Effects of Temperature and Extraguild Prey Density on Intraguild Predation of Coccinella septempunctata and Harmonia axyridis
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Insect Rearing
2.2. Effects of Temperature and EG Prey Density on IGP and EG Prey Consumption
2.3. Data Analysis
3. Results
3.1. Effects of Temperature and EG Prey Density on IGP
3.1.1. IGP by First Instar Larvae
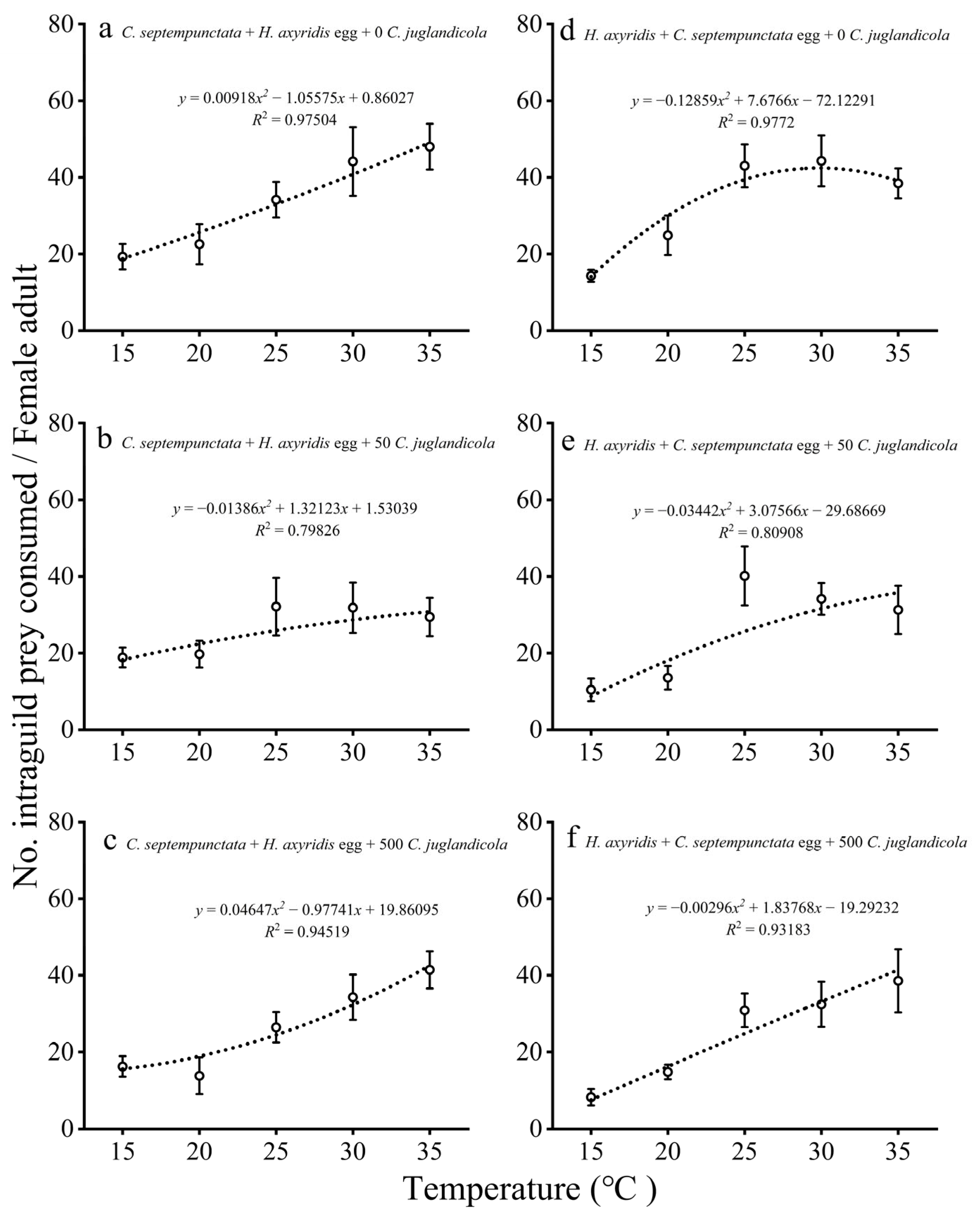
3.1.2. IGP by Adult Females
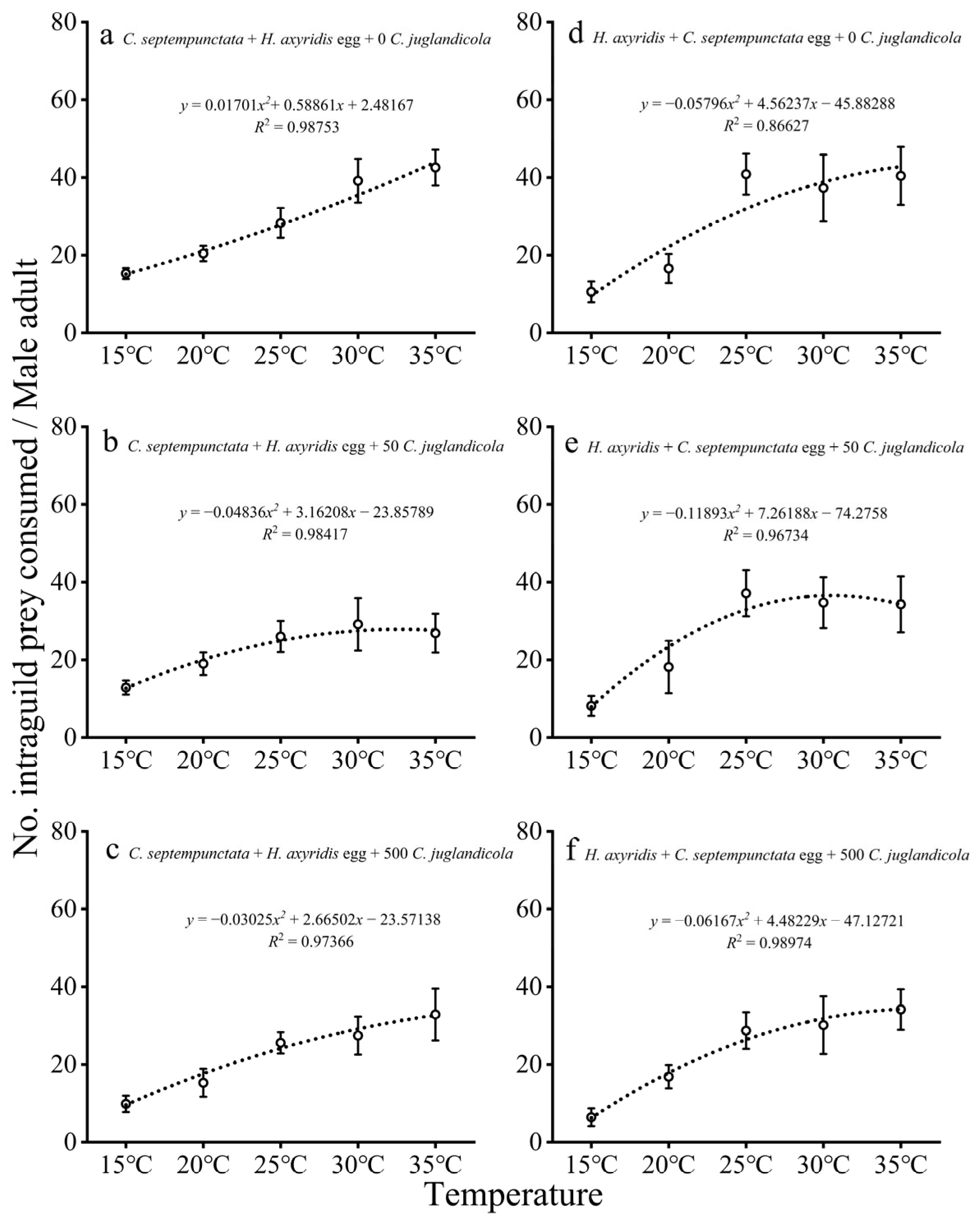
3.1.3. IGP by Adult Males
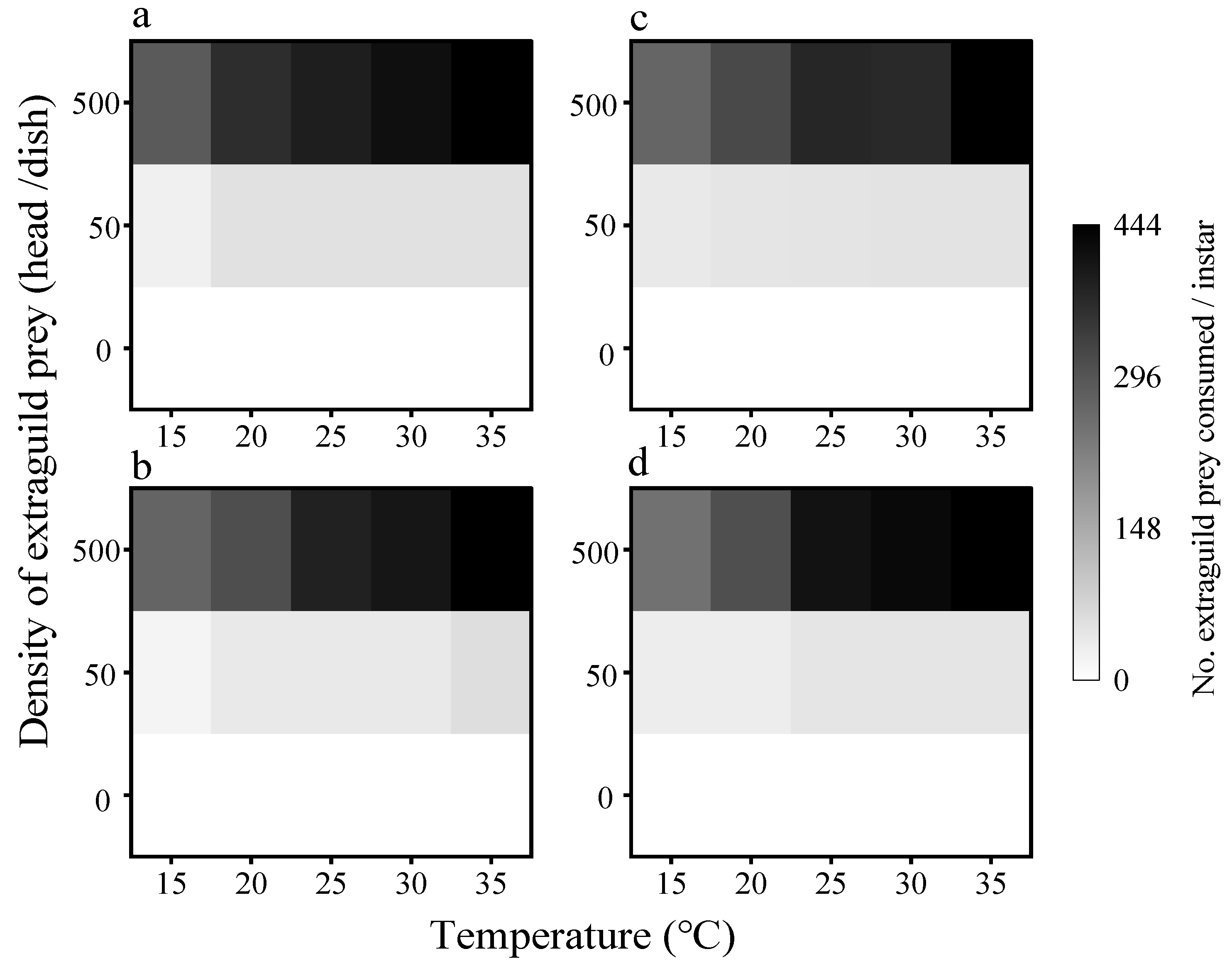
3.2. Consumption of EG Prey
3.2.1. Consumption of EG Prey by First Instar Larvae
3.2.2. Consumption of EG Prey by Adults
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Polis, G.A.; Holt, R.D. Intraguild predation: The dynamics of complex trophic interactions. Trends Ecol. Evol. 1992, 7, 151–154. [Google Scholar] [CrossRef] [PubMed]
- Arim, M.; Marquet, P.A. Intraguild predation: A widespread interaction related to species biology. Ecol. Lett. 2004, 7, 557–564. [Google Scholar] [CrossRef]
- Polis, G.A.; Myers, C.A.; Holt, R.D. The ecology and evolution of intraguild predation: Potential competitors that eat each other. Annu. Rev. Ecol. Syst. 1989, 20, 297–330. [Google Scholar] [CrossRef]
- Felix, S.; Soares, A.O. Intraguild predation between the aphidophagous ladybird beetles Harmonia axyridis and Coccinella undecimpunctata (Coleoptera: Coccinellidae): The role of body weight. Eur. J. Entomol. 2004, 101, 237–242. [Google Scholar] [CrossRef]
- Liu, S.Q. Population dynamic of Chromaphis juglandicola and Control Effect of Dominant Natural Enemies to Chromaphis juglandicola. Master’s Thesis, Xinjiang Agricultural University, Urumqi, China, 2020. [Google Scholar]
- Roy, H.E.; Brown, P.M.; Adriaens, T.; Berkvens, N.; Borges, I.; Clusella-Trullas, S. The harlequin ladybird, Harmonia axyridis: Global perspectives on invasion history and ecology. Biol. Invasions 2016, 18, 997–1044. [Google Scholar] [CrossRef]
