Silencing the cyp314a1 and cyp315a1 Genes in the Aedes albopictus 20E Synthetic Pathway for Mosquito Control and Assessing Algal Blooms Induced by Recombinant RNAi Microalgae
Simple Summary
Abstract
1. Introduction
2. Methods
2.1. Bioinformatic Analysis
2.2. Mosquito Maintenance
2.3. Microalgae Strains and Plasmids
2.4. Construction of RNAi Vectors
2.5. Microalgae Transfection
2.6. Mosquito Feeding Experiments
2.7. Measurement of Larval Superoxide Dismutase (SOD), Peoxidase (POD) and Catalase (CAT) Activities
2.8. The 20-Hydroxyecdysone(20E) Compensation Test
2.9. RT-qPCR
2.10. Simulated Field Trials
2.11. Sample Preparation and DNA Extraction of the Test Water
2.12. Amplification and Sequencing of the Hypervariable Region
2.13. Statistical Analyses
3. Results
3.1. CYP314A1 and CYP315A1 Proteins
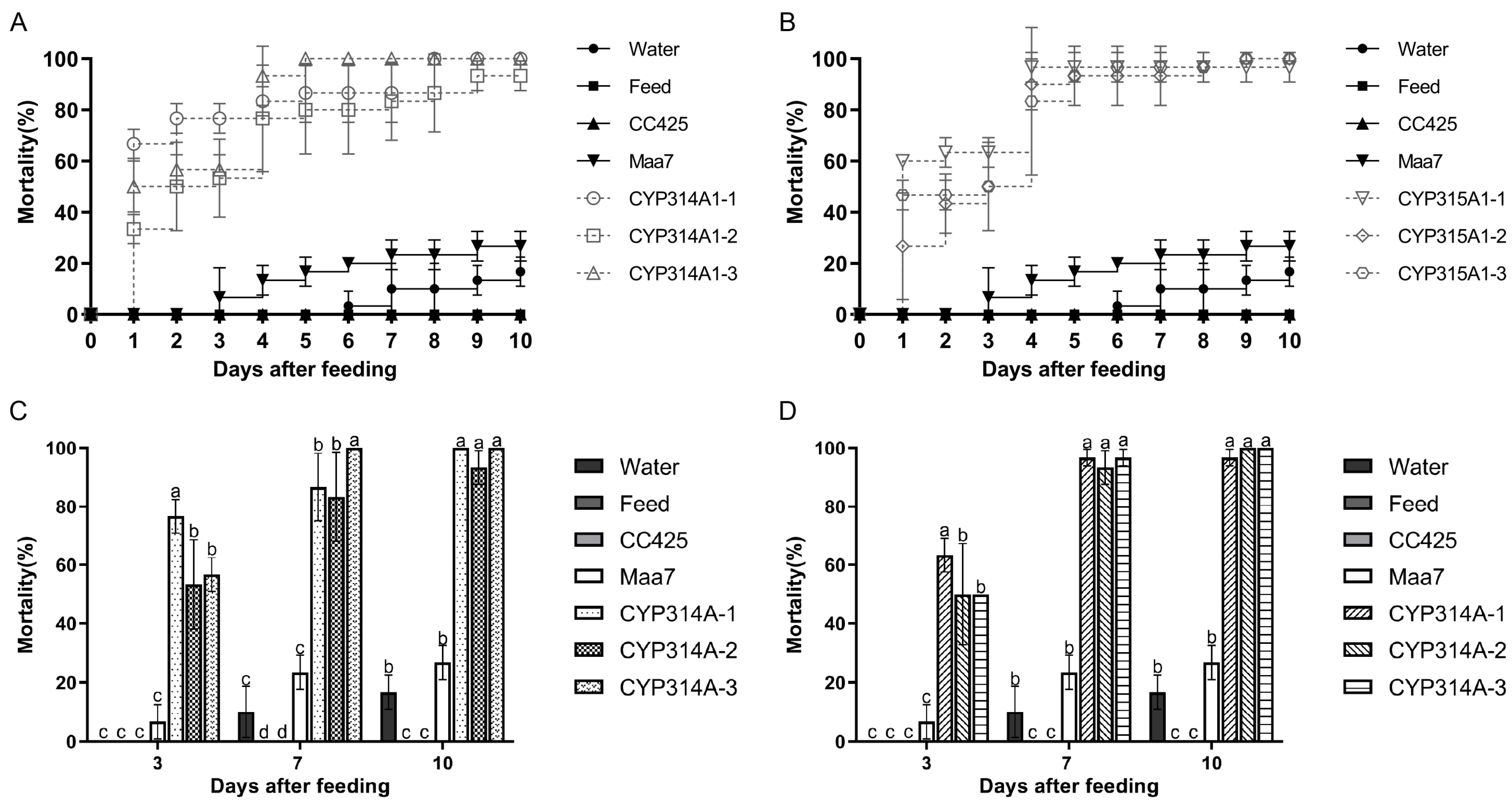
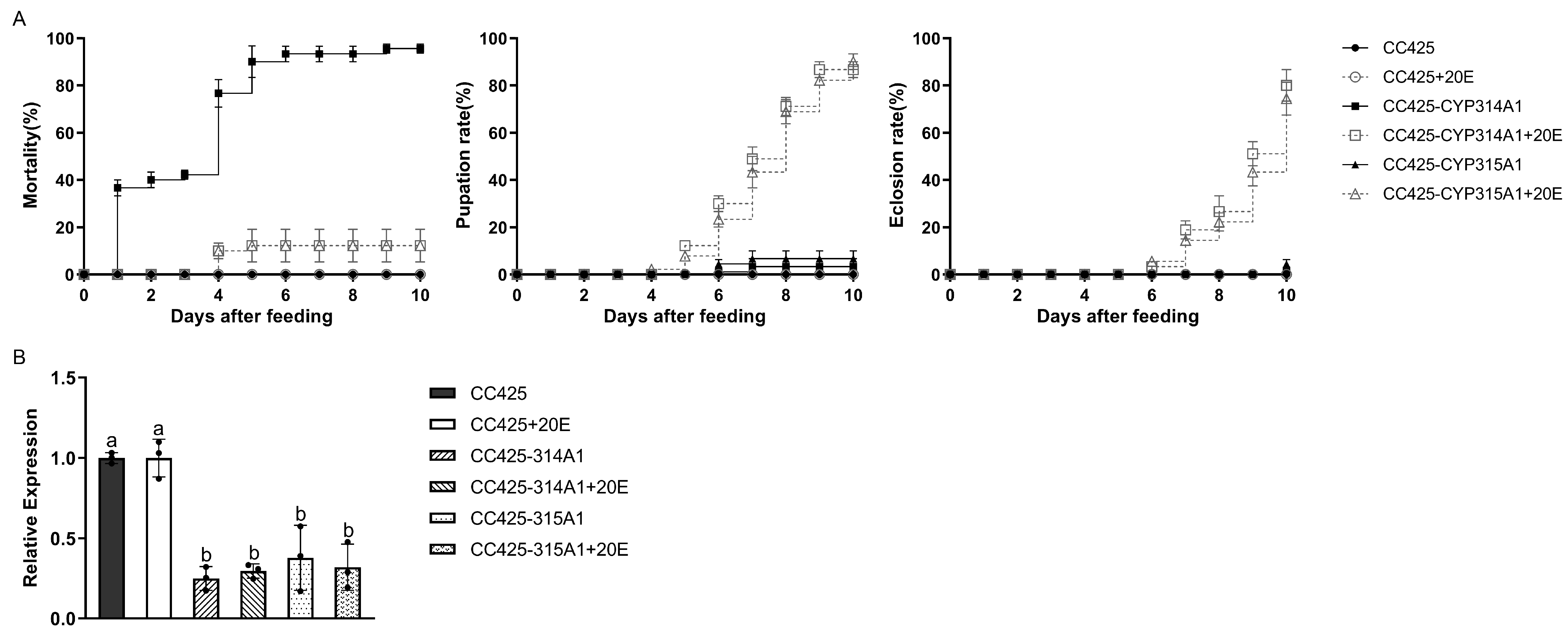
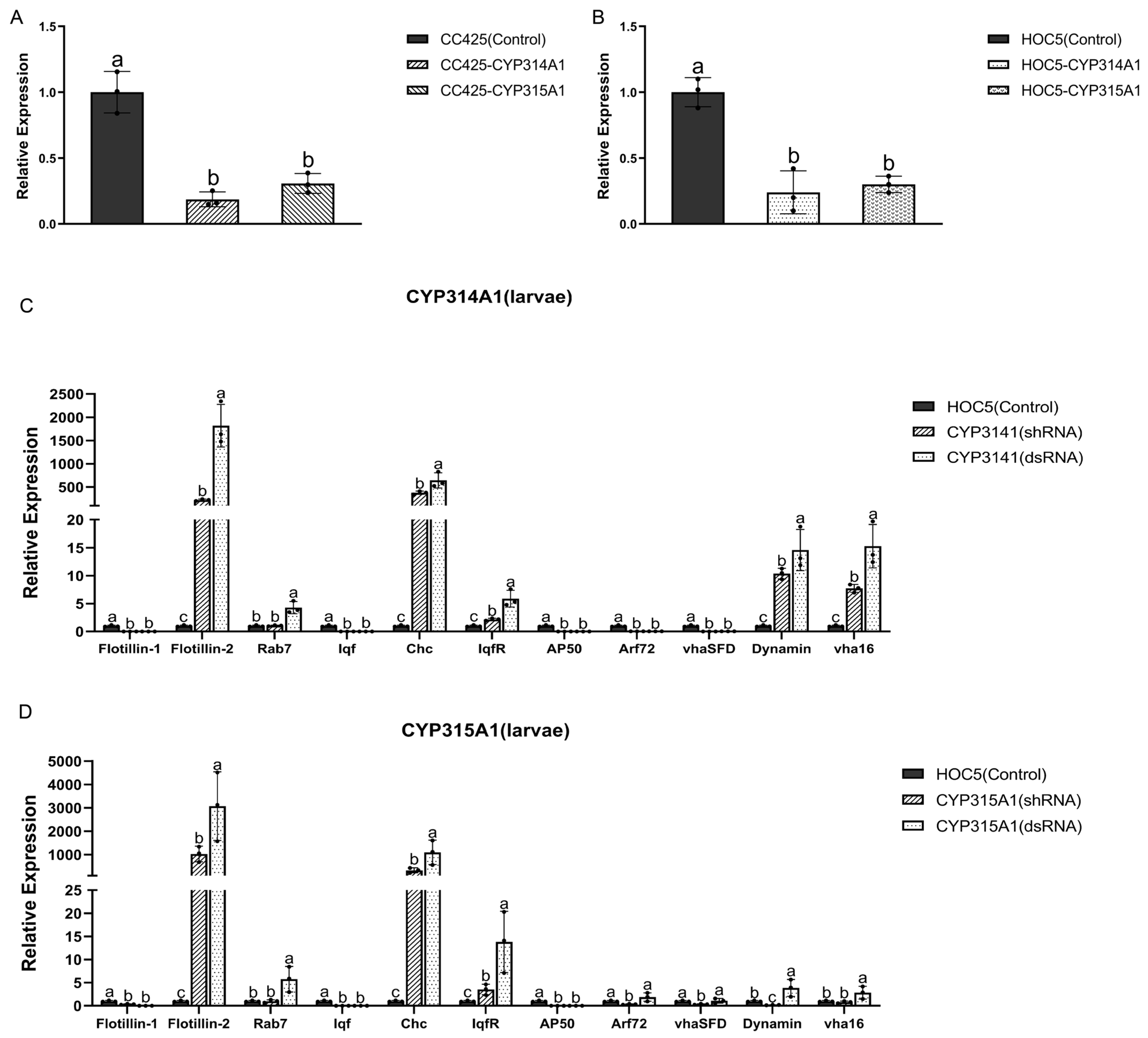
3.2. Cyp314a1 and cyp315a1 RNAi Recombinant Chlamydomonas/Chlorella Are Lethal to Ae. albopictus
3.3. Effects of RNAi Recombinant Chlorella on Larval Development, and Redox Stress and 20E Compensation Assay
3.4. The Expression of the RNAi Target Genes and the Genes in the Clathrin-Mediated Endocytosis (CME) Pathway
3.5. Simulated-Field Trials
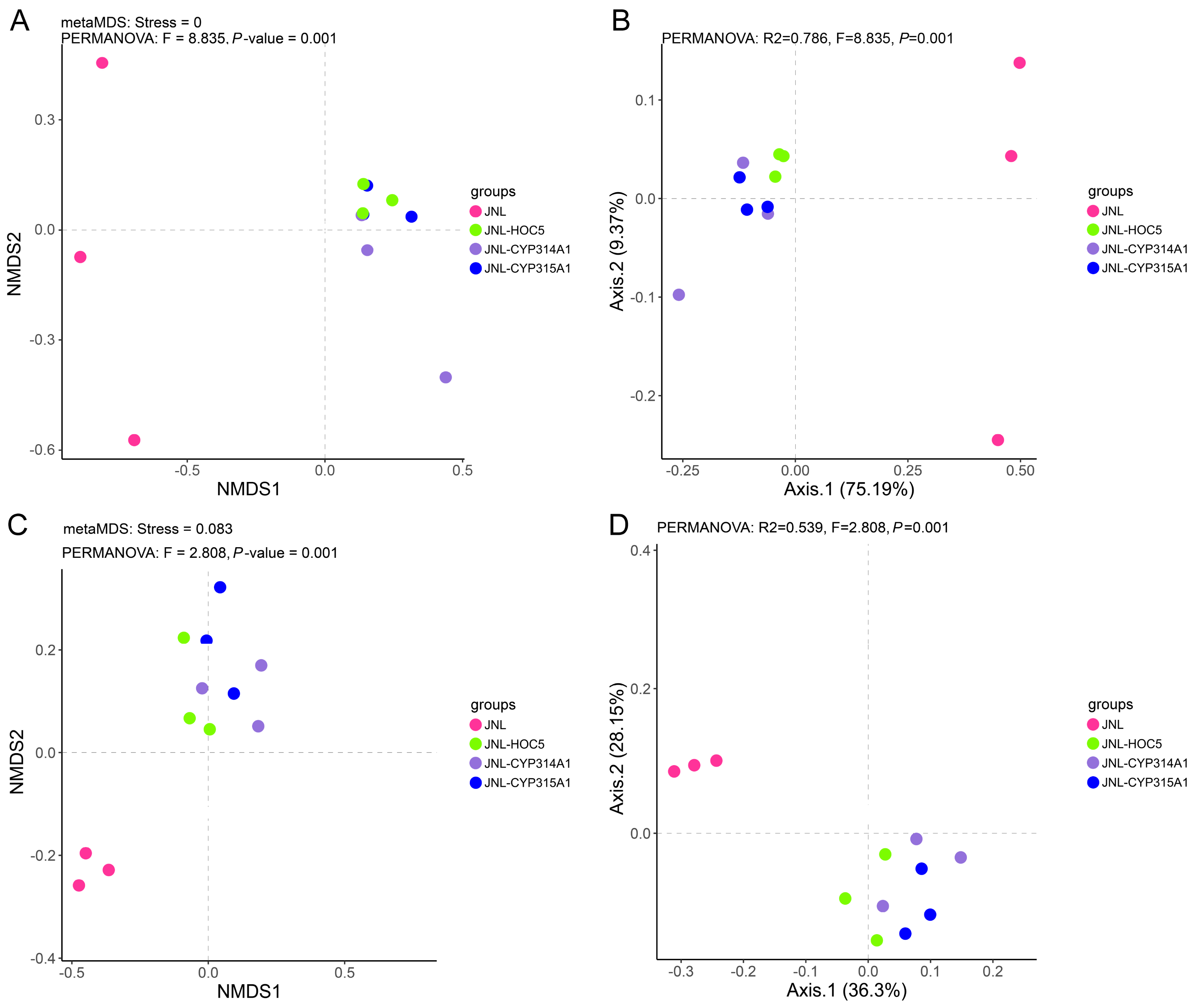
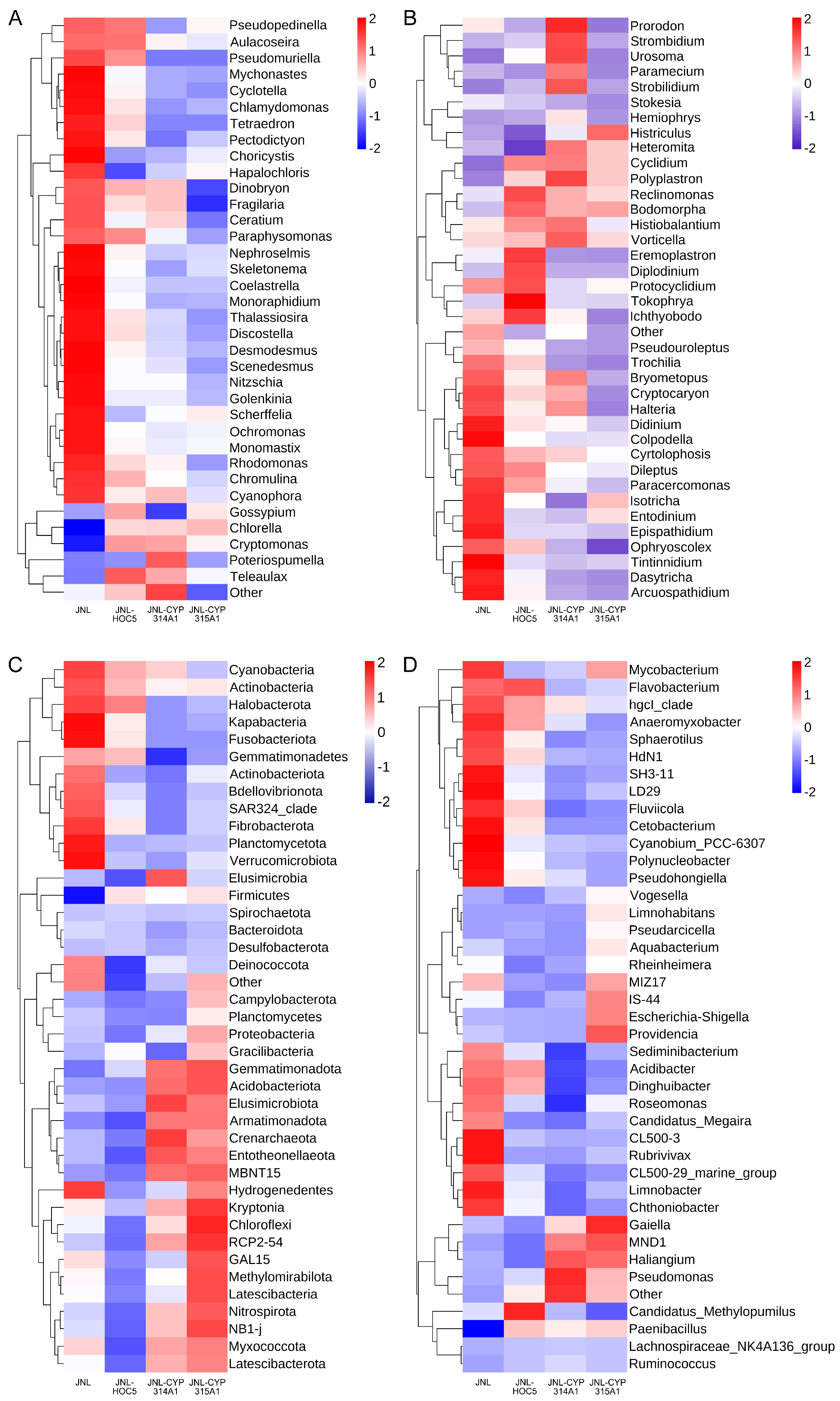
3.6. Plankton Abundance and Diversity in Simulated Field Test Waters
3.7. Changes in Plankton in Simulated Field Test Waters
3.8. Changes in Prokaryotes in Simulated Field Test Waters
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Waggoner, J.J.; Gresh, L.; Vargas, M.J. Viremia and Clinical Presentation in Nicaraguan Patients Infected with Zika Virus, Chikungunya Virus, and Dengue Virus. Clin. Infect. Dis. 2016, 63, 1584–1590. [Google Scholar] [CrossRef]
- Bhatt, S. The global distribution and burden of dengue. Nature 2013, 496, 504–507. [Google Scholar] [CrossRef]
- WHO. Ten Threats to Global Health in 2019; WHO: Geneva, Switzerland, 2019. [Google Scholar]
