Host Adaptability and Genetic Mechanisms of the Rice Strain of Fall Armyworm (Spodoptera frugiperda)
Simple Summary
Abstract
1. Introduction
- (1)
- Host Range Hypothesis: The RS possesses superior dietary adaptability compared to the CS, particularly manifesting as enhanced growth performance on non-preferred hosts (rice and ryegrass), attributable to more efficient detoxification metabolic pathways.
- (2)
- Specialization Hypothesis: The CS demonstrates optimal growth and development specifically on its preferred host (corn), with significantly reduced performance on alternative hosts, reflecting evolutionary specialization and narrower physiological adaptation.
- (3)
- Hybridization Hypothesis: Contrary to previous reports of reproductive incompatibility, the two biotypes retain the capacity for successful hybridization under controlled conditions, with hybrid offspring exhibiting transgressive traits and dominant inheritance of RS-like host adaptability characteristics.
2. Materials and Methods
2.1. Insect Collection and Rearing Conditions
2.2. Biotype Analysis
2.3. Host Preference Bioassay
2.4. Mating Success Rates
2.5. Hybridization and Feeding Preference
2.6. Statistical Analysis
3. Results
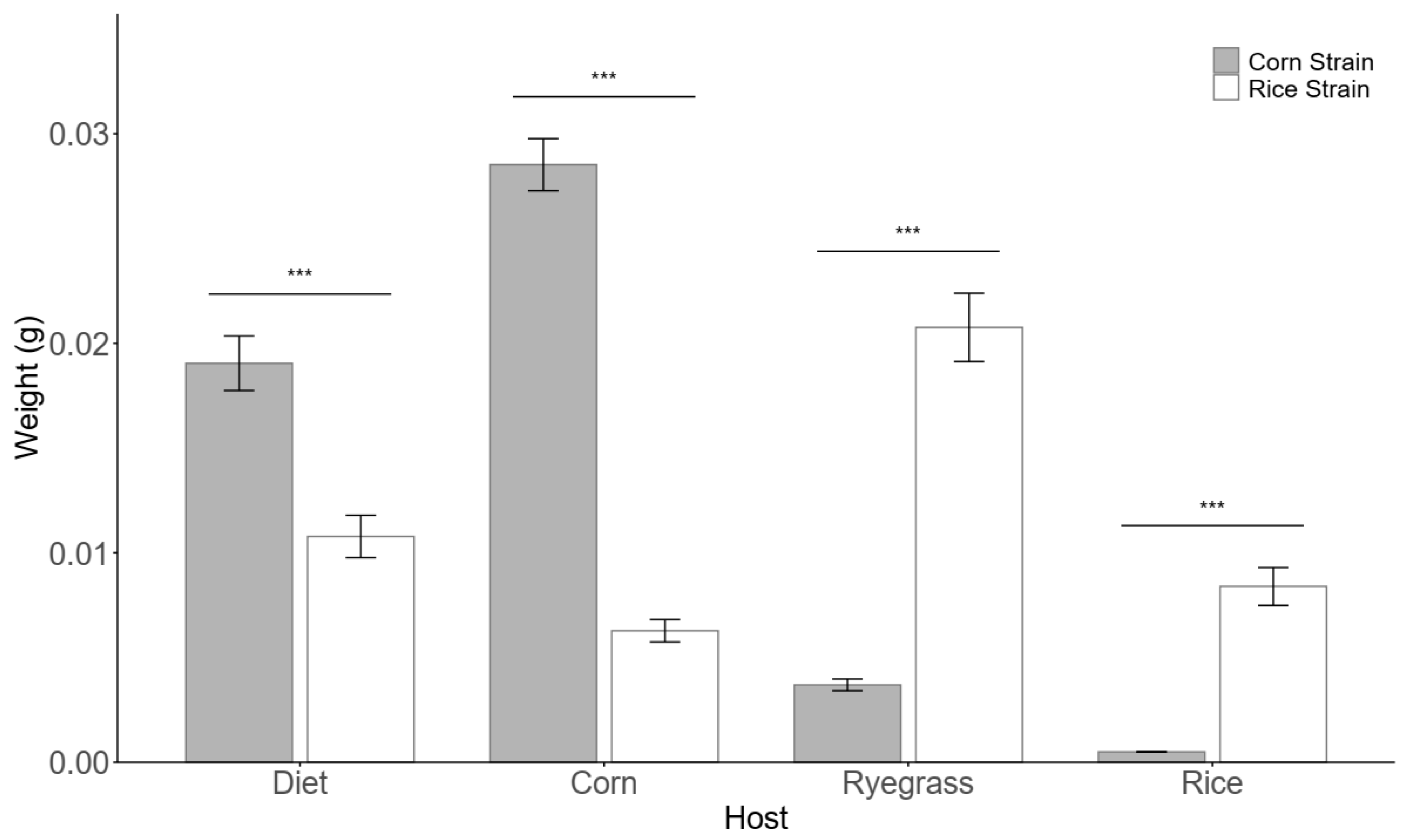
3.1. Growth and Development Rates of Rice Strain (RS) and Corn Strain (CS) Fall Armyworm on Different Host Plants
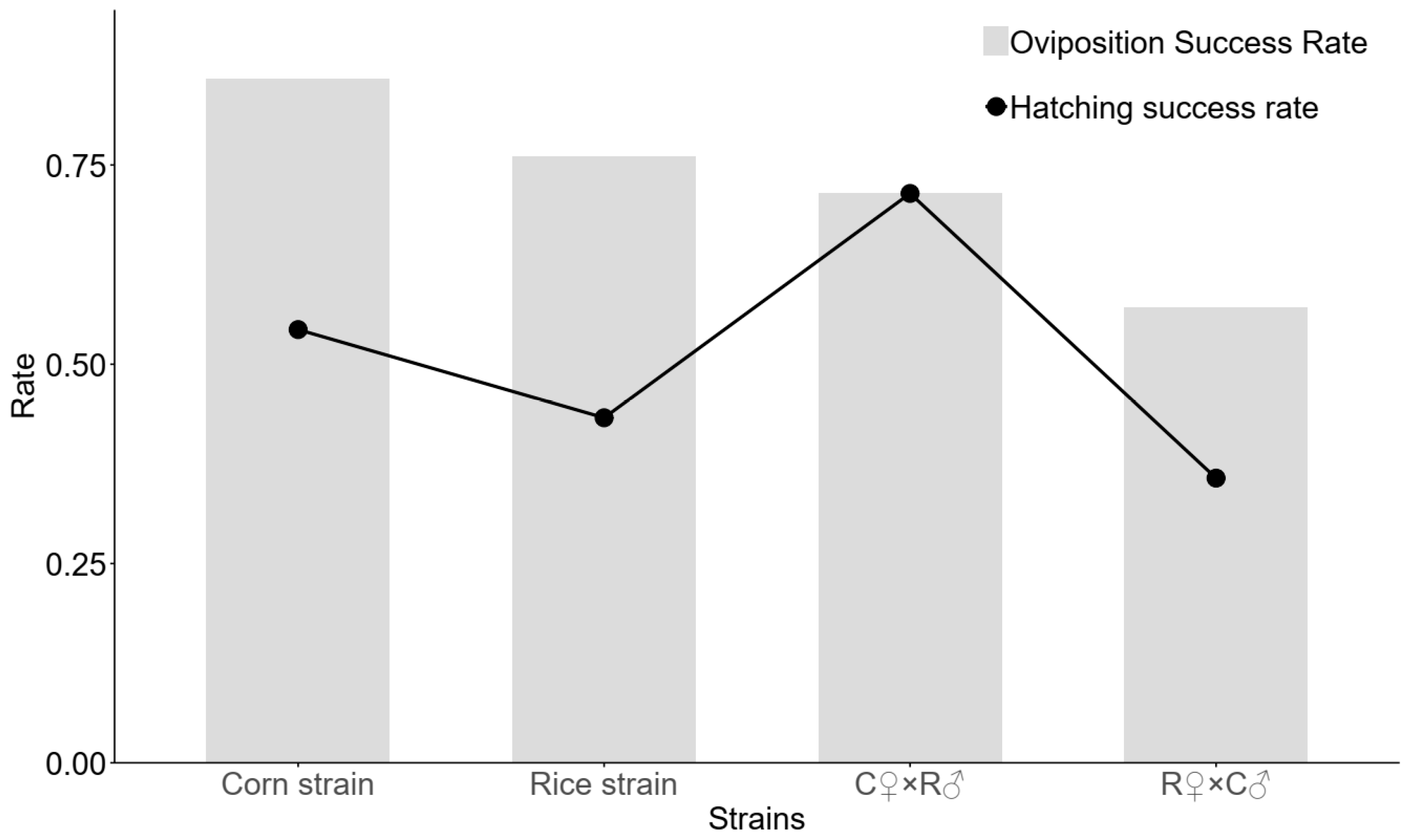
3.2. Interstrain and Intrastrain Mating Success Rates of RS and CS Strains
3.3. Inheritance of Detoxification Metabolism in the Rice Strain
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gouin, A.; Bretaudeau, A.; Nam, K.; Gimenez, S.; Aury, J.-M.; Duvic, B.; Hilliou, F.; Durand, N.; Montagné, N.; Darboux, I.; et al. Two Genomes of Highly Polyphagous Lepidopteran Pests (Spodoptera frugiperda, Noctuidae) with Different Host-Plant Ranges. Sci. Rep. 2017, 7, 11816. [Google Scholar] [CrossRef]
- Nagoshi, R.N.; Fleischer, S.; Meagher, R.L.; Hay-Roe, M.; Khan, A.; Murúa, M.G.; Silvie, P.; Vergara, C.; Westbrook, J. Fall Armyworm Migration across the Lesser Antilles and the Potential for Genetic Exchanges between North and South American Populations. PLoS ONE 2017, 12, e0171743. [Google Scholar] [CrossRef]