- Koch, R.L. The multicolored Asian lady beetle, Harmonia axyridis: A review of its biology, uses in biological control, and non-target impacts. J. Insect Sci. 2003, 3, 32. [Google Scholar] [CrossRef]
- Hamid, M.F.; Hameed, Y.; Sarmad, M.; Abbas, K.; Shahzaib, M.; Zakria, M.; Zaka, S.M. Functional Response of Coccinella septempunctata (Coleoptera: Coccinellidae) to Different Species of Aphids (Hemiptera: Aphididae). J. Kansas Entomol. Soc. 2021, 93, 313–326. [Google Scholar] [CrossRef]
- Kalushkov, P.; Hodek, I. The effects of thirteen species of aphids on some life history parameters of the ladybird Coccinella septempunctata. BioControl 2004, 49, 21–32. [Google Scholar] [CrossRef]
- Adachi-Hagimori, T.; Shibao, M.; Tanaka, H.; Seko, T.; Miura, K. Control of Myzus persicae and Lipaphis erysimi (Hemiptera: Aphididae) by adults and larvae of a flightless strain of Harmonia axyridis (Coleoptera: Coccinellidae) on non-heading Brassica cultivars in the greenhouse. BioControl 2011, 56, 207–213. [Google Scholar] [CrossRef]
- Alarcón, C.J.; Yanqui, D.F.; Moreno, L.S.M.; Nuñez, F.A.; Arostegui, L.E.; Buendía, M.M.A.; Garay, E. Is the seven-point ladybird (Coccinella septempunctata) effective in the biological control of the whitefly (Trialeurodes vaporariorum). Sci. Agropec. 2019, 10, 489–495. [Google Scholar] [CrossRef]
- Tan, X.; Hu, N.; Zhang, F.; Ramirez-Romero, R.; Desneux, N.; Wang, S.; Ge, F. Mixed release of two parasitoids and a polyphagous ladybird as a potential strategy to control the tobacco whitefly Bemisia tabaci. Sci. Rep. 2016, 6, 28245. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Costamagna, A.C.; Landis, D.A.; Difonzo, C.D. Suppression of soybean aphid by generalist predators results in a trophic cascade in soybeans. Ecol. Appl. 2007, 17, 441–451. [Google Scholar] [CrossRef] [PubMed]
- Obrycki, J.J.; Harwood, J.D.; Kring, T.J.; O’Neil, R.J. Aphidophagy by Coccinellidae: Application of biological control in agroecosystems. Biol. Control 2009, 51, 244–254. [Google Scholar] [CrossRef]
