Comparison of Hard Tick (Acari: Ixodidae) Fauna in Natural and Anthropogenic Habitats in Croatia
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Field Work and Identification of Sampled Ticks
2.3. Faunal Similarity and Climatic Conditions Assessment
3. Results
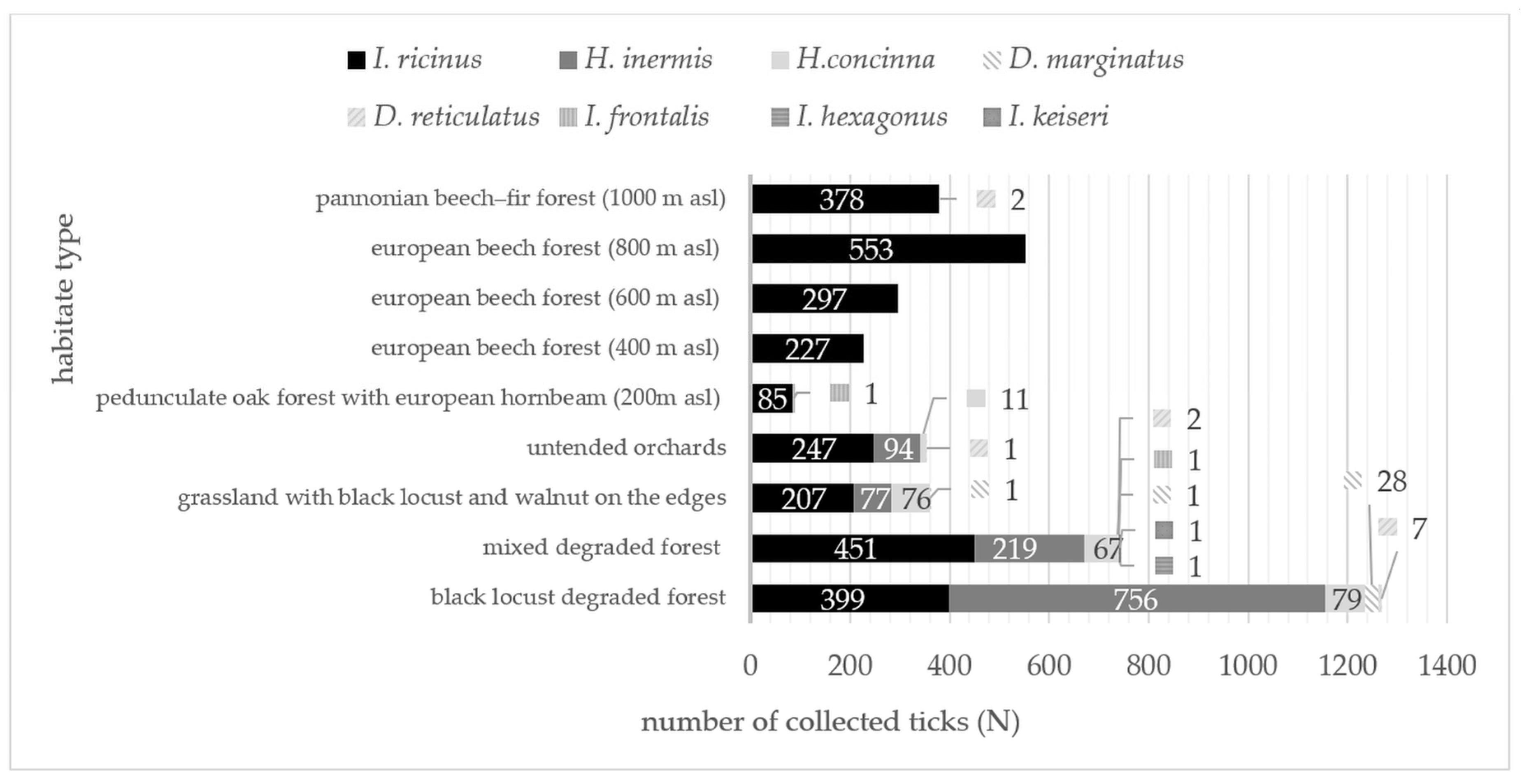
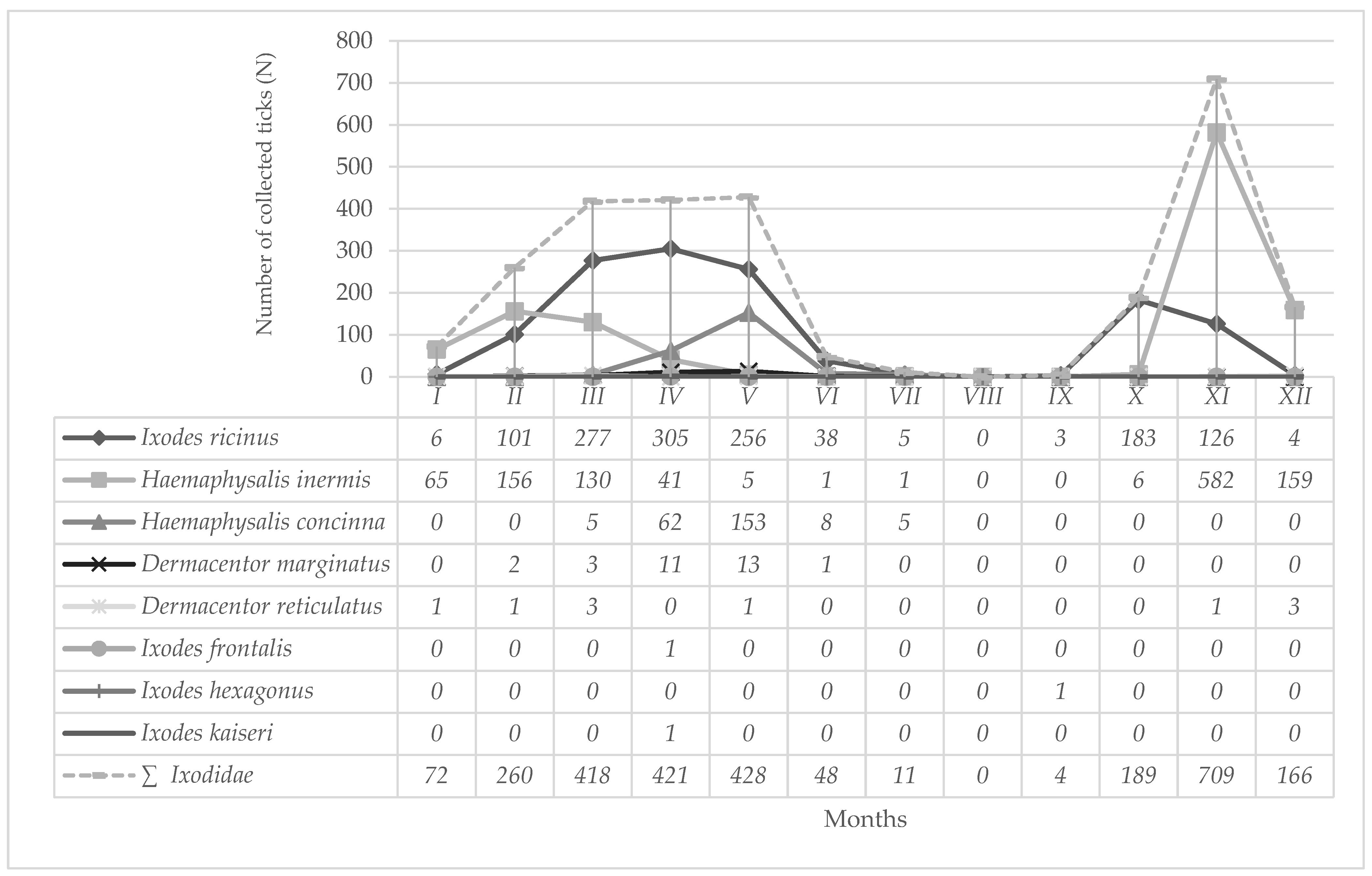
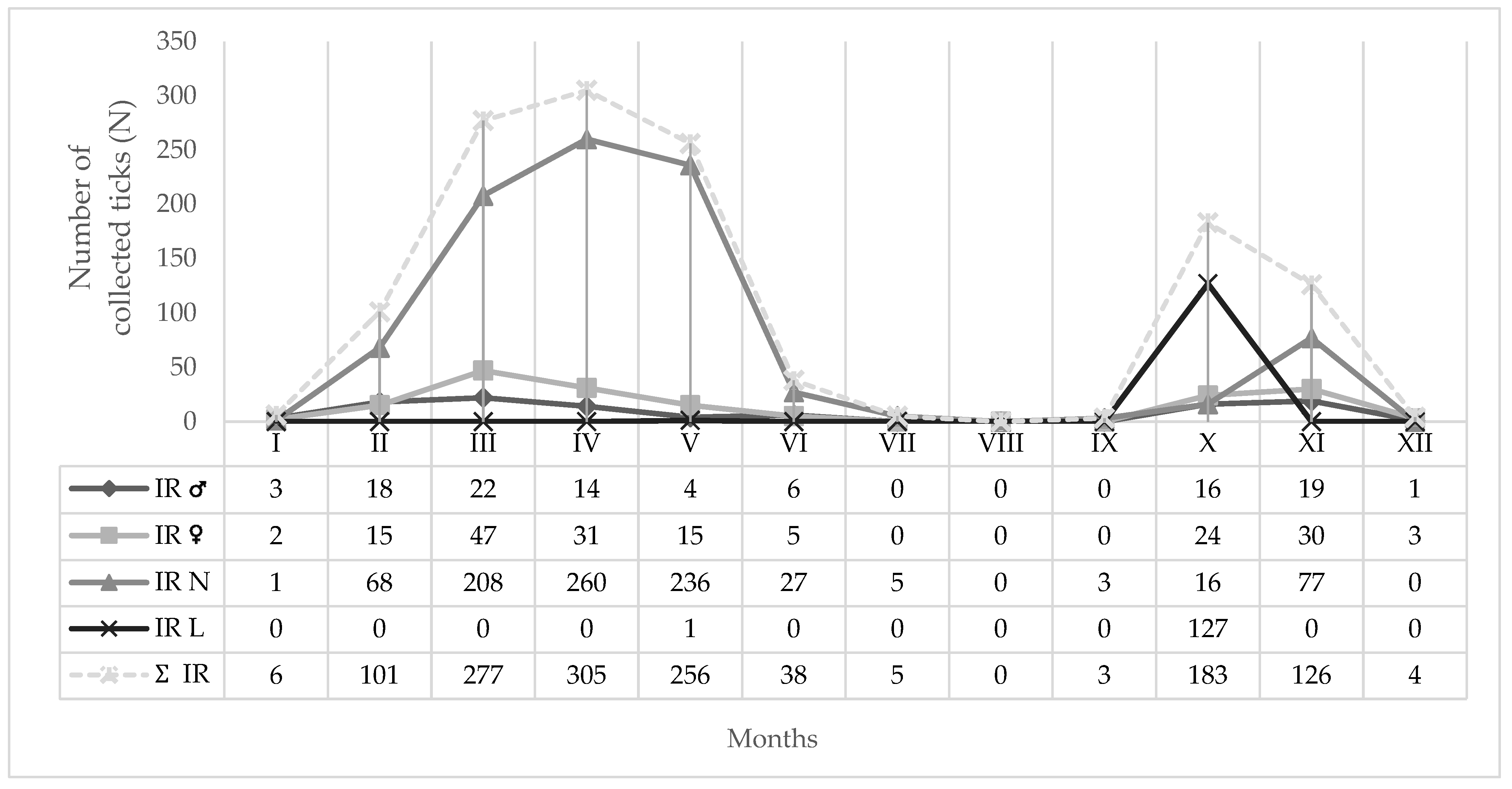
3.1. Identified Tick Species
3.1.1. Faunal Similarity
3.1.2. Correlation Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- de la Fuente, J.; Estrada-Peña, A.; Venzal, J.M.; Kocan, K.M.; Sonenshine, D.E. Overview: Ticks as vectors of pathogens that cause disease in humans and animals. Front. Biosci. 2008, 13, 6938–6946. [Google Scholar] [CrossRef]
- Jongejan, F.; Uilenberg, G. The global importance of ticks. Parasitology 2004, 129, 3–14. [Google Scholar] [CrossRef]
- de la Fuente, J.; Estrada-Peña, A.; Rafael, M.; Almazán, C.; Bermúdez, S.; Abdelbaset, A.E.; Kasaija, P.D.; Kabi, F.; Akande, F.A.; Ajagbe, D.O.; et al. Perception of ticks and tick-borne diseases worldwide. Pathogens 2023, 12, 1258. [Google Scholar] [CrossRef]
- Estrada-Peña, A.; de la Fuente, J. Machine learning algorithms for the evaluation of risk by tick-borne pathogens in Europe. Ann. Med. 2024, 56, 2405074. [Google Scholar] [CrossRef]
- Daniel, M.; Materna, J.; Hönig, V.; Metelka, L.; Danielová, V.; Harčarik, J.; Kliegrová, S.; Grubhoffer, L. Vertical distribution of the tick Ixodes ricinus and tick-borne pathogens in the Northern Moravian mountains correlated with climate warming (Jeseníky Mts.), Czech Republic). Cent. Eur. J. Public Health 2009, 17, 139–145. [Google Scholar] [CrossRef]
- Daniel, M.; Danielová, V.; Kříž, B.; Jirsa, A.; Nožička, J. Shift of the tick Ixodes ricinus and tick-borne encephalitis to higher altitudes in Central Europe. Eur. J. Clin. Microbiol. Infect. Dis. 2003, 22, 327–328. [Google Scholar] [CrossRef] [PubMed]
- Smith, R.; Takkinen, J. Editorial team Collective. Lyme borreliosis: Europe-wide coordinated surveillance and action needed? Euro Surveill. 2006, 11, 2977. [Google Scholar]
- Mysterud, A.; Jore, S.; Østerås, O.; Viljugrein, H. Emergence of tick-borne diseases at northern latitudes in Europe: A comparative approach. Sci. Rep. 2017, 7, 16316. [Google Scholar] [CrossRef]
- Petrović, A.; Stanić, A.; Popović, A.; Ivanović, I.; Supić, D.; Marinković, D.; Bursić, V. Seasonal Dynamics and Physiological Age of Ixodid Ticks Collected from Dogs. Animals 2023, 13, 3026. [Google Scholar] [CrossRef] [PubMed]
- Orkun, Ö.; Karaer, Z.; Çakmak, A.; Nalbantoglu, S. Identification of Tick-Borne Pathogens in Ticks Feeding on Humans in Turkey. PloS Negl. Trop. Dis. 2014, 8, e3067. [Google Scholar] [CrossRef] [PubMed]
