3.1.2. Morphological Description
Female (
n = 3). Body length 2.1–2.3 mm (mean 2.2 mm).
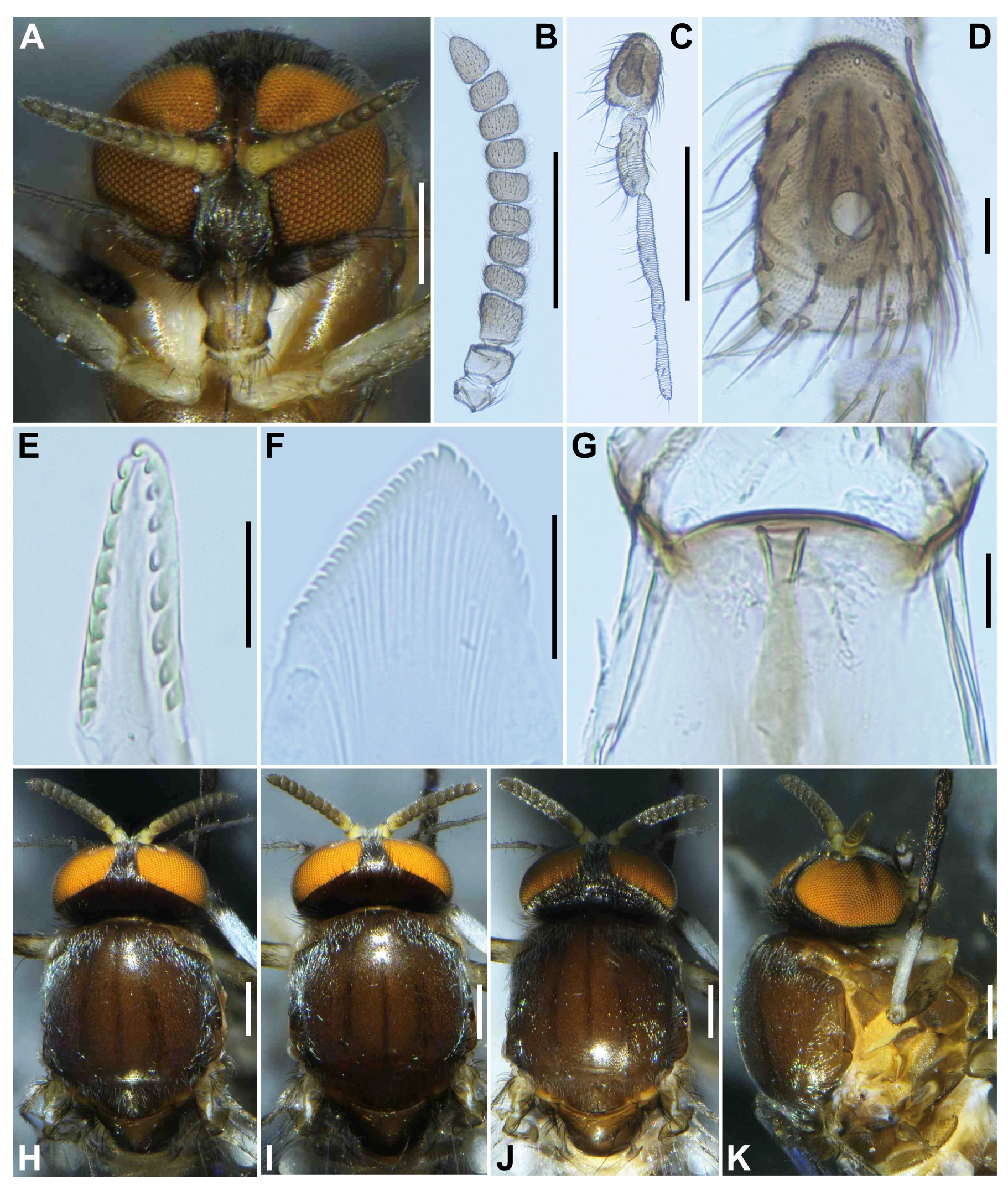
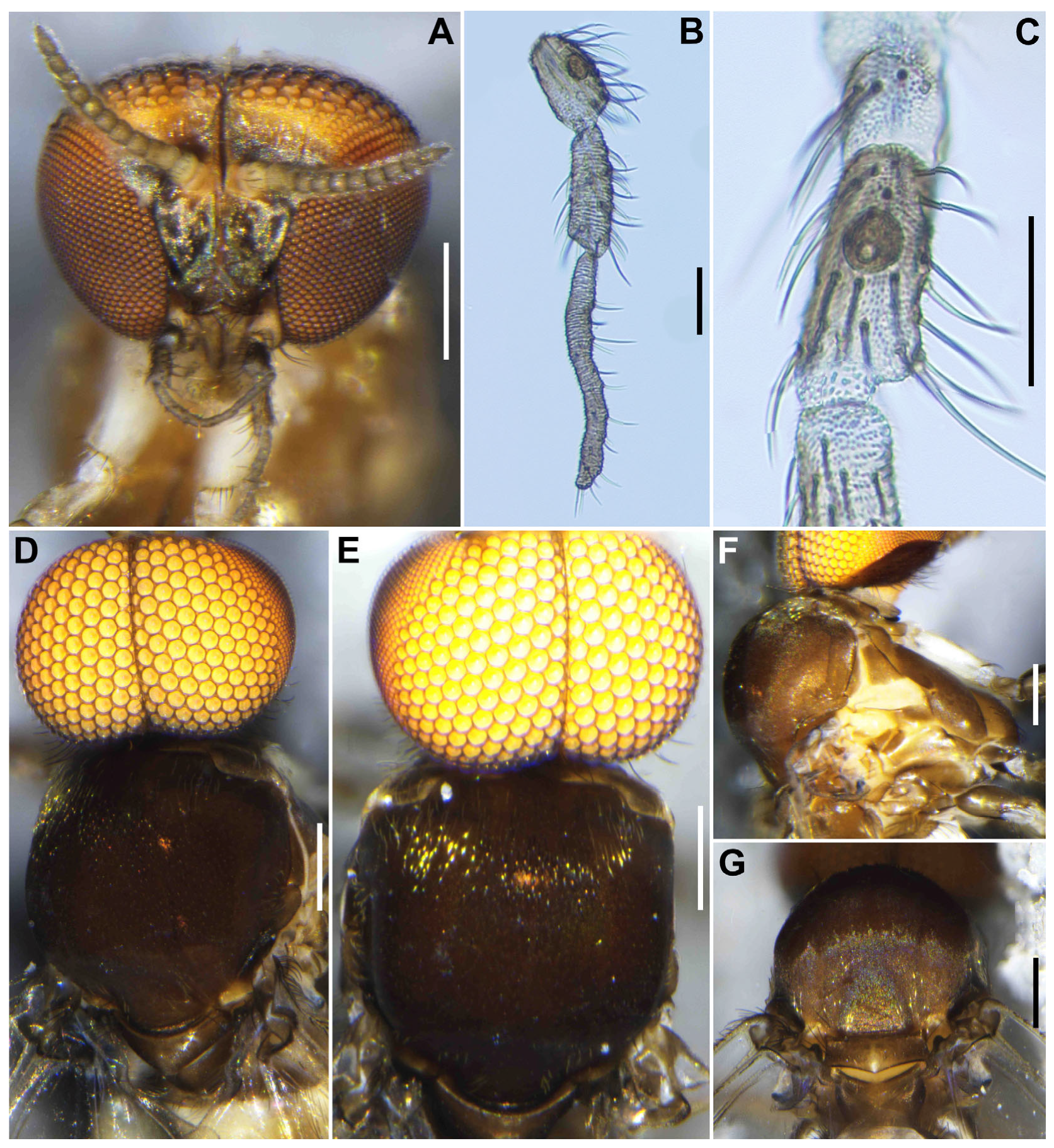
Head (
Figure 1A). Slightly narrower than width of thorax. Frons brownish black to black, moderately covered with yellowish white scale-like recumbent short hairs interspersed with several dark longer hairs along each lateral margin; frontal ratio 1.3–1.4:1.0:2.1–2.2; frons-head ratio 1.0:4.6–4.7. Fronto-ocular area well developed, narrow, directed dorsolaterally. Clypeus brownish black to black, shiny, silver pruinose, brilliantly iridescent when illuminated, densely covered with yellowish white short hairs interspersed with dark longer hair on each side. Labrum 0.6–0.7 times as long as clypeus. Antenna (
Figure 1A,B) composed of scape, pedicel, and 9 flagellomeres, brownish black except scape, pedicel, and anterior surface of first and second flagellomere yellow, first flagellomere 1.7–1.8 times as long as second. Maxillary palpus composed of 5 segments, medium brown, proportional lengths of third, fourth, and fifth segments 1.0:1.0–1.1:2.7–2.8; third segment (
Figure 1C) swollen; sensory vesicle (
Figure 1D) elongate, 0.65 times as long as third segment, with medium-sized opening. Maxillary lacinia (
Figure 1E) with 8 inner and 11 outer teeth. Mandible (
Figure 1F) with 17 or 18 inner and 8 or 9 outer teeth. Cibarium (
Figure 1G) medially forming sclerotized plate folded forward from posterior margin, with dark mediolongitudinal ridge having bifid apex.
