New Insight and Confrontation of the Internal Structure and Sensilla of the Mouthparts of Cicadomorpha (Insecta: Hemiptera)
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
3. Results
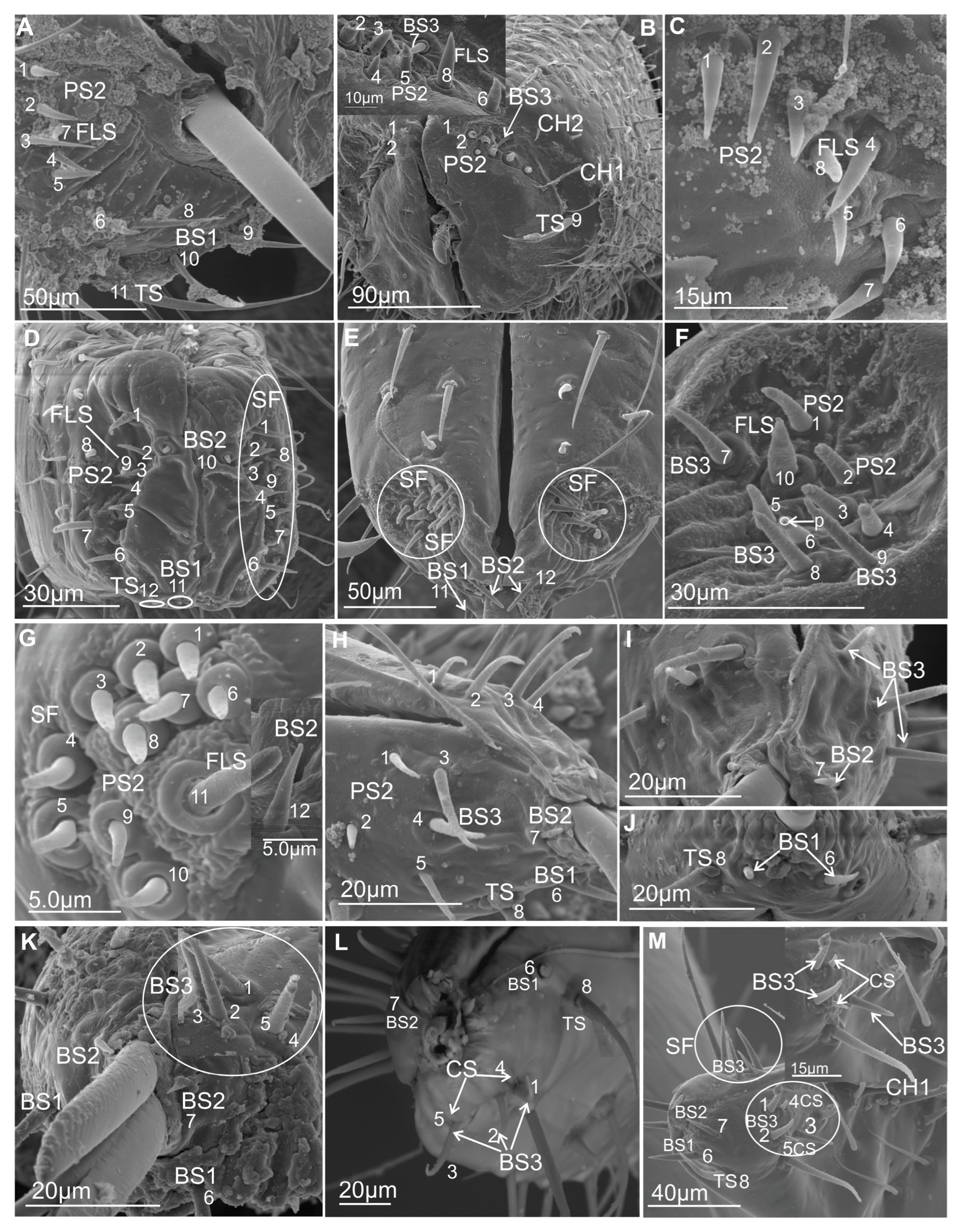
3.1. External Structure of the Terminal Labial Segment
3.2. External Shape and Size of the Maxillary and Mandibular Stylets
3.3. Types of Labial Sensilla
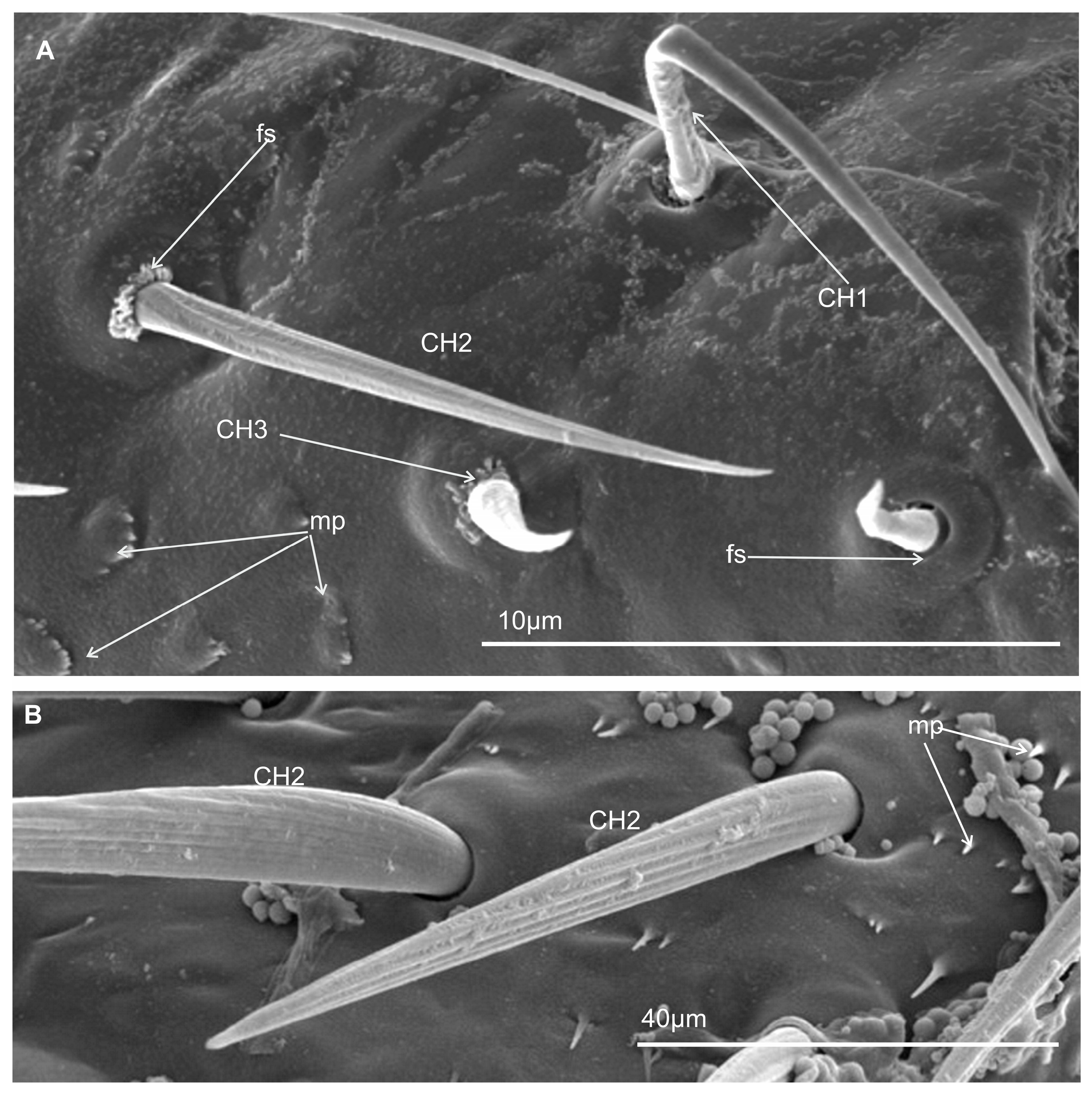
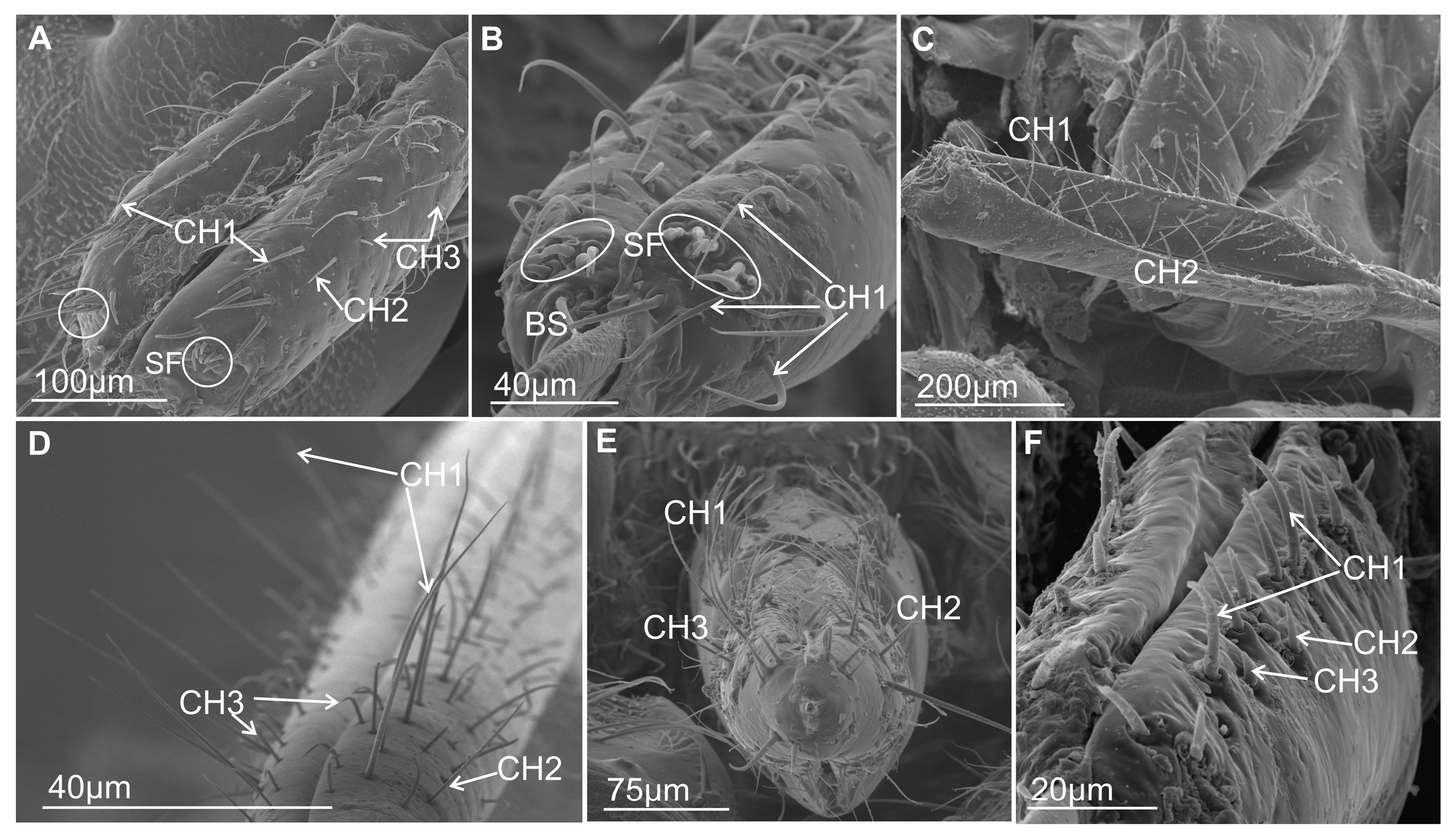
3.3.1. Mechanosensilla
- Chaetica type 1 (CH–1) (Figure 6A) are long (their length above 20 µm) and slender with shallow longitudinal grooves and a pointed tip slightly curved to the rostrum surface.
