Spotted Lanternflies Respond to Natural Pheromone Lures for Mate-Finding and Oviposition
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
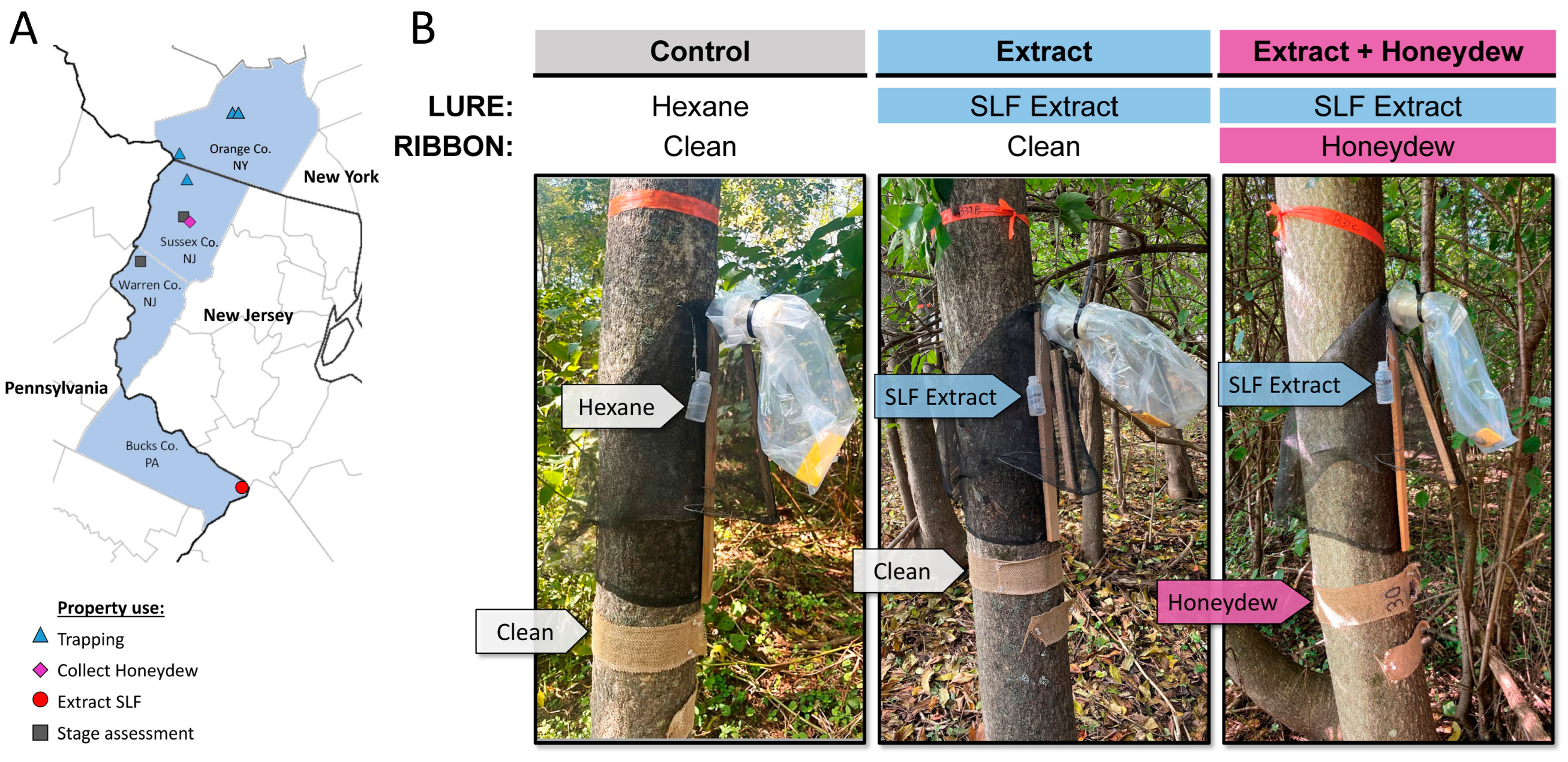
2.1. Field Sites and Experimental Design
2.2. Burlap Ribbons Laden with Crude Honeydew
2.3. Extract Diffusers
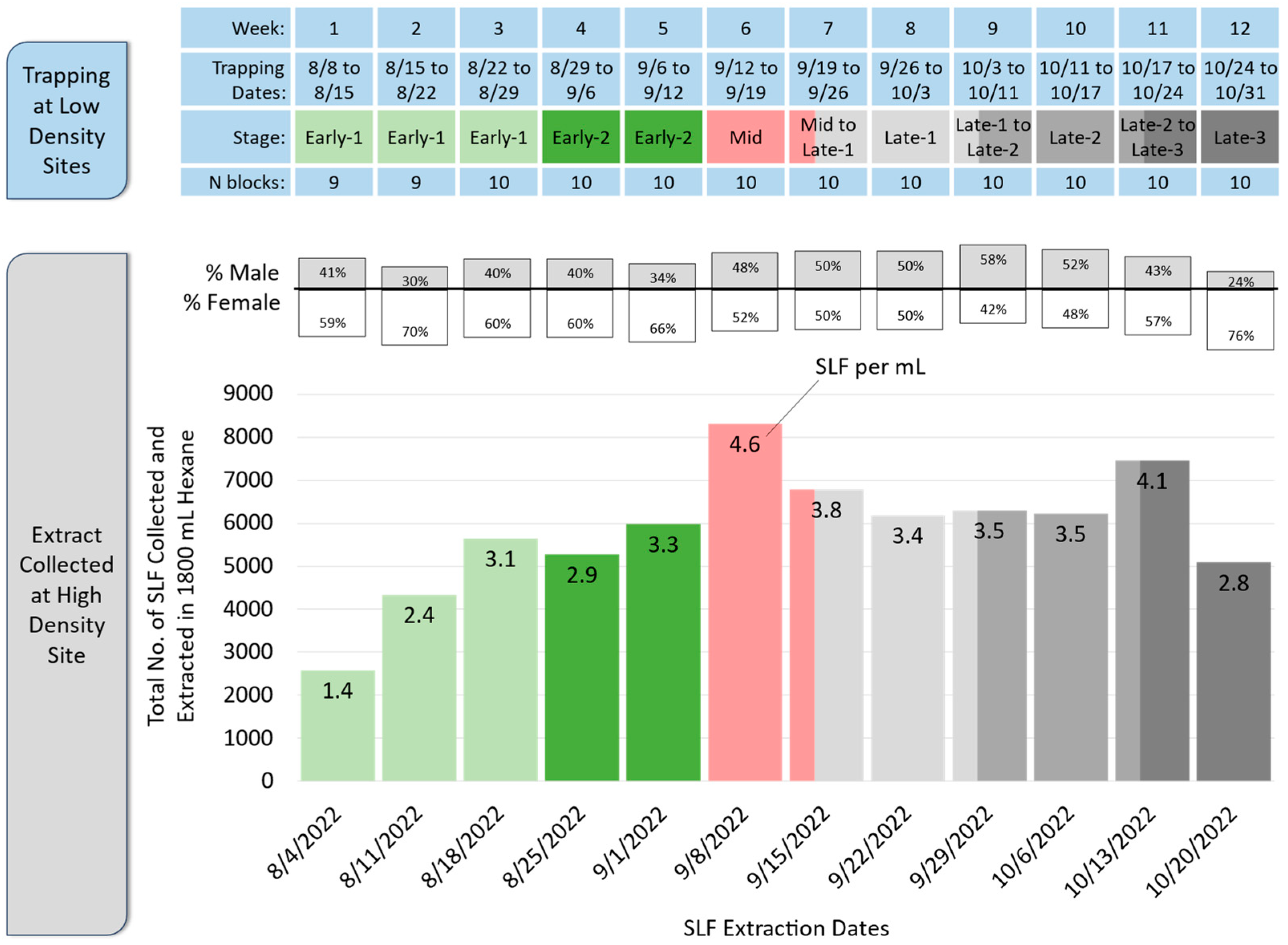
2.4. SLF Whole-Body Extract
2.5. Statistical Analysis
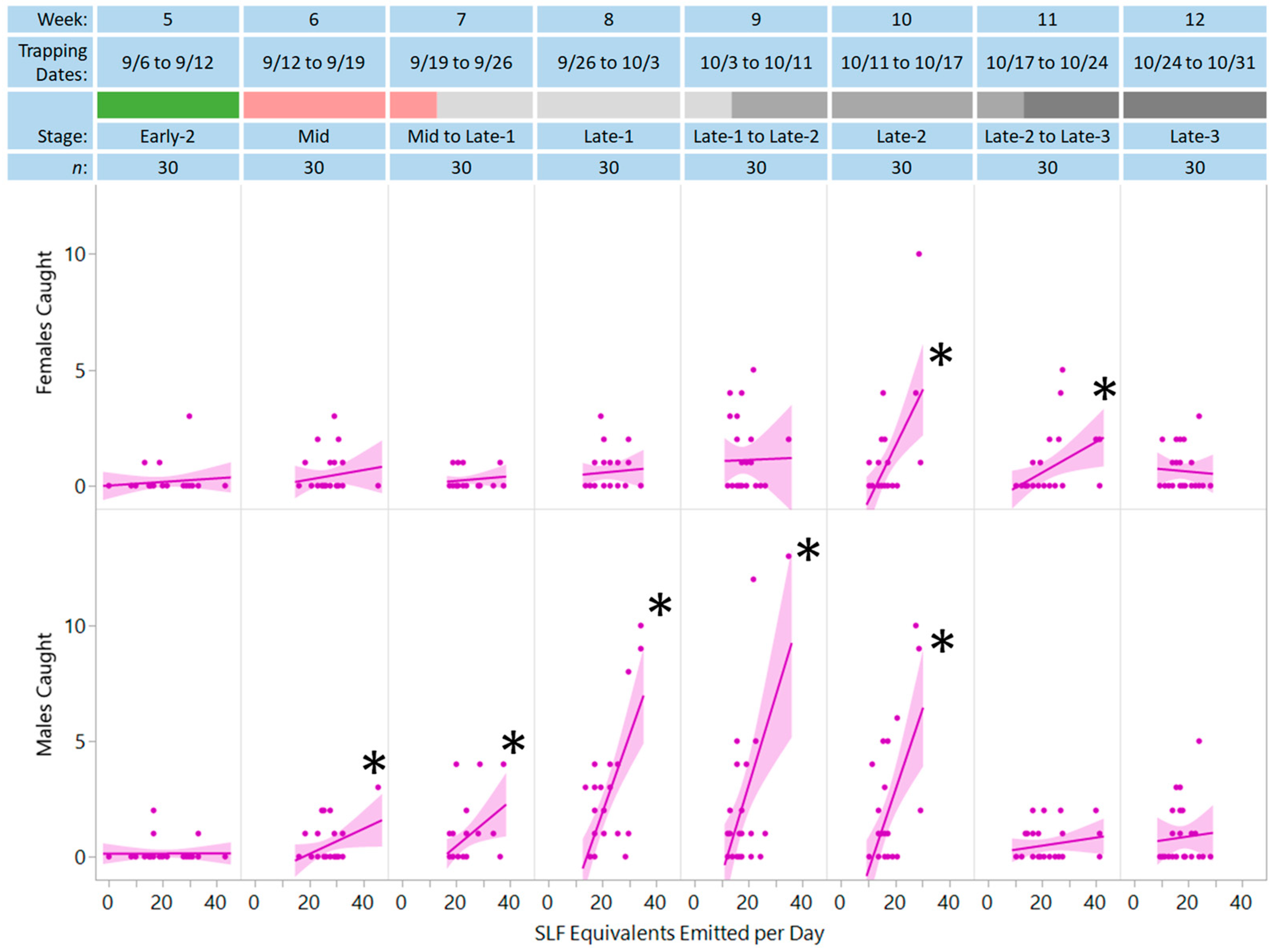
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Harner, A.D.; Leach, H.L.; Briggs, L.; Centinari, M. Prolonged phloem feeding by the spotted lanternfly, an invasive planthopper, alters resource allocation and inhibits gas exchange in grapevines. Plant Direct 2022, 6, e452. [Google Scholar] [CrossRef]
- Barringer, L.; Ciafré, C.M. Worldwide Feeding Host Plants of Spotted Lanternfly, with Significant Additions from North America. Environ. Entomol. 2020, 49, 999–1011. [Google Scholar] [CrossRef]
- Song, S.; Kim, S.; Kwon, S.W.; Lee, S.-I.; Jablonski, P.G. Defense sequestration associated with narrowing of diet and ontogenetic change to aposematic colours in the spotted lanternfly. Sci. Rep. 2018, 8, 16831. [Google Scholar] [CrossRef]
- Cooperband, M.F.; Murman, K. Responses of adult spotted lanternflies to artificial aggregations composed of all males or females. Front. Insect Sci. 2022, 2, 981832. [Google Scholar] [CrossRef]
- Cooperband, M.; Wickham, J.; Warden, M. Factors guiding the orientation of nymphal spotted lanternfly, Lycorma delicatula. Insects 2023, 14, 279. [Google Scholar] [CrossRef]
- Urban, J.M. Perspective: Shedding light on spotted lanternfly impacts in the USA. Pest Manag. Sci. 2020, 76, 10–17. [Google Scholar] [CrossRef]
- Cooperband, M.F.; Wickham, J.; Cleary, K.; Spichiger, S.-E.; Zhang, L.; Baker, J.; Canlas, I.; Derstine, N.; Carrillo, D. Discovery of three kairomones in relation to trap and lure development for spotted lanternfly (Hemiptera: Fulgoridae). J. Econ. Entomol. 2019, 112, 671–682. [Google Scholar] [CrossRef]
