Thermal Melanism in Pachnoda iskuulka (Coleoptera: Scarabaeidae: Cetoniinae)
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Rearing and Breeding
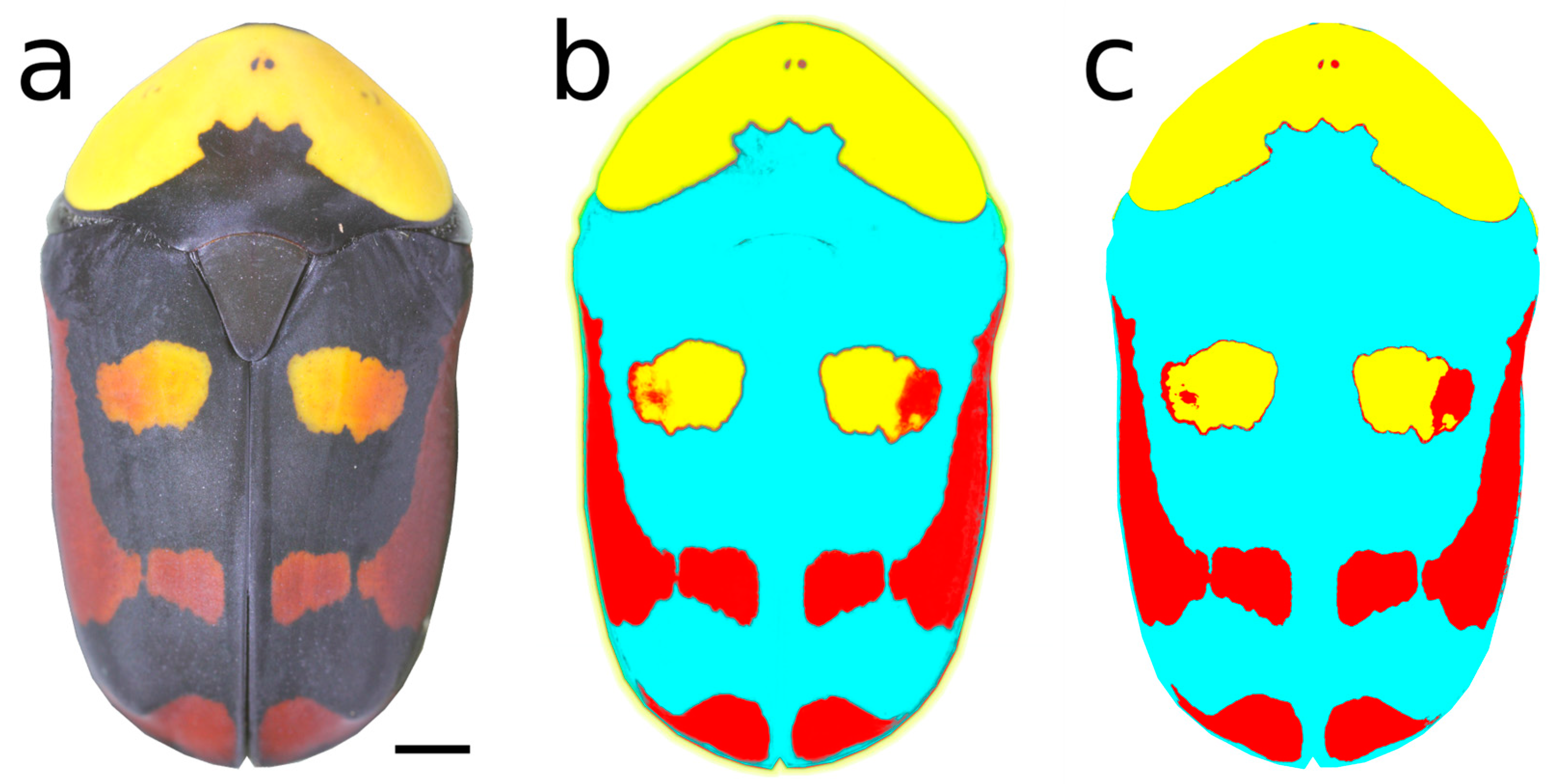
2.2. Photography and Image Analysis
2.3. Statistical Analysis
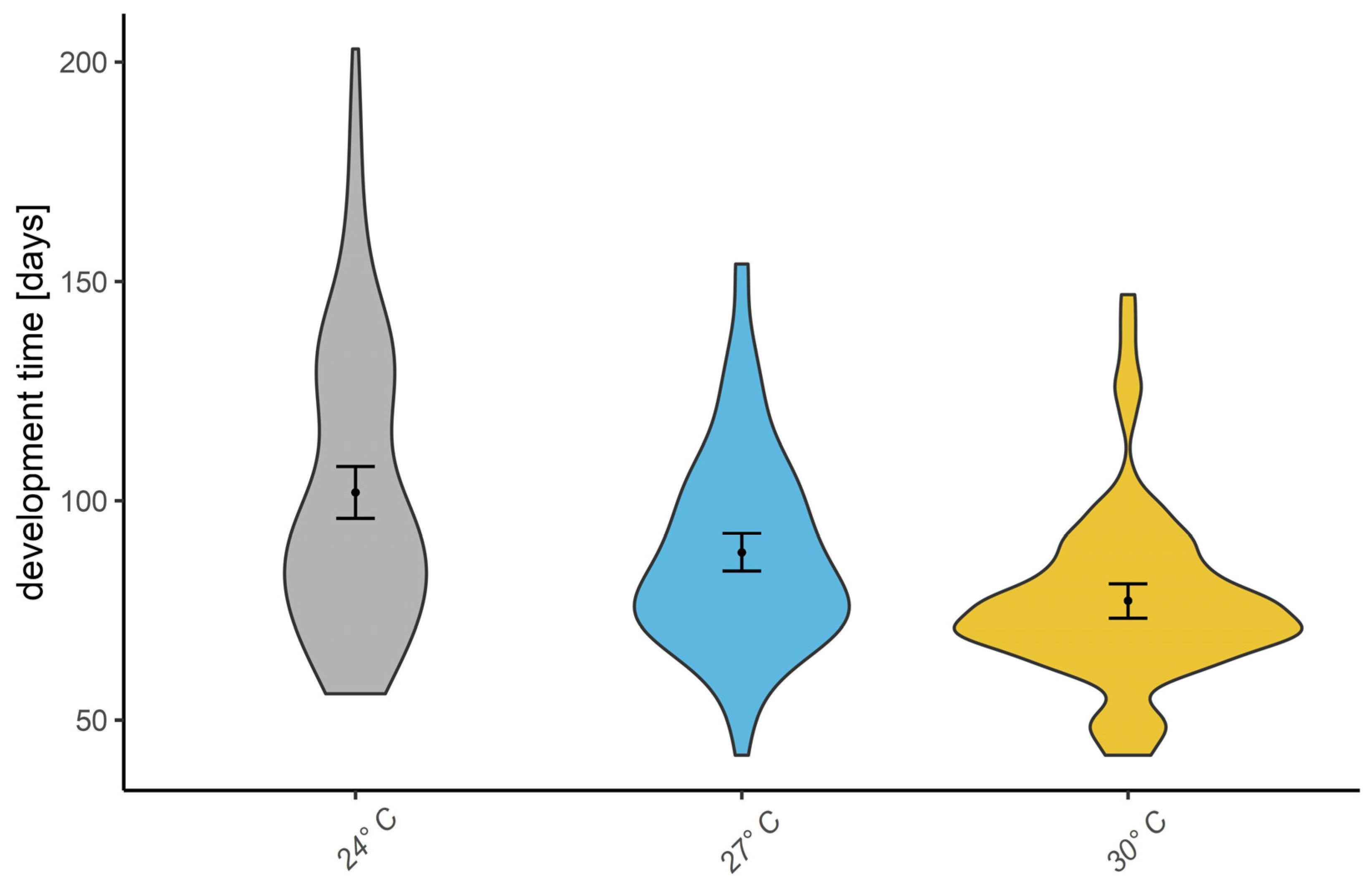
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Klein, E.A. Comprehensive Etymological Dictionary of the English Language; Elsevier Publishing Company: Amsterdam, The Netherlands, 1969; ISBN 0444409300. [Google Scholar]
- Danks, H.V. Insect Life Cycle Polymorphism: Theory, Evolution and Ecological Consequences for Seasonality; Springer Science & Business Media: Berlin, Germany, 1994; ISBN 9048144019. [Google Scholar]
- Lo, N.; Simpson, S.J.; Sword, G.A. Polyphenism in Insects. Cur. Biol. 2011, 21, 738–749. [Google Scholar]
- Kingsolver, J.G.; Wiernasz, D.C. Seasonal Polyphenism in Wing-Melanin Pattern and Thermoregulatory Adaptation in Pieris Butterflies. Am. Nat. 1991, 137, 816–830. [Google Scholar] [CrossRef]
- Clusella-Trullas, S.; Van Wyk, J.H.; Spotila, J.R. Thermal melanism in ectotherms. J. Therm. Biol. 2007, 32, 235–245. [Google Scholar] [CrossRef]
- Forsman, A. Rethinking the thermal melanism hypothesis: Rearing temperature and coloration in pygmy grasshoppers. Evol. Ecol. 2011, 25, 1247–1257. [Google Scholar] [CrossRef]
- Andrén, C.; Nilson, G. Reproductive success and risk of predation in normal and melanistic colour morphs of the adder, Vipera berus. Biol. J. Linn. Soc. 1981, 15, 235–246. [Google Scholar] [CrossRef]
- Tanaka, S.; Saeki, S.; Nishide, Y.; Sugahara, R.; Shiotsuki, T. Body-color and behavioral responses by the mid-instar nymphs of the desert locust, Schistocerca gregaria (Orthoptera: Acrididae) to crowding and visual stimuli. Entomol. Sci. 2016, 19, 391–400. [Google Scholar] [CrossRef]