- Chaetica type 2 (CH–2) (Figure 6B) are generally of middle length (ranging from 10 to 20 µm) with longitudinal grooves.
- Chaetica type 3 (CH–3) (Figure 6A) are short (their length ranged from 4.0 to 9.0 µm) and cone-shaped with longitudinal grooves, with a curved end.
3.3.2. Arrangement of the Labial Mechanosensilla
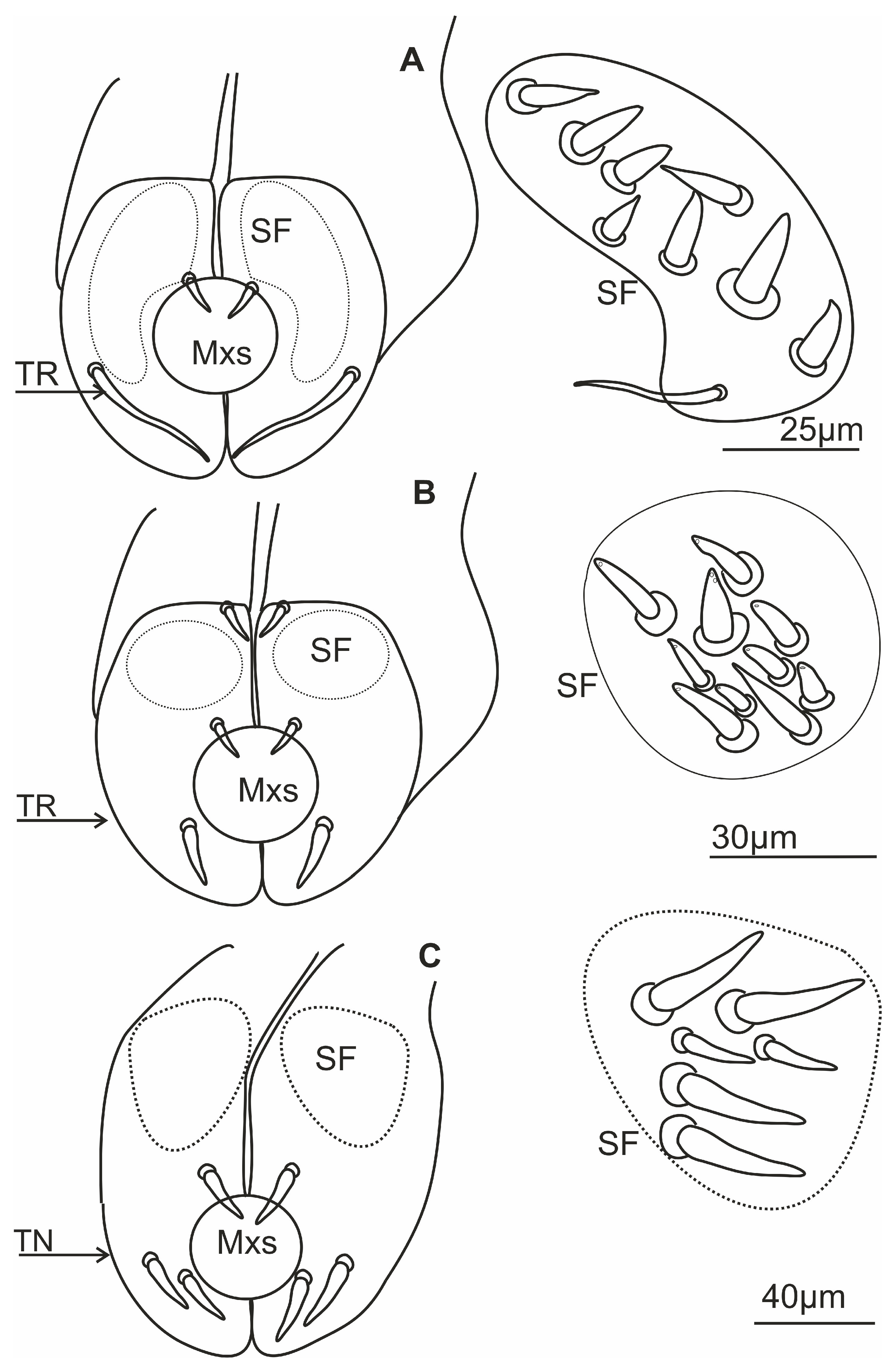
3.3.3. Chemosensilla
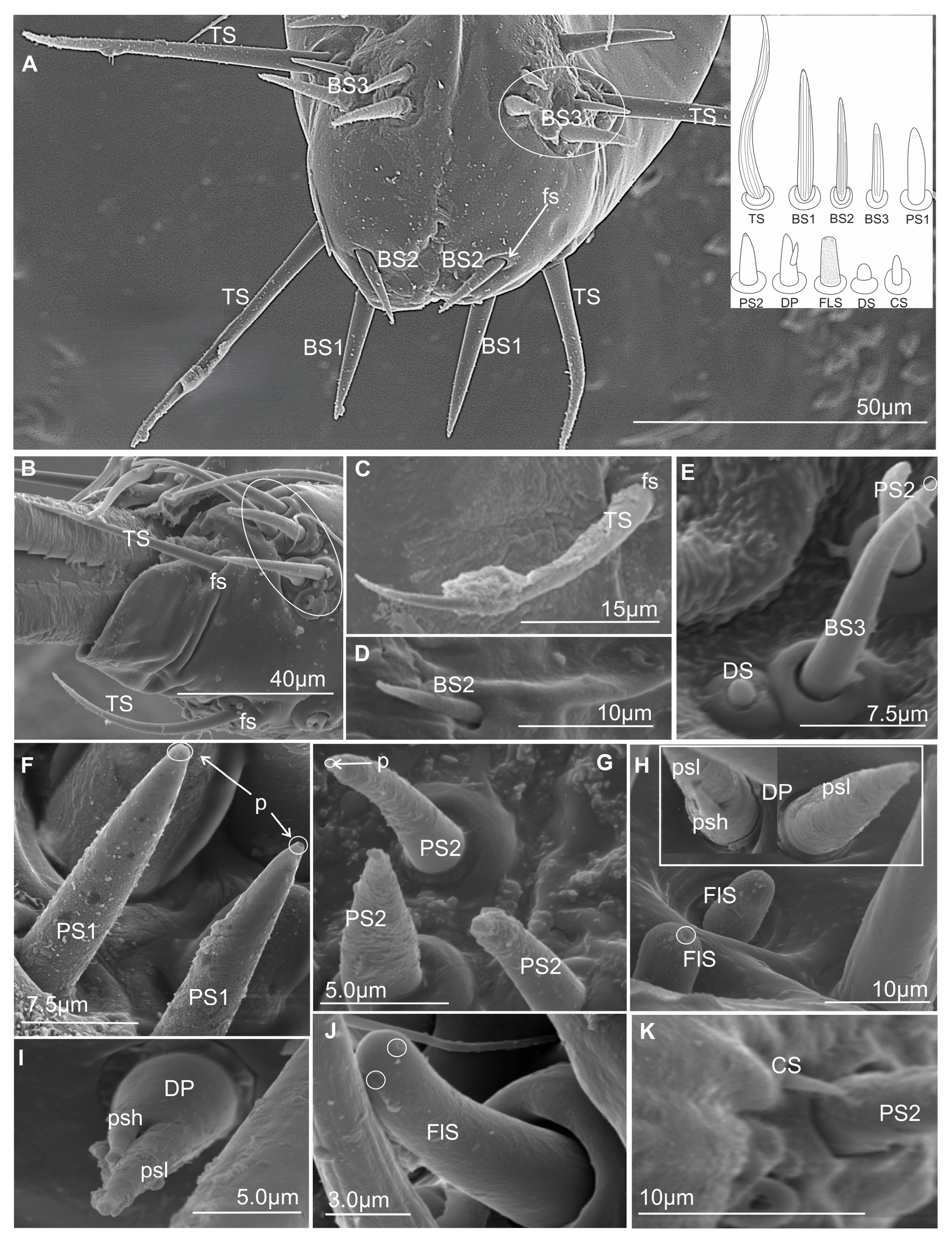
3.3.4. Contact Chemoreceptor Sensilla
- TS–sensillum trichoidea, uniporous (Figure 8A–C) are the longest of these sensilla (ranging from 25.0 to 95 µm in length) and the slenderest of labial tip sensilla, with a curved shaft from a regular and flexible socket. It is difficult to observe the terminal pore, but the characteristics and location of the hairs suggest that they could be uniporous sensilla (chemosensilla) of the labial tip. These sensilla, located only on the middle and lower surface of the tip of the labium, are not numerous (one or two pairs were noticed).