- Derstine, N.T.; Meier, L.; Canlas, I.; Murman, K.; Cannon, S.; Carrillo, D.; Wallace, M.; Cooperband, M.F. Plant volatiles help mediate host plant selection and attraction of the spotted lanternfly, Lycorma delicatula (Hemiptera: Fulgoridae): A generalist with a preferred host. Environ. Entomol. 2020, 49, 1049–1062. [Google Scholar] [CrossRef]
- Moon, S.-R.; Cho, S.-R.; Jeong, J.-W.; Shin, Y.-H.; Yang, J.-O.; Ahn, K.-S.; Yoon, C.; Kim, G.-H. Attraction response of spot clothing wax cicada, Lycorma delicatula (Hemiptera: Fulgoridae) to spearment oil. J. Korean Soc. Appl. Biol. Chem. 2011, 54, 558–567. [Google Scholar] [CrossRef]
- Yoon, C.; Moon, S.-R.; Jeong, J.-W.; Shin, Y.-H.; Cho, S.-R.; Ahn, K.-S.; Yang, J.-O.; Kim, G.-H. Repellency of lavender oil and linalool against spot clothing wax cicada, Lycorma delicatula (Hemiptera: Fulgoridae) and their electrophysiological responses. J. Asia-Pac. Entomol. 2011, 14, 411–416. [Google Scholar] [CrossRef]
- Faal, H.; Meier, L.R.; Canlas, I.J.; Murman, K.; Wallace, M.S.; Carrillo, D.; Cooperband, M.F. Volatiles from male honeydew excretions attract conspecific male spotted lanternflies, Lycorma delicatula (Hemiptera: Fulgoridae). Front. Insect Sci. 2022, 2, 982965. [Google Scholar] [CrossRef] [PubMed]
- Faal, H.; Canlas, I.; Carrillo, D.; Cooperband, M.F. Evidence of pheromone use in a fulgorid, spotted lanternfly. Forests 2022, 13, 1639. [Google Scholar] [CrossRef]
- Faal, H.; Canlas, I.J.; Cossé, A.; Jones, T.H.; Carrillo, D.; Cooperband, M.F. Investigating photodegredation of spotted lanternfly body volatiles as a potential pheromone synthesis pathway. Insects 2023, 14, 551. [Google Scholar] [CrossRef] [PubMed]
- Faal, H.; Cooperband, M.F. Antennal sensitivity of spotted lanternflies, Lycorma delicatula: Differential electrophysiological responses of males and females to compounds derived from host plants and conspecifics. Insects 2024, 15, 162. [Google Scholar] [CrossRef] [PubMed]