- Brahma, S.; Sharma, D.; Kundu, M.; Saha, N.; Saha, G.K.; Aditya, G. Intraguild predation in Heteroptera: Effects of density and predator identity on dipteran prey. Neotrop. Entomol. 2015, 44, 374–384. [Google Scholar] [CrossRef] [PubMed]
- Mirande, L.; Desneux, N.; Haramboure, M.; Schneider, M.I. Intraguild predation between an exotic and a native coccinellid in Argentina: The role of prey density. J. Pest Sci. 2015, 88, 155–162. [Google Scholar] [CrossRef]
- Frances, D.N.; McCauley, S.J. Warming drives higher rates of prey consumption and increases rates of intraguild predation. Oecologia 2018, 187, 585–596. [Google Scholar] [CrossRef]
- Salas Gervassio, N.G.; Pérez–Hedo, M.; Luna, M.G.; Urbaneja, A. Intraguild predation and competitive displacement between Nesidiocoris tenuis and Dicyphus maroccanus, two biological control agents in tomato pests. Insect Sci. 2017, 24, 809–817. [Google Scholar] [CrossRef]
- Liu, S.Q.; Wang, Q.; Gao, G.Z. Harmonia axyridis and Coccinella septempunctata: Predation Comparison to Chromaphis juglandicola. Chin. Agric. Sci. Bul. 2020, 36, 118–122. [Google Scholar]
- Sluss, R.R. Population dynamics of the walnut aphid, Chromaphis juglandicola (Kalt.) in Northern California. Ecology 1967, 48, 41–58. [Google Scholar] [CrossRef]
- Nowierski, R.M.; Gutierrez, A.P. Microhabitat distribution and spatial dispersion patterns of the walnut aphid, Chromaphis juglandicola (Homoptera: Aphididae), in California. Environ. Entomol. 1986, 15, 555–561. [Google Scholar] [CrossRef]
- Jaskiewicz, B.; Kmiec, K. The occurrence of Panaphis juglandis [Goetze] and Chromaphis juglandicola [Kalt.] on walnut under the urban conditions of Lublin. Acta Sci. Pol. Hortorum Cultus 2007, 6, 15–26. [Google Scholar]
- Aqaverdi, N.I.; Inqilab, N.G. Some bioecological pecularities of Panaphis juglandis (Goeze, 1778) and Chromaphis juglandicola (Kaltenbach, 1843) (Hemiptera, Aphididae) the pests of Persian walnut (Juglans regia L.) in Azerbaijan. J. Entomol. Zool. Stud. 2018, 6, 800–803. [Google Scholar]
- Michelbacher, A.E.; Ortega, J.C. A technical study of insects and related pests attacking walnuts. Calif. Agric. Exp. Sta. Bull. 1958, 764, 1–86. [Google Scholar]