- BS1–sensillum basiconicum uniporous (Figure 8A) are of medium length (approximately 20–45 µm) and are scattered on the ventral surface below the stylet bundles (Md and Mx). The surface of their wall is smooth; a tip is slightly tapered and possesses a terminal pore. On one side, one or two pairs of sensilla are present.
- BS2–sensillum basiconicum uniporous (Figure 8A,D) are short cones with an average length of 7–20 µm, which is always shorter than that of the sensilla basiconica (BS1) (Figure 8A). Their base is wider (2.0 µm), but the diameter measures 0.9 µm by the first quarter of the sensillum and remains this size up to the apex. These sensilla possess longitudinal ridges along their entire length or are smooth and probably have a terminal pore.
3.3.5. Gustatory Sensilla
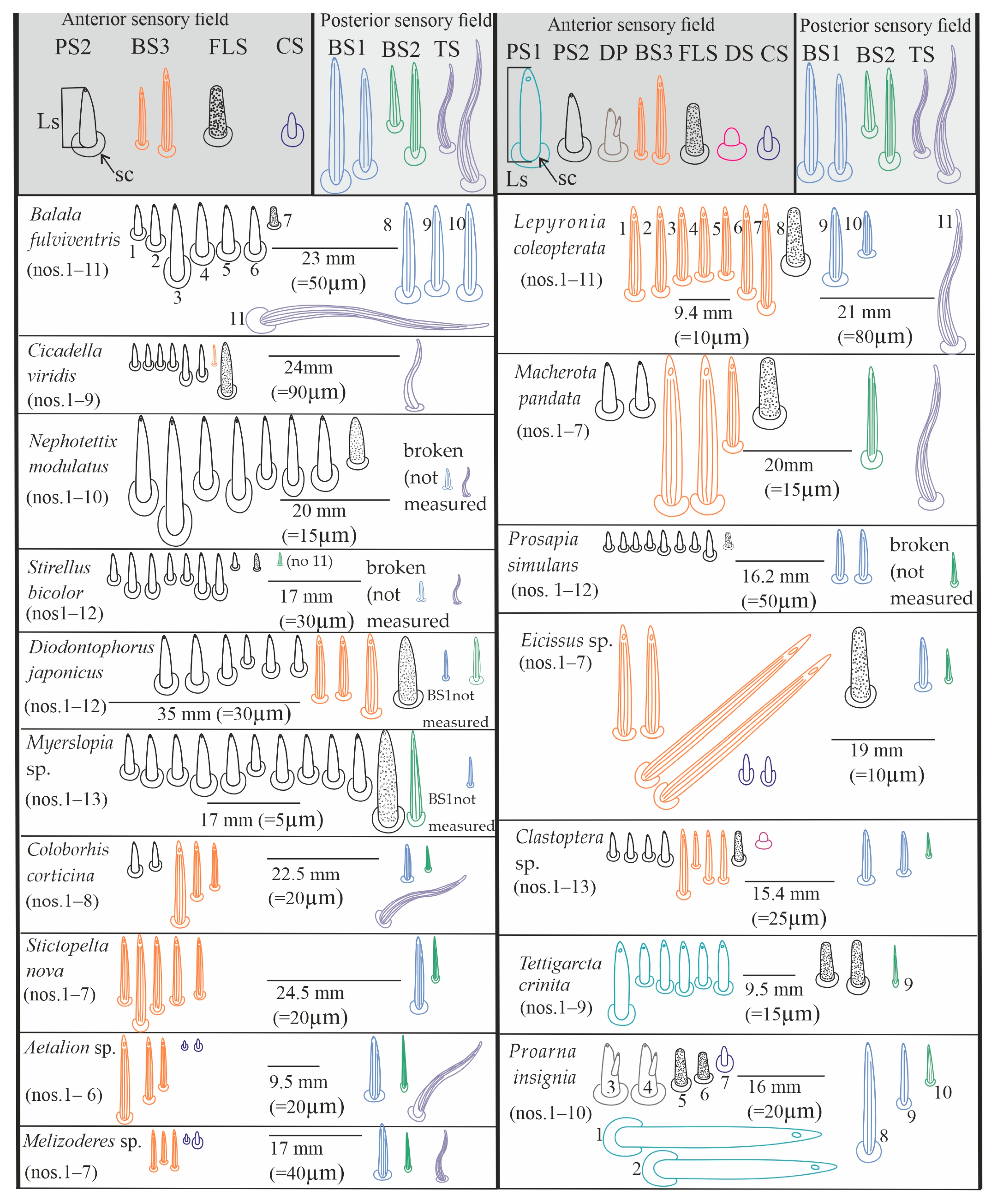
- BS3–sensillum basiconicum uniporous (Figure 8E) is slim and of middle length (6–20 µm), with slightly longitudinal ridges along its whole length and a terminal pore. In most species, the average length of the sensilla is around 20 µm, while the shortest are found in Cicadella (6 µm), in contrast to the longest, which are found only in Aetalion sp. (24 µm) (Table 2). These sensilla are longer than the sensillum peg and, in several numbers, are present on the anterion sensory fields.
- PS–sensillum peg uniporous (Figure 8E–G) are uniporous cones with slightly rounded tips. The surface of these sensilla is smooth along their entire length, and the terminal pore is clearly visible (Figure 8F,G). The base of the sensillum is wider than the blunted tip. Based on their different lengths, two subtypes are recognised: PS1 and PS2. PS1 is longer, at around 20.0 µm (Figure 8F), and the longest are observed in (P. insignis) (Table 2). PS2 has a length ranging from 2.0 to 19.0 µm. Sensilla are arranged in the sensory field, and there are numerous sensilla in one field, from 1 to 10.
- DP–sensillum peg, double uniporous (Figure 8H,I), has a base of the cone with a long (psl) and a short (psh) peg. Probably both pegs of this sensillum possess the terminal pore. This sensillum was found only in one species (P. insignis).
3.3.6. Olfactory Sensilla
- FlS–sensillum finger-like (Figure 8H,J) is a thick cone sunken in a shallow socket. They can be compared to short sensilla pegs but with a strong, rounded tip (Figure 8H). One or two pairs of sensilla are present in some species. Their surface is covered with undulated grooves and pitted. Terminally, the rounded tip of these sensilla possesses one or two larger pores than wall multipores (Figure 8H,J). Usually, these sensilla ranged from 6 to 10 µm in length; however, in one species (Cicadella viridis), an excessive length of 36.7 µm was observed (Table 2).
3.3.7. Thermo-Hygrosensilla
- DS–sensillum dome-shaped (Figure 8E) is low with a rounded tip. This type of sensillum is situated on the sensory field near basiconica sensilla. This sensillum was found only in species of Clastoptera.
- CS–sensillum coleoconicum (Figure 8K), the thin pegs are inserted in a shallow cavity on the sensory field. This type of sensillum is very short (about 3 to 5 µm) and placed near the sensillum basiconicum.