- Gray, J.S.; Dautel, H.; Estrada-Peña, A.; Kahl, O.; Lindgren, E. Effects of Climate Change on Ticks and Tick-Borne Diseases in Europe. Inter. Perspect. Infect. Dis. 2009, 2009, 593232. [Google Scholar] [CrossRef]
- Daniel, M.; Danielová, V.; Fialová, A.; Malý, M.; Kríž, B.; Nuttall, P.A. Increased Relative Risk of Tick-Borne Encephalitis in Warmer Weather. Front. Cell Infect. Microbiol. 2018, 8, 90. [Google Scholar] [CrossRef]
- Vilibić-Čavlek, T.; Bogdanić, M.; Savić, V.; Barbić, L.; Stevanović, V.; Kaić, B. Tick-borne Human Diseases around the Globe. In The TBE Book; Dobler, G., Erber, W., Bröker, M., Chitimia-Dobler, L., Schmitt, H.J., Eds.; Global Health Press: Singapore, 2024; Chapter 1; pp. 1–7. [Google Scholar]
- Ogden, N.H.; Tsao, J.I. Biodiversity and Lyme disease: Dilution or amplification? Epidemics 2009, 1, 196–206. [Google Scholar] [CrossRef]
- Swei, A.; Ostfeld, R.S.; Lane, R.S.; Briggs, C.J. Impact of the experimental removal of lizards on Lyme disease risk. Proc. Biol. Sci. 2011, 278, 2970–2978. [Google Scholar] [CrossRef] [PubMed]
- Mikačić, D. A contribution to the study of the biocoenology of ticks (Ixodidae) in north-western Croatia. Vet. Arhiv 1968, 38, 23–27. [Google Scholar]
- Tovornik, D.; Vesenjak-Hirjan, J. A revision of ticks belonging to the Rhipicephalus sanguineus complex (Latreille), collected in the Yugoslav Coastal Region. Biol. Vestn. 1988, 36, 77–84. [Google Scholar]
- Vilibić-Čavlek, T.; Barbić, L.; Mrzljak, A.; Brnić, D.; Klobučar, A.; Ilić, M.; Janev-Holcer, N.; Bogdanić, M.; Jemeršić, L.; Stevanović, V.; et al. Emerging and neglected viruses of zoonotic importance in Croatia. Pathogens 2021, 10, 73. [Google Scholar] [CrossRef] [PubMed]
- Vilibić-Čavlek, T.; Janev-Holcer, N.; Bogdanić, M.; Ferenc, T.; Vujica Ferenc, M.; Krčmar, S.; Savić, V.; Stevanović, V.; Ilić, M.; Barbić, L. Current status of vector-borne diseases in Croatia: Challenges and future prospects. Life 2023, 13, 1856. [Google Scholar] [CrossRef]
- Vucelja, M.; Krčmar, S.; Habuš, J.; Mojčec Perko, V.; Boljfetić, M.; Bjedov, L.; Margaletić, J. Altitudinal distribution, seasonal dynamics and Borrelia burgdorferi sensu lato infections in hard ticks (Acari: Ixodidae) in different forest communities in inland Croatia. Sustainability 2023, 15, 4862. [Google Scholar] [CrossRef]
- Komadina, I. Effect of the Settlements on the Natural Values of Medvednica Nature Park. Undergraduate Thesis, University of Zagreb Faculty of Forestry, Zagreb, Croatia, 2016. Available online: https://urn.nsk.hr/urn:nbn:hr:108:425048 (accessed on 28 August 2025).