Thorax. Scutum (
Figure 1H–K) brownish black to black except anterolateral calli light brown, whitish-pruinose and shiny when illuminated at certain angles, with 3 longitudinal vitae (1 narrow median and 2 broad submedian), densely covered with whitish scale-like recumbent short hairs sparsely intermixed with brown similar short hairs mainly on longitudinal vittae (
Figure 1I). Scutellum (
Figure 1J) dark brown, shiny when illuminated at certain angles, covered with dark brown long hairs. Postnotum (
Figure 1J) dark brown, gray-pruinose, shiny when illuminated at certain angles, and bare. Pleural membrane (
Figure 1K) whitish yellow and bare. Katepisternum dark brown, longer than deep, shiny when illuminated at certain angles, and moderately covered with dark brown hairs.
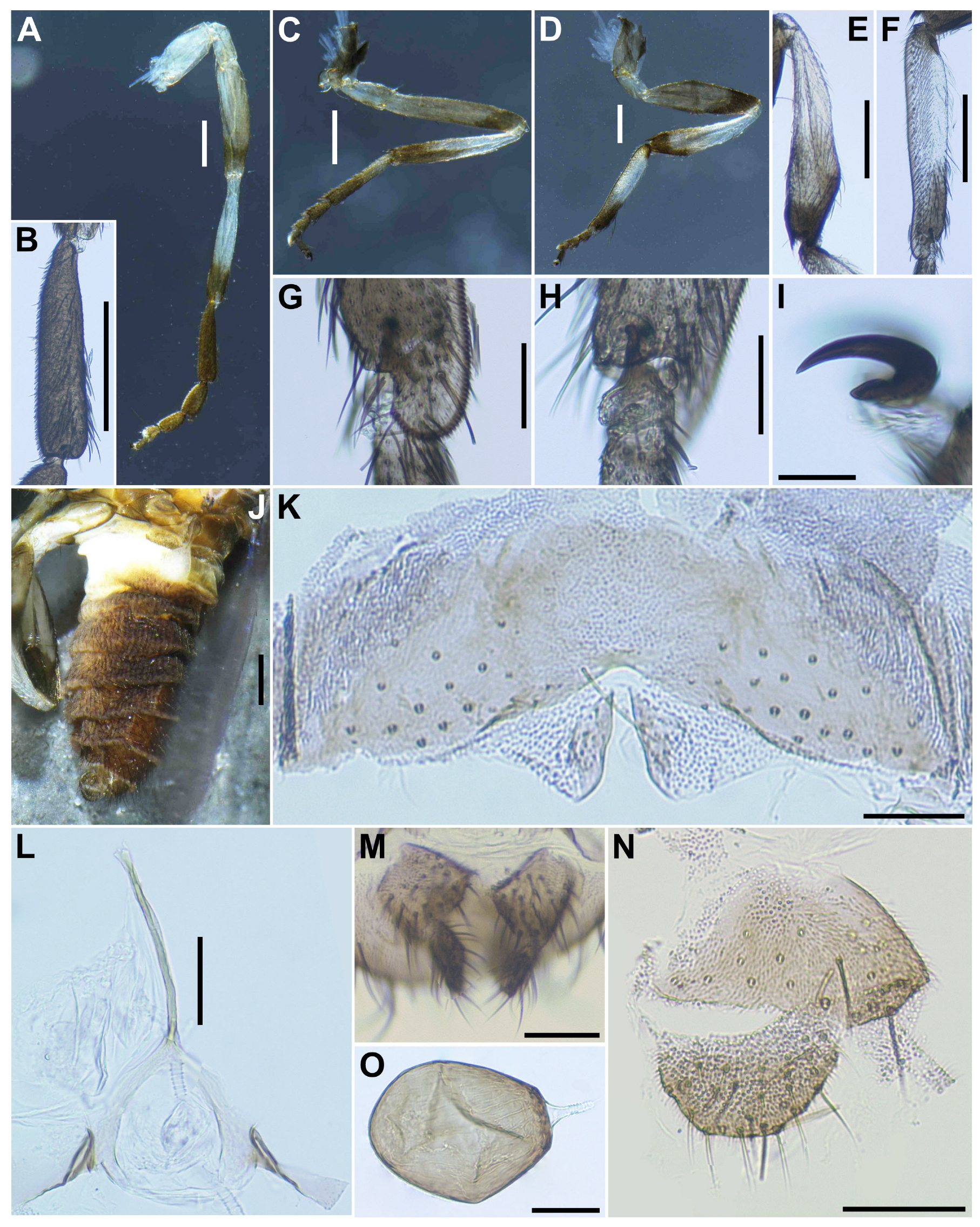
Legs. Foreleg (
Figure 2A): coxa whitish yellow; trochanter light brown; femur light brown with apex medium brown (though extreme tip yellow); tibia white with distal 1/3 brownish black, outer surface largely white iridescent when illuminated; tarsus brownish black; basitarsus (
Figure 2B) moderately dilated, 4.5 times as long as its greatest width. Midleg (
Figure 2C): coxa medium brown except posterolateral surface dark brown; trochanter light brown; femur light to medium brown with apex medium to dark brown (though extreme tip yellow); tibia white on little more than basal half, and light to dark brown on rest; tarsus brownish black, except basal half of basitarsus light brown, though its border not well defined. Hind leg (
Figure 2D): coxa light brown; trochanter whitish yellow; femur medium brown with base whitish yellow and apical cap dark brown; tibia (
Figure 2E) whitish on basal half or little more, and light brown to brownish black on apical covered with whitish-yellow fine hairs on outer and posterior surfaces of basal three-fourths and white sheen on posterior surface of basal three-fourths when illuminated at certain angles; tarsus brownish black except basal two-thirds of basitarsus (though base light brown and narrow portion along anterior margin slightly darkened), and basal one-third of second tarsomere white; basitarsus (
Figure 2F) narrow, nearly parallel-sided, 5.4 times as long as wide, and 0.6 and 0.5 times as wide as greatest widths of tibia and femur, respectively; calcipala (
Figure 2G) nearly as long as wide at base, and 0.42 times as wide as greatest width of basitarsus. Pedisulcus (
Figure 2H) well defined. Claw (
Figure 2I) with large basal tooth 0.45 times length of claw.
Wing. (
n = 4). Length 1.9–2.1 mm (mean 2.0 mm). Costa with dark spinules and dark hairs. Subcosta with dark hairs except near apex bare. Base of radial vein with tuft of dark brown hairs. Basal portion of radius fully haired; R
1 with dark spinules and hairs; R
2 with hairs only. Basal cell absent.
Halter. White except basal stem darkened.
Abdomen (
Figure 2J). Basal scale light brown, with fringe of whitish-yellow hairs. Dorsal surface of abdomen brownish black except segment 2 whitish brown, moderately covered with dark short to long hairs and yellow fine short hairs; tergites of segments 6–9 shiny when illuminated at certain angles; ventral surface of segment 2 whitish, and those of other segments medium to dark brown. Sternal plate on segment 7 undeveloped.
Terminalia. Sternite 8 (
Figure 2K) bare medially, with 12–17 medium-long to long hairs, together with 2 or 3 slender short hairs on each side. Ovipositor valves (
Figure 2K) triangular, thin, membranous, each moderately covered with micro-setae interspersed with 1 or 2 short hairs; inner margins slightly sinuous, sclerotized, and somewhat separated from each other. Genital fork (
Figure 2L) of usual inverted-Y form, with slender and thick stem; arms of moderate width and moderately folded medially, and lateral plate of each arm with thin lobe projection directed posteromedially. Paraproct in ventral view (
Figure 2M) somewhat widened posteromedially, with 5 or 6 sensilla on anteromedial surface; paraproct in lateral view (
Figure 2N) slightly produced ventrally, 0.42–0.44 times as long as wide, with 18–20 medium-long to long hairs on ventral and lateral surfaces. Cercus in lateral view (
Figure 2N) short, rounded posteriorly, 0.54–0.56 times as long as wide. Spermatheca (
Figure 2O) ellipsoidal, 1.5 times as long as its greatest width, dark brown except duct unpigmented, and with many fissures on surface; internal setae absent.