- Xiang, B.; Gao, P.; Kang, Y.F. Importation of Zika virus in China: A significant risk in southern China. J. Infect. 2017, 74, 328–330. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Lu, H.Z. Yellow fever in China is still an imported disease. Biosci. Trends 2016, 10, 158–162. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Sun, F.J.; Tong, Y.G. Rift Valley fever virus imported into China from Angola. Lancet Infect. Dis. 2016, 16, 1226. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Wu, J.; Zhang, Q.L. Chikungunya outbreak in Guangdong province, China, 2010. Emerg. Infect. Dis. 2012, 18, 493–495. [Google Scholar] [CrossRef]
- Hemingway, J. Averting a malaria disaster: Will insecticide resistance derail malaria control? Lancet 2016, 387, 1785–1788. [Google Scholar] [CrossRef]
- Van, H. Global trends in the production and use of DDT for control of malaria and other vector-borne diseases. Malar. J. 2012, 11, 401. [Google Scholar]
- Bian, G.; Zhou, G.; Lu, P.; Xi, Z. Replacing a native Wolbachia with a novel strain results in an increase in endosymbiont load and resistance to dengue virus in a mosquito vector. PLoS Negl. Trop. Dis. 2013, 7, e2250. [Google Scholar] [CrossRef]
- Xue, L.; Fang, X.; Hyman, J.M. Comparing the effectiveness of different strains of Wolbachia for controlling chikungunya, dengue fever, and zika. PLoS Negl. Trop. Dis. 2018, 12, e0006666. [Google Scholar] [CrossRef]
- Flores, H.A.; Neill, S.L. Controlling vector-borne diseases by releasing modified mosquitoes. Nat. Rev. Microbiol. 2018, 16, 508–518. [Google Scholar] [CrossRef] [PubMed]
- Bargielowski, I.; Alphey, L.; Koella, J.C. Cost of mating and insemination capacity of a genetically modified mosquito Aedes aegypti OX513A compared to its wild type counterpart. PLoS ONE 2011, 6, e26086. [Google Scholar] [CrossRef] [PubMed]
- Curtis, Z.; Matzen, K.; Neira, O.M. Assessment of the Impact of Potential Tetracycline Exposure on the Phenotype of Aedes aegypti OX513A: Implications for Field Use. PLoS Negl. Trop. Dis. 2015, 9, e0003999. [Google Scholar] [CrossRef] [PubMed]
- Fei, X.; Zhang, Y.; Ding, L.; Xiao, S.; Xie, X.; Li, Y.; Deng, X. Development of an RNAi-based microalgal larvicide for the control of Aedes aegypti. Parasit. Vectors 2021, 14, 387. [Google Scholar] [CrossRef]
- Fei, X.; Huang, X.; Li, Z.; Li, X.; He, C.; Xiao, S.; Li, Y.; Zhang, X.; Deng, X. Effect of marker-free transgenic Chlamydomonas on the control of Aedes mosquito population and on plankton. Parasit. Vectors 2023, 16, 18. [Google Scholar] [CrossRef]