- Ćiković, D.; Tutiš, V.; Kralj, J.; Barišić, S.; Kirin, T. Zajednice Ptica, Danje i Noćne Grabljivice Šumskih Ekosustava Parka Prirode Medvednica s Preporukama za Gospodarenje Šumama; Zavod za Ornitologiju HAZU: Zagreb, Croatia, 2007. [Google Scholar]
- Mulić, R.; Petković, B.; Klišmanić, Z.; Jerončić, I. Bolesti koje se prenose krpeljima na području Hrvatske. Liječnički Vjesn. 2011, 133, 89–95. [Google Scholar]
- Bognar, A.; Crkvenčić, I.; Pepeonik, Z.; Riđanović, J.; Roglić, J.; Sić, M.; Šegota, T.; Vresk, M. Geography of SR Croatia. In Eastern Croatia, 1st ed.; Školska Knjiga: Zagreb, Croatia, 1975; pp. 1–256. [Google Scholar]
- Purger, D.; Csiky, J. Biljke Banskog Brda, 1st ed.; University of Pécs: Pécs, Hungary, 2008; pp. 1–58. [Google Scholar]
- Duić, Ž.; Briški, M. Učinak geoloških struktura na hidrogeološke značajke kvartarnih naslaga u Baranji. Rud.-Geološko-Naft. Zb. 2010, 22, 1–9. [Google Scholar]
- Bilušić Dumbović, B.; Kušan, V.; Birov, T.; Rapić, S.; Mesić, Z.; Stresec, D. Krajobrazna Studija Zagrebačke Županije za Razinu Obrade Krajobraznih Tipova/Područja; Arhikon d.o.o., Oikon d.o.o. Institute of Applied Ecology: Zagreb, Croatia, 2013. [Google Scholar]
- Kušan, V. Karta Poljoprivrednog Zemljišta Republike Hrvatske Mjerila 1:5.000; Oikon d.o.o.—Institut za Primijenjenu Ekologiju: Zagreb, Croatia, 2020; pp. 1–285. [Google Scholar]
- Vukelić, J.; Rauš, Đ. Forest Phytocenology and Forest Communities in Croatia, 1st ed.; Faculty of Forestry, University of Zagreb: Zagreb, Croatia, 1998; pp. 173–177. [Google Scholar]
- Topić, J.; Vukelić, J. Priručnik za Određivanje Kopnenih Staništa u Hrvatskoj Prema Direktivi o Staništima EU; Državni Zavod za Zaštitu Prirode: Zagreb, Croatia, 2009; pp. 1–376. [Google Scholar]
- Farkaš-Topolnik, N.; Malić-Limari, S.; Ban Ćurić, T.; Sović, P.; Jurjević Varga, M.; Slukan, D.; Vlašić, A.; Ocvirek, M.; Rosandić, H.; Šantek, S.; et al. Plan Upravljanja Parka Prirode Medvednica 2011–2020; Javna Ustanova Park Prirode Medvednica: Zagreb, Croatia, 2010. [Google Scholar]
- Vrbek, B.; Pernar, N.; Bakšić, D.; Turk, K.; Ivanković, D. Karta Tala Parka Prirode Medvenica, M 1:25.000; Hrvatski Šumarski Institut: Jastrebarsko, Croatia, 2009. [Google Scholar]
- Nikolić, T. Vaskularna Flora Parka Prirode Medvednica. Rasprostranjenost Odabranih Svojti: Ugrožene, Endemične, Proljetnice, Alergogene i dr; Hrvatsko Botaničko Društvo: Zagreb, Croatia, 2004. [Google Scholar]
- Zaninović, K.; Gajić-Čapka, M.; Perčec Tadić, M.; Vučetić, M.; Milković, J.; Bajić, A.; Cindrić, K.; Cvitan, L.; Katušin, Z.; Kaučić, D.; et al. Climate Atlas of Croatia 1961–1990, 1971–2000, 1st ed.; Državni Hidrometeorološki Zavod Zagreb: Zagreb, Croatia, 2008; pp. 1–200. [Google Scholar]
- Šegota, T.; Filipčić, A. Köppenova podjela klima i hrvatsko nazivlje. Geoadria 2003, 8, 17–31. [Google Scholar] [CrossRef]
- Vukelić, J. Croatian Forest Vegetation, 1st ed.; Faculty of Forestry, University of Zagreb: Zagreb, Croatia, 2012; pp. 81–101. [Google Scholar]
- Falco, R.; Fish, D. The comparison of methods for sampling the deer tick, Ixodes dammini, in a Lyme disease endemic area. Exp. Appl. Acarol. 1992, 14, 165–173. [Google Scholar] [CrossRef]
- Estrada-Peña, A.; Gray, J.S.; Kahl, O.; Lane, R.S.; Nijhof, A.M. Research on the ecology of ticks and tick-borne pathogens—Methodological principles and caveats. Front. Cell. Infect. Microbiol. 2013, 3, 29. [Google Scholar] [CrossRef] [PubMed]
- Hillyard, P.D. Ticks of North-West Europe: Keys and Notes for Identification of the Species, 1st ed.; Synopses of the British Fauna (New Series) (52); Field Studies Council: Shrewsbury, UK, 1996; pp. 1–178. [Google Scholar]
- Estrada-Peña, A.; Bouattour, A.; Camicas, J.L.; Walker, A.R. Ticks of domestic animals in the Mediterranean region. In A Guide to Identification of Species, 1st ed.; University of Zaragoza: Zaragoza, Spain, 2004; pp. 1–131. [Google Scholar]
- Estrada- Peña, A.; Mihalca, A.D.; Petney, T.N. Ticks of Europe and North Africa: A Guide to Species Identification, 1st ed.; Springer International Publishing AG: Cham, Szwitzerland, 2017; pp. 1–404. [Google Scholar]
- Hornok, S.; Sándor, A.D.; Beck, R.; Farkas, R.; Beati, L.; Kontschán, J.; Takács, N.; Földvári, G.; Silaghi, C.; Meyer-Kayser, E.; et al. Contributions to the phylogeny of Ixodes (Pholeoixodes) canisuga, I. (Ph.) kaiseri, I. (Ph.) hexagonus and a simple pictorial key for the identification of their females. Parasit. Vectors 2017, 10, 545. [Google Scholar] [CrossRef]
- Durbešić, P. Upoznavanje i istraživanje kopnenih člankonožaca. In Mala Ekološka Biblioteka, Book 4, 1st ed.; Hrvatsko Ekološko Društvo: Zagreb, Croatia, 1988; pp. 1–71. [Google Scholar]
- CMHS (Croatian Meteorological and Hydrological Service). Climate Data. Available online: https://meteo.hr/index_en.php (accessed on 26 June 2025).
- Cindrić, K.; Juras, J.; Pasarić, Z. On precipitation monitoring with theoretical statistical distributions. Theor. Appl. Climatol. 2019, 136, 145–156. [Google Scholar] [CrossRef]
- TIBCO Software Inc. Statistica, version 14.1.0.8.; TIBCO Software Inc.: San Ramon, CA, USA, 2023; Available online: https://tibco.com (accessed on 18 July 2025).
- Evans, J.D. Straightforward Statistics for the Behavioral Sciences; Brooks/Cole Publishing: Pacific Grove, CA, USA, 1996. [Google Scholar]
- Radović, J.; Čivić, K.; Topić, R. Biodiversity of Croatia; State Institute for Nature Protection, Ministry of Culture: Zagreb, Croatia, 2006; ISBN 953-7169-20-0. [Google Scholar]
- Estrada-Peña, A. Distribution, abundance, and habitat preferences of Ixodes ricinus (Acari: Ixodidae) in northern Spain. J. Med. Entomol. 2001, 38, 361–370. [Google Scholar] [CrossRef]
- Walker, A.R.; Alberdi, M.P.; Urquhart, K.A.; Rose, H. Risk factors in habitats of the tick Ixodes ricinus influencing human exposure to Ehrlichia phagocytophila bacteria. Med. Vet. Entomol. 2001, 15, 40–49. [Google Scholar] [CrossRef] [PubMed]
- Čavlović, J. Prva Nacionalna Inventura Šuma Republike Hrvatske; Ministarstvo Regionalnog Razvoja, Šumarstva i Vodnog Gospodarstva, Suizdavač Šumarski Fakultet Sveučilišta u Zagrebu: Zagreb, Croatia, 2010; pp. 91–102. [Google Scholar]
- Krčmar, S. Hard ticks (Acari, Ixodidae) of Croatia. ZooKeys 2012, 234, 19–57. [Google Scholar] [CrossRef]
- Krčmar, S. Diversity, ecology, and seasonality of hard ticks (Acari: Ixodidae) in eastern Croatia. J. Vector Ecol. 2019, 44, 18–29. [Google Scholar] [CrossRef]
- Krčmar, S.; Vereš, M.; Trilar, T. Fauna of hard ticks (Acari: Ixodidae) in different habitats in Croatian part of Baranja. Šumarski List 2014, 138, 309–314. [Google Scholar]
- Cvek, M.; Vucelja, M.; Puškadija, D.; Poljuha, D.; Broznić, D.; Pustijanac, E.; Šantić, M.; Tomić Linšak, D. Ticks as vectors of zoonotic pathogens: Public health aspects in the Istria County. Šumarski List 2025, 149, 11–12. [Google Scholar]
- Mičetić, A. Fauna of Hard Ticks (Fam. Ixodidae) in Private Forests at Vicinity of Ponikve (Municipality Bakar). Master’s Thesis, Universitiy of Zagreb Faculty of Ofrestry and Wood Technology, Zagreb, Croatia, 2021. Available online: https://urn.nsk.hr/urn:nbn:hr:108:673658 (accessed on 18 July 2025).