- Barnes, M.M.; Moffitt, H.R. A five-year study of the effects of the walnut aphid and the European red mite on Persian walnut productivity in coastal orchards. J. Econ. Entomol. 1978, 7, 71–74. [Google Scholar] [CrossRef]
- Singh, R.; Singh, G. Aphids. In Polyphagous Pests of Crops; Omkar, Ed.; Springer: Singapore, 2021; pp. 105–182. [Google Scholar]
- Michaud, J.P. The ecological significance of aphid cornicles and their secretions. Annu. Rev. Entomol. 2022, 67, 65–81. [Google Scholar] [CrossRef]
- Le Ralec, A.; Anselme, C.; Outreman, Y.; Poirié, M.; Van Baaren, J.; Le Lann, C.; Jacques, J.M. Evolutionary ecology of the interactions between aphids and their parasitoids. Comptes Rendus Biol. 2010, 333, 554–565. [Google Scholar] [CrossRef]
- Carletto, J.; Martin, T.; Vanlerberghe-Masutti, F.; Brévault, T. Insecticide resistance traits differ among and within host races in Aphis gossypii. Pest Manag. Sci. 2010, 66, 301–307. [Google Scholar] [CrossRef]
- Bass, C.; Puinean, A.M.; Zimmer, C.T.; Denholm, I.; Field, L.M.; Foster, S.P.; Gutbrod, O.; Nauen, R.; Slater, R.; Williamson, M.S. The evolution of insecticide resistance in the peach potato aphid, Myzus persicae. Insect Biochem. Mol. Biol. 2014, 51, 41–51. [Google Scholar] [CrossRef]
- Kaleem Ullah, R.M.; Gao, F.; Sikandar, A.; Wu, H. Insights into the Effects of Insecticides on Aphids (Hemiptera: Aphididae): Resistance Mechanisms and Molecular Basis. Int. J. Mol. Sci. 2023, 24, 6750. [Google Scholar] [CrossRef]
- Perrin, M.; Borowiec, N.; Thaon, M.; Siegwart, M.; Delattre, T.; Moiroux, J. Differential influence of temperature on the toxicity of three insecticides against the codling moth Cydia pomonella (L.) and two natural enemies. J. Pest Sci. 2024, 97, 229–241. [Google Scholar] [CrossRef]
- Mohapatra, S.; Padhi, J.; Singh, S. Enhancing yield and economic benefits through sustainable pest management in Okra cultivation. Sci. Rep. 2024, 14, 22220. [Google Scholar] [CrossRef] [PubMed]