- Köhler, G.; Samietz, J.; Schnielzeth, H. Morphological and colour morph clines along an altitudinal gradient in the meadow grasshopper Pseudochorthippus parallelus. PLoS ONE 2017, 12, e0189815. [Google Scholar] [CrossRef]
- Berry, A.J.; Willmer, P.G. Temperature and the colour polymorphism of Philaenus spumarius (Homoptera: Aphrophoridae). Ecol. Entomol. 1986, 11, 251–259. [Google Scholar] [CrossRef]
- Quartau, J.A.; Borges, P.A. On the colour polymorphism of Philaenus spumarius (L.) (Homoptera, Cercopidae) in Portugal. Misc. Zool. 1997, 20, 19–30. [Google Scholar]
- Brakefield, P.M. Ecological studies on the polymorphic ladybird Adalia bipunctata in the Netherlands. I. Population biology and geographical variation of melanism. J. Anim. Ecol. 1984, 53, 761–774. [Google Scholar] [CrossRef]
- Brakefield, P.M. Ecological studies on the polymorphic ladybird Adalia bipunctata in the Netherlands. II. Population dynamics, differential timing of reproduction and thermal melanism. J. Anim. Ecol. 1984, 53, 775–790. [Google Scholar] [CrossRef]
- Brakefield, P.M.; Willmer, P.G. The basis of thermal melanism in the ladybird Adalia bipunctata: Differences in reflectance and thermal properties between the morphs. Heredity 1985, 54, 9–14. [Google Scholar] [CrossRef]
- Michie, L.J.; Mallard, F.; Majerus, M.E.N.; Jigglins, F.M. Melanic through nature or nurture: Genetic polymorphism and phenotypic plasticity in Harmonia axyridis. J. Evol. Biol. 2010, 23, 1699–1707. [Google Scholar] [CrossRef]