- Milutinović, M.; Radulović, Ž. Ecological notes on ticks (Acari: Ixodidae) in Serbia (central regions). Acta Vet. 2002, 52, 49–58. [Google Scholar]
- Omeragić, J.; Kapo, N.; Goletić, Š.; Softić, A.; Terzić, I.; Šabić, E.; Škapur, V.; Klarić Soldo, D.; Goletić, T. Investigation of Tick-Borne Pathogens in Ixodes Ticks from Bosnia and Herzegovina. Animals 2024, 14, 2190. [Google Scholar] [CrossRef] [PubMed]
- Huitink, M.; de Rooij, M.; Montarsi, F.; Vittoria Salvati, M.; Obber, F.; Da Rold, G.; Sgubin, S.; Mazzotta, E.; di Martino, G.; Mazzucato, M.; et al. Habitat Suitability of Ixodes ricinus Ticks Carrying Pathogens in North-East Italy. Pathogens 2024, 13, 836. [Google Scholar] [CrossRef] [PubMed]
- Cull, B.; Vaux, A.G.C.; Ottowell, L.J.; Gillingham, E.L.; Medlock, J.M. Tick infestation of small mammals in an English woodland. J. Vector Ecol. 2017, 42, 74–83. [Google Scholar] [CrossRef]
- Milutinović, M.; Radulović, Ž.; Tomanović, S.; Tomanović, Ž. Seasonal distribution of Borreliae in Ixodes ricinus ticks in the Belgrade region, Serbia. Arch. Biol. Sci. Belgrade 2006, 58, 183–186. [Google Scholar] [CrossRef]
- Omeragić, J.; Šerić-Haračić, S.; Klarić Soldo, D.; Kapo, N.; Fejzić, N.; Škapur, V.; Medlock, J. Distribution of ticks in Bosnia and Herzegovina. Tick. Tick-Borne Dis. 2022, 13, 1–5. [Google Scholar] [CrossRef]
- Kovačević, J.; Bučanović, T.; Krčmar, S. Hard tick fauna (Acari: Ixodidae) in different types of habitats in the city of Osijek (Eastern Croatia). Nat. Croat. 2020, 29, 63–72. [Google Scholar] [CrossRef]
- Vucelja, M.; Bjedov, L.; Boljfetić, M.; Klobučar, A.; Krčmar, S.; Borak, S.; Modrić, M.; Juričić, K.; Peleš, V.; Margaletić, J.; et al. Monitoring of Hard Ticks at Urban Recreational Sites in the City of Zagreb from 2016 to 2018. Infektol. Glasn. 2019, 39, 33–39. [Google Scholar] [CrossRef]
- Kahl, O.; Gray, J.S. The biology of Ixodes ricinus with emphasis on its ecology. Ticks Tick Borne Dis. 2023, 14, 102114. [Google Scholar] [CrossRef] [PubMed]
- Burri, C.; Moran Cadenas, F.; Douet, V.; Moret, J.; Gern, L. Ixodes ricinus density and infection prevalence of Borrelia burgdorferi sensu lato along a north-facing altitudinal gradient in the Rhône Valley (Switzerland). Vector Borne Zoonotic Dis. 2007, 7, 50–58. [Google Scholar] [CrossRef]
- Perret, J.L.; Guerin, P.M.; Diehl, P.A.; Vlimant, M.; Gern, L. Darkness induces mobility, and saturation deficit limits questing duration, in the tick Ixodes ricinus. J. Exp. Biol. 2003, 206, 1809–1815. [Google Scholar] [CrossRef]
- PIMNP (Public Institution “Medvednica Nature Park”). Management Plan of Medvednica Nature Park and Associated Protected Areas and Ecological Network Area (PU 5000); Ministry of Economy and Sustainable Development: Zagreb, Croatia, 2023; p. 39. [Google Scholar]
- Krapinec, K. Program Zaštite Divljači za dio Parka Prirode “Medvednica”—Grad Zagreb, za Razdoblje 2010./2011.–2019./2020. Grad Zagreb, Gradski Ured za Poljoprivredu i Šumarstvo: Zagreb, Croatia, 2010; pp. 107–108. [Google Scholar]
- Čehulić, I.J. The Applicability of Digital Sensor Cameras for the Purpose of Recording the Activities of Urban Wildlife Populations in the Medvednica Nature Park. Master’s Thesis, University of Zagreb Faculty of Forestry and Wood Technology, Zagreb, Croatia, 2024. Available online: https://urn.nsk.hr/urn:nbn:hr:108:003271 (accessed on 7 July 2025).
- Gray, J.; Kahl, O.; Zintl, A. What do we still need to know about Ixodes ricinus? Ticks Tick Borne Dis. 2021, 12, 101682. [Google Scholar] [CrossRef] [PubMed]
- Zubriková, D.; Blañarová, L.; Hrklová, G.; Syrota, Y.; Macko, J.; Blahútová, D.; Blažeková, V.; Stanko, M.; Švirlochová, K.; Vichová, B. The Impact of Altitude on Tick-Borne Pathogens at Two Mountain Ranges in Central Slovakia. Pathogens 2024, 13, 586. [Google Scholar] [CrossRef]
- Grillon, A.; Sauleau, E.; Boulanger, N. Risk of Ixodes ricinus Bites in a Population of Forestry Workers in an Endemic Region in France. Pathogens 2024, 13, 696. [Google Scholar] [CrossRef]
- Rizzoli, A.; Silaghi, C.; Obiegala, A.; Rudolf, I.; Hubalek, Z.; Foldvari, G.; Plantard, O.; Vayssier-Taussat, M.; Bonnet, S.; Spitalska, E.; et al. Ixodes ricinus and its transmitted pathogens in urban and peri-urban areas in Europe: New hazards and relevance for public health. Front. Public Health 2014, 2, 251. [Google Scholar] [CrossRef]
- Hansford, K.M.; Wheeler, B.W.; Tschirren, B.; Medlock, J.M. Questing Ixodes ricinus ticks and Borrelia spp. in urban green space across Europe: A review. Zoon Public Health 2022, 69, 153–166. [Google Scholar] [CrossRef]
- Estrada-Peña, A.; Venzal, J.M. Changes in habitat suitability for the tick Ixodes ricinus (Acari: Ixodidae) in Europe (1900–1999). EcoHealth 2006, 3, 154–162. [Google Scholar] [CrossRef]
- Sawczyn-Domańska, A.; Wójcik-Fatla, A. Detection and prevalence of Anaplasma phagocytophilum in Ixodes ricinus ticks in eastern Poland. Ann. Agric. Environ. Med. 2024, 31, 439–441. [Google Scholar] [CrossRef] [PubMed]
- Tytar, V.; Kozynenko, I.; Pupins, M.; Škute, A.; Čeirãns, A.; Nekrasova, O. Species distribution modeling of the castor bean tick, Ixodes ricinus (Linnaeus, 1758), under current and future climates, with a special focus on Ukraine and Latvia. Climate 2024, 12, 184. [Google Scholar] [CrossRef]