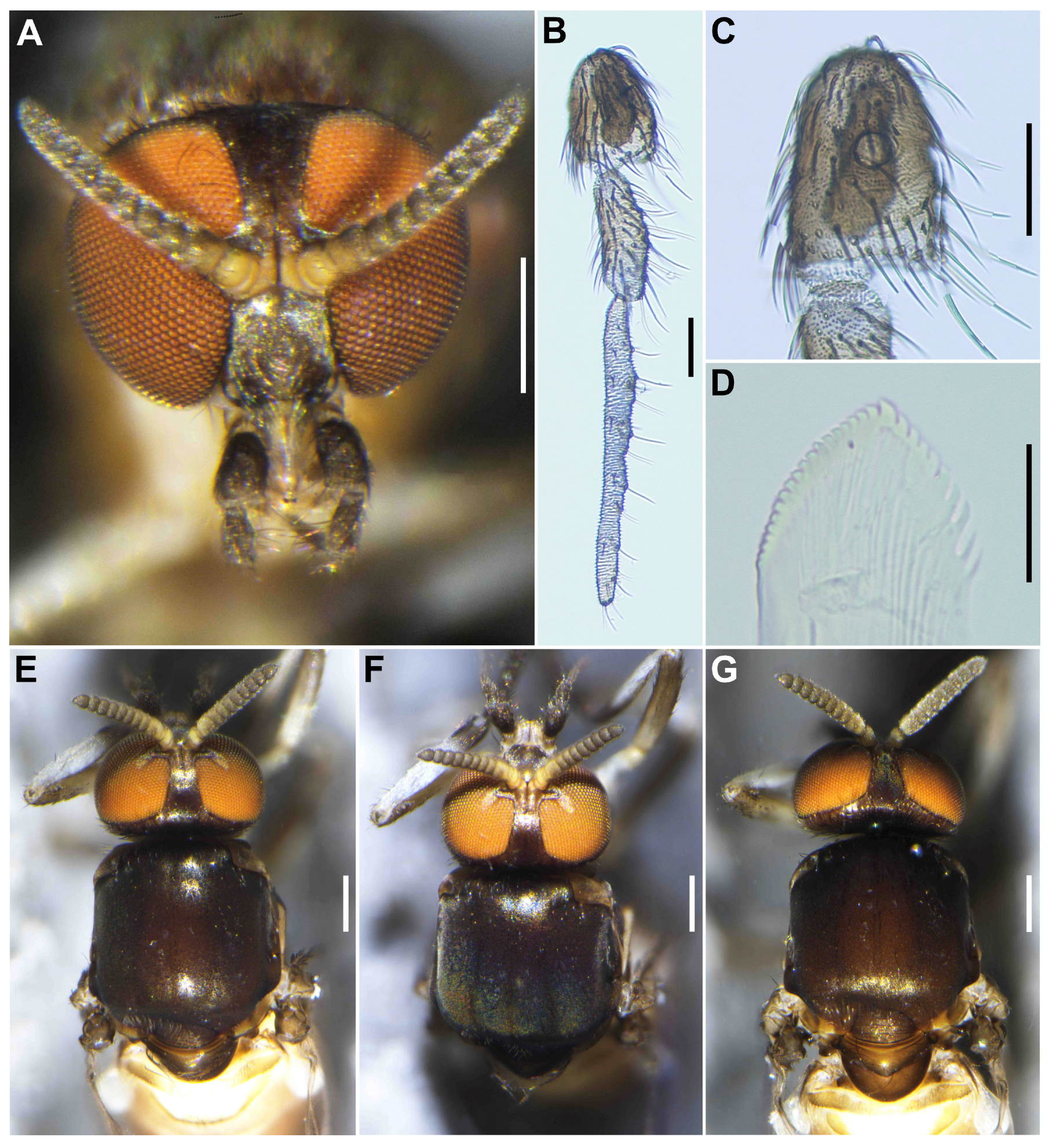
Male (
n = 3). Body length 2.6–2.7 mm (mean 2.65 mm).
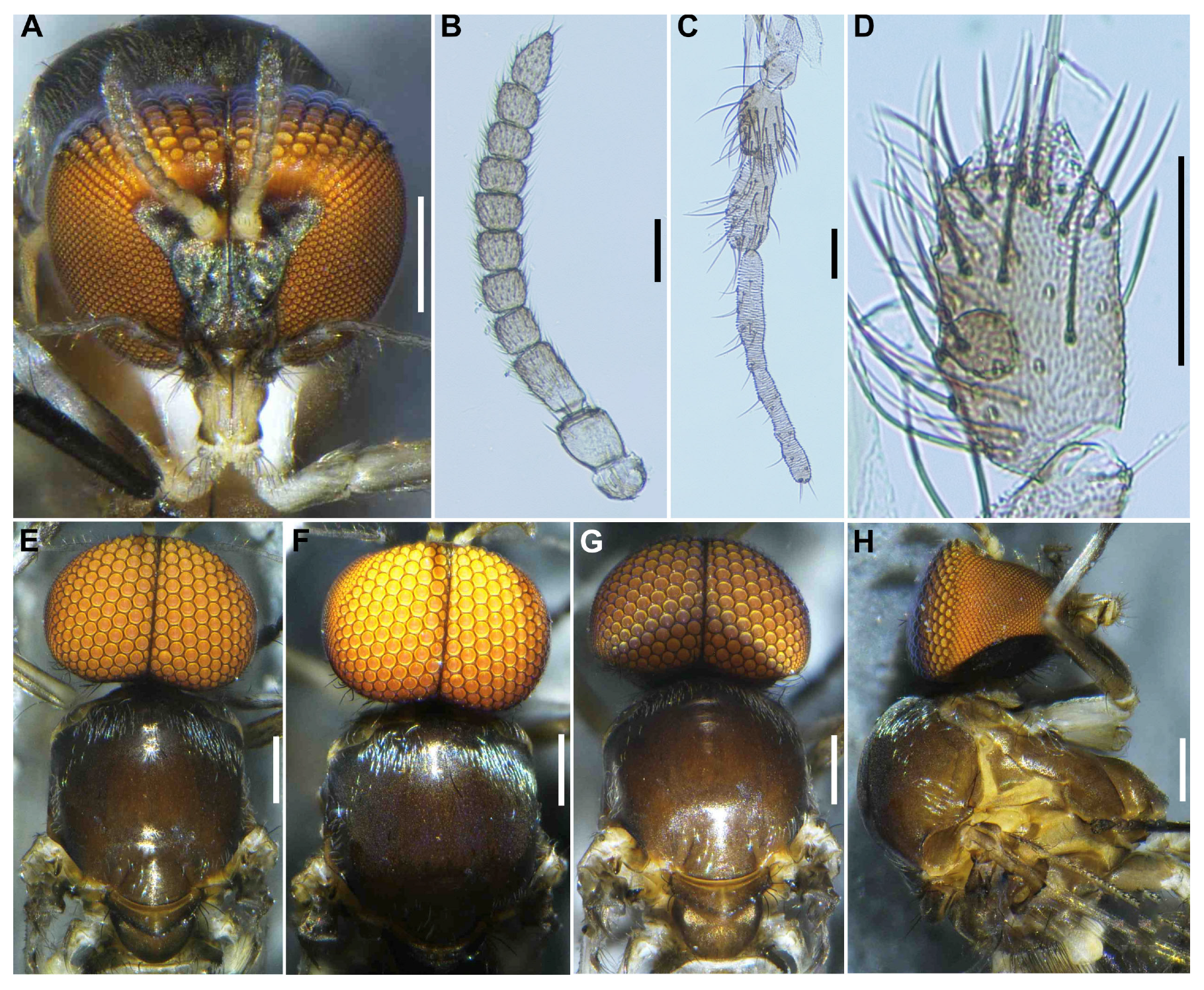
Head (
Figure 3A). Wider than thorax. Upper eye medium brown, consisting of large facets in 10 vertical columns and 12 horizontal rows. Face brownish black, white-pruinose. Clypeus brownish black, whitish-pruinose, densely covered with golden-yellow scale-like medium-long hairs (mostly directed upward) interspersed with several light-brown simple longer hairs. Antenna (
Figure 3A,B) composed of scape, pedicel, and 9 flagellomeres, medium to dark brown except scape, pedicel, and base of first flagellomere yellow; first flagellomere elongate, 1.7–1.8 times length of second (
Figure 3B). Maxillary palpus (
Figure 3C) light to medium brown, with 5 segments, proportional lengths of third, fourth, and fifth segments 1.0:1.1:3.0–3.1; third segment slightly widened apically; sensory vesicle (
Figure 3D) globular, small, 0.17 times length of third segment, and with small opening.
Thorax. Scutum (
Figure 3E–H) brownish black, except shoulders light brown, scutum white pruinose on each shoulder, along lateral margins and on prescutellar area, when illuminated at various angles, scutum densely covered with whitish-yellow recumbent short hairs (
Figure 3E–G). Scutellum dark brown with whitish-pruinose on posterior margin, with dark upright hairs and whitish short hairs (
Figure 3E,G). Postnotum (
Figure 3G), brownish black, with whitish-pruinose, shiny bare. Pleural membrane light brown, shiny when illuminated at certain angles and bare. Katepisternum (
Figure 3H) dark brown, shiny when illuminated at certain angles, and moderately covered with fine whitish-yellow hairs.