- Honek, A.; Brown, P.M.J.; Martinkova, Z.; Skuhrovec, J.; Brabec, M.; Burgio, G.; Evans, E.W.; Fournier, M.; Grez, A.A.; Kulfan, J.; et al. Factors determining variation in colour morph frequencies in invasive Harmonia axyridis populations. Biol. Inv. 2020, 22, 2049–2062. [Google Scholar] [CrossRef]
- Stuart-Fox, D.; Newton, E.; Clusella-Trulas, S. Thermal consequences of colour and near-infrared reflectance. Philosoph. Trans. Roy. Soc. B Biol. Sci. 2017, 372, 20160345. [Google Scholar] [CrossRef]
- Nedvěd, O. Jak “se dělá” tečkování u slunéček. Živa 2011, 2011, 34–37. [Google Scholar]
- Gross, J.; Schmolz, E.; Hilker, M. Thermal Adaptations of the Leaf Beetle Chrysomela lapponica (Coleoptera: Chrysomelidae) to Different Climes of Central and Northern Europe. Environ. Entomol. 2004, 33, 799–806. [Google Scholar] [CrossRef]
- Mason, C.W. Structural Colors in Insects II. J. Phys. Chem. 1927, 31, 321–354. [Google Scholar] [CrossRef]
- Davis, A.L.V.; Brink, D.J.; Scholtz, C.H.; Prinsloo, L.C.; Deschodt, C.M. Functional implications of temperature-correlated colour polymorphism in an iridescent, scarabaeine dung beetle. Ecol. Entomol. 2008, 33, 771–779. [Google Scholar] [CrossRef]
- Stanbrook, R.A.; Harris, W.E.; Wheater, C.; Jones, M. Evidence of phenotypic plasticity along an altitudinal gradient in the dung beetle Onthophagus proteus. PeerJ 2021, 9, e10798. [Google Scholar] [CrossRef]
- Tesař, Z. Mecynorrhina machulkai spec. n. (Col. Ceton.). Sborník entomologického oddělení Národního muzea v Praze 1935, 8, 101. [Google Scholar]
- Wu, L.W.; Chen, M.Y.; Li, C.L. Phylogenetic position and morphological polymorphism of the chafer, Clinterocera nigra (Coleoptera: Scarabaeidae: Cetoniinae) from Taiwan. Mitochondrial DNA Part B 2022, 7, 1513–1515. [Google Scholar] [CrossRef] [PubMed]