- Randolph, S.E.; Green, R.M.; Hoodless, A.N.; Peacey, M.F. An empirical quantitative framework for the seasonal population dynamics of the tick Ixodes ricinus. Int. J. Parasitol. 2002, 32, 979–989. [Google Scholar] [CrossRef] [PubMed]
- Egyed, L.; Elő, P.; Sréter-Láncz, Z.; Széll, Z.; Balogh, Z.; Sréter, T. Seasonal activity and tick-borne pathogen infection rates of Ixodes ricinus ticks in Hungary. Ticks Tick Borne Dis. 2012, 3, 90–94. [Google Scholar] [CrossRef]
- Vilibić-Čavlek, T.; Krčmar, S.; Bogdanić, M.; Tomljenović, M.; Barbić, L.; Rončević, D.; Sabadi, D.; Vucelja, M.; Santini, M.; Hunjak, B.; et al. An Overview of Tick-Borne Encephalitis Epidemiology in Endemic Regions of Continental Croatia, 2017–2023. Microorganisms 2024, 12, 386. [Google Scholar] [CrossRef]
- Schulz, M.; Mahling, M.; Pfister, K. Abundance and seasonal activity of questing Ixodes ricinus ticks in their natural habitats in southern Germany in 2011. J. Vector Ecol. 2014, 39, 56–65. [Google Scholar] [CrossRef]
- Ferroglio, E.; Vada, R.; Occhibove, F.; Fracchia, M.; de Cicco, F.; Palencia, P.; Varzandi, A.R.; Zanet, S. An Integrated Approach to an Emerging Problem: Implementing a Whole Year of Camera Trap Survey in Evaluating the Impact of Wildlife on Tick Abundance. Trans. Emerg. Dis. 2024, 2024, 4064855. [Google Scholar] [CrossRef]
- Hoch, T.; Madouasse, A.; Jacqout, M.; Wongnak, P.; Beugnet, F.; Bournez, L.; Cosson, J.F.; Huard, F.; Moutailer, S.; Plantard, O.; et al. Seasonality of host-seeking Ixodes ricinus nymph abundance in relation to climate. Peer Community J. 2024, 4, e2. [Google Scholar] [CrossRef]
- Jouda, F.; Perret, J.-L.; Gern, L. Ixodes ricinus density, and distribution and prevalence of Borrelia burgdorferi sensu lato infection along an altitudinal gradient. J. Med. Entomol. 2004, 41, 162–169. [Google Scholar] [CrossRef]
- Hornok, S.; Farkas, R. Influence of biotope on the distribution and peak activity of questing ixodid ticks in Hungary. Med. Vet. Entomol. 2009, 23, 41–46. [Google Scholar] [CrossRef]
- Dantas-Torres, F.; Otranto, D. Seasonal dynamics of Ixodes ricinus on ground level and higher vegetation in a preserved wooded area in southern Europe. Vet. Parasitol. 2013, 192, 253–258. [Google Scholar] [CrossRef] [PubMed]
- Walsh, R.; Gormally, M.; Williams, C.; Hamilton, O.; Carbeck, B.; Carlin, C. Ixodes ricinus in Ireland: Exploring the links between environmental factors, host species activity and tick abundance in an area of Europe with limited potential vertebrate hosts. Res. Sq. 2024, 32, 1–18. [Google Scholar] [CrossRef]
- Perez-Eid, C.; Macaigne, F.; Gilot, B. Ecological approach to biotopes of Haemaphysalis (Alloceraea) inermis Birula, 1895, in France. Acarologia 1993, 34, 205–209. [Google Scholar]
- Hornok, S. Allochronic seasonal peak activities of Dermacentor and Haemaphysalis spp. under continental climate in Hungary. Vet. Parasitol. 2009, 163, 366–369. [Google Scholar] [CrossRef]
- Rubel, F.; Brugger, K.; Walter, M.; Vogelgesang, J.R.; Didyk, Y.M.; Fu, S.; Kahl, O. Geographical distribution, climate adaptation and vector competence of the Eurasian hard tick Haemaphysalis concinna. Ticks Tick Borne Dis. 2018, 9, 1080–1089. [Google Scholar] [CrossRef]
- Széll, Z.; Sréter-Lancz, Z.; Márialigeti, K.; Sréter, T. Temporal distribution of Ixodes ricinus, Dermacentor reticulatus and Haemaphysalis concinna in Hungary. Vet. Parasitol. 2006, 141, 377–379. [Google Scholar] [CrossRef]
- Rubel, F.; Brugger, K.; Pfeffer, M.; Chitimia-Dobler, L.; Didyk, Y.M.; Leverenz, S.; Dautel, H.; Kahl, O. Geographical distribution of Dermacentor marginatus and Dermacentor reticulatus in Europe. Ticks Tick Borne Dis. 2016, 7, 224–233. [Google Scholar] [CrossRef]
- Estrada-Peña, A.; Santos-Silva, M.M. The distribution of ticks (Acari: Ixodidae) of domestic livestock in Portugal. Exp. Appl. Acarol. 2005, 36, 233–246. [Google Scholar] [CrossRef] [PubMed]
- Walter, M.; Brugger, K.; Rubel, F. The ecological niche of Dermacentor marginatus in Germany. Parasitol. Res. 2016, 115, 2165–2174. [Google Scholar] [CrossRef]
- Kubiak, K.; Sielawa, H.; Dziekoňska-Rynko, J.; Kubiak, D.; Rydzewska, M.; Dzika, E. Dermacentor reticulatus ticks (Acari: Ixodidae) distribution in north-eastern Poland: An endemic area of tick-borne diseases. Exp. Appl. Acarol. 2018, 75, 289–298. [Google Scholar] [CrossRef]
- Kubiak, K.; Szymańska, H.; Dziekońska-Rynko, J.; Tylkowska, A.; Dmitryjuk, M.; Dzika, E. Tick-borne pathogens in questing adults Dermacentor reticulatus from the Eastern European population (north-eastern Poland). Sci. Rep. 2024, 14, 698. [Google Scholar] [CrossRef]
- Földvári, G.; Široky, P.; Szekeres, S.; Majoros, G.; Sprong, H. Dermacentor reticulatus: A vector on the rise. Parasit. Vectors 2016, 9, 314. [Google Scholar] [CrossRef] [PubMed]
- Vucelja, M. Rodent Control (Rodentia, Murinae, Arvicolinae) in Pedunculate Oak Forests (Quercus robur L.)—Integrated Approach and Zoonotic Aspect, 1st ed.; Faculty of Forestry, University of Zagreb: Zagreb, Croatia, 2013; pp. 25–210. [Google Scholar]