3.4. Distribution of the Labial Tip Sensilla Membracoidea
3.5. Distribution of the Labial Tip Sensilla of Cercopoidea and Cicadoidea
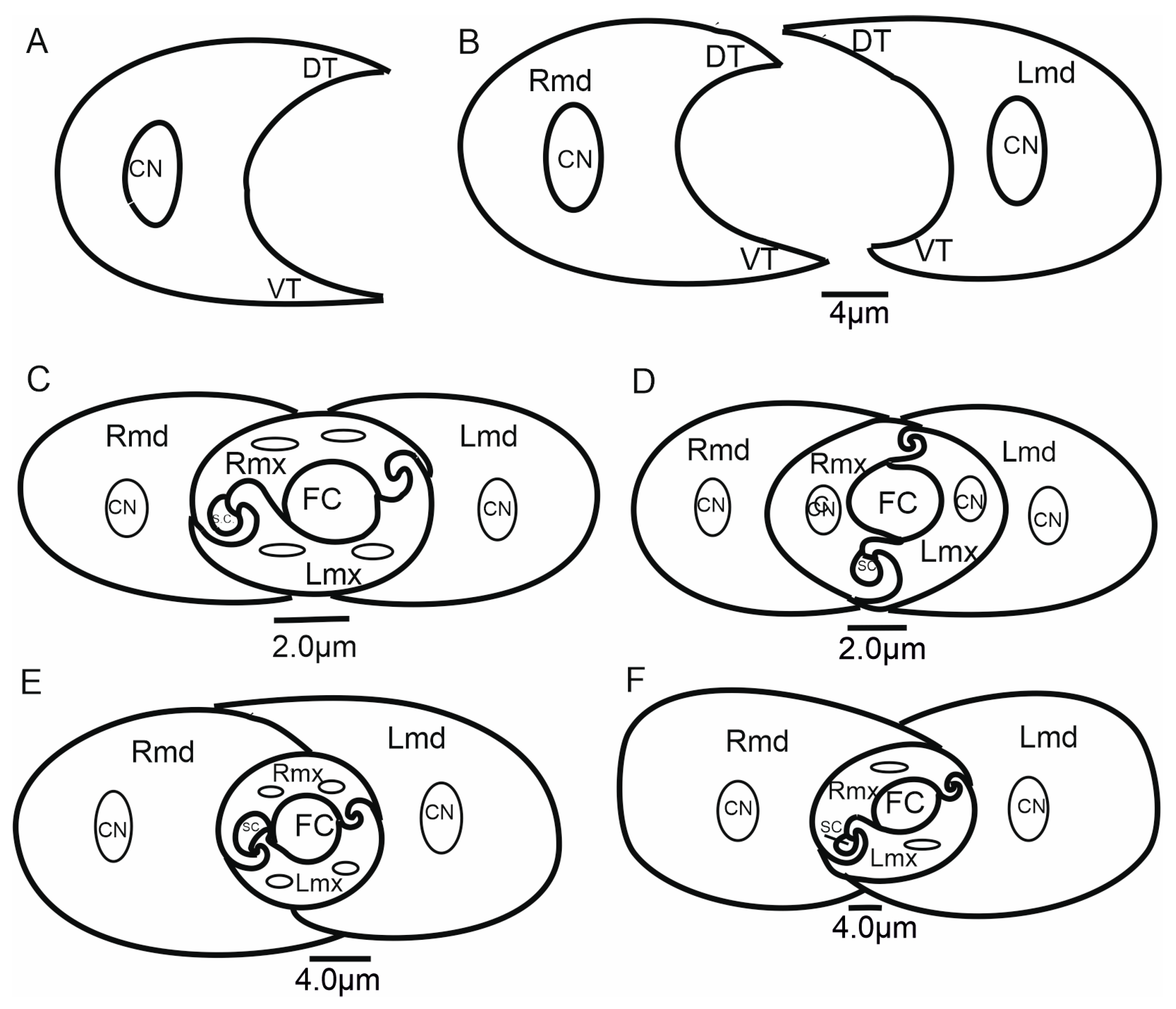
3.6. Scheme of the Connecting System of the Mandible and Maxillae
3.6.1. Maxillae and Mandibles in Cross-Section
- Diagonal: The dorsal and ventral locks are turned to the left in relation to a perpendicular line (Figure 14B,F). Observed only in Ledrinae and Iassinae in the proximal part of the stylets.
3.6.2. Salivary and Food Canals
4. Discussion
4.1. Labium-Shaped and Stylet Bundles
4.2. Labial Tip Sensilla
4.3. The Cross-Section of the Stylets
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sorensen, J.T.; Campbell, B.C.; Gill, R.J.; Steffen-Campbell, J.D. Non-monophyly of Auchenorrhyncha (“Homoptera”), based upon 18S rDNA phylogeny: Eco-evolutionary and cladistics implications within pre-Heteropterodea Hemiptera (s.l.) and a proposal for new monophyletic suborders. Pan-Pac. Entomol. 1995, 71, 31–60. [Google Scholar]
- Bourgoin, T.; Campbell, B.C. Inferring a phylogeny for Hemiptera: Falling into the ‘autapomorphic trap’. Denisia 2002, 4, 67–82. [Google Scholar]
- Hennig, W. Die Stammesgeschichte der Insekten; WaldemarKramer: Frankfurt am Main, Germany, 1969; p. 436. [Google Scholar]
- Cobben, R.H. Evolutionary trends in Heteroptera. Part II. Mouthpart—Structures and feeding strategies. Meded. Landbouwhogesch. Wagening. 1978, 78, 5–401. [Google Scholar]
- Carver, M.; Gross, G.F.; Woodward, T.E. Hemiptera. In CSIRO, Division of Entomology. Insects of Australia, 2nd ed.; Cornell University Press: Ithaca, NY, USA, 1991; Volume 2, pp. 429–509. [Google Scholar]
- Kristensen, N.P. Phylogeny of extant Hexapods. In CSIRO, Division of Entomology. Insects of Australia, 2nd ed.; Cornell University Press: Ithaca, NY, USA, 1991; Volume 2, pp. 125–140. [Google Scholar]
- Cryan, J.R. Molecular phylogeny of Cicadomorpha (Insecta: Hemiptera: Cicadoidea, Cercopoidea and Membracoidea): Adding evidence to the controversy. Syst. Entomol. 2005, 30, 563–574. [Google Scholar] [CrossRef]
- Dietrich, C.H. Evolution of Cicadomorpha (Insecta, Hemiptera). Denisia 2002, 4, 155–170. [Google Scholar]
- Dietrich, C.H. “Auchenorrhyncha”: (Cicadas, Spittlebugs, Leafhoppers, Treehoppers, and Planthoppers). In Encyclopedia of Insects; Resh, V., Cardé, R., Eds.; Academic Press, Inc.: San Diego, CA, USA, 2009; pp. 56–64. [Google Scholar]
- Paladini, A.; Takiya, D.M.; Urban, J.M.; Cryan, J.R. New World spittlebugs (Hemiptera: Cercopidae: Ischnorhininae): Dated molecular phylogeny, classification, and evolution of aposematic coloration. Mol. Phylogenet. Evol. 2018, 120, 321–334. [Google Scholar] [CrossRef] [PubMed]
- Smith, T.R.; Capinera, J.L. Host preferences and habitat associations of some Florida grasshoppers (Orthoptera: Acrididae). Environ. Entomol. 2005, 34, 210–224. [Google Scholar] [CrossRef]
- McGavin, G.C. Bugs of the World; Blandford Press: London, UK, 1993; p. 192. [Google Scholar]
- Leopold, R.A.; Freeman, T.P.; Buckner, J.S.; Nelson, D.R. Mouthpart morphology and stylet penetration of host plants by the glassywinged sharpshooter, Homalodisca coagulata, (Homoptera: Cicadellidae). Arthropod Struct. Dev. 2003, 32, 189–199. [Google Scholar] [CrossRef] [PubMed]
- Zhai, Z.C.; Wang, J.J.; Dietrich, C.H.; Huang, M. SEM study of the mouthparts of Nacolus tuberculatus (Walker) (Hemiptera: Cicadellidae) with comparative notes on other Hemiptera. Zoomorphology 2023, 142, 35–49. [Google Scholar] [CrossRef]
- Carlson, E.K.; Davis, L.J.; Ware, J.L.; Gonzalez-Mozo, L. Comparative study of the integumental fine structure of Membracidae (Hemiptera: Auchenorrhyncha). Zoomorphology 2025, 144, 19. [Google Scholar] [CrossRef]
- Tavella, L.; Arzone, A. Comparative morphology of mouth parts of Zyginidia pullula, Empoasca vitis, and Graphocephala fennahi (Homoptera, Auchenorrhyncha). Boll. Zool. 1993, 60, 33–39. [Google Scholar] [CrossRef][Green Version]
- Zhang, Y.; Dietrich, C.H.; Duan, Y. Structure and sensilla of the mouthparts of Alobaldia tobae, Maiestas dorsalis and Stirellus indrus (Hemiptera: Cicadellidae: Deltocephalinae). Zoomorphology 2020, 139, 189–198. [Google Scholar] [CrossRef]