- Zhang, L.; Li, Z.; Peng, Y.; Liang, X.; Wilson, K.; Chipabika, G.; Karangwa, P.; Uzayisenga, B.; Mensah, B.A.; Kachigaba, D.L.; et al. Global Genomic Signature Reveals the Evolution of Fall Armyworm in the Eastern Hemisphere. Mol. Ecol. 2023, 32, 5463–5478. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, B.; Zheng, W.; Liu, C.; Zhang, D.; Zhao, S.; Li, Z.; Xu, P.; Wilson, K.; Withers, A.; et al. Genetic Structure and Insecticide Resistance Characteristics of Fall Armyworm Populations Invading China. Mol. Ecol. Resour. 2020, 20, 1682–1696. [Google Scholar] [CrossRef] [PubMed]
- Sagar, D.; Suroshe, S.S.; Keerthi, M.C.; Poorani, J.; Gupta, A.; Chandel, R.K. Native Parasitoid Complex of the Invasive Fall Armyworm, Spodoptera frugiperda (J. E. Smith) from Northern India. Int. J. Trop. Insect Sci. 2022, 42, 2773–2778. [Google Scholar] [CrossRef]
- Mendesil, E.; Tefera, T.; Blanco, C.A.; Paula-Moraes, S.V.; Huang, F.N.; Viteri, D.M.; Hutchison, W.D. The Invasive Fall Armyworm, Spodoptera frugiperda, in Africa and Asia: Responding to the Food Security Challenge, with Priorities for Integrated Pest Management Research. J. Plant Dis. Prot. 2023, 130, 1175–1206. [Google Scholar] [CrossRef]
- Juárez, M.L.; Schöfl, G.; Vera, M.T.; Vilardi, J.C.; Murúa, M.G.; Willink, E.; Hänniger, S.; Heckel, D.G.; Groot, A.T. Population Structure of Spodoptera frugiperda Maize and Rice Host Forms in South America: Are They Host Strains? Entomol. Exp. Appl. 2014, 152, 182–199. [Google Scholar] [CrossRef]
- Pashley, D.P.; Martin, J.A. Reproductive Incompatibility between Host Strains of the Fall Armyworm (Lepidoptera: Noctuidae). Ann. Entomol. Soc. Am. 1987, 80, 731–733. [Google Scholar] [CrossRef]
- Schöfl, G.; Heckel, D.G.; Groot, A.T. Time-Shifted Reproductive Behaviours among Fall Armyworm (Noctuidae: Spodoptera frugiperda) Host Strains: Evidence for Differing Modes of Inheritance. J. Evol. Biol. 2009, 22, 1447–1459. [Google Scholar] [CrossRef]
- Ingber, D.A.; McDonald, J.H.; Mason, C.E.; Flexner, L. Oviposition Preferences, Bt Susceptibilities, and Tissue Feeding of Fall Armyworm (Lepidoptera: Noctuidae) Host Strains. Pest Manag. Sci. 2021, 77, 4091–4099. [Google Scholar] [CrossRef]