- Rubel, F.; Brugger, K.; Belova, O.A.; Kholodilov, I.S.; Didyk, Y.M.; Kurzrock, L.; García-Pérez, A.L.; Kahl, O. Vectors of disease at the northern distribution limit of the genus Dermacentor in Eurasia: D. reticulatus and D. silvarum. Exp. Appl. Acarol. 2020, 82, 95–123. [Google Scholar] [CrossRef]
- Dobec, M.; Golubić, D.; Punda-Polić, V.; Kaeppeli, F.; Sievers, M. Rickettsia helvetica in Dermacentor reticulatus Ticks. Emerg. Infect. Dis. 2009, 15, 98–100. [Google Scholar] [CrossRef]
- Krčmar, S.; Klobučar, A.; Vucelja, M.; Boljfetić, M.; Kučinić, M.; Madić, J.; Cvek, M.; Bruvo Mađarić, B. DNA barcoding of hard ticks (Ixodidae), notes on distribution of vector species and new faunal record for Croatia. Ticks Tick Borne Dis. 2022, 13, 101920. [Google Scholar] [CrossRef] [PubMed]
- Cunze, S.; Glock, G.; Kochmann, J.; Klimpel, S. Ticks on the move—Climate change-induced range shifts of three tick species in Europe: Current and future habitat suitability for Ixodes ricinus in comparison with Dermacentor reticulatus and Dermacentor marginatus. Parasitol. Res. 2022, 121, 2241–2252. [Google Scholar] [CrossRef]
- Zygner, W.; Górski, P.; Wedrychowicz, H. New localities of Dermacentor reticulatus tick (vector of Babesia canis canis) in central and eastern Poland. Pol. J. Vet. Sci. 2009, 12, 549–555. [Google Scholar] [PubMed]
- Jemeršić, L.; Dežđek, D.; Brnić, D.; Prpić, J.; Janicki, Z.; Keros, T.; Roić, B.; Slavica, A.; Terzić, S.; Konjević, D.; et al. Detection and genetic characterization of tick-borne encephalitis virus (TBEV) derived from ticks removed from red foxes (Vulpes vulpes) and isolated from spleen samples of red deer (Cervus elaphus) in Croatia. Ticks Tick Borne Dis. 2014, 5, 7–13. [Google Scholar] [CrossRef]
- Tovornik, D. On the bionomics of the Ixodes (Pholeoixodes) hexagonus Leach, 1815 in Slovenia (Yugoslavia). Biol Vestn. 1987, 35, 101–120. [Google Scholar]
- Tovornik, D. Ixodes (Eschatocephalus) vespertilionis Koch, 1844 (Arachn., Ixodidae) regarding its specific hosts and natural habitats (Slovenia, Yugoslavia). Acta Entomol. Jugosl. 1990, 23, 1–2. [Google Scholar]
- Matak, P. Hard Tick Fauna (Acari: Ixodidae) in Different Types of Habitats in the City of Vinkovci. Master’s Thesis, Sveučilište Josipa Jurja Strossmayera u Osijeku, Odjel za biologiju, Osijek, Croatia, 2023. Available online: https://urn.nsk.hr/urn:nbn:hr:181:936364 (accessed on 18 June 2025).
- Palomar, A.M.; Santibáñez, P.; Mazuelas, D.; Roncero, L.; Santibáñez, S.; Portillo, A.; Oteo, J.A. Role of birds in dispersal of etiologic agents of tick-borne zoonoses, Spain, 2009. Emerg. Infect. Dis. 2012, 18, 1188–1191. [Google Scholar] [CrossRef] [PubMed]
- Heylen, D.J.A.; Krawczyk, A.; Lopes de Carvalho, I.; Núncio, M.S.; Sprong, H.; Norte, A.C. Bridging of cryptic Borrelia cycles in European songbirds. Environ. Microbiol. 2017, 19, 1857–1867. [Google Scholar] [CrossRef]
- Bona, M.; Stanko, M. First records of the tick Ixodes frontalis (Panzer, 1795) (Acari, Ixodidae) in Slovakia. Ticks Tick-Borne Dis. 2013, 4, 478–481. [Google Scholar] [CrossRef] [PubMed]
- Hoogstraal, H.; Kaiser, M.N.; Traylor, M.A.; Guindy, E.; Gaber, S. Ticks (Ixodidae) on birds migrating from Europe and Asia to Africa 1959-61. Bull. World Health Organ. 1963, 28, 235–262. [Google Scholar]
- Norte, A.C.; de Carvalho, I.L.; Ramos, J.A.; Gonçalves, M.; Gern, L.; Núncio, M.S. Diversity and seasonal patterns of ticks parasitizing wild birds in western Portugal. Exp. Appl. Acarol. 2012, 58, 327–339. [Google Scholar] [CrossRef]
- Drehmann, M.; Chitimia-Dobler, L.; Lindau, A.; Frank, A.; Mai, S.; Fachet, K.; Hauck, D.; Knoll, S.; Strube, C.; Lühken, R.; et al. Ixodes frontalis: A neglected but ubiquitous tick species in Germany. Exp. Appl. Acarol. 2019, 78, 79–91. [Google Scholar] [CrossRef]
- Tovornik, D.; Matjašič, M. Infestacija malih sesalcev z iksodidnimi klopi kot funkcija pokrajinskih razmer. Zdrav. Var. 1991, 30, 199–204. [Google Scholar]
- Monks, D.; Fisher, M.; Forbes, N.A. Ixodes frontalis and avian tick-related syndrome in the United Kingdom. J. Small Anim. Pract. 2006, 47, 451–455. [Google Scholar] [CrossRef]
- Gray, J.S. The development and seasonal activity of the tick Ixodes ricinus: A vector of Lyme borreliosis. Rev. Med. Vet. Entomol. 1991, 79, 323–333. [Google Scholar]
- Perret, J.-L.; Rais, O.; Gern, L. Influence of climate on the proportion of Ixodes ricinus nymphs and adults questing in a tick population. J. Med. Entomol. 2004, 41, 361–365. [Google Scholar] [CrossRef]
- Eisen, R.J.; Eisen, L.; Castro, M.B.; Lane, R.S. Environmentally related variability in risk of exposure to Lyme disease spirochetes in northern California: Effect of climatic conditions and habitat type. Environ. Entomol. 2003, 32, 1010–1018. [Google Scholar] [CrossRef]
- Randolph, S.E.; Storey, K. Impact of microclimate on immature tick-rodent host interactions (Acari: Ixodidae): Implications for parasite transmission. J. Med. Entomol. 1999, 36, 741–748. [Google Scholar] [CrossRef] [PubMed]
- Knülle, W.; Rudolph, D. Humidity relationships and water balance of ticks. In Physiology of Ticks; Obenchain, F.D., Galun, R., Eds.; Pergamon Press: Oxford, UK, 1982; pp. 43–70. [Google Scholar]
- Perret, J.L. Computer-Assisted Laboratory and Field Studies of The Host-Finding Behaviour of the Tick Ixodes ricinus (Acarina: Ixodidae): Ecological Implications of Climate and Light. Ph.D. Thesis, Université de Neuchâtel, Neuchâtel, Switzerland, 2002. [Google Scholar]