- Rohde, B.B.; Cooperband, M.F.; Canlas, I.; Mankin, R.W. Evidence of receptivity to vibroacoustic stimuli in the spotted lanternfly. J. Econ. Entomol. 2022, 115, 2116–2120. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.-R.; Liu, J.-J.; Li, X.-Y.; Liang, A.-P.; Bourgoin, T. Relating antennal sensilla diversity and possible species behaviour in the planthopper pest Lycorma delicatula (Hemiptera: Fulgoromorpha: Fulgoridae). PLoS ONE 2018, 13, e0194995. [Google Scholar] [CrossRef] [PubMed]
- Dweck, H.K.M.; Rutledge, C.E. The subapical labial sensory organ of spotted lanternfly Lycorma delicatula. Open Biol. 2024, 14, 230438. [Google Scholar] [CrossRef] [PubMed]
- Hao, Y.; Dietrich, C.H.; Dai, W. Structure and Sensilla of the Mouthparts of the Spotted Lanternfly Lycorma delicatula (Hemiptera: Fulgoromorpha: Fulgoridae), a Polyphagous Invasive Planthopper. PLoS ONE 2016, 11, e0156640. [Google Scholar] [CrossRef] [PubMed]
- Francese, J.A.; Cooperband, M.F.; Murman, K.M.; Cannon, S.L.; Booth, E.G.; Devine, S.M.; Wallace, M.S. Developing traps for the spotted lanternfly, Lycorma delicatula (Hemiptera: Fulgoridae). Environ. Entomol. 2020, 49, 269–276. [Google Scholar] [CrossRef]
- Francese, J.A.; Cooperband, M.F.; Booth, E.G.; Devine, S.M.; Murman, K.M.; Cannon, S.L.; Wallace, M.S. Developing traps for the spotted lanternfly. In Proceedings of the 30th USDA Interagency Research Forum on Invasive Species, Annapolis, MD, USA, 14–17 January 2020; pp. 18–19. [Google Scholar]
- Nixon, L.J.; Leach, H.; Barnes, C.; Urban, J.; Kirkpatrick, D.M.; Ludwick, D.C.; Short, B.; Pfeiffer, D.G.; Leskey, T.C. Development of behaviorally based monitoring and biosurveillance tools for the invasive spotted lanternfly (Hemiptera: Fulgoridae). Environ. Entomol. 2020, 49, 1117–1126. [Google Scholar] [CrossRef]
- Siderhurst, M.S.; Murman, K.M.; Kaye, K.T.; Wallace, M.S.; Cooperband, M.F. Radio telemetry and harmonic radar tracking of the spotted lanternfly, Lycorma delicatula (White) (Hemiptera: Fulgoridae). Insects 2024, 15, 17. [Google Scholar] [CrossRef] [PubMed]
- Aho, K.; Derryberry, D.; Peterson, T. Model selection for ecologists: The worldviews of AIC and BIC. Ecology 2014, 95, 631–636. [Google Scholar] [CrossRef] [PubMed]