- Jiang, J.; Dietrich, C.; Li, C.; Song, Y. Comparative morphology of part of the integumental fine structure of two Erythroneurine species: Singapora shinshana (Matsumura, 1932) and Empoasca nara Sipra Dworakowska, 1980 (Hemiptera, Cicadellidae, Typhlocybinae). Zookeys 2022, 1103, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Polard, D.G. The stylet structure of leafhopper (Eupteryx melisae Curtis: Homoptera, Cicadellidae). J. Nat. Hist. 1972, 6, 261–271. [Google Scholar] [CrossRef]
- Backus, E.A. Anatomical and sensory mechanisms of leafhopper and planthopper feeding behavior. In The Planthoppers and Leafhoppers; Nault, L.R., Ed.; John Wiley and Sons: Rodriguez, New York, 1985; pp. 163–194. [Google Scholar]
- Pollard, D.G. Stylet penetration and feeding damage of Eupteryx mellissae CURTIS (Hemiptera: Cicadellidae) on sage. Bull. Entomol. Res. 1968, 58, 55–71. [Google Scholar] [CrossRef]
- Sogawa, K. Feeding of the rice plant- and leafhoppers. Rev. Plant Prot. Res. 1973, 6, 31–43. [Google Scholar]
- Forbes, A.R.; Raine, J. The stylets of the six–spotted leafhopper, Macrosteles fascifrons (Homoptera: Cicadellidae). Can. Entomologist. 1973, 105, 559–567. [Google Scholar] [CrossRef]
- Zhao, L.; Dai, W.; Zhang, C.; Zhang, Y. Morphological characterization of the mouthparts of the vector leafhopper Psammotettix striatus (L.) (Hemiptera: Cicadellidae). Micron 2010, 41, 754–759. [Google Scholar] [CrossRef] [PubMed]
- Pollard, D.G. Directional control of the stylets in phytophagous Hemiptera. Proc. R. Entomol. Soc. Lond. Ser. B 1969, 44, 173–185. [Google Scholar] [CrossRef]
- Wensler, R.J. The fine structure of distal receptors on the labium of the aphid, Brevicoryne brassicae L. (Homoptera). Cell Tissue Res. 1977, 181, 409–422. [Google Scholar] [CrossRef]
- Backus, E.A.; McLena, D. The sensory systems and feeding behavior of leafhoppers. The aster leafhopper, Macrosteles fascifrons Stal (Homoptera, Cicadellidae). J. Morphol. 1982, 172, 361–379. [Google Scholar] [CrossRef]
- Foster, S.; Goodman, L.J.; Duckett, J.G. Sensory receptors associated with the stylets and cibarium of the rice brown planthopper, Nilaparvata lugens. Cell Tissue Res. 1983, 232, 111–119. [Google Scholar] [CrossRef] [PubMed]
- Brożek, J.; Herczek, A. Modifications in the inner structure of the mouth parts of selects species Cicadellidae (Hemiptera). Acta Entomol. Silesiana 2001, 7–8, 19–25. [Google Scholar]
- Brożek, J.; Herczek, A. Internal structure of the mouthparts of true bugs (Hemiptera: Heteroptera). Pol. J. Entomol. 2004, 73, 79–106. [Google Scholar]
- Brożek, J.; Bourgoin, T.; Szwedo, J. The interlocking mechanism of maxillae and mandibles in Fulgoroidea (Insecta: Hemiptera: Fulgoromorpha). Pol. J. Entomol. 2006, 75, 239–253. [Google Scholar]
- Brożek, J. Internal structure of the mouthparts in Coccinea (Hemiptera: Sternorrhyncha). Pol. J. Entomol. 2006, 75, 255–265. [Google Scholar]
- Brożek, J. Labial sensillae and the internal structure of the mouthparts of Xenophyes cascus (Bergroth 1924) (Peloridiidae: Coleorrhyncha: Hemiptera) and their significance in evolutionary studies on the Hemiptera. Monogr. Aphids Other Hemipterous Insects 2007, 13, 35–42. [Google Scholar]
- Emeljanov, A.F. The phylogeny of cicadoids (Homoptera, Cicadina) based on comparative morphological data. Tr. Vseoyuznogho Entomol. Obs. 1987, 69, 19–109. (In Russian) [Google Scholar]
- Emeljanov, A.F. Evolutionary scenario of rostrum formation in the Rhynchota. Entomol. Rev. 2002, 82, 1197–1206. [Google Scholar]
- Miles, P. The Saliva of Hemiptera. Adv. Insect Physiol. 1972, 9, 183–255. [Google Scholar]
- Snodgrass, R.E. The mouth parts of the cicada. Proc. Entomol. Soc. Wash. 1921, 23, 1–15. [Google Scholar]
- Snodgrass, R.E. The head and mouth parts of the cicada. Proc. Entomol. Soc. Wash. 1927, 29, 1–16. [Google Scholar]
- Snodgrass, R.E. The loral plates and the hypopharynx of Hemiptera. Proc. Entomol. Soc. Wash. 1938, 40, 228–236. [Google Scholar]
- Arora, G.L.; Singh, S. Morphology and musculature of the head and mouth-parts of Idiocerus atkinsoni Leth. (Jassidae, Homoptera). J. Morphol. 1962, 110, 131–140. [Google Scholar] [CrossRef]
- Dietrich, C.H.; McKamey, S.H.; Deitz, L.L. Morphology- based phylogeny of the treehopper family Membracidae (Hemiptera: Cicadomorpha: Membracoidea). Syst. Entomol. 2001, 26, 231–239. [Google Scholar] [CrossRef]