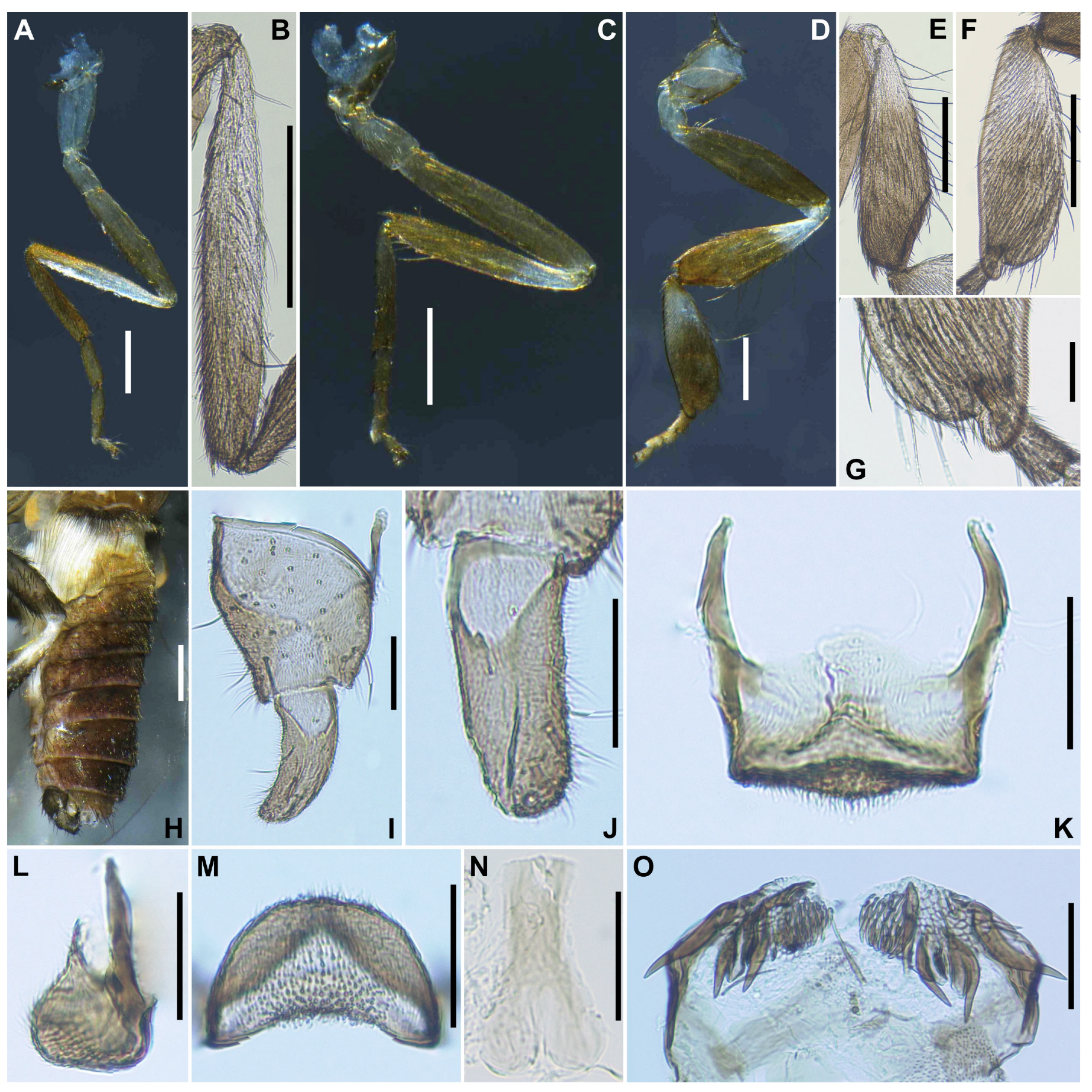
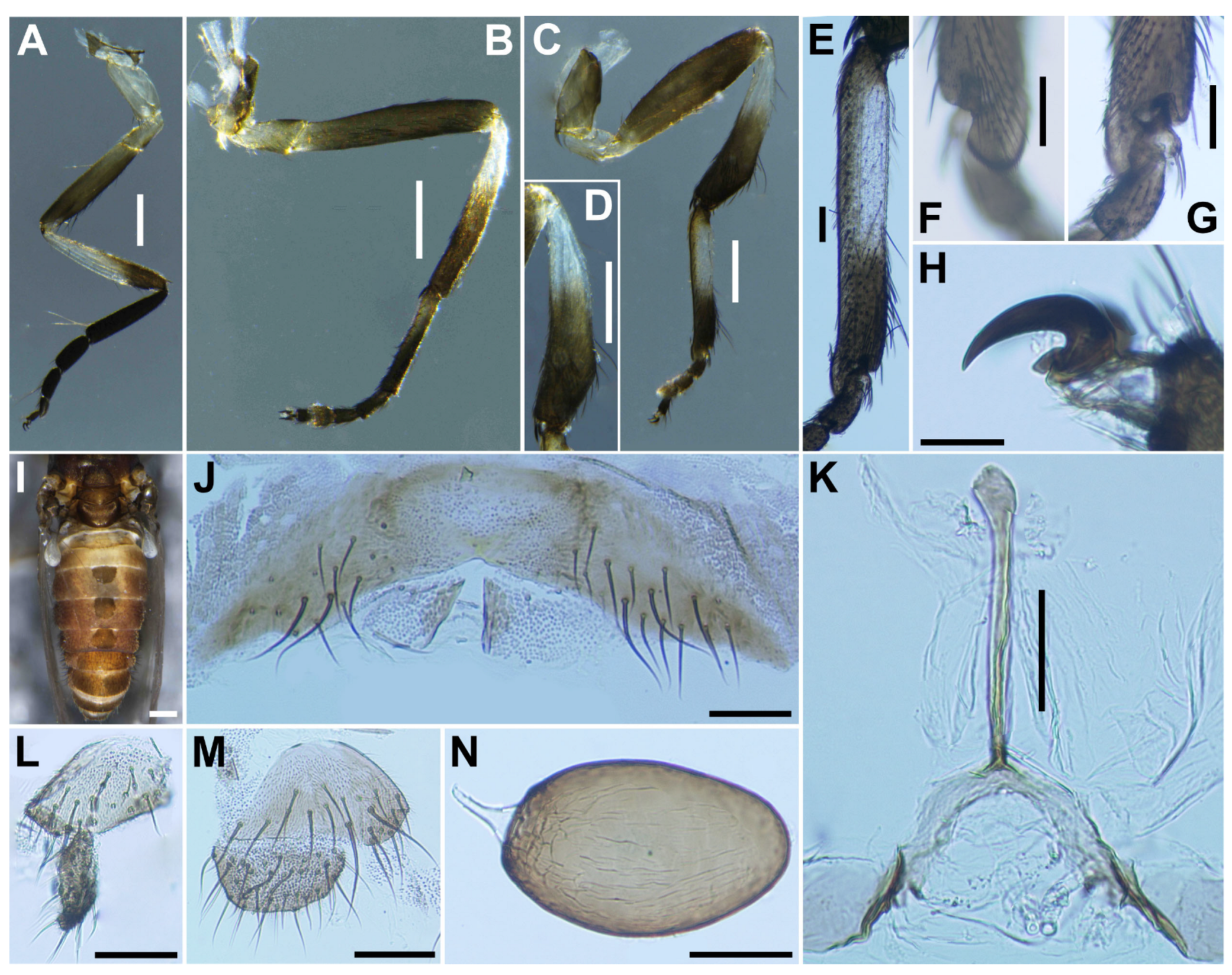
Legs. Foreleg (
Figure 4A), coxa whitish-yellow; trochanter light brown; femur light brown with apex medium brown; tibia (
Figure 4B) light brown on extreme base, dark brown on the rest, outer surface and center area largely white, iridescent when illuminated; tarsus brownish black; basitarsus nearly parallel-sided, 6.0 times as long as its greatest width. Midleg (
Figure 4C), coxa medium brown except posterolateral surface dark brown; trochanter light brown; femur light brown with apex medium to dark brown (though extreme tip yellow); tibia whitish-yellow on basal one-fifth, and medium to dark brown on rest; tarsus dark brown to brownish black except basal one-third of basitarsus light to medium brown though its border not well defined. Hind leg (
Figure 4D), coxa light to medium brown; trochanter whitish brown; femur medium brown with basal extreme whitish-yellow and apical cap dark brown; tibia (
Figure 4E) dark brown to brownish black except basal one-third whitish; tarsus medium to dark brown except basal one-third or little more of basitarsus white (though base medium brown), and basal one-third of second tarsomere white; basitarsus (
Figure 4F) enlarged, wedge-shaped, 3.1 times as long as wide, and 0.94 and 1.07 times as wide as greatest width of tibia and femur, respectively; calcipala (
Figure 4G) as long as basal width, and 0.27 times as wide as greatest width of basitarsus. Pedisulcus well defined.
Wing (
n = 4). Length 1.6–1.7 mm (mean 1.65 mm); other characteristics as in female, except subcosta without hairs.
Halter. White except basal stem darkened.
Abdomen (
Figure 4H). Basal scale dark brown, with fringe of dark brown long hairs. Dorsal surface of abdomen medium brown to brownish black, except segment 2 light brown, moderately covered with dark short to long hairs and yellow short hairs; segments 2 and 5–8 each with pair of shiny dorsolateral patches.
Genitalia. Coxite in ventral view (
Figure 4I) nearly rectangular, 1.4 times as long as its greatest width. Style in ventral view (
Figure 4I) gently bent inward, nearly parallel-sided and with apical spine; style in ventrolateral view (
Figure 4J) 2.4 times as long as its greatest width at base, 0.85 times length of coxite, and nearly parallel-sided on basal one-third and gently narrowed toward apex. Ventral plate in ventral view (
Figure 4K) with body transverse, 0.5 times as long as wide, with posterior half or little more markedly narrower than basal width, with anterior margin produced anteromedially, and posterior margin slightly convex medially, densely covered with micro-setae on ventral surface; basal arms of moderate length, slightly divergent from base to middle, then somewhat convergent apically; ventral plate in lateral view (
Figure 4L) moderately produced ventrally; ventral plate in caudal view (
Figure 4M) rounded ventrally, about half as high as basal width, and densely covered with micro-setae on posterior surface. Median sclerite (
Figure 4N) wide, plate-like, thin, and well sclerotized on base to middle area, with widened apex. Parameres (
Figure 4O) each with 4 long and several short ones. Aedeagal membrane moderately covered with micro-setae. Cercus small, rounded, and encircled with 12–13 hairs.