- Akaike, H. Information theory and an extension of the maximum likelihood principle. In Selected Papers of Hirotugu Akaike; Springer: Berlin/Heidelberg, Germany, 1998; pp. 199–213. [Google Scholar]
- Rai, M.; Hassanali, A.; Saini, R.; Odongo, H.; Kahoro, H. Identification of components of the oviposition aggregation pheromone of the gregarious desert locust, Schistocerca gregaria (Forskal). J. Insect Physiol. 1997, 43, 83–87. [Google Scholar] [CrossRef] [PubMed]
- McCall, P. Oviposition aggregation pheromone in the Simulium damnosum complex. Med. Vet. Entomol. 1995, 9, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Ha, N.; Challita, E.; Harrison, J.; Clark, E.; Cooperband, M.; Bhamla, S. Superfast excretion of viscous particle-laden droplets in phloem feeding insects. Bull. Am. Phys. Soc. 2024. [Google Scholar]
- Challita, E.J.; Sehgal, P.; Krugner, R.; Bhamla, M.S. Droplet superpropulsion in an energetically constrained insect. Nat. Commun. 2023, 14, 860. [Google Scholar] [CrossRef] [PubMed]
- Reddy, G.V.; Guerrero, A. Interactions of insect pheromones and plant semiochemicals. Trends Plant Sci. 2004, 9, 253–261. [Google Scholar] [CrossRef]
- Lewis, P.; Davila-Flores, A.; Wallis, E. An effective trap for spotted lanternfly egg masses. Front. Insect Sci. 2023, 3, 24. [Google Scholar] [CrossRef] [PubMed]
- Hung, K.Y.; Michailides, T.J.; Millar, J.G.; Wayadande, A.; Gerry, A.C. House fly (Musca domestica L.) attraction to insect honeydew. PLoS ONE 2015, 10, e0124746. [Google Scholar] [CrossRef]
- Peach, D.A.; Gries, R.; Young, N.; Lakes, R.; Galloway, E.; Alamsetti, S.K.; Ko, E.; Ly, A.; Gries, G. Attraction of female Aedes aegypti (L.) to aphid honeydew. Insects 2019, 10, 43. [Google Scholar] [CrossRef]
- Meiners, J.M.; Griswold, T.L.; Harris, D.J.; Ernest, S.M. Bees without flowers: Before peak bloom, diverse native bees find insect-produced honeydew sugars. Am. Nat. 2017, 190, 281–291. [Google Scholar] [CrossRef]
- Brown, R.L.; El-Sayed, A.M.; Unelius, C.R.; Beggs, J.R.; Suckling, D.M. Invasive Vespula wasps utilize kairomones to exploit honeydew produced by sooty scale insects, Ultracoelostoma. J. Chem. Ecol. 2015, 41, 1018–1027. [Google Scholar] [CrossRef]