- Fei, X.; Xiao, S.; Huang, X.; Li, Z.; Li, X.; He, C.; Li, Y.; Zhang, X.; Deng, X. Control of Aedes mosquito populations using recombinant microalgae expressing short hairpin RNAs and their effect on plankton. PLoS Negl. Trop. Dis. 2023, 17, e0011109. [Google Scholar] [CrossRef]
- Li, X.; Zhang, H. Endocytic pathway mediates refractoriness of insect Bactrocera dorsalis to RNA interference. Sci. Rep. 2015, 5, 8700. [Google Scholar] [CrossRef]
- Xu, L.; Xu, S.; Sun, L. Synergistic action of the gut microbiota in environmental RNA interference in a leaf beetle. Microbiome 2021, 9, 98. [Google Scholar] [CrossRef]
- Zhang, J.; Khan, S.A.; Hasse, C. Full crop protection from an insect pest by expression of long double-stranded RNAs in plastids. Science 2015, 347, 991–994. [Google Scholar] [CrossRef]
- Xu, W.; Zhang, M.; Li, Y. Complete protection from Henosepilachna vigintioctopunctata by expressing long double-stranded RNAs in potato plastid. J. Integr. Plant Biol. 2023, 65, 1003–1011. [Google Scholar] [CrossRef]
- Nelson, D.R.; Goldstone, J.V.; Stegeman, J.J. The cytochrome P450 genesis locus: The origin and evolution of animal cytochrome P450s. Philos. Trans. R. Soc. B Biol. Sci. 2013, 368, 20120474. [Google Scholar] [CrossRef]
- Ventura, T.; Bose, U.; Fitzgibbon, Q.P.; Smith, G.G.; Shaw, P.N.; Cummins, S.F.; Elizur, A. CYP450s analysis across spiny lobster metamorphosis identifies a long sought missing link in crustacean development. J. Steroid Biochem. Mol. Biol. 2017, 171, 262–269. [Google Scholar] [CrossRef]
- Dermauw, W.; Van, L.T.; Feyereisen, R. Diversity and evolution of the P450 family in arthropods. Insect Biochem. Mol. Biol. 2020, 127, 103490. [Google Scholar] [CrossRef] [PubMed]
- Feyereisen, R. Evolution of insect P450. Biochem. Soc. Trans. 2006, 34, 1252–1255. [Google Scholar] [CrossRef]
- Feyereisen, R. Arthropod CYPomes illustrate the tempo and mode in P450 evolution. Biochim. Biophys. Acta 2011, 1814, 19–28. [Google Scholar] [CrossRef]
- Zhang, H.; Gao, H.; Lin, X.; Yang, B.; Wang, J.; Yuan, X.; Zhang, Z.; He, T.; Liu, Z. Akt-FoxO signaling drives co-adaptation to insecticide and host plant stresses in an herbivorous insect. J. Adv. Res. 2025, 75, 53–64. [Google Scholar] [CrossRef]