- Li, H.; Liang, X.; Peng, Y.; Liu, Z.X.; Zhang, L.; Wang, P.; Jing, M.H.; Wilson, K.; Garvin, M.R.; Wu, K.M.; et al. Novel Mito-Nuclear Combinations Facilitate the Global Invasion of a Major Agricultural Crop Pest. Adv. Sci. 2024, 11, e2305353. [Google Scholar] [CrossRef]
- Nagoshi, K.L.; Allan, S.A.; Meagher, R.L. Assessing the Use of Wing Morphometrics to Identify Fall Armyworm (Lepidoptera: Noctuidae) Host Strains in Field Collections. J. Econ. Entomol. 2020, 113, 800–807. [Google Scholar] [CrossRef]
- Montezano, D.G.; Specht, A.; Sosa-Gómez, D.R.; Roque-Specht, V.F.; Sousa-Silva, J.C.; Paula-Moraes, S.V.; Peterson, J.A.; Hunt, T.E. Host Plants of Spodoptera frugiperda (Lepidoptera: Noctuidae) in the Americas. Afr. Entomol. 2018, 26, 286–300. [Google Scholar] [CrossRef]
- Saldamando, C.I.; Vélez-Arango, A.M. Host Plant Association and Genetic Differentiation of Corn and Rice Strains of Spodoptera frugiperda Smith (Lepidoptera: Noctuidae) in Colombia. Neotrop. Entomol. 2010, 39, 921–929. [Google Scholar] [CrossRef] [PubMed]
- Hay-Roe, M.M.; Meagher, R.L.; Nagoshi, R.N. Effects of Cyanogenic Plants on Fitness in Two Host Strains of the Fall Armyworm (Spodoptera frugiperda). J. Chem. Ecol. 2011, 37, 1314–1322. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.C.; Dong, J.F.; Tang, Q.B.; Yan, Y.H.; Gelbic, I.; Van Loon, J.J.A.; Wang, C.Z. Hybridization between Helicoverpa armigera and Helicoverpa assulta (Lepidoptera: Noctuidae): Development and Morphological Characterization of F1 Hybrids. Bull. Entomol. Res. 2005, 95, 409–416. [Google Scholar] [CrossRef]
- Saveer, A.M.; Becher, P.G.; Birgersson, G.; Hansson, B.S.; Witzgall, P.; Bengtsson, M. Mate Recognition and Reproductive Isolation in the Sibling Species Spodoptera littoralis and Spodoptera litura. Front. Ecol. Evol. 2014, 2, 18. [Google Scholar] [CrossRef]
- Dumas, P.; Legeai, F.; Lemaitre, C.; Scaon, E.; Orsucci, M.; Labadie, K.; Gimenez, S.; Clamens, A.-L.; Henri, H.; Vavre, F.; et al. Spodoptera frugiperda (Lepidoptera: Noctuidae) Host-Plant Variants: Two Host Strains or Two Distinct Species? Genetica 2015, 143, 305–316. [Google Scholar] [CrossRef]