- Tena, A.; Wäckers, F.L.; Heimpel, G.E.; Urbaneja, A.; Pekas, A. Parasitoid nutritional ecology in a community context: The importance of honeydew and implications for biological control. Curr. Opin. Insect Sci. 2016, 14, 100–104. [Google Scholar] [CrossRef]
- Nelson, A.S.; Mooney, K.A. The evolution and ecology of interactions between ants and honeydew-producing hemipteran insects. Annu. Rev. Ecol. Evol. Syst. 2022, 53, 379–402. [Google Scholar] [CrossRef]
- Peñalver-Cruz, A.; Satour, P.; Jaloux, B.; Lavandero, B. Honeydew Is a Food Source and a Contact Kairomone for Aphelinus mali. Insects 2023, 14, 426. [Google Scholar] [CrossRef]
- Hågvar, E.; Hofsvang, T. Effect of honeydew and hosts on plant colonization by the aphid parasitoid Ephedrus cerasicola. Entomophaga 1989, 34, 495–501. [Google Scholar] [CrossRef]
- Shaltiel, L.; Ayal, Y. The use of kairomones for foraging decisions by an aphid parasitoid in small host aggregations. Ecol. Entomol. 1998, 23, 319–329. [Google Scholar] [CrossRef]
- Choi, M.Y.; Roitberg, B.D.; Shani, A.; Raworth, D.A.; Lee, G.H. Olfactory response by the aphidophagous gall midge, Aphidoletes aphidimyza to honeydew from green peach aphid, Myzus persicae. Entomol. Exp. Appl. 2004, 111, 37–45. [Google Scholar] [CrossRef]
- Baoyu, H.; Chengsong, Z. Rhythm of honeydew excretion by the tea aphid and its attraction to various natural enemies. Acta Ecologica Sinica 2007, 27, 3637–3643. [Google Scholar] [CrossRef]
- Leroy, P.D.; Heuskin, S.; Sabri, A.; Verheggen, F.J.; Farmakidis, J.; Lognay, G.; Thonart, P.; Wathelet, J.P.; Brostaux, Y.; Haubruge, E. Honeydew volatile emission acts as a kairomonal message for the Asian lady beetle Harmonia axyridis (Coleoptera: Coccinellidae). Insect Sci. 2012, 19, 498–506. [Google Scholar] [CrossRef]
- Ide, T.; Suzuki, N.; Katayama, N. The use of honeydew in foraging for aphids by larvae of the ladybird beetle, Coccinella septempunctata L.(Coleoptera: Coccinellidae). Ecol. Entomol. 2007, 32, 455–460. [Google Scholar] [CrossRef]