- Mao, Y.; Cai, W.; Wang, J.; Hong, G.; Tao, X.; Wang, L. Silencing a cotton bollworm P450 monooxygenase gene by plant-mediated RNAi impairs larval tolerance of gossypol. Nat. Biotechnol. 2007, 25, 1307–1313. [Google Scholar] [CrossRef] [PubMed]
- Ma, K.; Li, F.; Tang, Q.; Liang, P.; Liu, Y.; Zhang, B. CYP4CJ1-mediated gossypol and tannic acid tolerance in Aphis gossypii Glover. Chemosphere 2019, 219, 961–970. [Google Scholar] [CrossRef] [PubMed]
- Bass, C.; Hebsgaard, M.; Hughes, J. Genomic resources for the brown planthopper, Nilaparvata lugens: Transcriptome pyrosequencing and microarray design. Insect Sci. 2011, 19, 1–12. [Google Scholar] [CrossRef]
- Bass, C.; Carvalho, R.; Oliphant, L.; Puinean, A.; Field, L.; Nauen, R. Overexpression of a cytochrome P450 monooxygenase, CYP6ER1, is associated with resistance to imidacloprid in the brown planthopper, Nilaparvata lugens. Insect Mol. Biol. 2011, 20, 763–773. [Google Scholar] [CrossRef]
- Mao, K.; Zhang, X.; Ali, E.; Liao, X.; Jin, R.; Ren, Z. Characterization of nitenpyram resistance in Nilaparvata lugens (Stal). Pestic. Biochem. Physiol. 2019, 157, 26–32. [Google Scholar] [CrossRef]
- Matsuda, K.; Ihara, M.; Sattelle, D. Neonicotinoid insecticides: Molecular targets, resistance, and toxicity. Annu. Rev. Pharmacol. Toxicol. 2020, 60, 241–255. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Gong, Y.; Ali, S.; Hou, M. Mechanisms of resistance to thiamethoxam and dinotefuran compared to imidacloprid in the brown planthopper: Roles of cytochrome P450 monooxygenase and a P450 gene CYP6ER1. Pestic. Biochem. Physiol. 2018, 150, 17–26. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Liao, X.; Mao, P.; Yang, D.; Li, E.; Alia, E. The role of detoxifying enzymes in field-evolved resistance to nitenpyram in the brown planthopper Nilaparvata lugens in China. Crop Prot. 2017, 94, 106–114. [Google Scholar] [CrossRef]
- Tan, Q.M.; Chen, W.W.; Li, H.H.; Liao, S.C.; Yi, G.Q.; Mei, Y.; Luo, J.; Tan, H.H.; Li, X.S. Adipokinetic hormone signaling regulates cytochrome P450-mediated chlorantraniliprole sensitivity in Spodoptera frugiperda (Lepidoptera: Noctuidae). Pest. Manag. Sci. 2022, 78, 2618–2628. [Google Scholar] [CrossRef]