- Hill, M.P.; Macfadyen, S.; Nash, M.A. Broad spectrum pesticide application alters natural enemy communities and may facilitate secondary pest outbreaks. PeerJ 2017, 5, e4179. [Google Scholar] [CrossRef] [PubMed]
- Majerus, M.E.N. Ladybird; Harper Collins Publisher: London, UK, 1994. [Google Scholar]
- Ma, K.Z.; Hao, S.G.; Zhao, H.Y.; Kang, L. Intraguild predation in the insect communities. Intraguild predation in the insect communities. Chin. J. Appl. Entomol. 2004, 41, 191–197. [Google Scholar]
- Woodward, G.U.Y.; Hildrew, A.G. Body-size determinants of niche overlap and intraguild predation within a complex food web. J. Anim. Ecol. 2002, 71, 1063–1074. [Google Scholar] [CrossRef]
- Gagnon, A.È.; Brodeur, J. Impact of plant architecture and extraguild prey density on intraguild predation in an agroecosystem. Entomol. Exp. Appl. 2014, 152, 165–173. [Google Scholar] [CrossRef]
- Nóia, M.; Borges, I.; Soares, A.O. Intraguild predation between the aphidophagous ladybird beetles Harmonia axyridis and Coccinella undecimpunctata (Coleoptera: Coccinellidae): The role of intra and extraguild prey densities. Biol. Control 2008, 46, 140–146. [Google Scholar] [CrossRef]
- Hindayana, D.; Meyhöfer, R.; Scholz, D.; Poehling, H.M. Intraguild predation among the hoverfly Episyrphus balteatus de Geer (Diptera: Syrphidae) and other aphidophagous predators. Biol. Control 2001, 20, 236–246. [Google Scholar] [CrossRef]
- Yu, X.L.; Feng, Y.; Fu, W.Y.; Sun, Y.X.; Liu, T.X. Intraguild predation between Harmonia axyridis and Aphidius gifuensis: Effects of starvation period, plant dimension and extraguild prey density. BioControl 2019, 64, 55–64. [Google Scholar] [CrossRef]
- Yu, X.L.; Zhang, Y.J.; Zuo, J.F.; Luo, X.; Zhang, L.; Danzeng, Z.M.; Wang, B.; Xia, L.P.; Zhang, S.Z.; Liu, T.X.; et al. Rising temperatures affect the interspecific interference competition between Harmonia axyridis and Propylea japonica, and their predation rate on Myzus persicae. J. Pest Sci. 2023, 96, 695–709. [Google Scholar] [CrossRef]
- Rogers, T.L.; Gouhier, T.C.; Kimbro, D.L. Temperature dependency of intraguild predation between native and invasive crabs. Ecology 2018, 99, 885–895. [Google Scholar] [CrossRef]