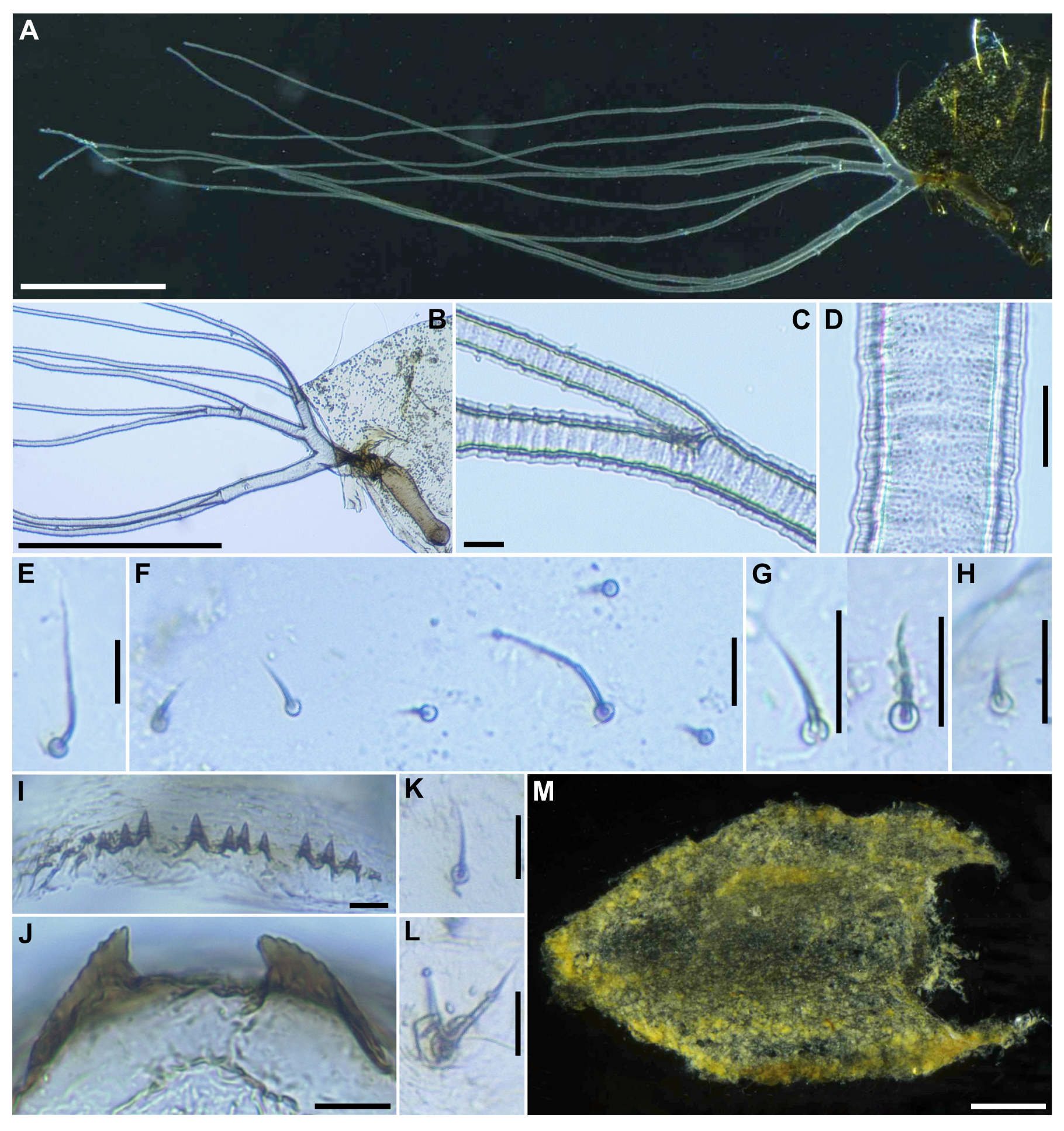
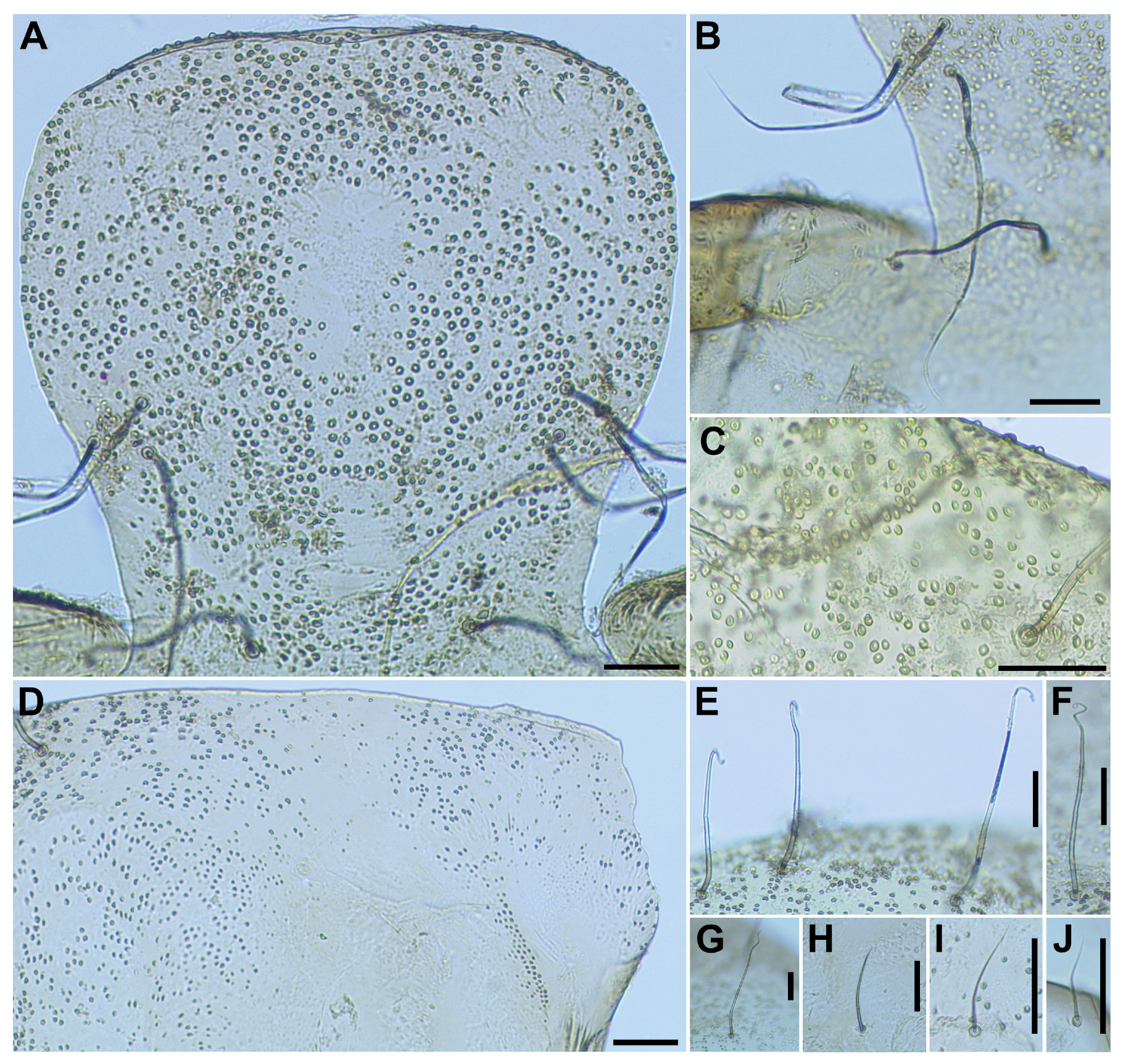
Pupa (
n = 6). Body length 3.1–3.4 mm (mean 3.3 mm).
Head. Integument yellow (
Figure 5A), densely covered with small round tubercles, except antennal sheaths. Frons with 3 pairs of unbranched long trichomes (
Figure 5B); face with pair of unbranched long trichomes (
Figure 5B); 3 frontal trichomes on each side arising close together, subequal in length to one another, and somewhat as long as facial one.
Thorax. Integument yellow (
Figure 5C), moderately covered by round tubercles on anterior lateral portion and posterior half somewhat sparsely covered with small tubercles, with 3 long dorsomedial trichomes (
Figure 5D), 2 long anterolateral trichomes (
Figure 5E), 1 unbranched (
Figure 5F) or branched (
Figure 5G) medium-long mediolateral trichome, and 3 ventrolateral trichomes with uncoiled apices (1 medium-long (
Figure 5H), 2 short (
Figure 5I)) on each side; all trichomes unbranched.