- Ullah, F.; Gul, H.; Tariq, K.; Desneux, N.; Gao, X.; Song, D. Functional analysis of cytochrome P450 genes linked with acetamiprid resistance in melon aphid, Aphis gossypii. Pestic. Biochem. Physiol. 2020, 170, 104687. [Google Scholar] [CrossRef]
- Chen, W.; Li, Z.; Zhou, C.; Ali, A.; Ali, S.; Wu, J. RNA interference in cytochrome P450 monooxygenase (CYP) gene results in reduced insecticide resistance in Megalurothrips usitatus Bagnall. Front. Physiol. 2023, 14, 1130389. [Google Scholar] [CrossRef]
- Wang, X.P.; Ye, M.Z.; Tu, W.T.; Zhao, X.F. 20-hydroxyecdysone via upregulating 4EBP expression and inhibiting its phosphorylation represses cell proliferation to permit insect larval molting. Insect Sci. 2025, in press. [Google Scholar] [CrossRef]
- Yuan, D.; Zhou, S.; Liu, S.; Li, K.; Zhao, H.; Long, S.; Liu, H.; Xie, Y.; Su, Y.; Yu, F.; et al. The AMPK-PP2A axis in insect fat body is activated by 20-hydroxyecdysone to antagonize insulin/IGF signaling and restrict growth rate. Proc. Natl. Acad. Sci. USA 2020, 117, 9292–9301. [Google Scholar] [CrossRef]
- Guo, S.; Tian, Z.; Wu, Q.W.; King-Jones, K.; Liu, W.; Zhu, F.; Wang, X.P. Steroid hormone ecdysone deficiency stimulates preparation for photoperiodic reproductive diapause. PLoS Genet. 2021, 17, e1009352. [Google Scholar] [CrossRef]
- Nian, X.; Wang, B.; Holford, P.; Beattie, G.; Tan, S.; Yuan, W.; Cen, Y.; He, Y.; Zhang, S. Neuropeptide Ecdysis-Triggering Hormone and Its Receptor Mediate the Fecundity Improvement of ‘Candidatus Liberibacter Asiaticus’-Infected Diaphorina citri Females and CLas Proliferation. Adv. Sci. 2025, 12, e2412384. [Google Scholar] [CrossRef]
- Moulos, P.; Alexandratos, A.; Nellas, I.; Dedos, S.G. Refining a steroidogenic model: An analysis of RNA-seq datasets from insect prothoracic glands. BMC Genom. 2018, 19, 537. [Google Scholar] [CrossRef]
- Liu, S.; He, C.; Liang, J.; Su, Q.; Hua, D.; Wang, S.; Wu, Q.; Xie, W.; Zhang, Y. Molecular characterization and functional analysis of the Halloween genes and CYP18A1 in Bemisia tabaci MED. Pestic. Biochem. Physiol. 2020, 167, 104602. [Google Scholar] [CrossRef]