- Aeschlimann, A. Ixodes ricinus, Linné, 1758 (Ixodoidae, Ixodidae). Essai préliminaire de synthèse sur la biologie de cette espèce en Suisse. Acta Trop. 1972, 29, 321–340. [Google Scholar]
- Gray, J.S. Biology of Ixodes species ticks in relation to tick-borne zoonoses. Wien. Klin. Wochenschr. 2002, 114, 473–478. [Google Scholar]
- García-Álvarez, L.; Palomar, A.M.; Oteo, J.A. Prevention and prophylaxis of tick-bites and tick-borne related diseases. Am. J. Infect. Dis. 2013, 9, 104–116. [Google Scholar] [CrossRef]
- Beck, R.; Vojta, L.; Mrljak, V.; Marinculić, A.; Beck, A.; Živičnjak, T.; Caccio, S.M. Diversity of Babesia and Theileria species in symptomatic and asymptomatic dogs in Croatia. Int. J. Parasitol. 2009, 39, 843–848. [Google Scholar] [CrossRef] [PubMed]
- Beck, A.; Huber, D.; Polkinghorne, A.; Gudan Kurilj, A.; Benko, V.; Mrljak, V.; Reljić, S.; Kusak, J.; Reil, I.; Beck, R. The prevalence and impact of Babesia canis and Theileria sp. in free-ranging grey wolf (Canis lupus) populations in Croatia. Parasit. Vectors 2017, 10, 168. [Google Scholar] [CrossRef]
- Randolph, S.E.; EDEN-TBD Sub-Project Team. Human activities predominate in determining changing incidence of tick-borne encephalitis in Europe. Eurosurveillance 2010, 15, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Dautel, H.; Dippel, C.; Oehme, R.; Hartelt, K.; Schettler, E. Evidence for an increased geographical distribution of Dermacentor reticulatus in Germany and detection of Rickettsia sp. Int. J. Med. Microbiol. 2006, 296, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Karbowiak, G. The occurrence of the Dermacentor reticulatus tick—Its expansion to new areas and possible causes. Ann. Parasitol. 2014, 60, 37–47. [Google Scholar]
- Rieg, S.; Schmoldt, S.; Theilacker, C.; de With, K.; Wölfel, S.; Kern, W.V.; Dobler, G. Tick-borne lymphadenopathy (TIBOLA) acquired in Southwestern Germany. BMC Infect. Dis. 2011, 11, 167. [Google Scholar] [CrossRef]
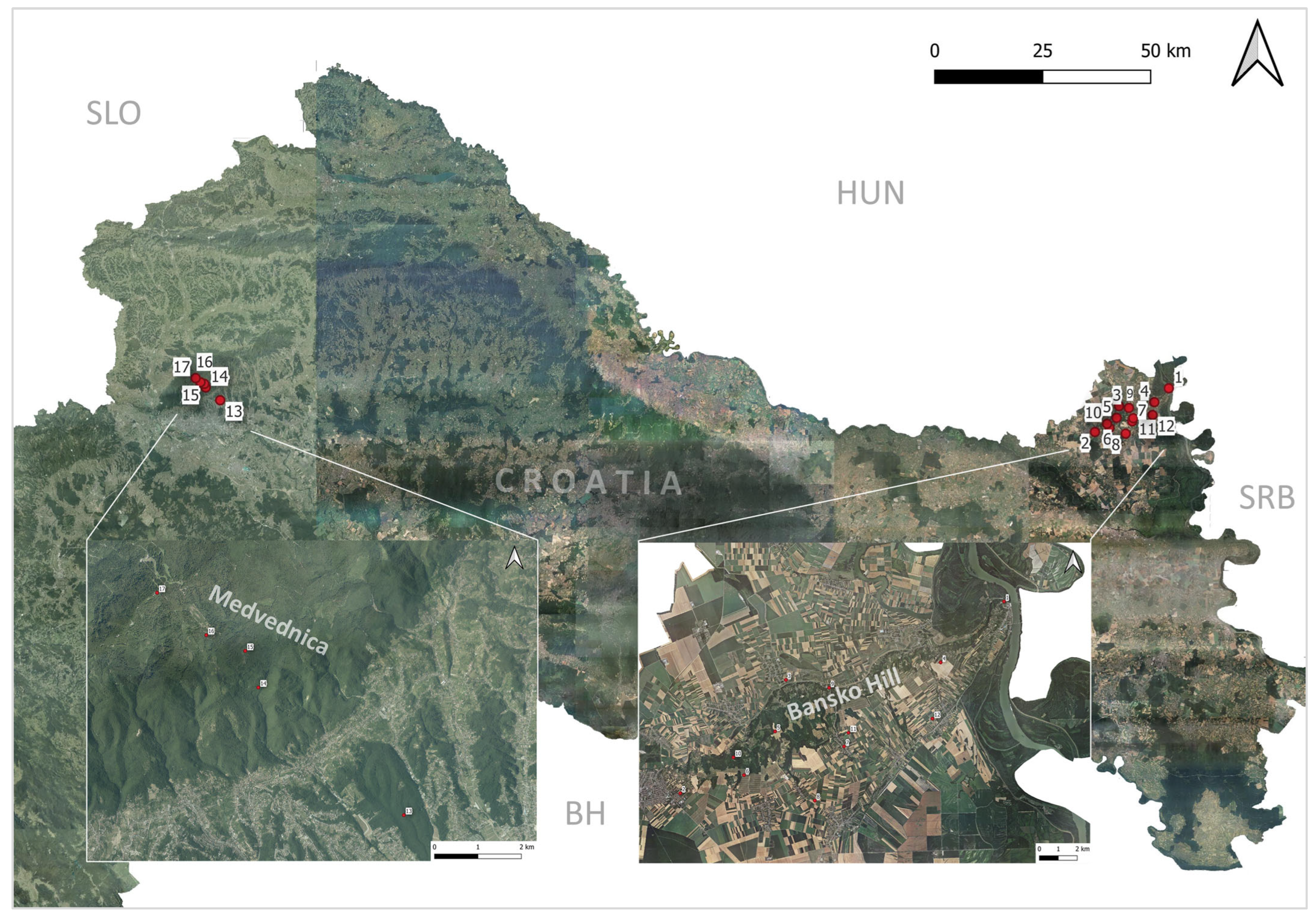
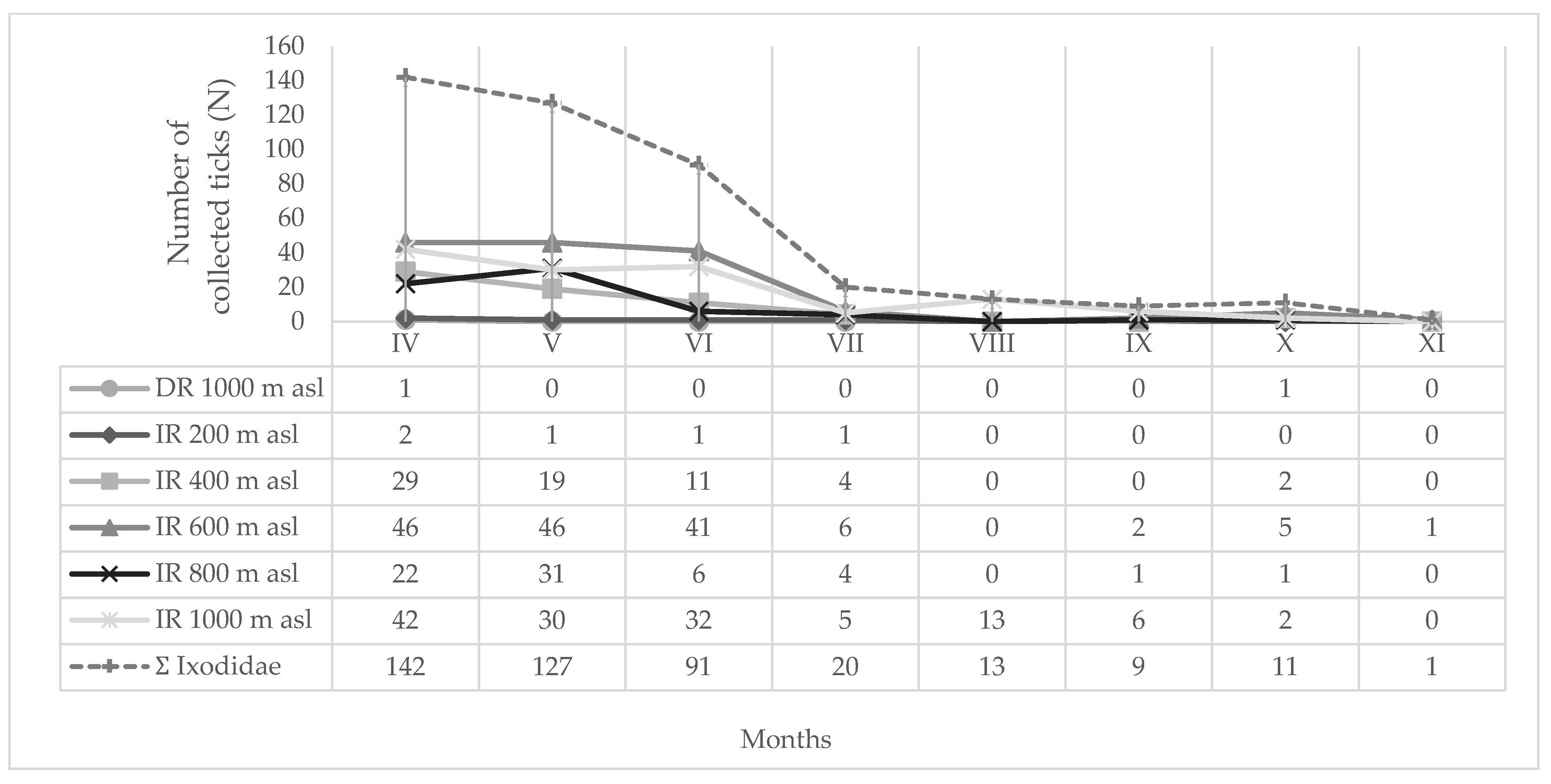
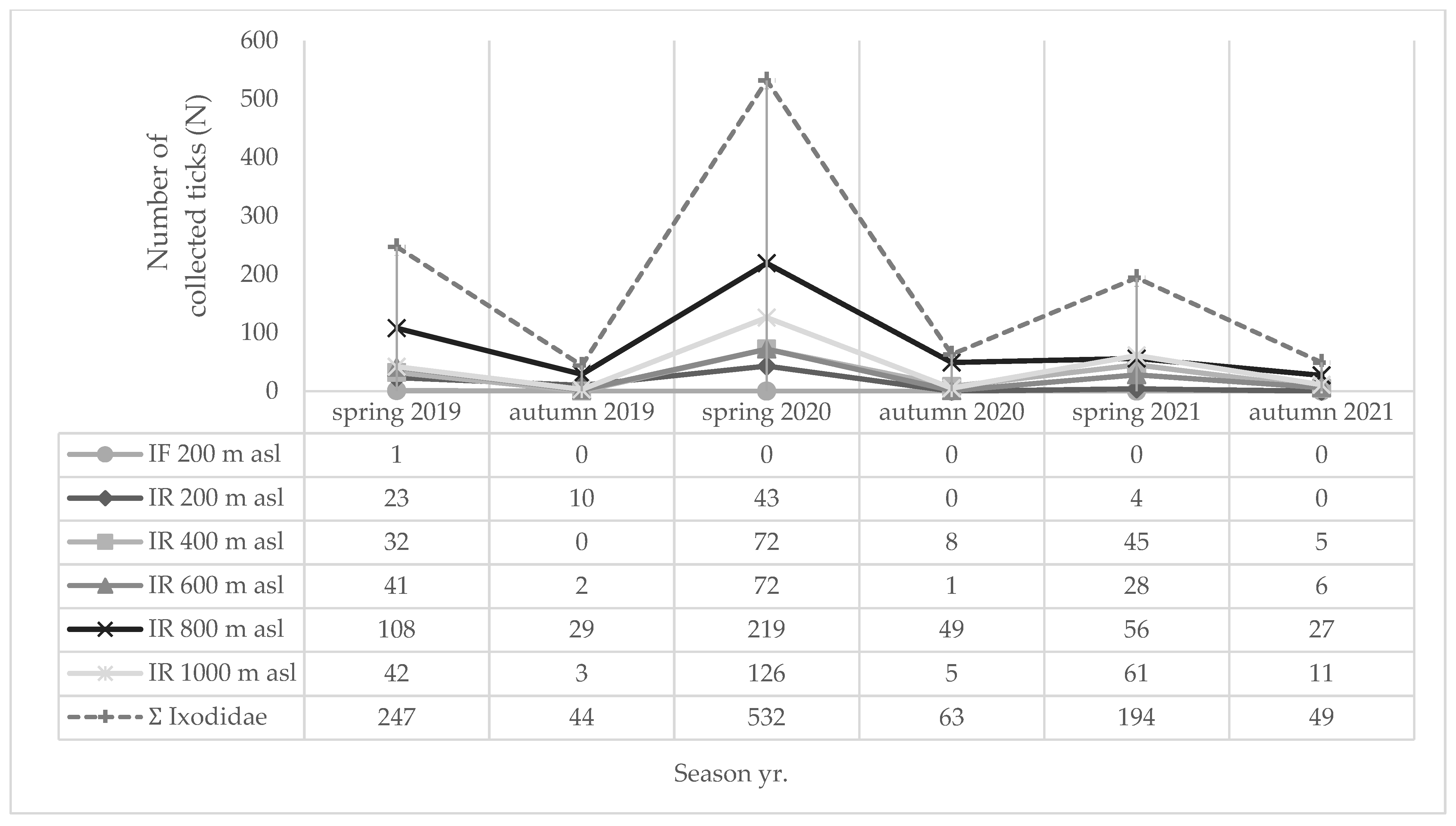
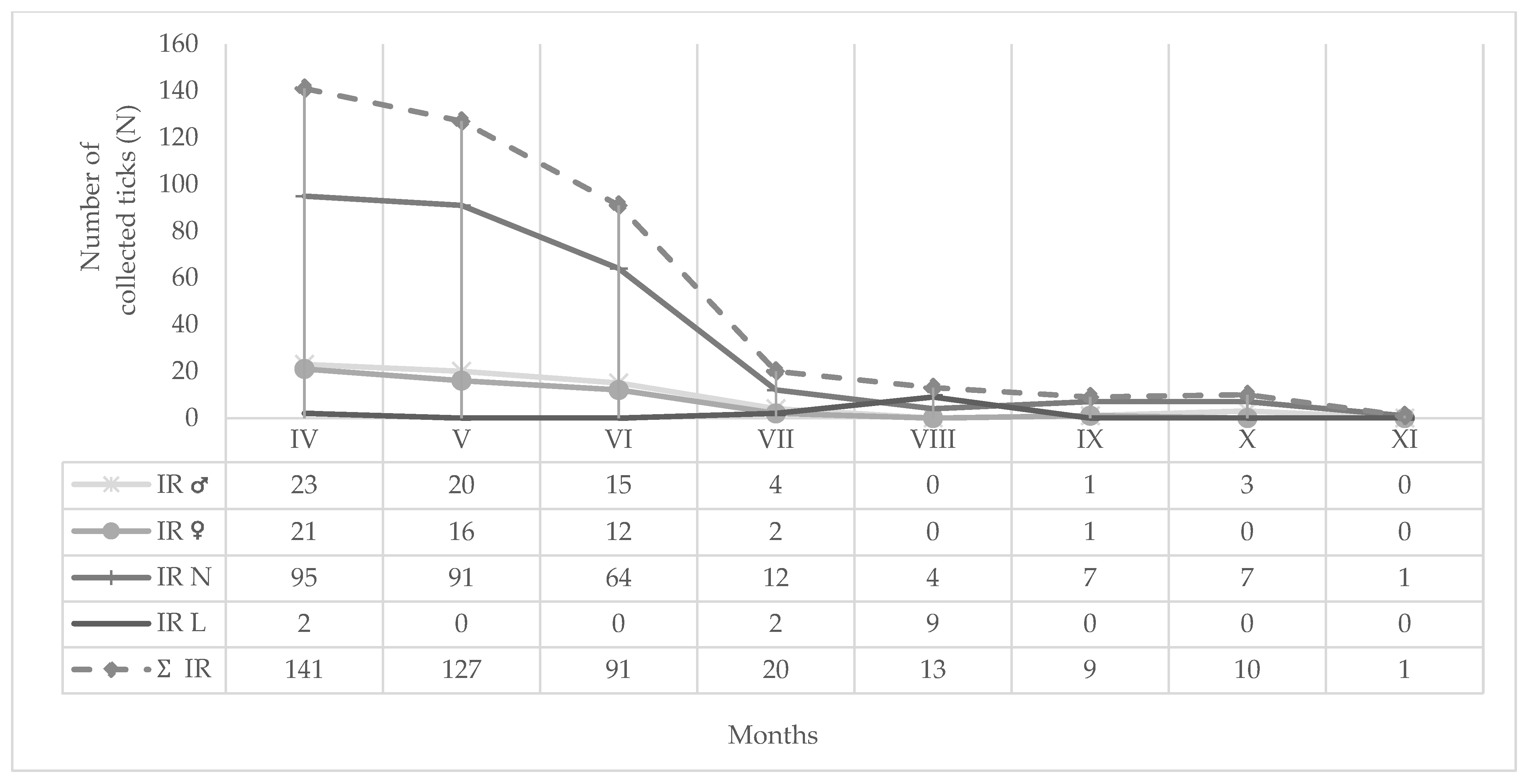
| Location | Tick Sampling Site | Altitude, Latitude (Degree/min/s) | Altitude (m a.s.l.) | Habitat Type |
|---|---|---|---|---|
| Bansko Hill | 01 Batina | N 45.854651 E 18.849288 | 128 | Untended orchards |
| 02 Beli Manastir | N 45.767953 E 18.625718 | 129 | Black locust degraded forest | |
| 03 Branjina | N 45.820343 E 18.699563 | 86 | Grassland with black locust and walnut on the edges | |
| 04 Draž (Vidikovac Trojnaš) | N 45.826566 E 18.805027 | 194 | Untended orchards | |
| 05 Kamenac (Odašiljač Belje) | N 45.796069 E 18.691250 | 239 | Black locust degraded forest | |
| 06 Karanac (Vidikovac Belje) | N 45.775711 E 18.669302 | 199 | Black locust degraded forest | |
| 07 Kotlina | N 45.788050 E 18.737783 | 115 | Mixed degraded forest | |
| 08 Kneževi Vinogradi | N 45.762711 E 18.716909 | 105 | Untended orchards | |
| 09 Podolje | N 45.816064 E 18.728725 | 97 | Untended orchards | |
| 10 Popovac (Rudnik) | N 45.784287 E 18.662404 | 231 | Black locust degraded forest | |
| 11 Suza | N 45.794460 E 18.741029 | 138 | Mixed degraded forest | |
| 12 Zmajevac | N 45.800027 E 18.798309 | 122 | Mixed degraded forest | |
| Medvednica mountain | 13 Medvednica 200 m a.s.l. | N 45 51.174 E 16 01.172 | 229 | Pedunculate oak forest with European hornbeam 1 |
| 14 Medvednica 400 m a.s.l. | N 45 52.748 E 15 58.568 | 422 | European beech forest 2 | |
| 15 Medvednica 600 m a.s.l. | N 45 53.201 E 15 58.330 | 611 | European beech forest 2 | |
| 16 Medvednica 800 m a.s.l. | N 45 53.394 E 15 57.634 | 810 | European beech forest 2 | |
| 17 Medvednica 1000 m a.s.l. | N 45 53.916 E 15 56.753 | 1003 | Pannonian beech–fir forest 3 |
| Species/ Locality | Ixodes ricinus N; % | Haemaphysalis inermis N; % | Haemaphysalis concinna N; % | Dermacentor marginatus N; % | Dermacentor reticulatus N; % | Ixodes frontalis N; % | Ixodes hexagonus N; % | Ixodes kaiseri N; % | Σ Σ N; % |
|---|---|---|---|---|---|---|---|---|---|
| Popovac | 232; 41.7 | 287; 51.6 | 22; 4.0 | 14; 2.5 | 1; 0.2 | 0; 0.0 | 0; 0.0 | 0; 0.0 | 556; 13.0 |
| Zmajevac | 234; 64.3 | 97; 26.6 | 29; 8.0 | 0; 0.0 | 2; 0.5 | 1; 0.3 | 1; 0.3 | 0; 0.0 | 364; 8.5 |
| Branjina | 207; 57.3 | 77; 21.3 | 76; 21.1 | 1; 0.3 | 0; 0.0 | 0; 0.0 | 0; 0.0 | 0; 0.0 | 361; 8.5 |
| Karanac | 91; 25.3 | 222; 61.8 | 43; 12.0 | 3; 0.8 | 0; 0.0 | 0; 0.0 | 0; 0.0 | 0; 0.0 | 359: 8.4 |
| Beli Manastir | 62; 19.6 | 227; 71.8 | 11; 3.5 | 10; 3.2 | 6; 1.9 | 0; 0.0 | 0; 0.0 | 0; 0.0 | 316; 7.4 |
| Suza | 152; 53.1 | 95; 33.2 | 38; 13.3 | 0; 0.0 | 0; 0.0 | 0; 0.0 | 0; 0.0 | 1; 0.3 | 286; 6.7 |
| Draž | 170; 92.9 | 13; 7.1 | 0; 0.0 | 0; 0.0 | 0; 0.0 | 0; 0.0 | 0; 0.0 | 0; 0.0 | 183; 4.3 |
| Podolje | 30; 29.1 | 65; 63.1 | 7; 6.8 | 0; 0.0 | 1; 1.0 | 0; 0.0 | 0; 0.0 | 0; 0.0 | 103; 2.4 |
| Kotlina | 65; 69.9 | 27; 29.0 | 0; 0.0 | 1; 1.1 | 0; 0.0 | 0; 0.0 | 0; 0.0 | 0; 0.0 | 93; 2.2 |
| Kamenac | 14; 36.8 | 20; 52.6 | 3; 7.9 | 1; 2.6 | 0; 0.0 | 0; 0.0 | 0; 0.0 | 0; 0.0 | 38; 0.9 |
| Kneževi Vinogradi | 21; 61.8 | 9; 26.5 | 4; 11.8 | 0; 0.0 | 0; 0.0 | 0; 0.0 | 0; 0.0 | 0; 0.0 | 34; 0.8 |