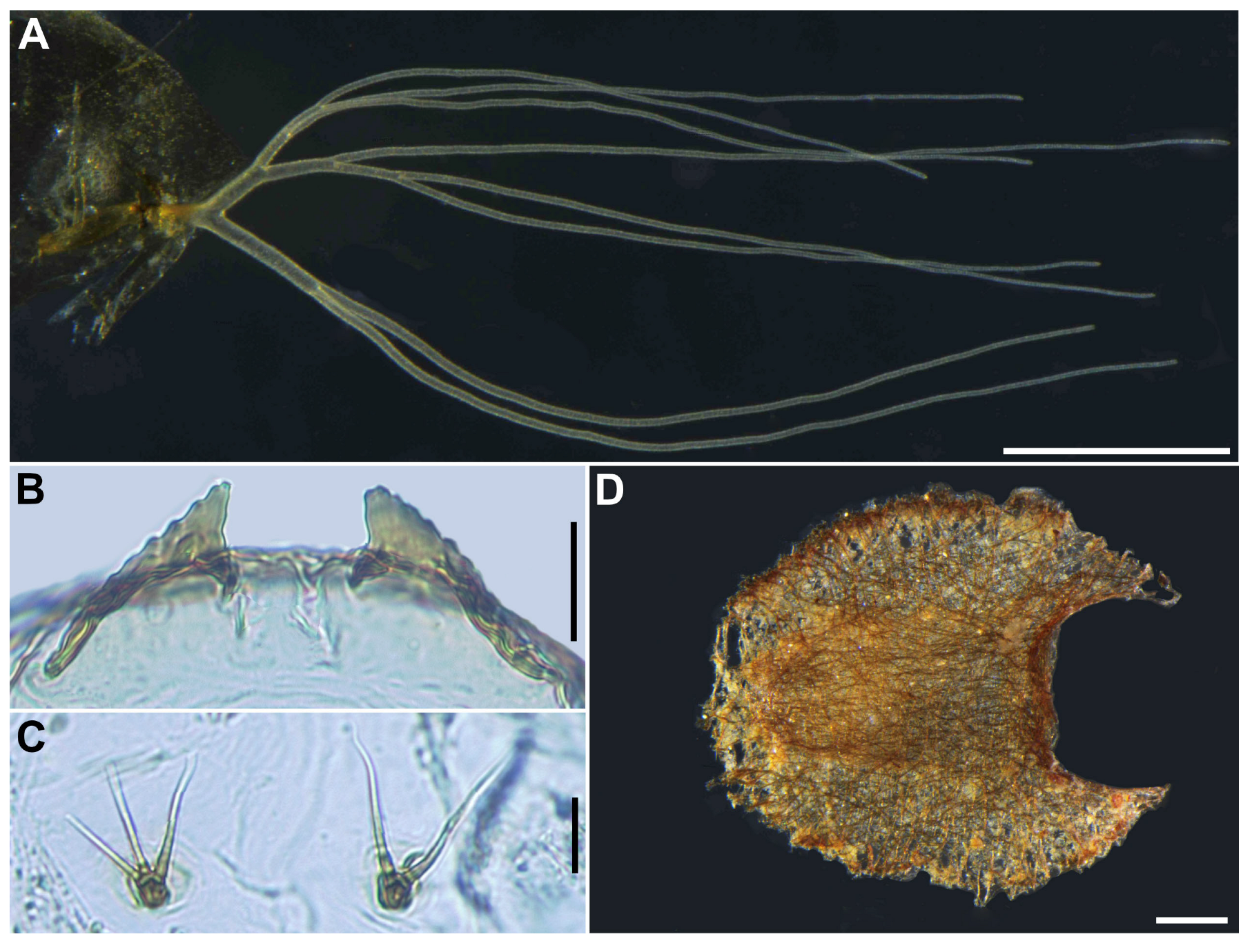
Gill (
Figure 6A) composed of 8 slender thread-like filaments, arranged as [(2+1)+(1+2)]+2 from dorsal to ventral, with short common basal stalk (
Figure 6B) having somewhat swollen transparent basal fenestra at base; common basal stalk 0.67 times length of interspiracular trunk (
Figure 6B); dorsal and middle triplets sharing short stalk; dorsal triplet (
Figure 6B) composed of 2 paired and 1 individual filaments with short primary and secondary stalks; middle triplet (
Figure 6B) composed of 1 individual and 2 paired filaments with medium-long primary and short secondary stalks; ventral paired filaments (
Figure 6B) with long stalk 1.4–1.6 times length of common basal stalk and as long as interspiracular trunk; all filaments light yellow, gradually tapered toward apex; 2 filaments of ventral pair subequal in length (ca. 3.2 mm including their own stalk) to each other, 6 filaments of dorsal and middle triplets subequal in length to one another, and slightly shorter than 2 filaments of ventral pair; filaments of ventral pair subequal in thickness to each other; primary stalk of dorsal triplet lying against stalk of ventral pair at angle of about 80 degrees when viewed laterally; cuticle of all filaments with well-defined annular ridges (
Figure 6C), densely covered with micro-tubercles (
Figure 6D).
Abdomen. Dorsally, all segments unpigmented or light yellow, without micro-tubercles; first segment with 1 slender medium long hair-like seta (
Figure 6E) on each side; segment 2 with 1 slender long hair-like seta and 5 short spinous setae submedially on each side (
Figure 6F), all setae unbranched; segments 3 and 4 each with 4 hooked spines and 1 unbranched short seta (
Figure 6G) on each side; segment 5 with 3 short setae (
Figure 6H) submedially on each side; segments 6–9 each with spine-combs in transverse row (
Figure 6I) (though those on segment 9 somewhat smaller than those on all other segments) and comb-like groups of micro-spines on each side; segment 9 with pair of wide, plate-like terminal hooks (
Figure 6J), with their outer margins 3.3–3.5 times as long as their inner margins and crenulated. Ventrally, segment 4 with 1 unbranched slender short seta (
Figure 6K) on each side; segment 5 with pair of bifid hooks (
Figure 6L) submedially and few unbranched slender short setae on each side; segments 6 and 7 each with pair of bifid inner and unbranched outer hooks, and few unbranched slender short setae on each side; segments 4–8 with comb-like groups of micro-spines. Each side of segment 9 with 3 grapnel-shaped hooklets.
Cocoon (
n = 6). Length 2.8–3.1 mm (mean 3.0 mm), width 1.9–2.2 mm (mean 2.6 mm) (
Figure 6M). Wall pocket-shaped, moderately woven, somewhat extended ventrolaterally and without anterodorsal portion; posterior half with floor roughly or moderately woven; individual threads visible or not.
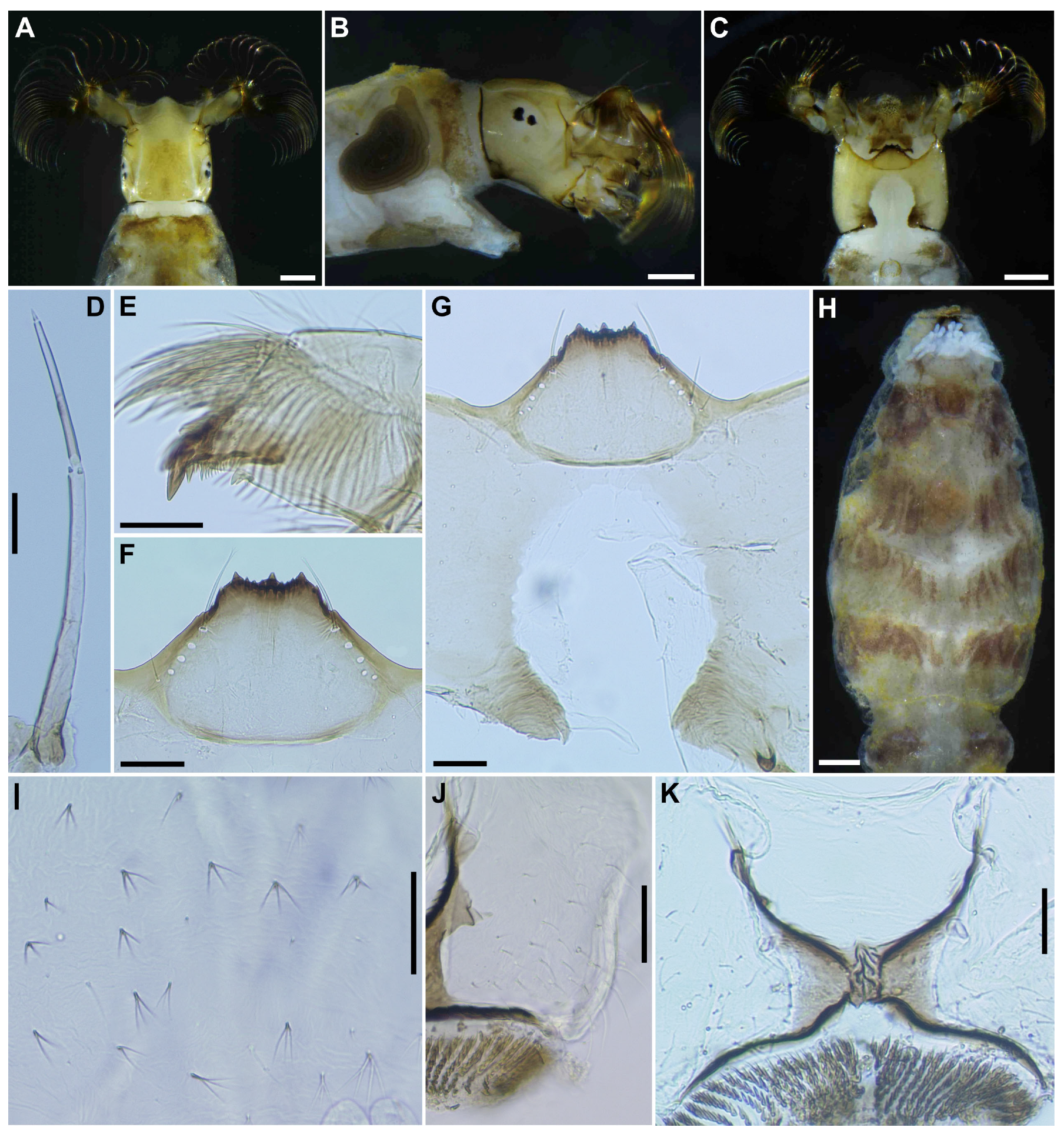
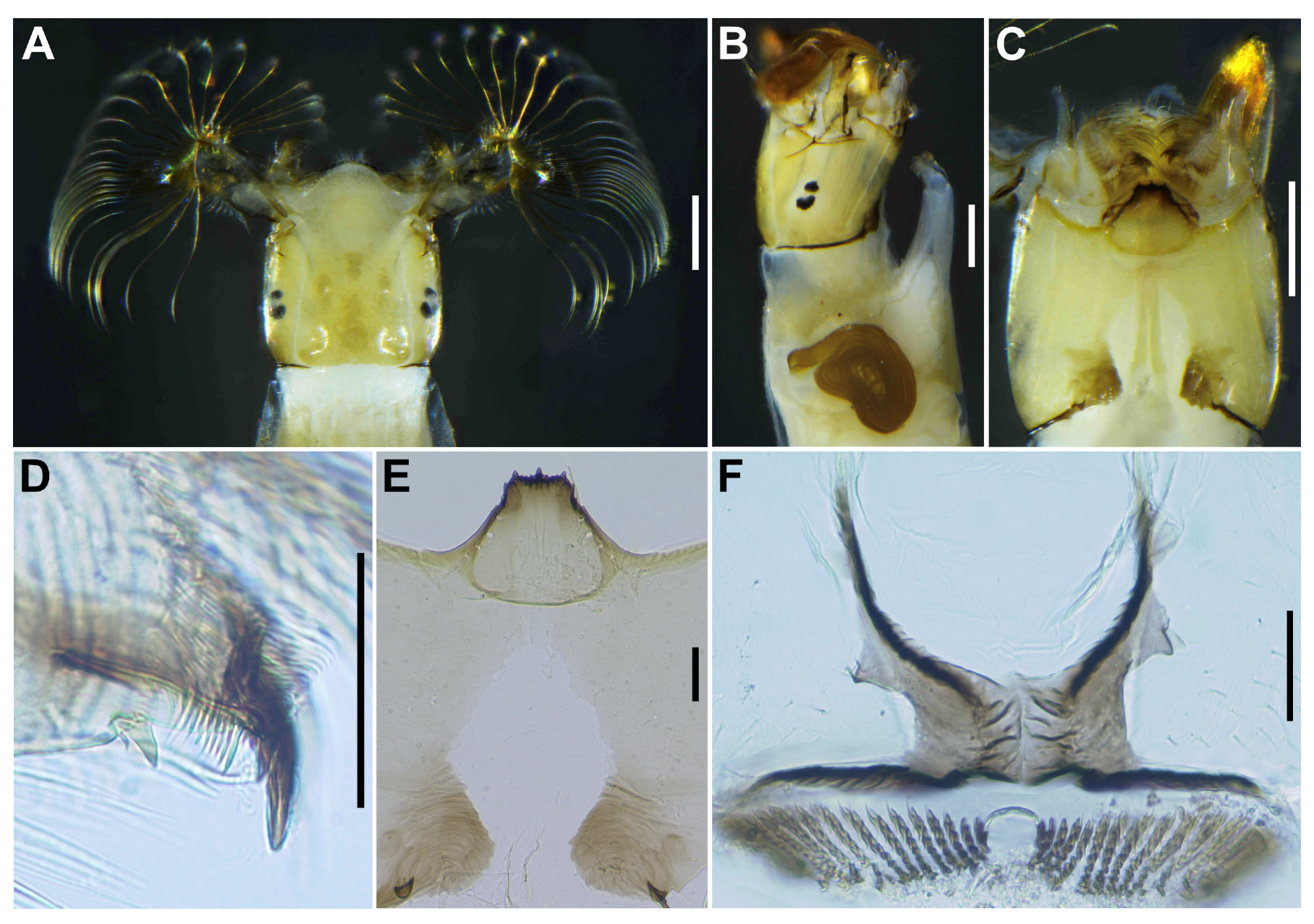
Mature larva (
n = 7). Body length 4.3–4.8 mm. (mean 4.5 mm) (