- Szwedo, J. A new genus and six new species of ground-dwelling leafhoppers from Chile and New Zealand (Hemiptera: Cicadomorpha: Myerslopiidae). Zootaxa 2004, 424, 1–20. [Google Scholar] [CrossRef]
- Knight, W.J. Ulopinae of New Zealand (Homoptera: Cicadellidae). N. Z. J. Sci. 1973, 16, 971–1007. [Google Scholar]
- Nielson, M.W. A new species of Myerslopia from chile (Homoptera: Cicadellidae). Entomol. News 1996, 107, 322–326. [Google Scholar]
- Rakitov, R. Observations on the Biology and Anatomy of Myerslopiidae (Hemiptera, Membracoidea). Psyche J. Entomol. 2015, 2015, 898063. [Google Scholar] [CrossRef]
- McIver, S.B. Structure of cuticular mechanoreceptors of arthropods. Annu. Rev. Entomol. 1975, 20, 381–397. [Google Scholar] [CrossRef] [PubMed]
- Altner, H.; Prillinger, L. Ultrastructure of invertebrate chemo-, termo-, and hygroreceptors and its functional significance. Int. Rev. Cytol. 1980, 67, 69–139. [Google Scholar]
- Walters, B.D.; Albert, P.J.; Zacharuk, R.Y. Morphology and ultrastructure of sensilla on the proboscis of the adult sprucebudworm, Choristoneura fumiferana (Clem.) (Lepidoptera:Tortricidae). Can. J. Zool. 1998, 76, 466–479. [Google Scholar] [CrossRef]
- Chapman, R.F. Mechanoreception. Chemoreception. In The Insects, Structure and Function, 4th ed.; Cambridge University Press: Cambridge, UK, 1998; pp. 610–652. [Google Scholar]
- Brożek, J.; Bourgoin, T. Morphology and distribution of the external labial sensilla in Fulgoromorpha (Insecta: Hemiptera). Zoomorphology 2013, 132, 33–65. [Google Scholar] [CrossRef]
- Foster, S.; Goodman, L.J.; Duckett, J.G. Ultrastructure of sensory receptors on the labium of the rice brown planthopper. Cell Tissue Res. 1983, 230, 353–366. [Google Scholar] [CrossRef]
- Hao, Y.; Dietrich, C.; Dai, W. Structure and Sensilla of the Mouthparts of the Spotted Lanternfly Lycorma delicatula (Hemiptera: Fulgoromorpha: Fulgoridae), a Polyphagous Invasive Planthopper. PLoS ONE 2016, 11, e0156640. [Google Scholar] [CrossRef] [PubMed]
- Backus, E.A. Sensory systems and behaviours which mediate hemipteran plant-feeding: A taxonomic overview. J. Insect Physiol. 1988, 34, 151–165. [Google Scholar] [CrossRef]
- Wang, T.; Pan, L.; Zhang, Y.; Dai, W. Morphology of the mouthparts of the spittlebug Philagra albinotata Uhler (Hemiptera: Cercopoidea: Aphrophoridae). Arthropod Struct. Dev. 2015, 44, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Zacharuk, R.Y. Ultrastructure and function of insect chemosensilla. Annu. Rev. Entomol. 1980, 25, 27–47. [Google Scholar] [CrossRef]
- Chapman, R.F. Chemoreception: The significance of receptor numbers. Adv. Insect Physiol. 1982, 16, 247–336. [Google Scholar]
- Ventura, M.U.; Panizzi, A.R. Morphology of olfactory sensilla and its role in host plant recognition by Neomegalotomus parvus (Westwood) (Heteroptera: Alydidae). Braz. Arch. Biol. Technol. 2005, 48, 589–597. [Google Scholar] [CrossRef]
- Shields, V.D.C. High resolution ultrastructural investigation of insect sensory organs using field emission scanning electron microscopy. In Microscopy: Science, Technology, Applications and Education; Méndez-Vilas, A., Díaz, J., Eds.; Formatex: Badajoz, Spain, 2010; pp. 321–328. [Google Scholar]
- Slifer, E. The structure of arthropod chemoreceptors. Annu. Rev. Entomol. 1970, 15, 121–142. [Google Scholar] [CrossRef]
- Steinbrecht, R.A. Chemo-, hygro-, and thermoreceptors. In Biology of the Integument Invertebrates; Bereiter-Hahn, J., Matoltsy, A.G., Richards, K.S., Eds.; Springer: Berlin, Germany, 1984; Volume 1, pp. 521–553. [Google Scholar]
- Hallberg, E.; Hansson, B.S.; Lofstedt, C. Lepidoptera, moths and butterflies: Morphology, physiology and development. In Sensilla and Proprioceptors; Kristensen, N.P., Ed.; Walter de Gruyter: Berlin, Germany, 2003; Volume 2, pp. 267–288. [Google Scholar]
- Kristoffersen, L.; Hallberg, E.; Walle, N.R.; Anderbrant, O. Sparse sensilla array on Trioza apicalis (Homoptera: Triozidae) antennae—An adaptation to high stimulus level. Arthropod Struct. Dev. 2006, 35, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Walker, G.P.; Gordh, G. The occurrence of apical labial sensilla in the Aleyrodidae and evidence for a contact chemosensory function. Entomol. Exp. Appl. 1989, 51, 215–224. [Google Scholar] [CrossRef]