- Král, D.; Hrůzová, L.; Šípek, P.; Awale, A.I.; Hurre, A.A.; Sommer, D. Pachnoda iskuulka (Coleoptera: Scarabaeidae. Zootaxa 2019, 4604, 482–496. [Google Scholar] [CrossRef] [PubMed]
- Vendl, T.; Kratochvíl, L.; Šípek, P. Ontogeny of sexual size dimorphism in the hornless rose chafer Pachnoda marginata (Coleoptera: Scarabaeidae: Cetoniinae). Zoology 2016, 119, 481–488. [Google Scholar] [CrossRef]
- Vendl, T.; Šípek, P.; Kouklík, O.; Kratochvíl, L. Hidden complexity in the ontogeny of sexual size dimorphism in male-larger beetles. Sci. Rep. 2018, 8, 5871. [Google Scholar] [CrossRef]
- Fletcher, T.J. Farmed deer: New domestic animals defined by controlled breeding. Reprod. Fert. Develop. 2001, 13, 511–516. [Google Scholar] [CrossRef]
- Driscoll, C.A.; MacDonald, D.W.; O’Brien, S.J. From wild animals to domestic pets, an evolutionary view of domestication. Proc. Nat. Acad. Sci. USA 2009, 106, 9971–9978. [Google Scholar] [CrossRef]
- Jakubec, P.; Qubaiová, J.; Novák, M.; Růžička, J. Developmental Biology of Forensically Important Beetle, Necrophila (Calosilpha) brunnicollis (Coleoptera: Silphidae). J. Med. Entomol. 2020, 20, 1–7. [Google Scholar] [CrossRef]
- Vendl, T.; Šípek, P. Immature stages of giants: Morphology and growth characteristics of Goliathus Lamarck, 1801 larvae indicate a predatory way of life (Coleoptera, Scarabaeidae, Cetoniinae). Zookeys 2016, 27, 25–44. [Google Scholar]
- Rueden, C.T.; Schindelin, J.; Hiner, M.C.; Dezonia, B.E.; Walter, A.E.; Arena, E.T.; Eliceiri, K.W. ImageJ2: ImageJ for the next generation of scientific image data. BMC Bioinform. 2017, 18, 529. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2023; Available online: https://www.R-project.org (accessed on 15 December 2024).