Figure 7A,B). Body gray except dorsal surface of thorax and part of proleg whitish-gray, ventral surface of thoracic segments 2 and 3 grayish, first thoracic segment with broad brown transverse band unconnected ventrally. Abdominal segments 1 with small reddish-brown spot on each dorsolateral portion, abdominal segments 2–4 each with pair of reddish-brown dorsolateral markings, and abdominal segment 4 also with faint reddish-brown marking ventrally, segments 5–8 each with transverse reddish-brown band on dorsal and dorsolateral surfaces, although those bands on abdominal segments 6–8 often disconnected dorsomedially.
Head. Head capsule width 0.42–0.47 mm. (mean 0.45 mm), length 0.50–0.56 mm. (mean 0.52 mm). Cephalic apotome (
Figure 8A) whitish-yellow, head spots faintly positive, sparsely covered with colorless fine setae. Lateral surface (
Figure 8B) of head capsule yellow, except posterior portion light brown, eye-spot region whitish, sparsely covered with colorless fine setae. Ventral surface of head capsule (
Figure 8C) light yellow, except basal portions of both sides of postgenal cleft dark brown and sparsely covered with minute colorless setae. Antenna (
Figure 8D) unpigmented, except first article mostly light brown, composed of 3 articles and apical sensillum, little longer than stem of labral fan; proportional lengths of first, second, and third articles 1.00:1.04–1.06:1.08–1.10. Labral fan with 34–36 primary rays. Mandible (
Figure 8E) with 3 comb-teeth decreasing in length from first tooth to third; mandibular serration composed of 1 medium-size and 1 small teeth, major tooth at acute angle against mandible on apical side; supernumerary serration absent. Hypostoma (
Figure 8F) with row of 9 apical teeth, of which median tooth nearly as long as or slightly longer than each corner tooth; lateral margin smooth; 5 hypostomal bristles per side lying nearly parallel to lateral margin. Postgenal cleft (
Figure 8G) deep, 13–15 times as long as postgenal bridge. Cervical sclerites composed of pair of small light brown rod-like pieces.
Thorax and
Abdomen. Thoracic and abdominal cuticle almost bare, except abdominal segments 5–8 (
Figure 8H) moderately covered with dark setae, each with 2–4 branches (
Figure 8I) and unbranched colorless fine setae dorsolaterally; last abdominal segment moderately covered with long unbranched colorless minute setae (
Figure 8J) on dorsolateral surface of each side of anal sclerite. Rectal papilla compound, each of 3 lobes with 6–8 finger-like secondary lobules per lobe. Anal sclerite (
Figure 8K) of usual X-form, with anterior arms 0.8–1.0 times as long as posterior ones, broadly sclerotized at base; no sensilla on broad base and posterior to posterior arms; accessory sclerite absent. Last abdominal segment with pair of large conical ventral papillae. Posterior circlet with 69–72 rows of hooklets, with up to 12–13 hooklets per row.
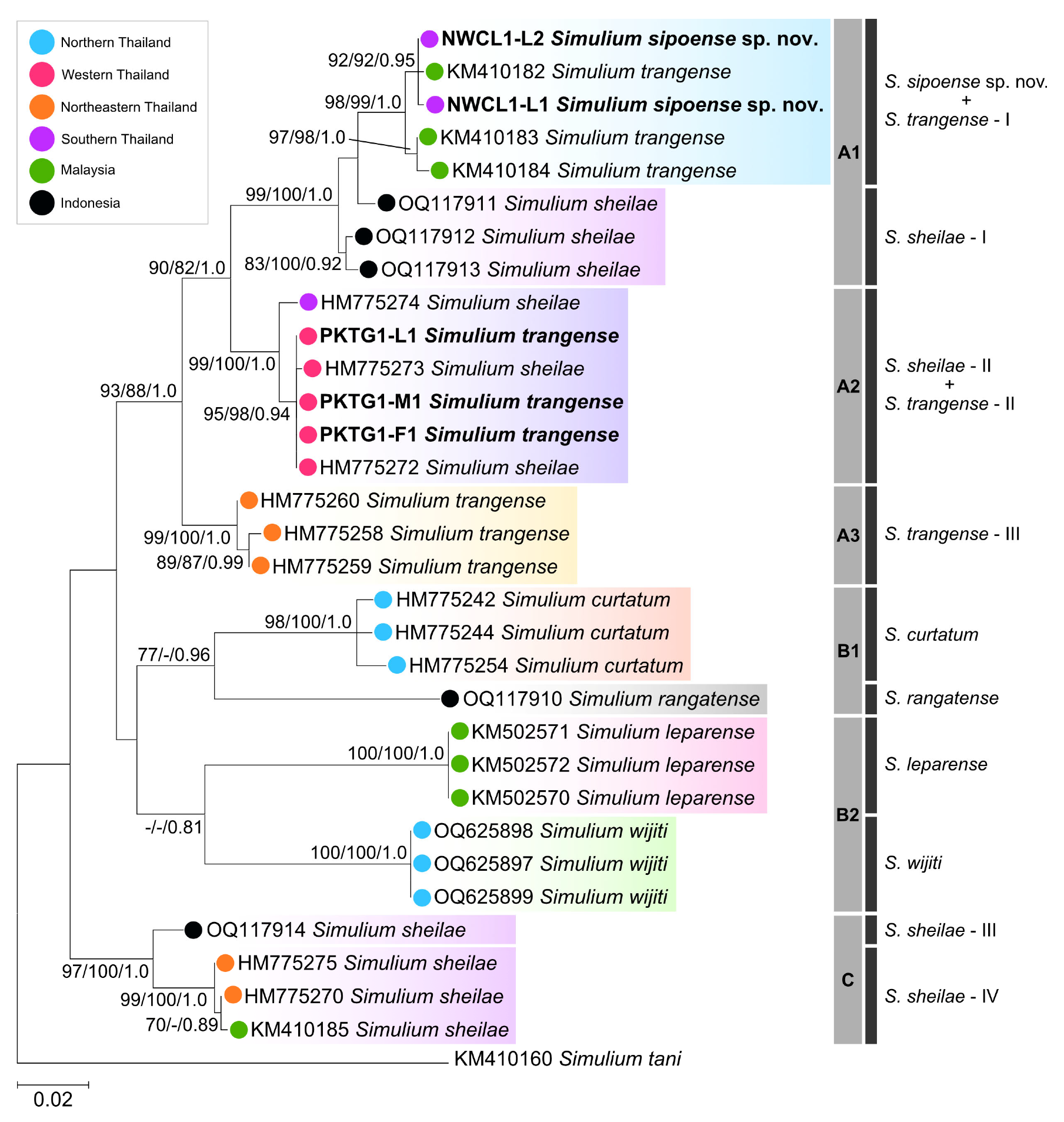
3.1.6. Remarks
This new species belongs to the
S. ceylonicum species-group of the subgenus
Gomphostilbia in the genus
Simulium, redefined by Takaoka [
1]. It is characterized by the dark hair tuft at the base of the radial vein and yellow fore coxae in both female and male. Male also possesses enlarged hind basitarsi (
Figure 4F) and ventral plate, with lateral margins nearly parallel-sided or slightly rounded (
Figure 4K) when viewed ventrally. The postgenal cleft of the larva is deep, with its apex close to the posterior margin of the hypostoma (
Figure 8G).