- Nagoshi, R.N.; Koffi, D.; Agboka, K.; Tounou, K.A.; Banerjee, R.; Jurat-Fuentes, J.L.; Meagher, R.L. Comparative Molecular Analyses of Invasive Fall Armyworm in Togo Reveal Strong Similarities to Populations from the Eastern United States and the Greater Antilles. PLoS ONE 2017, 12, e0181982. [Google Scholar] [CrossRef]
- Nagoshi, R.N.; Brambila, J.; Meagher, R.L. Use of DNA Barcodes to Identify Invasive Armyworm Spodoptera Species in Florida. J. Insect Sci. 2011, 11, 154. [Google Scholar] [CrossRef]
- Nagoshi, R.N. Improvements in the Identification of Strains Facilitate Population Studies of Fall Armyworm Subgroups. Ann. Entomol. Soc. Am. 2012, 105, 351–358. [Google Scholar] [CrossRef]
- Vilaplana, L.; Wilson, K.; Redman, E.M.; Cory, J.S. Pathogen Persistence in Migratory Insects: High Levels of Vertically-Transmitted Virus Infection in Field Populations of the African Armyworm. Evol. Ecol. 2009, 24, 147–160. [Google Scholar] [CrossRef]
- Meagher, R.L.; Nagoshi, R.N. Differential Feeding of Fall Armyworm (Lepidoptera: Noctuidae) Host Strains on Meridic and Natural Diets. Ann. Entomol. Soc. Am. 2012, 105, 462–470. [Google Scholar] [CrossRef]
- Pashley, D.P.; Hardy, T.N.; Hammond, A.M. Host Effects on Developmental and Reproductive Traits in Fall Armyworm Strains (Lepidoptera: Noctuidae). Ann. Entomol. Soc. Am. 1995, 88, 748–755. [Google Scholar] [CrossRef]
- Jeschke, V.; Kearney, E.E.; Schramm, K.; Kunert, G.; Shekhov, A.; Gershenzon, J.; Vassão, D.G. How Glucosinolates Affect Generalist Lepidopteran Larvae: Growth, Development and Glucosinolate Metabolism. Front. Plant Sci. 2017, 8, 1995. [Google Scholar] [CrossRef] [PubMed]
- Mason, C.J.; Peiffer, M.; Chen, B.; Hoover, K.; Felton, G.W. Opposing Growth Responses of Lepidopteran Larvae to the Establishment of Gut Microbiota. Microbiol. Spectr. 2022, 10, e0194122. [Google Scholar] [CrossRef] [PubMed]
- Tessnow, A.E.; Raszick, T.J.; Porter, P.; Sword, G.A. Patterns of Genomic and Allochronic Strain Divergence in the Fall Armyworm, Spodoptera frugiperda (J.E. Smith). Ecol. Evol. 2022, 12, e8706. [Google Scholar] [CrossRef]