- Pohlert, T. PMCMRplus: Calculate Pairwise Multiple Comparisons of Mean Rank Sums Extended. R Package Version 1.9.8. 2023. Available online: https://CRAN.R-project.org/package=PMCMRplus (accessed on 15 December 2024).
- Neter, J.; Kutner, M.; Wasserman, W.; Nachtsheim, C. Applied Linear Statistical Models, 4th ed.; McGraw-Hill/Irwin: New York, NY, USA, 1996; ISBN 007310874X. [Google Scholar]
- Fox, J.; Weisberg, S. An R Companion to Applied Regression, 3rd ed.; Sage: Thousand Oaks, CA, USA, 2019; ISBN 9781544336473. [Google Scholar]
- Régnière, J.; Powell, J.; Bentz, B.; Nealis, V. Effects of temperature on development, survival and reproduction of insects: Experimental design, data analysis and modeling. J. Ins. Physiol. 2013, 58, 634–647. [Google Scholar] [CrossRef] [PubMed]
- Zvereva, E.L.; Kozlov, M.V.; Neuvonen, S. Decrease in feeding niche breadth of Melasoma lapponica (Coleoptera: Chrysomelidae) with increase in pollution. Oecologia 1995, 104, 323–329. [Google Scholar] [CrossRef] [PubMed]
- Zvereva, E.L.; Kozlov, M.V.; Kruglova, O. Colour polymorphism in relation to population dynamics of the leaf beetle, Chrysomela lapponica. Evol. Ecol. 2002, 16, 523–539. [Google Scholar] [CrossRef]
- Zverev, V.; Kozlov, M.V.; Forsman, A.; Zvereva, E.L. Ambient temperatures differently influence colour morphs of the leaf beetle Chrysomela lapponica: Roles of thermal melanism and developmental plasticity. J. Therm. Biol. 2018, 74, 100–109. [Google Scholar] [CrossRef]
- Kozlov, M.V.; Oudendijk, Z.; Forsman, A.; Lanta, V.; Barclay, M.V.; Gusarov, V.I.; Zvereva, E.L. Climate shapes the spatio-temporal variation in colour morph diversity and composition across the distribution range of Chrysomela lapponica leaf beetle. Ins. Sci. 2022, 93, 942–955. [Google Scholar] [CrossRef]
- Knapp, M.; Nedvěd, O. Gender and Timing during Ontogeny Matter: Effects of a Temporary High Temperature on Survival, Body Size and Colouration in Harmonia axyridis. PLoS ONE 2013, 8, e74984. [Google Scholar] [CrossRef]
- Su, W.; Michaud, J.P.; Xiaoling, T.; Murray, L.; Fan, Z. Melanism in a Chinese Population of Harmonia axyridis (Coleoptera: Coccinellidae): A Criterion for Male Investment with Pleiotropic Effects on Behavior and Fertility. J. Ins. Behav. 2013, 26, 679–689. [Google Scholar] [CrossRef]
- De Souza, A.R.; Mayorquin, A.Z.; Sarmiento, C.E. Paper wasps are darker at high elevation. J. Therm. Biol. 2020, 89, 102535. [Google Scholar] [CrossRef]