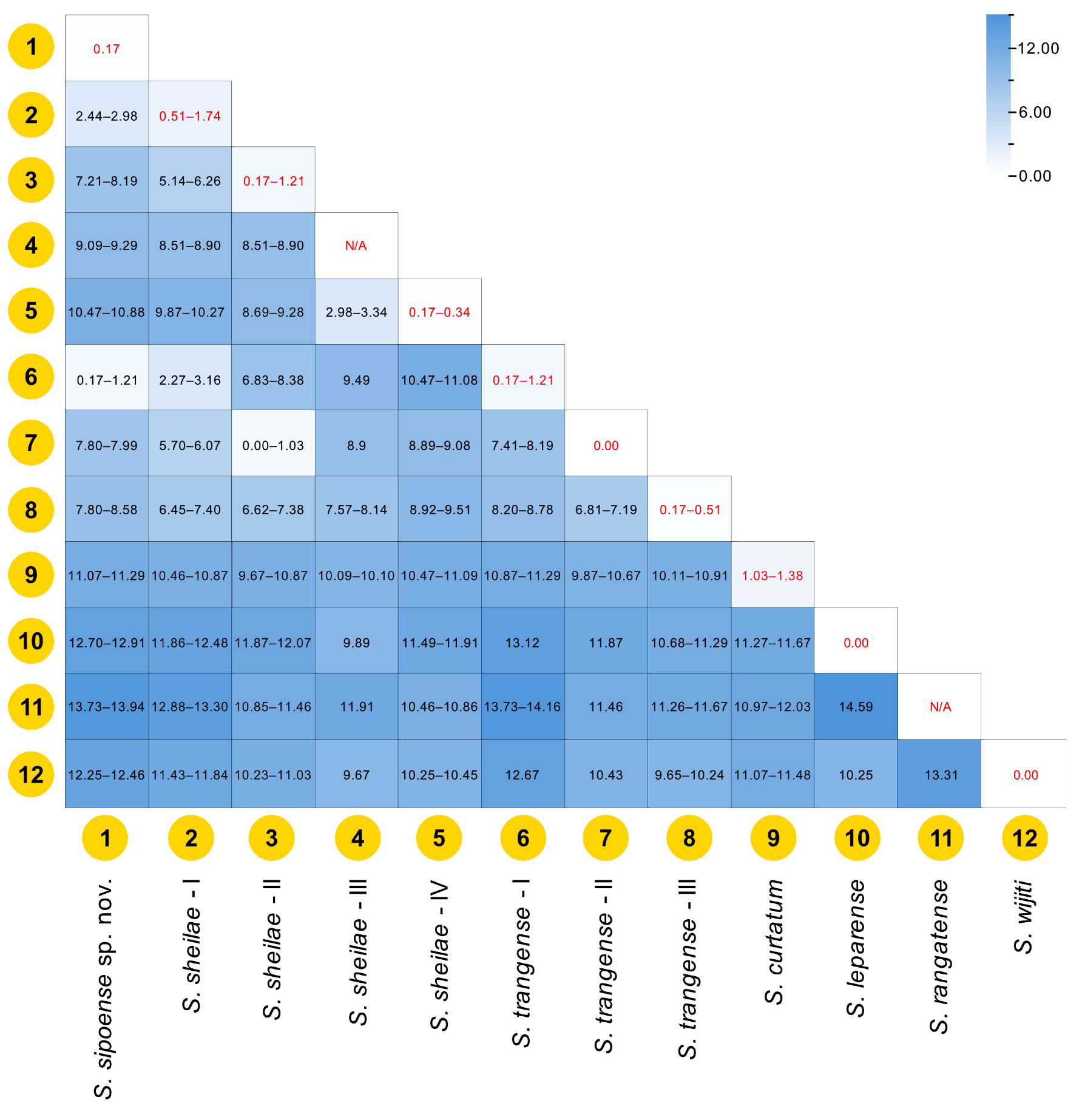
Simulium sipoense sp. nov. is conspecific with the species redescribed as
S. trangense, based on specimens collected from Langkawi Island, Malaysia [
6]. This conclusion is supported by both morphological and genetic similarities. However, Thai specimens (type specimens) exhibit slight morphological differences from the Malaysian specimens (shown in parentheses), particularly in the following numerical features: the length ratio of the female sensory vesicle to the third palpal segment is 0.65 time (0.55–0.59 time), and the ratio of the length of the male hind basitarsus to its greatest width is 3.1 time (3.5–3.9 time).
Simulium sipoense sp. nov. is closely similar to
S. sheilae in sharing the following characteristics: in the female, large sensory vesicle, scutum pattern and claw with large basal tooth; in the male, similar number of upper-eye large facets, small sensory vesicle and ventral plate with its posterior margin slightly convex medially; and in the pupa, similar 8 gill filament arranged as 3+3+2 from dorsal to ventral. However, it is distinguished from
S. sheilae in the female by the number of teeth on maxillary lacinia, 8 inner and 11 outer teeth (cf. 10 and 13 in
S. sheilae), and length ratio of the hind basitarsus with its widest 5.4 time (6.3 time in
S. sheilae); in the male, by the length ratio of the hind basitarsus against its greatest width 3.1 time (3.5 time); in the pupa, by the wide, plate-like terminal hook (cone shaped in
S. sheilae), cocoon without an anterodorsal projection or bulge when view dorsally (with an anterodorsal projection or bulge in
S. sheilae); and in the larva, by the number of primary fan rays 34–36 primary rays (cf. 47 primary rays in
S. sheilae) and first thoracic segment, with dark gray broad transverse band (cf. with a reddish brown broad band) [
21].
The new species is distinguished from the three other Thai members (
S.
curtatum,
S.
pangsidaense, and
S.
wijiti) of the
S. ceylonicum species-group by the following characteristics: in the female, by the length ratio of the sensory vesicle against the third palpal segment 0.65 time (0.60 in
S.
curtatum, 0.57–0.63 in
S.
pangsidaense, and 0.25–0.32 in
S.
wijiti) and the length ratio of the hind basitarsus against its greatest width 5.4 times (5.9 in
S.
pangsidaense, 6.3–6.4 in
S.
wijiti, and data unavailable for
S.
curtatum); in the male, by the upper-eye (large) facets arranged in 10 vertical columns and 12 horizontal rows, (13 columns and 14–16 rows in
S.
curtatum and
S.
pangsidaense; and 15 columns and 15 or 16 rows in
S.
wijiti), hind tibia dark brown to brownish black except the basal one-third whitish (light to medium brown, except the base whitish yellow and the apical cap brownish black in
S. pangsidaense and yellowish on little more than the basal one-third with an ochreous subbasal marking, and light to medium brown on the rest, except the apical cap brownish black in
S. wijiti and data unavailable for
S.
curtatum), the length ratio of the hind basitarsus against its greatest width is 3.1, compared to 3.8–4.6 in
S.
pangsidaense and
S.
wijiti (data unavailable for
S.
curtatum); in the pupa, by the terminal hook wide and plate-like with its outer margin crenulated (same in
S. pangsidaense, triangular terminal hooks with their outer margins smooth in
S. wijiti, and data unavailable for
S.
curtatum), and ninth abdominal segment unpigmented or light yellow (dark yellow in
S. pangsidaense); and in the larva, by the number of the primary rays of the labral fan, 34–36 compared to 27–32 in
S.
wijiti and
S.
pangsidaense (data unavailable for
S.
curtatum), and the first abdominal segment with a small reddish-brown spot on each dorsolateral surface (a reddish-brown transverse band in
S. pangsidaense and a greenish transverse band in
S. curtatum and
S. wijiti) [
5,
7,
21,
22].
The new species is distinguished from
S. doisaketense Jitklang, Kuvangkadilok, Baimai, Takaoka & Adler, 2008, reported from northern Thailand by Jitklang et al. [
7], in the larval stage by the presence of reddish-brown transverse bands on abdominal segments 2–4 (cf.
S. doisaketense has greenish transverse bands on abdominal segments 1–4), and a deep postgenal cleft that is 13–15 times as long as the postgenal bridge (cf.
S. doisaketense has a medium-long postgenal cleft (1.5–2.8 times as long as postgenal bridge)).
The new species is similar to
S. leparense from Malaysia in many characteristics, such as the relative length of the female claw tooth; the shape and color of the male hind basitarsus; and a short common stalk of the pupal gill, with 8 filaments and terminal hooks that are wide, plate-like, and have crenulated outer margins. However,
S. sipoense sp. nov. can be differentiated from
S. leparense by the following characteristics (characteristics of
S. leparense in parentheses): in the female, by the length ratio of the sensory vesicle to the third palpal segment of 0.65 (0.55), the color of short hairs on the scutum, which is whitish (dark brown), and scutum pattern with 3 longitudinal vittae (absent); in the male, by the number of upper-eye large facets arranged in 10 vertical columns and 12 horizontal rows (7 or 8 vertical columns and 11 horizontal rows) and length ratio of the sensory vesicle against third palpal segment 0.17 time (0.14 time); and in the pupa, by the presence of 3 grapnel-shaped hooklets on each side of abdominal segment 9 (absent) [
23].