 0 | 0 | 53 | 11 | 0 | 0 | 0 | 0 | 130 | 3.71 |
| Cx. (Mel.) vomerifer | 0 | 0 | 0 | 0 | 0 | 0 | 4 | 1 | 0 | 0 | 1 | 0 | 6 | 0.17 |
| Hg. (Hag.) albomaculatus | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0.06 |
| Hg. (Hag.) janthinomys | 0 | 1 | 1 | 0 | 0 | 0 | 2 | 2 | 0 | 0 | 0 | 0 | 6 | 0.17 |
| Hg. (Hag.) sp. | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0.03 |
| Li. durhamii | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0.06 |
| Limatus spp. | 6 | 0 | 0 | 0 | 6 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 12 | 0.34 |
| Ma. (Man.) indubitans | 0 | 0 | 5 | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 8 | 0.23 |
| Ma. (Man.) pseudotitillans | 0 | 0 | 0 | 0 | 0 | 0 | 3 | 0 | 0 | 0 | 0 | 0 | 3 | 0.09 |
| Ma. (Man.) spp. | 0 | 1 | 267 | 70 | 0 | 0 | 0 | 6 | 0 | 0 | 2 | 0 | 346 | 9.89 |
| Ma. (Man.) titillans | 1 | 1 | 0 | 0 | 24 | 83 | 67 | 27 | 0 | 0 | 0 | 0 | 203 | 5.80 |
| Ps. (Jan.) albipes | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0.06 |
| Ps. (Jan.) ferox | 11 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 12 | 0.34 |
| Ps. (Jan.) sp. | 2 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 3 | 0.09 |
| Sa. (Sab.) tarsopus | 0 | 0 | 0 | 0 | 0 | 7 | 0 | 0 | 0 | 0 | 0 | 0 | 7 | 0.20 |
| Sa. (Sab.) amazonicus | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0.06 |
| Sa. (Sab.) belisarioi | 0 | 2 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 4 | 0.11 |
| Sa. (Sab.) cyaneus | 0 | 2 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 3 | 0.09 |
| Sa. (Sab.) quasicyaneus | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0.06 |
| Sa. (Sbo.) chloropterus | 1 | 2 | 0 | 0 | 0 | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 6 | 0.17 |
| Sabethes spp. | 0 | 0 | 0 | 0 | 0 | 4 | 0 | 0 | 0 | 0 | 0 | 0 | 4 | 0.11 |
| Tr. (Trc.) digitatum | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0.06 |
| Ur. (Ura.) ditaeniota | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0.03 |
| Ur. (Ura.) geometrica | 0 | 0 | 0 | 0 | 0 | 0 | 11 | 1 | 0 | 0 | 0 | 0 | 12 | 0.34 |
| Uranotaenia (Ura.) spp. | 0 | 0 | 0 | 1 | 0 | 0 | 35 | 0 | 0 | 0 | 102 | 24 | 162 | 4.63 |
| Wy. negrensis/occulta | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0.06 |
| Wyeomyia spp. | 16 | 0 | 0 | 0 | 7 | 0 | 4 | 1 | 0 | 0 | 1 | 0 | 29 | 0.83 |
| Abundance | 1079 | 2172 | 249 | 3500 | ||||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nascimento, R.E.d.; Dias, D.D.; Nascimento, B.L.S.d.; Costa, T.S.d.; Souto, R.N.P.; Casseb, L.M.N.; Neto, J.P.N.; Carvalho, V.L. Culicidae Fauna (Diptera: Culicomorpha) of the Municipality of Mazagão, Amapá, in the Brazilian Amazon. Insects 2025, 16, 1036. https://doi.org/10.3390/insects16101036
Nascimento REd, Dias DD, Nascimento BLSd, Costa TSd, Souto RNP, Casseb LMN, Neto JPN, Carvalho VL. Culicidae Fauna (Diptera: Culicomorpha) of the Municipality of Mazagão, Amapá, in the Brazilian Amazon. Insects. 2025; 16(10):1036. https://doi.org/10.3390/insects16101036
Chicago/Turabian StyleNascimento, Rafael Espíndola do, Daniel Damous Dias, Bruna Lais Sena do Nascimento, Tiago Silva da Costa, Raimundo Nonato Picanço Souto, Livia Medeiros Neves Casseb, Joaquim Pinto Nunes Neto, and Valeria Lima Carvalho. 2025. "Culicidae Fauna (Diptera: Culicomorpha) of the Municipality of Mazagão, Amapá, in the Brazilian Amazon" Insects 16, no. 10: 1036. https://doi.org/10.3390/insects16101036
APA StyleNascimento, R. E. d., Dias, D. D., Nascimento, B. L. S. d., Costa, T. S. d., Souto, R. N. P., Casseb, L. M. N., Neto, J. P. N., & Carvalho, V. L. (2025). Culicidae Fauna (Diptera: Culicomorpha) of the Municipality of Mazagão, Amapá, in the Brazilian Amazon. Insects, 16(10), 1036. https://doi.org/10.3390/insects16101036