- Sun, J.Q.; Wang, G.H.; Ren, Y.D.; Chen, Q.; Bai, Y.Y. Effect of temperature change on the predation of predatory arthropods from winter-spring fallow waterlogged paddy fields on their prey and the cold tolerance of the predators. Acta Ecol. Sin. 2022, 42, 2943–2961. [Google Scholar]
- Gvoždík, L.; Boukal, D.S. Impacts of predator–induced behavioural plasticity on the temperature dependence of predator–prey activity and population dynamics. J. Anim. Ecol. 2021, 90, 503–514. [Google Scholar] [CrossRef] [PubMed]
- Schmitz, O.J.; Rosenblatt, A.E.; Smylie, M. Temperature dependence of predation stress and the nutritional ecology of a generalist herbivore. Ecology 2016, 97, 3119–3130. [Google Scholar] [CrossRef] [PubMed]
- Reim, C.; Teuschl, Y.; Blanckenhorn, W.U. Size-dependent effects of larval and adult food availability on reproductive energy allocation in the yellow dung fly. Funct. Ecol. 2006, 20, 1012–1021. [Google Scholar] [CrossRef]
- Ingels, B.; De Clercq, P. Effect of size, extraguild prey and habitat complexity on intraguild interactions: A case study with the invasive ladybird Harmonia axyridis and the hoverfly Episyrphus balteatus. BioControl 2011, 56, 871–882. [Google Scholar] [CrossRef]
- Guo, Y.; Lv, J.; Jiang, X.; Wang, B.; Gao, Y.; Wang, E.; Xu, X. Intraguild predation between Amblyseius swirskii and two native Chinese predatory mite species and their development on intraguild prey. Sci. Rep. 2016, 6, 22992. [Google Scholar] [CrossRef]
- Kulkarni, S.S.; Evenden, M.L. Functional response of larval and adult Coccinella septempunctata to eggs and larvae of Plutella xylostella on canola. Entomol. Exp. Appl. 2024, 172, 334–344. [Google Scholar] [CrossRef]
- Schwarz, T.; Frank, T. Aphid feeding by lady beetles: Higher consumption at higher temperature. BioControl 2019, 64, 323–332. [Google Scholar] [CrossRef]
- McCoull, C.J.; Swain, R.; Barnes, R.W. Effect of temperature on the functional response and components of attack rate in Naucoris congrex Stål (Hemiptera: Naucoridae). Aust. J. Entomol. 1998, 37, 323–327. [Google Scholar] [CrossRef]