- Rohr, J.; Sarkar, N.; Balenger, S.; Jeong, B.R.; Cerutti, H. Tandem inverted repeat system for selection of effective transgenic RNAi strains in Chlamydomonas. Plant J. 2004, 40, 611–621. [Google Scholar] [CrossRef]
- Wang, Y.L.; Xu, Z.D.; Jiang, S.J.; Huang, K.Y. Screening of Chlorella cell wall-deficient mutants and establishment of transformation system. Acta Hydrobiol. Sin. 2016, 40, 370–377. [Google Scholar]
- Liu, J.; He, X.C.; Chen, X.; Ma, B.; Wei, H.T.; Yang, M.F. An efficient method for electrotransformation of Chlamydomonas reinhardtii. J. Beijing Univ. Agric. 2019, 34, 9–13. [Google Scholar]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Ukeda, H. Novel spectrophotometric assay for superoxide dismutase (SOD) activity using water-soluble tetrazolium salts. Biosci. Biotechnol. Biochem. 2002, 66, 941–943. [Google Scholar]
- Chance, B.; Maehly, A.C. Assay of catalases and peroxidases. Methods Enzymol. 1955, 2, 764–775. [Google Scholar]
- Beers, R.F.; Sizer, I.W. A spectrophotometric method for measuring the breakdown of hydrogen peroxide by catalase. J. Biol. Chem. 1952, 195, 133–140. [Google Scholar] [CrossRef]
- Guo, S. 20-Hydroxyecdysone regulates larval-pupal transition via insulin-like signaling in the mosquito Aedes aegypti. Insect Biochem. Mol. Biol. 2020, 127, 103477. [Google Scholar]
- Zhang, Q. Ecdysone signaling mediates the trade-off between immunity and reproduction in Aedes albopictus. PLoS Pathog. 2021, 17, e1009632. [Google Scholar]
- Chomczynski, P.; Sacchi, N. The single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction: Twenty-something years on. Nat. Protoc. 2006, 1, 581–585. [Google Scholar] [CrossRef]
- Dzaki, N. Evaluation of reference genes at different developmental stages for quantitative real-time PCR in Aedes aegypti. Sci. Rep. 2017, 7, 43618. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Mysore, K.; Li, P.; Wang, C.W.; Hapairai, L.K.; Scheel, N.D.; Realey, J.S.; Sun, L. Characterization of a yeast interfering RNA larvicide with a target site conserved in the synaptotagmin gene of multiple disease vector mosquitoes. PLoS Negl. Trop. Dis. 2019, 13, e0007422. [Google Scholar] [CrossRef]
- GB 3838-2002; Environmental Quality Standards for Surface Water. Ministry of Ecology and Environment of China; Environmental Science Press: Beijing, China, 2002.