- Rani, P.U.; Madhavendra, S.S. Morphology and distribution of antennal sense organs and diversity of mouthpart structures in Odontopus nigricornis (Stall) and Nezera viridula L. (Hemiptera). Int. J. Insect Morphol. Embryol. 1995, 24, 119–132. [Google Scholar] [CrossRef]
- Schoonhoven, L.M.; Henstra, S. Morphology of some rostrum receptors in Dysdercus spp. Neth. J. Zool. 1972, 22, 343–346. [Google Scholar]
- Avé, D.; Frazier, J.L.; Hatfield, L.D. Contact chemoreception in the tarnished plant bug Lygus lineolaris. Entomol. Exp. Appl. 1978, 24, 17–27. [Google Scholar] [CrossRef]
- Hatfield, L.D.; Frazier, J.L. Ultrastructure of the labial tip sensilla of the tarnished plant bug, Lygus lineolaris (P. de Beauvois) (Hemiptera: Miridae). Int. J. Insect Morphol. Embryol. 1980, 9, 59–66. [Google Scholar] [CrossRef]
- Gaffal, K.P. Terminal sensilla on the labium of Dysdercus intermedius Distant (Heteroptera: Pyrrhocoridae). Int. J. Insect Morphol. Embryol. 1981, 10, 1–6. [Google Scholar] [CrossRef]
- Rani, P.U. Sensillary morphology on the rostral apex and their possible role in prey location behaviour of the carnivorous stinkbug, Eocanthecona furcellata (Wolff) (Heteroptera: Pentatomidae). Acta Zool. 2009, 90, 246–256. [Google Scholar] [CrossRef]
- Baker, G.T.; Chen, X.P.; Ma, P.W.K. Labial tip sensilla of Blissus leucopterus (Hemiptera: Blissidae): Ultrastructure and behavior. Insect Sci. 2008, 15, 271–275. [Google Scholar] [CrossRef]
- Garzo, E.; Bonani, J.P.; Lopes, J.R.S.; Fereres, A. Morphological description of the mouthparts of the Asian citrus psyllid, Diaphorina citri Kuwayama (Hemiptera: Psyllidae). Arthropod Struct. Dev. 2012, 41, 79–86. [Google Scholar] [CrossRef] [PubMed]
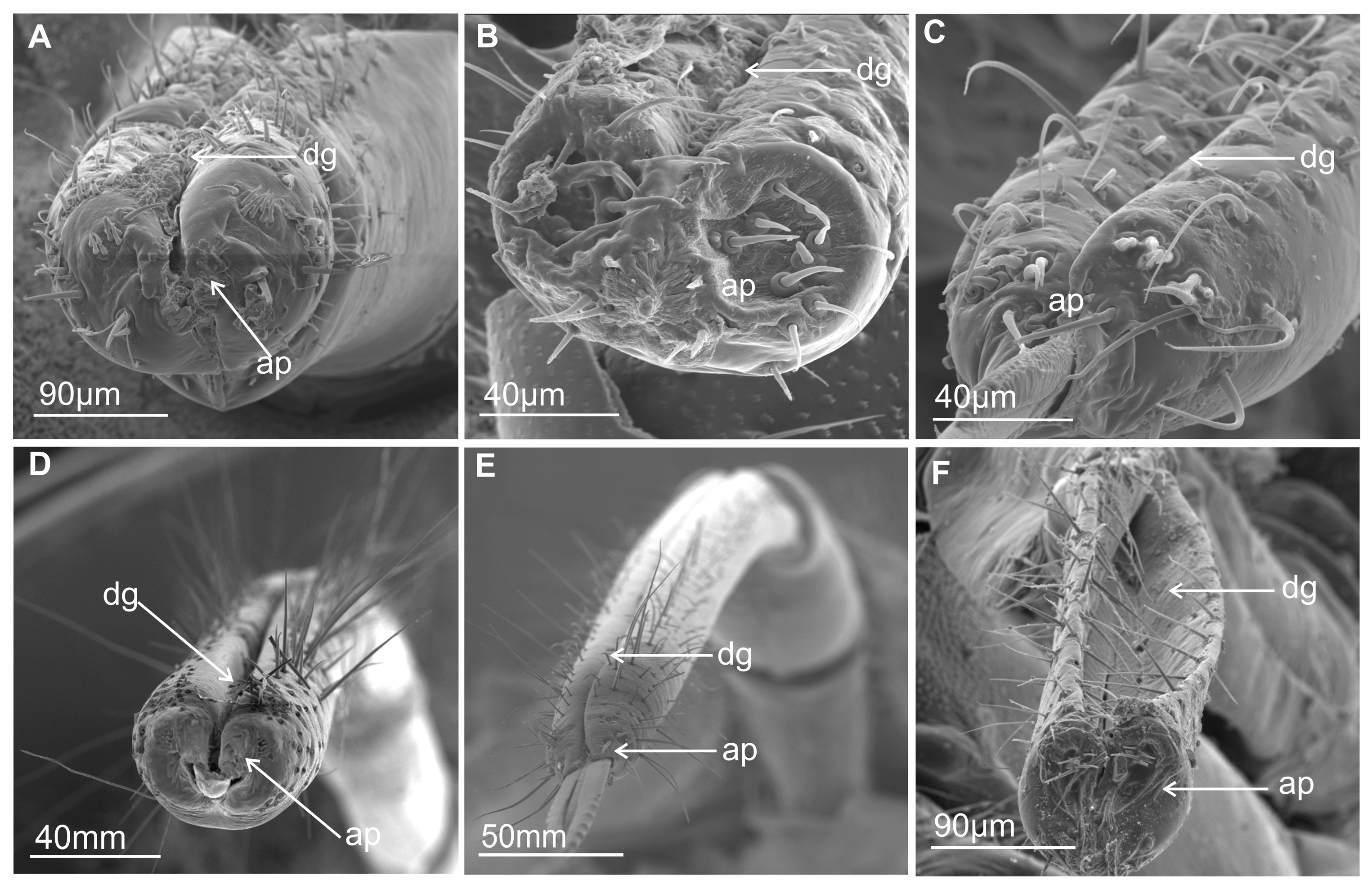

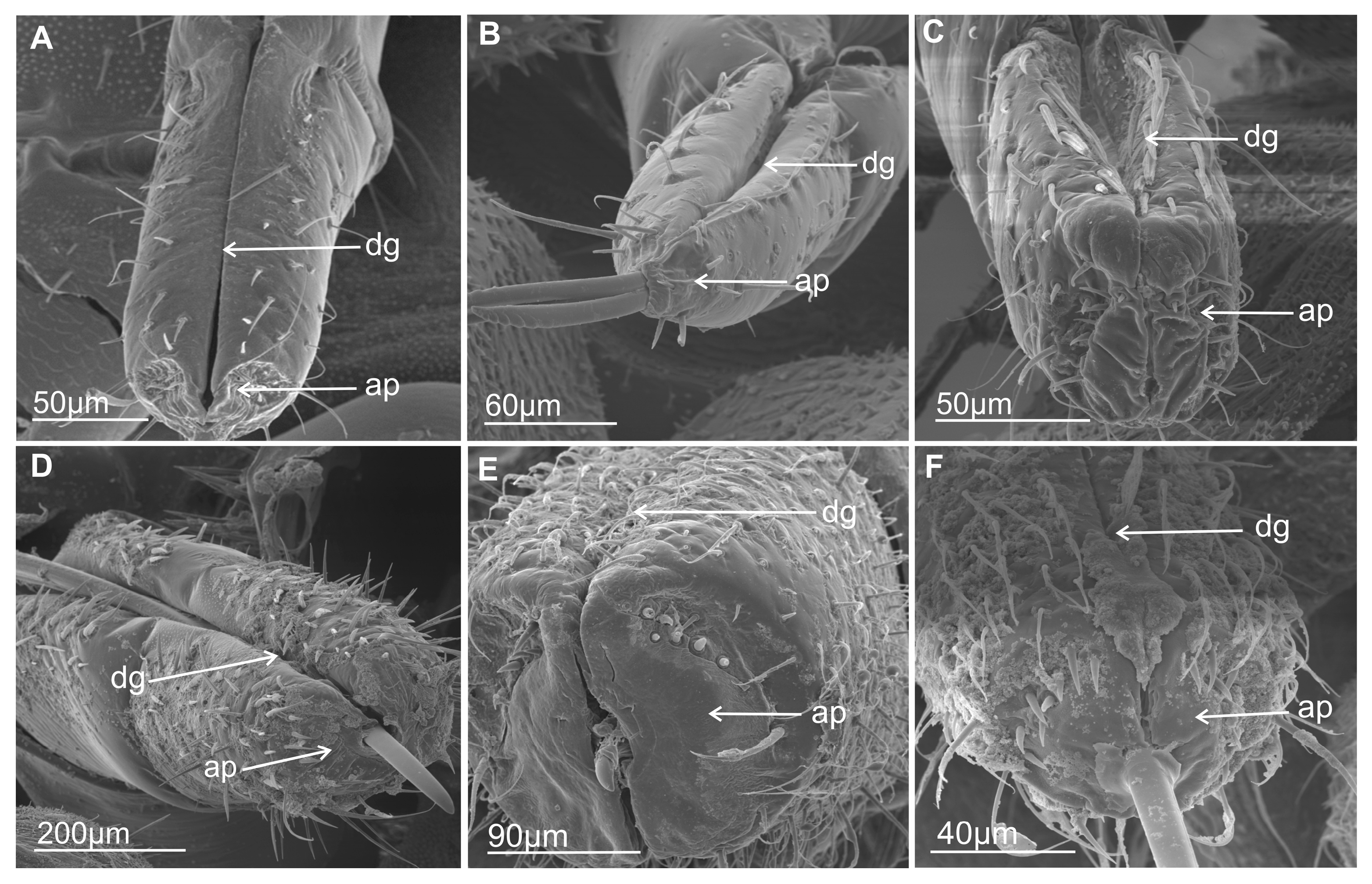
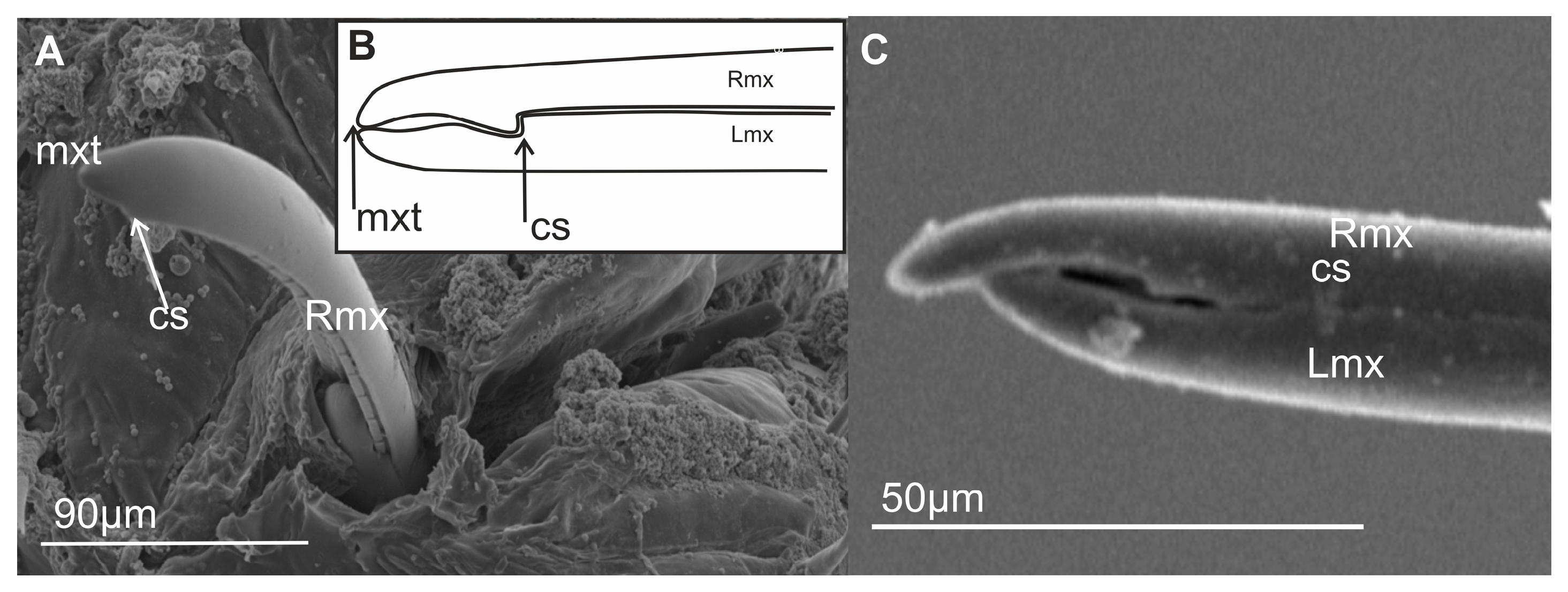
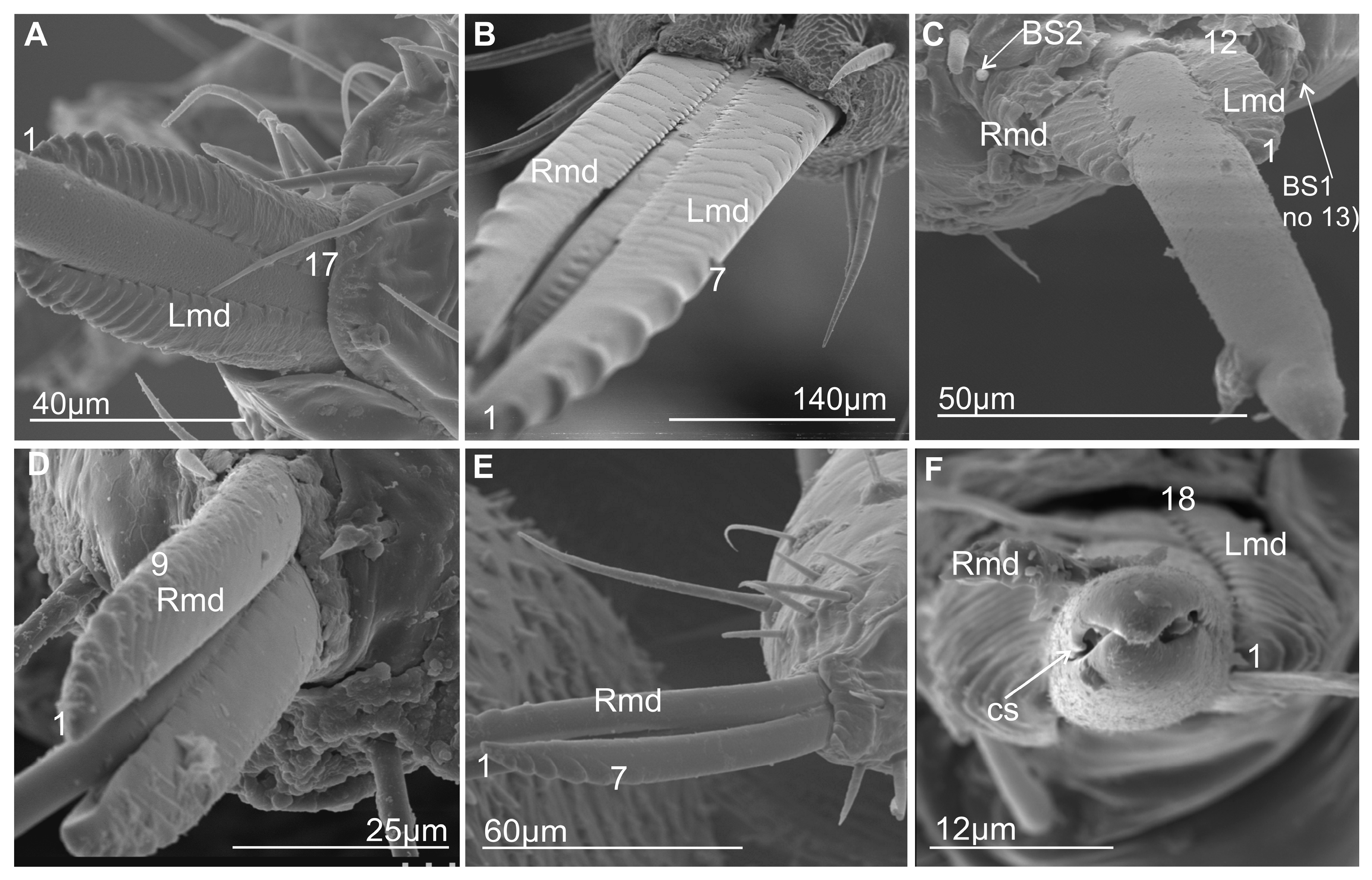




| Superfamily | Family | Species |
|---|---|---|
| Cicadoidea | Tettigarctidae | Tettigarcta crinita Disant, 1883 Proarna insignis Distant, 1881 |
| Cicadidae | Cicadetta podolica (Eichwald 1830) | |
| Cercopoidea | Aphrophoridae | Lepyronia coleoptrata (Linnaeus, 758) Aphrophora costalis Matsumura, 1903 |
| Cercopidae | Prosapia simulans (Walker, 1858) | |
| Clastopteridae | Clastoptera sp. Germar, 1839 | |
| Epipygidae | Eicissus decipiens Fowler, 1897 | |
| Machaerotidae | Machaerota pandata Distant, 1916 | |
| Membracoidea | Melizoderidae | Melizoderes sp. Spinola, 1850 |
| Myerslopiidae | Myerslopia sp. Evans, 1947 | |
| Aetalionidae | Aetalion sp. Latreille, 1810 | |
| Membracidae | Stictopelta nova Goding, 1892 | |
| Tolania sp. Stål, 1858 | ||
| Ulopidae | Ulopa reticulata (Fabricius, 1794) | |
| Coloborrhis corticina Germar, 1850 | ||
| Cicadellidae | Diodontophorus japonicus (Ishihara, 1957) | |
| Stirellus bicolor (Van Duzee, 1892) | ||
| Balala fulviventris (Walker 1851) Ledra aurita (Linnaeus, 1758) Cicadella viridis (Linnaeus, 1758) Nephotettix modulatus Melichar, 1912 Neotituria kongosana (Matsumura, 1915) Idiocerus stigmaticalis Lewis, 1834 Psammotettix alienus (Dahlbom, 1850) Iassus lanio (Linnaeus, 1761) Macropsis fuscinervis (Boheman, 1845) Strogglyocephalus agrestis (Fallén, 1806) Kyboasca bipunctata (Oshanin, 1871) | ||
| Edwardsiana gratiosa (Boheman, 1852) Eupteryx vitatta (Linnaeus, 1758) Streptanus sordidus (Zetterstedt, 1828) Doratura stylata (Boheman, 1847) |
| PS2 | BS3 | FLS | CS | PS1 | DP | BS1 | BS2 | TS | |
|---|---|---|---|---|---|---|---|---|---|
| species | mm/ µm | mm/ µm | mm/ µm | mm/ µm | mm/ µm | mm/ µm | mm/ µm | mm/ µm | mm/ µm |
| Balala fulviventris | (1) 5.4/11.7 (2) 7.4/16.0 (3) 13.8/30 (4) 9.4/20.0 (5) 8.2/17.0 (6) 8.6/18.6 SA= 8.7/18.8 | ----- | (7) 3.2/6.9 | ----- | ----- | ----- | (8) 16.8/36.5 (9) 15.8/34.4 (10) 16.6/33.9 SA= 16.4/34.9 | Not observed | (11) 44/95 |
| Cicadella viridis | (1) 4.0/6.8 (2) 4.0/6.8 (3) 4.0/6.8 (4) 4.3/6.9 (5) 6.7/11.0 (6) 5.8/9.4 SA= 4.8/7.9 | (7) 3.5/6.0 | (8) 9.7/36.3 | ----- | ----- | ----- | ----- | ----- | (9) 12/45 |