- Finke, D.L.; Denno, R.F. Intraguild predation diminished in complex-structured vegetation: Implications for prey suppression. Ecology 2002, 83, 643–652. [Google Scholar] [CrossRef]


| IG Predator Stage | IG Prey Density | EG Prey Density | Temperature |
|---|---|---|---|
| First instar larvae | 10 eggs | 0, 3, 9 | 15 °C, 20 °C, 25 °C, 30 °C, 35 °C |
| Female adults | 100 eggs | 0, 50, 500 | 15 °C, 20 °C, 25 °C, 30 °C, 35 °C |
| Male adults | 100 eggs | 0, 50, 500 | 15 °C, 20 °C, 25 °C, 30 °C, 35 °C |
| Intraguild Predator | Intraguild Predator Stage | Intraguild Prey Density | Source of Variation | df | F | p |
|---|---|---|---|---|---|---|
| C. septempunctata | First instar larvae | Temperature | 4 | 9.19 | <0.0001 | |
| 10 H. axyridis eggs | EG prey density | 2 | 4.50 | 0.0052 | ||
| Temperature × EG prey density | 8 | 0.26 | 0.9789 | |||
| Female adults | Temperature | 4 | 11.79 | <0.0001 | ||
| 100 H. axyridis eggs | EG prey density | 2 | 2.32 | 0.0918 | ||
| Temperature × EG prey density | 8 | 0.29 | 0.9733 | |||
| Male adults | Temperature | 4 | 14.02 | <0.0001 | ||
| 100 H. axyridis eggs | EG prey density | 2 | 4.2 | 0.0080 | ||
| Temperature × EG prey density | 8 | 0.64 | 0.8234 | |||
| H. axyridis | First instar larvae | Temperature | 4 | 10.4 | <0.0001 | |
| 10 C. septempunctata eggs | EG prey density | 2 | 5.48 | 0.0016 | ||
| Temperature × EG prey density | 8 | 0.21 | 0.9899 | |||
| Female adults | Temperature | 4 | 18.71 | <0.0001 | ||
| 100 C. septempunctata eggs | EG prey density | 2 | 3.71 | 0.0118 | ||
| Temperature × EG prey density | 8 | 0.50 | 0.9595 | |||
| Male adults | Temperature | 4 | 15.26 | <0.0001 | ||
| 100 C. septempunctata eggs | EG prey density | 2 | 1.36 | 0.2419 | ||
| Temperature × EG prey density | 8 | 0.21 | 0.9895 |
| Intraguild Predator | Intraguild Predator Stage | Intraguild Prey Density | Source of Variation | df | F | p |
|---|---|---|---|---|---|---|