- Maruyama, T.; Fuerst, P.A. Population bottlenecks and nonequilibrium models in population genetics. II. Number of alleles in a small population that was formed by a recent bottleneck. Genetics 1985, 111, 675–689. [Google Scholar] [CrossRef]
- Clark, R.; Crowe, T.J. The Genus Pachnoda in Ethiopia: Identification, Pest Status and Control of the Species. Institute of Agricultural Research: Addis Abeba, Ethiopia, 1977. [Google Scholar]
- Willi, Y.; Van Buskirk, J.; Hoffmann, A.A. Limits to the Adaptive Potential of Small Populations. Ann. Rev. Ecol. Evol. Syst. 2006, 37, 433–458. [Google Scholar] [CrossRef]
- Mallet, J. Causes and Consequences of a Lack of Coevolution in Müllerian mimicry. Evol. Ecol. 1999, 13, 777–806. [Google Scholar] [CrossRef]
- Bocek, M.; Kusy, D.; Motyka, M.; Bocak, L. Persistence of multiple patterns and intraspecific polymorphism in multi-species Müllerian communities of net-winged beetles. Front. Zool. 2019, 16, 38. [Google Scholar] [CrossRef] [PubMed]
- Asgari, M.; Alderete, N.A.; Lin, Z.; Benavides, R.; Espinosa, H.D. A matter of size? Material, structural and mechanical strategies for size adaptation in the elytra of Cetoniinae beetles. Acta Biomater. 2021, 122, 236–248. [Google Scholar] [CrossRef] [PubMed]
- True, J.R. Insect melanism: The molecules matter. Trends Ecol. Evol. 2003, 18, 640–647. [Google Scholar] [CrossRef]

| DF | Sum of Square | Mean Square | F Value | p Value | R2 | |
|---|---|---|---|---|---|---|
| Area | 1 | 1077.04 | 1077.04 | 111.36 | 2.0 × 10−16 | 27.7% |
| Temperature | 2 | 63.59 | 31.79 | 3.29 | 0.03878 | 1.6% |
| Residuals | 283 | 2737.10 | 9.67 |
| 24 °C | 27 °C | 30 °C | |
|---|---|---|---|
| Black | 64.98 | 64.94 | 63.22 |
| Yellow | 19 | 19.16 | 20.06 |
| Red | 14.38 | 14.11 | 14.92 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bogusch, P.; Petřík, O.; Hlaváček, A.; Šebesta, O.; Šípek, P. Thermal Melanism in Pachnoda iskuulka (Coleoptera: Scarabaeidae: Cetoniinae). Insects 2025, 16, 61. https://doi.org/10.3390/insects16010061
Bogusch P, Petřík O, Hlaváček A, Šebesta O, Šípek P. Thermal Melanism in Pachnoda iskuulka (Coleoptera: Scarabaeidae: Cetoniinae). Insects. 2025; 16(1):61. https://doi.org/10.3390/insects16010061
Chicago/Turabian StyleBogusch, Petr, Oto Petřík, Antonín Hlaváček, Ondřej Šebesta, and Petr Šípek. 2025. "Thermal Melanism in Pachnoda iskuulka (Coleoptera: Scarabaeidae: Cetoniinae)" Insects 16, no. 1: 61. https://doi.org/10.3390/insects16010061
APA StyleBogusch, P., Petřík, O., Hlaváček, A., Šebesta, O., & Šípek, P. (2025). Thermal Melanism in Pachnoda iskuulka (Coleoptera: Scarabaeidae: Cetoniinae). Insects, 16(1), 61. https://doi.org/10.3390/insects16010061