| Nephotettix modulatus | (1) 15.0/11.2 (2) 21.0/15.7 (3) 13.0/9.7 (4) 14.4/10.8 (5) 11.0/8.2 (6) 12.3/9.2 (7) 11.0/8.2 SA= 13.9/10.4 | ----- | (8) 7.8/5.8 | ----- | ----- | ----- | (9) broken (not measured) | ----- | (10) broken (not measured) |
| Stirellus bicolor | (1) 4.9/8.6 (2) 7.3/12.8 (3) 7.1/12.5 (4) 4.9/8.6 (5) 4.9/8.6 (6) 7.2/12.4 (7) 7.4/12.9 (8) 2.6/4.5 SA= 5.7/10.1 | ----- | (9) 2.8/4.9 | ----- | ----- | ----- | (10) broken (not measured) | (11) 1.9/3.3 | (12) broken (not measured) |
| Diodontophorus japonicus | (1) 9.3/7.9 (2) 9.0/8.2 (3) 7.6/6.5 (4) 5.1/4.3 (5) 6.7/5.7 (6) 6.5/5.5 SA= 7.3/6.3 | (7) 11.2/9.6 (8) 9.1/10.7 (9) 14.3/12.5 SA= 1.5/10.9 | (10) 12.1/10.3 | ----- | ----- | ----- | (11) (not measured) (Figure 10E) | (12) 7.7/21 | ----- |
| Myerslopia sp. | (1) 6.8/2.0 (2) 8.2/2.4 (3) 7.8/2.3 (4) 9.5/2.8 (5) 9.0/2.6 (6) 6.4/1.9 (7) 8.4/2.5 (8) 7.5/2.2 (9) 8.5/2.5 (10) 10.1/3.0 SA= 8.2/2.1 | ----- | (11) 18/5.2 | ----- | ----- | ----- | (13) broken (not measured) (Figure 5C) | (12) 16.5/8.7 | ----- |
| Coloborrhis corticina | (1) 5.7/5.0 (2) 4.7/4.1 SA= 5.2/4.5 | (3) 15.2/13.5 (4) 10.4/9.2 (5) 8.4/7.4 SA= 11.3/10.3 | ----- | ----- | ----- | ----- | (6) 6.7/5.9 | (7) 4.4/3.8 | (8) 17.0/25 |
| Stictopelta nova | ----- | (1) 11.8/9.6 (2) 16.2/13.2 (3) 13.4/11.0 (4) 12.1/9.9 (5) 10.7/8.7 SA= 12.8/10.4 | ----- | ----- | ----- | ----- | (6) 12.7/30.0 | (7) 7.3/6.0 | ----- |
| Aetalion sp. | ----- | (1) 8.8/18.0 (2) 11.0/23.0 (3) 15.0/31.0 SA= 8.6/24.0 | ----- | (4) 1.0/2.1 (5) 1.5/3.1 | ----- | ----- | (6) 11.8/25.0 | (7) 10.3/22 | (8) 18.0/38 |
| Melizoderes sp. | ----- | (1) 7.0/16.0 (2) 5.4/12.7 (3) 7.0/16.0 SA= 6.4/14.9 | ----- | (4) 1.0/2.3 (5) 2.4/5.6 | ----- | ----- | (6) 9.6/22.5 | (7) 6.2/14.5 | (8) 8.3/19.5 |
| Lepyronia coleopterata | ----- | (1) 16.6/17.3 (2) 15.5/16.3 (3) 12.7/13.3 (4) 12.2/12.8 (5) 12.6/13.3 (6) 16.0/16.8 (7) 19.5/20.5 SA= 15.0/15.7 | (8) 11.2/12 | ----- | ----- | ----- | (9) 13.0/49.0 (10) 6.5/24.7 | ----- | (11) 25.0/95.0 |
| Machaerota pandata | (1) 8.9/6.6 (2) 8.9/6.6 | (3) 29.1/21.8 (4) 29.1/21.8 (5) 15.1/11.2 SA= 24.0/18.2 | (6) 11.2/8.4 | ----- | ----- | ----- | ----- | (7) 29.5/22 | (8) 22.5/44 |
| Prosapia simulans | (1) 3.0/9.2 (2) 3.0/9.2 (3) 3.0/9.2 (4) 3.0/9.2 (5) 3.5/10.7 (6) 3.0/9.2 (7) 2.8/8.5 (8) 4.8/14.5 SA= 3.2/9.9 | ----- | (9) 10.3/7.3 | ----- | ----- | ----- | (10) 10.2/30.0 (11) 10.2/30.0 | (12) broken (not measured) | ----- |
| Eicissus sp. | ----- | (1) 19.9/10.4 (2) 19.3/10.1 (3) 42.0/22.1 (4) 42.9/22.1 SA= 30.0/16.1 | (7) 14.2/7.4 | (5) 4.7/2.4 (6) 4.5/2.3 | ----- | ----- | ----- | ----- | ----- |
| Clastoptera sp. | (1) 4.5/7.3 (2) 4.6/7.4 (3) 4.2/6.8 (4) 5.6/9.0 SA= 4.7/7.6 | (5) 11.8/19.0 (6) 6.5/10.5 (7) 9.0/14.6 (8) 9.0/14.6 SA= 9.0/14.6 | (9) 5.4/8.7 | ----- | ----- | ----- | (11) 9.3/15.0 (12) 7.7/12.0 | (13) 4.2/6.8 | ----- |
| (10) 1.0/2.6 Dome shaped sensi-llum | |||||||||
| Tettigarcta crinita | ----- | ----- | (7) 6.9/10.8 (8) 8.6/13.5 | ----- | (1) 14/22 (2) 7.2/11 (3) 7.8/12 (4) 8.9/14 (5) 7.6/12 (6) 8.9/14 SA= 9.0/17.8 | ----- | ----- | (9) 4.4/10 | ----- |
| Proarna insignis | ----- | ----- | (5) 6.7/8.3 (6) 4.6/5.7 | (7) 3.6/4.5 | (1) 32/40 (2) 37/46 SA= 34/43 | (3) 9.7/12 (4) 9.7/12 | (8) 20.3/64 (9) 12.7/40 | (10) 7.3/23 | ----- |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brożek, J.; Wegierek, P.; Webb, M.; Stroiński, A. New Insight and Confrontation of the Internal Structure and Sensilla of the Mouthparts of Cicadomorpha (Insecta: Hemiptera). Insects 2025, 16, 1026. https://doi.org/10.3390/insects16101026
Brożek J, Wegierek P, Webb M, Stroiński A. New Insight and Confrontation of the Internal Structure and Sensilla of the Mouthparts of Cicadomorpha (Insecta: Hemiptera). Insects. 2025; 16(10):1026. https://doi.org/10.3390/insects16101026
Chicago/Turabian StyleBrożek, Jolanta, Piotr Wegierek, Mick Webb, and Adam Stroiński. 2025. "New Insight and Confrontation of the Internal Structure and Sensilla of the Mouthparts of Cicadomorpha (Insecta: Hemiptera)" Insects 16, no. 10: 1026. https://doi.org/10.3390/insects16101026
APA StyleBrożek, J., Wegierek, P., Webb, M., & Stroiński, A. (2025). New Insight and Confrontation of the Internal Structure and Sensilla of the Mouthparts of Cicadomorpha (Insecta: Hemiptera). Insects, 16(10), 1026. https://doi.org/10.3390/insects16101026