| Batina | 26; 78.8 | 7; 21.2 | 0; 0.0 | 0; 0.0 | 0; 0.0 | 0; 0.0 | 0; 0.0 | 0; 0.0 | 33; 0.8 |
| Σ = 12 | 1304; 47.8 | 1146; 42.0 | 233; 8.5 | 30; 1.1 | 10; 0.4 | 1; 0.04 | 1; 0.04 | 1; 0.04 | 2726; 100.0 |
| Medvednica (200 m a.s.l.) | 85; 98.8 | 0; 0.0 | 0; 0.0 | 0; 0.0 | 0; 0.0 | 1; 1.2 | 0; 0.0 | 0; 0.0 | 86; 2.0 |
| Medvednica (400 m a.s.l.) | 227; 100.0 | 0; 0.0 | 0; 0.0 | 0; 0.0 | 0; 0.0 | 0; 0.0 | 0; 0.0 | 0; 0.0 | 227; 5.3 |
| Medvednica (600 m a.s.l.) | 297; 100.0 | 0; 0.0 | 0; 0.0 | 0; 0.0 | 0; 0.0 | 0; 0.0 | 0; 0.0 | 0; 0.0 | 297; 7.0 |
| Medvednica (800 m a.s.l.) | 553; 100.0 | 0; 0.0 | 0; 0.0 | 0; 0.0 | 0; 0.0 | 0; 0.0 | 0; 0.0 | 0; 0.0 | 553; 13.0 |
| Medvednica (1000 m a.s.l.) | 378; 99.5 | 0; 0.0 | 0; 0.0 | 0; 0.0 | 2; 0.5 | 0; 0.0 | 0; 0.0 | 0; 0.0 | 380; 8.9 |
| Σ = 5 | 1540; 99.8 | 0; 0.00 | 0; 0.00 | 0; 0.00 | 2; 0.01 | 1; 0.01 | 0; 0.00 | 0; 0.00 | 1543; 100.00 |
| Σ Σ = 17 | 2844; 66.62 | 1146; 26.84 | 233; 5.46 | 30; 0.70 | 12; 0.28 | 2; 0.05 | 1; 0.02 | 1; 0.02 | 4269; 100.00 |
| Species/Developmental Stage | Females N; % | Males N; % | Nymphs N; % | Larvae N; % | Total N; % |
|---|---|---|---|---|---|
| Ixodes ricinus | 172; 13.2 | 103; 7.9 | 901; 69.1 | 128; 9.8 | 1304; 47.8 |
| Haemaphysalis inermis | 828; 72.3 | 314; 27.4 | 4; 0.3 | 0; 0.0 | 1146; 42.0 |
| Haemaphysalis concinna | 10; 4.3 | 18; 7.7 | 166; 71.2 | 39; 16.7 | 233; 8.5 |
| Dermacentor marginatus | 21; 70.0 | 9; 30.0 | 0; 0.0 | 0; 0.0 | 30; 1.1 |
| Dermacentor reticulatus | 8; 80.0 | 2; 20.0 | 0; 0.0 | 0; 0.0 | 10; 0.04 |
| Ixodes frontalis | 1; 100.0 | 0; 0.0 | 0; 0.0 | 0; 0.0 | 1; 0.04 |
| Ixodes hexagonus | 1; 100.0 | 0; 0.0 | 0; 0.0 | 0; 0.0 | 1; 0.04 |
| Ixodes kaiseri | 1; 100.0 | 0; 0.0 | 0; 0.0 | 0; 0.0 | 1; 0.04 |
| ∑ = 8 | 1042; 38.22 | 446; 16.36 | 1071; 39.29 | 167; 6.13 | 2726; 100.00 |
| Species/Developmental Stage | Females N; % | Males N; % | Nymphs N; % | Larvae N; % | Total N; % |
|---|---|---|---|---|---|
| Ixodes ricinus | 107; 6.9 | 148; 9.6 | 961; 62.4 | 324; 21.0 | 1540; 99.8 |
| Dermacentor reticulatus | 1; 50.0 | 1; 50.0 | 0; 0.0 | 0; 0.0 | 2; 0.1 |
| Ixodes frontalis | 0; 0.0 | 0; 0.0 | 1, 100.0 | 0; 0.0 | 1; 0.1 |
| ∑ = 3 | 108; 7.0 | 149; 9.7 | 962; 62.3 | 324; 21.0 | 1543; 100.00 |
| Vegetation Type | Black Locust Degraded Forests | Mixed Degraded Forests | Untended Orchards | Semi-Natural Grasslands |
|---|---|---|---|---|
| Black locust degraded forests | - | 76.92 | 88.88 | 88.88 |
| Mixed degraded forests | - | - | 66.66 | 66.66 |
| Untended orchards | - | - | - | 75 |
| Vegetation Type | Pannonian Beech–Fir Forests | European Beech Forests | Pedunculate Oak Forest with European Hornbeam |
|---|---|---|---|
| Pannonian beech–fir forests | - | 66.66 | 50.00 |
| European beech forests | - | - | 66.66 |
| Species | Temperature (°C) | Relative Humidity (%) | ||
|---|---|---|---|---|
| r * | p-Value | r | p-Value | |
| Ixodes ricinus | −0.1016 | 0.7533 | −0.2538 | 0.4261 |
| Haemaphysalis inermis | −0.5931 | 0.0421 | 0.5551 | 0.0610 |
| Haemaphysalis concinna | 0.1910 | 0.5521 | −0.2667 | 0.4020 |
| Dermacentor marginatus | 0.1216 | 0.7065 | −0.3826 | 0.2197 |
| Dermacentor reticulatus | −0.6289 | 0.0285 | 0.4430 | 0.1492 |
| Ixodes frontalis | 0.0151 | 0.9628 | −0.3590 | 0.2518 |
| Ixodes hexagonus | 0.1594 | 0.6206 | −0.0531 | 0.8697 |
| Ixodes kaiseri | 0.0151 | 0.9628 | −0.3590 | 0.2518 |
| ∑ Ixodidae | −0.4518 | 0.1403 | 0.2143 | 0.5037 |
| I. ricinus Stages | Temperature (°C) | Relative Humidity (%) | I. ricinus m a.s.l | Temperature (°C) | Relative Humidity (%) | ||||
|---|---|---|---|---|---|---|---|---|---|
| r * | p-Value | r | p-Value | r * | p-Value | r | p-Value | ||
| ♂ | 0.1646 | 0.6968 | −0.5137 | 0.1928 | 200 | 0.2028 | 0.6301 | −0.7684 | 0.0259 |
| ♀ | 0.1692 | 0.6887 | −0.5439 | 0.1635 | 400 | 0.0776 | 0.8552 | −0.6473 | 0.0827 |
| N | 0.1887 | 0.6544 | −0.4731 | 0.2364 | 600 | 0.2156 | 0.6081 | −0.3921 | 0.3366 |
| L | 0.4780 | 0.2308 | −0.3515 | 0.3931 | 800 | 0.0255 | 0.9523 | −0.4344 | 0.2822 |
| Ʃ IR | 0.2099 | 0.6178 | −0.5149 | 0.1916 | 1000 | 0.3793 | 0.3541 | −0.5614 | 0.1476 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krčmar, S.; Vucelja, M.; Pezzi, M.; Boljfetić, M.; Margaletić, J.; Bjedov, L. Comparison of Hard Tick (Acari: Ixodidae) Fauna in Natural and Anthropogenic Habitats in Croatia. Insects 2025, 16, 1027. https://doi.org/10.3390/insects16101027
Krčmar S, Vucelja M, Pezzi M, Boljfetić M, Margaletić J, Bjedov L. Comparison of Hard Tick (Acari: Ixodidae) Fauna in Natural and Anthropogenic Habitats in Croatia. Insects. 2025; 16(10):1027. https://doi.org/10.3390/insects16101027
Chicago/Turabian StyleKrčmar, Stjepan, Marko Vucelja, Marco Pezzi, Marko Boljfetić, Josip Margaletić, and Linda Bjedov. 2025. "Comparison of Hard Tick (Acari: Ixodidae) Fauna in Natural and Anthropogenic Habitats in Croatia" Insects 16, no. 10: 1027. https://doi.org/10.3390/insects16101027
APA StyleKrčmar, S., Vucelja, M., Pezzi, M., Boljfetić, M., Margaletić, J., & Bjedov, L. (2025). Comparison of Hard Tick (Acari: Ixodidae) Fauna in Natural and Anthropogenic Habitats in Croatia. Insects, 16(10), 1027. https://doi.org/10.3390/insects16101027