| C. septempunctata | First instar larvae | 10 H. axyridis eggs | Temperature | 4 | 8.81 | <0.0001 |
| EG prey density | 2 | 184.86 | <0.0001 | |||
| Temperature × EG prey density | 8 | 4.15 | <0.001 | |||
| Female adults | 100 H. axyridis eggs | Temperature | 4 | 9.67 | <0.0001 | |
| EG prey density | 2 | 1239.16 | <0.0001 | |||
| Temperature × EG prey density | 8 | 7.00 | <0.0001 | |||
| Male adults | 100 H. axyridis eggs | Temperature | 4 | 10.10 | <0.0001 | |
| EG prey density | 2 | 856.15 | <0.0001 | |||
| Temperature × EG prey density | 8 | 7.39 | <0.0001 | |||
| H. axyridis | First instar larvae | 10 C. septempunctata eggs | Temperature | 4 | 7.71 | <0.0001 |
| EG prey density | 2 | 140.94 | <0.0001 | |||
| Temperature × EG prey density | 8 | 4.35 | <0.0001 | |||
| Female adults | 100 C. septempunctata eggs | Temperature | 4 | 16.97 | <0.0001 | |
| EG prey density | 2 | 1506.69 | <0.0001 | |||
| Temperature × EG prey density | 8 | 14.15 | <0.0001 | |||
| Male adults | 100 C. septempunctata eggs | Temperature | 4 | 18.14 | <0.0001 | |
| EG prey density | 2 | 1452.06 | <0.0001 | |||
| Temperature × EG prey density | 8 | 14.22 | <0.0001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wen, X.; Gao, G. Effects of Temperature and Extraguild Prey Density on Intraguild Predation of Coccinella septempunctata and Harmonia axyridis. Insects 2025, 16, 62. https://doi.org/10.3390/insects16010062
Wen X, Gao G. Effects of Temperature and Extraguild Prey Density on Intraguild Predation of Coccinella septempunctata and Harmonia axyridis. Insects. 2025; 16(1):62. https://doi.org/10.3390/insects16010062
Chicago/Turabian StyleWen, Xia, and Guizhen Gao. 2025. "Effects of Temperature and Extraguild Prey Density on Intraguild Predation of Coccinella septempunctata and Harmonia axyridis" Insects 16, no. 1: 62. https://doi.org/10.3390/insects16010062
APA StyleWen, X., & Gao, G. (2025). Effects of Temperature and Extraguild Prey Density on Intraguild Predation of Coccinella septempunctata and Harmonia axyridis. Insects, 16(1), 62. https://doi.org/10.3390/insects16010062