- American Public Health Association (APHA). Standard Methods for the Examination of Water and Wastewater, 23rd ed.; APHA: Washington, DC, USA, 2017. [Google Scholar]
- Liu, Y. Comparative analysis of water quality testing methods between GB and ISO standards. Environ. Monit. China 2015, 31, 45–52. [Google Scholar]
- Winnepenninckx, B.; Backeljau, T.; Wachter, R. Extraction of high molecular weight DNA from mollusks. Trends Genet. 1993, 9, 407. [Google Scholar] [PubMed]
- Cheung, M.K.; Au, C.H.; Chu, K.H.; Kwan, H.S.; Wong, C.K. Composition and genetic diversity of picoeukaryotes in subtropical coastal waters as revealed by 454 pyrosequencing. ISME J. 2010, 4, 1053–1059. [Google Scholar] [CrossRef]
- Zimmermann, J.; Jahn, R.; Gemeinholzer, B. Barcoding diatoms: Evaluation of the V4 subregion on the 18S rRNA gene, including new primers and protocols. Org. Divers. Evol. 2011, 11, 173–192. [Google Scholar] [CrossRef]
- Chen, H.; Jiang, W. Application of high-throughput sequencing in understanding human oral microbiome related with health and disease. Front. Microbiol. 2014, 5, 508. [Google Scholar] [CrossRef]
- Yuan, X.L.; Cao, M.; Liu, X.M.; Du, Y.M.; Shen, G.M.; Zhang, Z.F.; Li, J.H.; Zhang, P. Composition and genetic diversity of the Nicotiana tabacum microbiome in different topographic areas and growth periods. Int. J. Mol. Sci. 2018, 19, 3421. [Google Scholar] [CrossRef]
- Lozupone, C.; Hamady, M.; Knight, R. Unifrac-an online tool for comparing microbial community diversity in a phylogenetic context. BMC Bioinform. 2006, 7, 371. [Google Scholar] [CrossRef]
- Altman, D.G.; Bland, J.M. Standard deviations and standard errors. Br. Med. J. 2005, 331, 903. [Google Scholar] [CrossRef]
- Komárek, J.; Kaštovský, J.; Jezberová, J. Taxonomic classification of cyanoprokaryotes (cyanobacterial genera) using a polyphasic approach. Preslia 2014, 86, 295–335. [Google Scholar]
- Callieri, C. Picocyanobacteria in freshwater ecosystems: Biogeographic patterns and community assembly mechanisms. J. Limnol. 2012, 71, 1–16. [Google Scholar]
- Paerl, H.W.; Otten, T.G. Harmful cyanobacterial blooms: Causes, consequences, and controls. Microb. Ecol. 2013, 65, 995–1010. [Google Scholar] [CrossRef]
- Wu, Y. Dynamics of cyanobacterial communities and their environmental drivers in constructed wetlands. Environ. Sci. Technol. 2019, 53, 8854–8862. [Google Scholar]
- Ash, C. Molecular identification of rRNA group 3 bacilli (Ash, Farrow, Wallbanks and Collins) using a PCR probe test. Proposal for the creation of a new genus Paenibacillus. Antonie Van Leeuwenhoek 1993, 64, 253–260. [Google Scholar] [CrossRef]
- Grady, E.N. Current knowledge and perspectives of Paenibacillus: A review. Microb. Cell Factories 2016, 15, 203. [Google Scholar] [CrossRef]
- Huang, X. Biodegradation of phenanthrene by Paenibacillus sp. PRNK-6: Pathway characterization and biosurfactant production. Chemosphere 2019, 231, 529–538. [Google Scholar]
- Zhao, Y. Paenibacillus strains as bioagents for eutrophication control: Nutrient removal and microbial community modulation. Environ. Res. 2021, 200, 111751. [Google Scholar]
- Newton, R.J. A guide to the natural history of freshwater lake bacteria. Microbiol. Mol. Biol. Rev. 2011, 75, 14–49. [Google Scholar] [CrossRef]
- Salcher, M.M. In situ substrate preferences of abundant bacterioplankton populations in a prealpine freshwater lake. ISME J. 2011, 5, 896–907. [Google Scholar] [CrossRef] [PubMed]
- Henson, M.W. Expanding the diversity of bacterioplankton isolates and modeling seasonal niche preferences of freshwater Actinobacteria. mSystems 2018, 3, e00227-18. [Google Scholar]
- Ghai, R. Metagenomics uncovers a new group of low GC and ultra-small marine Actinobacteria. Sci. Rep. 2014, 4, 2471. [Google Scholar] [CrossRef]
- Liao, J. Ecological drivers of the microbial communities in interconnected freshwater and marine ecosystems. Microbiome 2021, 9, 217. [Google Scholar]
- Paupy, C. Aedes albopictus, an arbovirus vector: From the darkness to the light. Microbes Infect. 2009, 11, 1177–1185. [Google Scholar] [CrossRef]
- Zhu, K.Y.; Palli, S.R. Mechanisms, applications, and challenges of insect RNA interference. Annu. Rev. Entomol. 2020, 65, 293–311. [Google Scholar] [CrossRef]
- Tabashnik, B.E. Insect resistance to Bt crops: Lessons from the first billion acres. Nat. Biotechnol. 2013, 31, 510–521. [Google Scholar] [CrossRef] [PubMed]
- Marcombe, S. Insecticide resistance in Aedes albopictus: Current status and mechanisms. PLoS Neglected Trop. Dis. 2019, 13, e0007615. [Google Scholar]
- Bachman, P.M. Ecological risk assessment for RNA interference-based plant-protective products: An overview of challenges and proposed science-based considerations. Regul. Toxicol. Pharmacol. 2016, 81, 108–120. [Google Scholar]
- Joga, M.R. RNA interference-based insecticidal crops: Potential effects on non-target species. Bioscience 2016, 66, 657–665. [Google Scholar]
- Benelli, G.; Beier, J.C. Current vector control challenges in the fight against mosquito-borne diseases. Acta Trop. 2017, 174, 91–96. [Google Scholar] [CrossRef]