- Miller, A.C.; Tessnow, A.E.; Meagher, R.L.; Nagoshi, R.N.; Gilligan, T.M.; Sword, G.A. Assessing Fall Armyworm (Spodoptera frugiperda) Allochronic Behavior as a Predictor of Local Strain Composition in United States Populations. Front. Plant Sci. 2024, 15, 1380624. [Google Scholar] [CrossRef]
- Hänniger, S.; Dumas, P.; Schöfl, G.; Gebauer-Jung, S.; Vogel, H.; Unbehend, M.; Heckel, D.G.; Groot, A.T. Genetic Basis of Allochronic Differentiation in the Fall Armyworm. BMC Evol. Biol. 2017, 17, 68. [Google Scholar] [CrossRef]
- Tay, W.T.; Meagher, R.L., Jr.; Czepak, C.; Groot, A.T. Spodoptera frugiperda: Ecology, Evolution, and Management Options of an Invasive Species. Annu. Rev. Entomol. 2023, 68, 299–317. [Google Scholar] [CrossRef] [PubMed]
- Unbehend, M.; Hänniger, S.; Meagher, R.L.; Heckel, D.G.; Groot, A.T. Pheromonal Divergence between Two Strains of Spodoptera frugiperda. J. Chem. Ecol. 2013, 39, 364–376. [Google Scholar] [CrossRef]
- Nagoshi, R.N.; Fleischer, S.; Meagher, R.L. Demonstration and Quantification of Restricted Mating between Fall Armyworm Host Strains in Field Collections by SNP Comparisons. J. Econ. Entomol. 2017, 110, 2568–2575. [Google Scholar] [CrossRef]
- Nagoshi, R.N.; Meagher, R.L. The Spodoptera frugiperda Host Strains: What They Are and Why They Matter for Understanding and Controlling This Global Agricultural Pest. J. Econ. Entomol. 2022, 115, 1729–1743. [Google Scholar] [CrossRef]
- Sisay, B.; Tamiru, A.; Subramanian, S.; Weldon, C.W.; Khamis, F.; Green, K.K.; Anderson, P.; Torto, B. Pheromonal Variation and Mating between Two Mitotypes of Fall Armyworm (Spodoptera frugiperda) in Africa. Sci. Rep. 2024, 14, 3848. [Google Scholar] [CrossRef]
- Meagher, R.L.; Nagoshi, R.N. Population Dynamics and Occurrence of Spodoptera frugiperda Host Strains in Southern Florida. Ecol. Entomol. 2004, 29, 614–620. [Google Scholar] [CrossRef]
- Nagoshi, R.N.; Meagher, R.L. Behavior and Distribution of the Two Fall Armyworm Host Strains in Florida. Fla. Entomol. 2004, 87, 440–449. [Google Scholar] [CrossRef]
- Prasifka, J.R.; Bradshaw, J.D.; Meagher, R.L.; Nagoshi, R.N.; Steffey, K.L.; Gray, M.E. Development and Feeding of Fall Armyworm on Miscanthus × giganteus and Switchgrass. J. Econ. Entomol. 2009, 102, 2154–2159. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Pashley, D.P.; McMichael, M.; Silvain, J.-F. Multilocus Genetic Analysis of Host Use, Introgression, and Speciation in Host Strains of Fall Armyworm (Lepidoptera: Noctuidae). Ann. Entomol. Soc. Am. 2004, 97, 1034–1044. [Google Scholar] [CrossRef]