- Fand, B.B.; Amala, U.; Yadav, D.; Rathi, G.; Mhaske, S.; Upadhyay, A.; Ahammed Shabeer, T.; Kumbhar, D. Bacterial volatiles from mealybug honeydew exhibit kairomonal activity toward solitary endoparasitoid Anagyrus dactylopii. J. Pest Sci. 2020, 93, 195–206. [Google Scholar] [CrossRef]
- Liu, J.; Xiao, D.; Liu, Y.; Zhan, Y.; Francis, F.; Liu, Y. Chemical cues from honeydew-associated bacteria to enhance parasitism efficacy: From laboratory to field assay. J. Pest Sci. 2024, 97, 873–884. [Google Scholar] [CrossRef]
- Moghbeli Gharaei, A.; Ziaaddini, M.; Jalali, M.; Michaud, J. Sex-specific responses of Asian citrus psyllid to volatiles of conspecific and host-plant origin. J. Appl. Entomol. 2014, 138, 500–509. [Google Scholar] [CrossRef]
- Sevarika, M.; Rondoni, G.; Ganassi, S.; Pistillo, O.M.; Germinara, G.S.; De Cristofaro, A.; Romani, R.; Conti, E. Behavioural and electrophysiological responses of Philaenus spumarius to odours from conspecifics. Sci. Rep. 2022, 12, 8402. [Google Scholar] [CrossRef]
- Chen, X.; Liang, A.-P. Identification of a self-regulatory pheromone system that controls nymph aggregation behavior of rice spittlebug Callitettix versicolor. Front. Zool. 2015, 12, 10. [Google Scholar] [CrossRef]
| Week | Trapping Date Range | Stage | Treatment | N | Female SLF Caught | SE | Slope | R2 | F | d.f. | p-Value |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 8/8 to 8/15 | Early-1 | Control | 9 | 0.22 | 0.222 | 0.139 | 0.108 | 0.85 | 1, 7 | 0.388 |
| Extract | 9 | 0 | 0 | - | - | - | 1, 7 | - | |||
| Extract + Honeydew | 9 | 0.11 | 0.111 | −0.018 | 0.040 | 0.29 | 1, 7 | 0.608 | |||
| 2 | 8/15 to 8/22 | Early-1 | Control | 9 | 0.33 | 0.167 | 0 | 0 | - | 0, 8 | - |
| Extract | 9 | 0 | 0 | 0 | 0 | - | 1, 7 | - | |||
| Extract + Honeydew | 9 | 0 | 0 | 0 | - | - | 1, 7 | - | |||
| 3 | 8/22 to 8/29 | Early-1 | Control | 30 | 0.03 | 0.033 | −0.005 | 0.078 | 2.35 | 1, 28 | 0.136 |
| Extract | 30 | 0.03 | 0.033 | 0.005 | 0.053 | 1.56 | 1, 28 | 0.222 | |||
| Extract + Honeydew | 30 | 0 | 0 | 0 | - | - | 1, 28 | - | |||
| 4 | 8/29 to 9/6 | Early-2 | Control | 30 | 0.17 | 0.084 | 0.013 | 0.083 | 2.52 | 1, 28 | 0.124 |