- Gilbert, L.I. The regulation of ecdysteroid synthesis in insects: A multi-gene family approach. Insect Biochem. Mol. Biol. 2004, 34, 1245–1252. [Google Scholar]
- Rewitz, K.F. The insect neuropeptide PTTH activates receptor tyrosine kinase Torso to initiate metamorphosis. Science 2007, 316, 894–897. [Google Scholar] [CrossRef]
- Yoshiyama, T. Neverland is a conserved cholesterol-binding protein required for ecdysone synthesis in insects. Dev. Biol. 2006, 298, 555–570. [Google Scholar]
- Feinberg, E.H. Transport of dsRNA into cells by the transmembrane protein SID-1. Science 2003, 301, 1545–1547. [Google Scholar] [CrossRef]
- Xiao, D. Clathrin-dependent endocytosis plays a predominant role in cellular uptake of double-stranded RNA in the red flour beetle. Insect Biochem. Molec. 2015, 60, 68–77. [Google Scholar] [CrossRef]
- Cappelle, K. The involvement of clathrin-mediated endocytosis and two Sid-1-like transmembrane proteins in double-stranded RNA uptake in the Colorado potato beetle midgut. Insect Mol. Biol. 2016, 25, 315–323. [Google Scholar] [CrossRef]
- Abbasi, R. A novel paperclip double-stranded RNA structure demonstrates clathrin-independent uptake in the mosquito Aedes aegypti. Insect Biochem. Mol. Biol. 2020, 127, 103492. [Google Scholar] [CrossRef]
- Smith, V.H.; Crews, T. Ecological impacts of genetically modified microalgae: A review. Appl. Phycol. 2014, 26, 1–14. [Google Scholar]
- Litchman, E.; Klausmeier, C.A. Trait-based community ecology of phytoplankton. Annu. Rev. Ecol. Evol. Syst. 2008, 39, 615–639. [Google Scholar] [CrossRef]
- Grover, J.P. Phytoplankton competition and coexistence: Intrinsic ecosystem dynamics and human impacts. Hydrobiologia 2012, 698, 5–25. [Google Scholar]
- Jones, R.I.; Gowen, R.J. Vertical distribution and temporal variation of Cryptophyceae in a stratified lake. J. Phycol. 1990, 26, 472–478. [Google Scholar]
- Buchan, A. Master recyclers: Features and functions of bacteria associated with phytoplankton blooms. Nat. Rev. Microbiol. 2014, 12, 686–698. [Google Scholar] [CrossRef]
- Ettwig, K.F. Nitrite-driven anaerobic methane oxidation by oxygenic bacteria. Nature 2010, 464, 543–548. [Google Scholar] [CrossRef]
- Tang, X. Metagenomic insights into the microbial community and nutrient cycling in the subsurface layer of a stratified eutrophic reservoir. Microb. Ecol. 2018, 76, 125–138.101. [Google Scholar]
- Kraft, B. Oxygen and nitrogen production by an ammonia-oxidizing archaeon. Science 2022, 375, 97–100. [Google Scholar] [CrossRef] [PubMed]
- Liu, J. Gemmatimonadota as a key player in organic sulfur cycling in freshwater sediments. ISME Commun. 2022, 2, 1–11. [Google Scholar]
- Shi, L. Phytoplankton-derived organic matter shapes microbial community structure and function in eutrophic lakes. Environ. Microbiol. 2020, 22, 5163–5178. [Google Scholar]
- Peacock, J.P. Resource partitioning drives microbial niche differentiation in a eutrophic lake. mSystems 2021, 6, e01222-20. [Google Scholar]
- Mu, D.S. Genomic insights into the ecological role of Myxococcota in degrading algal-derived polysaccharides. Front. Microbiol. 2020, 11, 591738. [Google Scholar]
- Zhu, J.; Zou, N.; Zhong, H. Spatiotemporal characteristics and its environmental application of dissolved organic matter (DOM) in sediments from Chaohu Lake, a eutrophic lake. Acta Sci. Circumstantiae 2020, 40, 2528–2538. [Google Scholar]
- Knoll, L.B.; Vanni, M.J.; Renwick, W.H. Temperate reservoirs are large carbon sinks and small CO2 sources: Results from high-resolution carbon budget. Glob. Biogeochem. Cycles 2013, 27, 52–64. [Google Scholar] [CrossRef]
- Villacorte, L.O.; Ekowati, Y.; Neu, T.R. Characterisation of algal organic matter produced by bloom-forming marine and freshwater algae. Water Res. 2015, 73, 216–230. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deng, X.; He, C.; Xue, C.; Xu, D.; Li, J.; Fei, X. Silencing the cyp314a1 and cyp315a1 Genes in the Aedes albopictus 20E Synthetic Pathway for Mosquito Control and Assessing Algal Blooms Induced by Recombinant RNAi Microalgae. Insects 2025, 16, 1033. https://doi.org/10.3390/insects16101033
Deng X, He C, Xue C, Xu D, Li J, Fei X. Silencing the cyp314a1 and cyp315a1 Genes in the Aedes albopictus 20E Synthetic Pathway for Mosquito Control and Assessing Algal Blooms Induced by Recombinant RNAi Microalgae. Insects. 2025; 16(10):1033. https://doi.org/10.3390/insects16101033
Chicago/Turabian StyleDeng, Xiaodong, Changhao He, Chunmei Xue, Dianlong Xu, Juncai Li, and Xiaowen Fei. 2025. "Silencing the cyp314a1 and cyp315a1 Genes in the Aedes albopictus 20E Synthetic Pathway for Mosquito Control and Assessing Algal Blooms Induced by Recombinant RNAi Microalgae" Insects 16, no. 10: 1033. https://doi.org/10.3390/insects16101033
APA StyleDeng, X., He, C., Xue, C., Xu, D., Li, J., & Fei, X. (2025). Silencing the cyp314a1 and cyp315a1 Genes in the Aedes albopictus 20E Synthetic Pathway for Mosquito Control and Assessing Algal Blooms Induced by Recombinant RNAi Microalgae. Insects, 16(10), 1033. https://doi.org/10.3390/insects16101033