- Acevedo, F.E.; Peiffer, M.; Ray, S.; Meagher, R.; Luthe, D.S.; Felton, G.W. Intraspecific Differences in Plant Defense Induction by Fall Armyworm Strains. New Phytol. 2018, 218, 310–321. [Google Scholar] [CrossRef]
- Acevedo, F.E.; Stanley, B.A.; Stanley, A.; Peiffer, M.; Luthe, D.S.; Felton, G.W. Quantitative Proteomic Analysis of the Fall Armyworm Saliva. Insect Biochem. Mol. Biol. 2017, 86, 81–92. [Google Scholar] [CrossRef]
- Yao, P.H.; Mobarak, S.H.; Yang, M.F.; Hu, C.X. Differential Detoxification Enzyme Profiles in C-Corn Strain and R-Rice Strain of Spodoptera frugiperda by Comparative Genomic Analysis: Insights into Host Adaptation. BMC Genom. 2025, 26, 14. [Google Scholar] [CrossRef] [PubMed]
- Rios, D.A.; Specht, A.; Roque-Specht, V.F.; Sosa-Gómez, D.R.; Fochezato, J.; Malaquias, J.V.; Gonçalves, G.L.; Moreira, G.R.P. Helicoverpa armigera and Helicoverpa zea Hybridization: Constraints, Heterosis, and Implications for Pest Management. Pest Manag. Sci. 2022, 78, 955–964. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, H.; Wu, C.; Wilson, K.; Xiao, Y.; Liu, K. Host Adaptability and Genetic Mechanisms of the Rice Strain of Fall Armyworm (Spodoptera frugiperda). Insects 2025, 16, 1029. https://doi.org/10.3390/insects16101029
Wang H, Wu C, Wilson K, Xiao Y, Liu K. Host Adaptability and Genetic Mechanisms of the Rice Strain of Fall Armyworm (Spodoptera frugiperda). Insects. 2025; 16(10):1029. https://doi.org/10.3390/insects16101029
Chicago/Turabian StyleWang, Hanyue, Chao Wu, Kenneth Wilson, Yutao Xiao, and Kaiyu Liu. 2025. "Host Adaptability and Genetic Mechanisms of the Rice Strain of Fall Armyworm (Spodoptera frugiperda)" Insects 16, no. 10: 1029. https://doi.org/10.3390/insects16101029
APA StyleWang, H., Wu, C., Wilson, K., Xiao, Y., & Liu, K. (2025). Host Adaptability and Genetic Mechanisms of the Rice Strain of Fall Armyworm (Spodoptera frugiperda). Insects, 16(10), 1029. https://doi.org/10.3390/insects16101029