| Extract | 30 | 0.10 | 0.056 | −0.004 | 0.022 | 0.64 | 1, 28 | 0.432 | |||
| Extract + Honeydew | 30 | 0.13 | 0.063 | 0.003 | 0.006 | 0.17 | 1, 28 | 0.680 | |||
| 5 | 9/6 to 9/12 | Early-2 | Control | 30 | 0.07 | 0.067 | 0.010 | 0.060 | 1.76 | 1, 28 | 0.196 |
| Extract | 30 | 0.17 | 0.084 | 0.007 | 0.012 | 0.33 | 1, 28 | 0.568 | |||
| Extract + Honeydew | 30 | 0.17 | 0.108 | 0.008 | 0.012 | 0.33 | 1, 28 | 0.568 | |||
| 6 | 9/12 to 9/19 | Mid | Control | 30 | 0.23 | 0.092 | 0.002 | 0.001 | 0.02 | 1, 28 | 0.895 |
| Extract | 30 | 0.20 | 0.088 | −0.011 | 0.014 | 0.39 | 1, 28 | 0.538 | |||
| Extract + Honeydew | 30 | 0.40 | 0.141 | 0.020 | 0.021 | 0.60 | 1, 28 | 0.447 | |||
| 7 | 9/19 to 9/26 | Mid-to-Late-1 | Control | 30 | 0.40 | 0.113 | 0.020 | 0.070 | 2.11 | 1, 28 | 0.157 |
| Extract | 30 | 0.37 | 0.131 | −0.040 | 0.111 | 3.52 | 1, 28 | 0.071 | |||
| Extract + Honeydew | 30 | 0.23 | 0.079 | 0.001 | 0.015 | 0.43 | 1, 28 | 0.518 | |||
| 8 | 9/26 to 10/3 | Late-1 | Control | 30 | 0.30 | 0.119 | 0.007 | 0.003 | 0.08 | 1, 28 | 0.778 |
| Extract | 30 | 0.40 | 0.132 | −0.009 | 0.004 | 0.11 | 1, 28 | 0.745 | |||
| Extract + Honeydew | 30 | 0.57 | 0.141 | 0.011 | 0.007 | 0.19 | 1, 28 | 0.667 | |||
| 9 | 10/3 to 10/11 | Late-1-to-Late-2 | Control | 30 | 0.70 | 0.145 | −0.051 | 0.065 | 1.96 | 1, 28 | 0.172 |
| Extract | 30 | 0.50 | 0.115 | −0.016 | 0.012 | 0.33 | 1, 28 | 0.572 | |||
| Extract + Honeydew | 30 | 1.10 | 0.277 | 0.005 | 0.000 | 0.01 | 1, 28 | 0.931 | |||
| 10 | 10/11 to 10/17 | Late-2 | Control | 30 | 0.63 | 0.282 | 0.097 | 0.093 | 2.88 | 1, 28 | 0.101 |
| Extract | 30 | 0.70 | 0.160 | −0.032 | 0.017 | 0.49 | 1, 28 | 0.489 | |||
| Extract + Honeydew | 30 | 0.93 | 0.371 | 0.239 | 0.307 | 12.43 | 1, 28 | 0.002 * | |||
| 11 | 10/17 to 10/24 | Late-2-to-Late-3 | Control | 30 | 0.70 | 0.268 | 0.045 | 0.027 | 0.79 | 1, 28 | 0.382 |
| Extract | 30 | 0.80 | 0.246 | 0.006 | 0.001 | 0.04 | 1, 28 | 0.839 | |||
| Extract + Honeydew | 30 | 0.70 | 0.232 | 0.066 | 0.180 | 6.15 | 1, 28 | 0.019 * | |||
| 12 | 10/24 to 10/31 | Late-3 | Control | 30 | 1.77 | 0.660 | 0.280 | 0.063 | 1.89 | 1, 28 | 0.181 |
| Extract | 30 | 1.63 | 0.344 | −0.100 | 0.088 | 2.71 | 1, 28 | 0.005 * | |||
| Extract + Honeydew | 30 | 0.63 | 0.162 | −0.010 | 0.004 | 0.11 | 1, 28 | 0.749 |
| Week | Trapping Date Range | Stage | Treatment | N | Male SLF Caught | SE | Slope | R2 | F | d.f. | p-Value |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 8/8 to 8/15 | Early-1 | Control | 9 | 0.11 | 0.111 | 0.070 | 0.108 | 0.85 | 1, 7 | 0.388 |
| Extract | 9 | 0.11 | 0.111 | −0.022 | 0.067 | 0.50 | 1, 7 | 0.502 | |||
| Extract + Honeydew | 9 | 0 | 0 | 0 | - | - | 1, 7 | - | |||
| 2 | 8/15 to 8/22 | Early-1 | Control | 9 | 0 | 0 | 0 | - | - | 0, 8 | - |
| Extract | 9 | 0.22 | 0.147 | 0.061 | 0.139 | 1.13 | 1, 7 | 0.324 | |||
| Extract + Honeydew | 9 | 0 | 0 | 0 | - | - | 1, 7 | - | |||
| 3 | 8/22 to 8/29 | Early-1 | Control | 30 | 0.10 | 0.056 | −0.002 | 0.006 | 0.17 | 1, 28 | 0.682 |
| Extract | 30 | 0 | 0 | 0 | - | - | 1, 28 | - | |||
| Extract + Honeydew | 30 | 0.03 | 0.033 | −0.006 | 0.029 | 0.83 | 1, 28 | 0.369 | |||
| 4 | 8/29 to 9/6 | Early-2 | Control | 30 | 0.07 | 0.046 | 0.004 | 0.021 | 0.61 | 1, 28 | 0.442 |
| Extract | 30 | 0 | 0 | 0 | - | - | 1, 28 | - | |||
| Extract + Honeydew | 30 | 0.03 | 0.033 | −0.004 | 0.033 | 0.94 | 1, 28 | 0.340 | |||
| 5 | 9/6 to 9/12 | Early-2 | Control | 30 | 0.10 | 0.056 | 0.000 | 0.000 | 0.00 | 1, 28 | 0.991 |
| Extract | 30 | 0.13 | 0.080 | 0.003 | 0.003 | 0.08 | 1, 28 | 0.786 | |||
| Extract + Honeydew | 30 | 0.13 | 0.080 | 0.001 | 0.000 | 0.01 | 1, 28 | 0.917 | |||
| 6 | 9/12 to 9/19 | Mid | Control | 30 | 0.63 | 0.256 | 0.000 | 0.000 | 0.00 | 1, 28 | 0.952 |
| Extract | 30 | 0.13 | 0.079 | 0.002 | 0.000 | 0.01 | 1, 28 | 0.917 | |||
| Extract + Honeydew | 30 | 0.46 | 0.150 | 0.054 | 0.131 | 4.23 | 1, 28 | 0.049 * | |||
| 7 | 9/19 to 9/26 | Mid-to-Late-1 | Control | 30 | 0.87 | 0.234 | 0.015 | 0.010 | 0.27 | 1, 28 | 0.608 |
| Extract | 30 | 0.87 | 0.248 | −0.045 | 0.039 | 1.14 | 1, 28 | 0.294 | |||
| Extract + Honeydew | 30 | 0.70 | 0.226 | 0.097 | 0.174 | 5.91 | 1, 28 | 0.022 * | |||
| 8 | 9/26 to 10/3 | Late-1 | Control | 30 | 1.60 | 0.433 | 0.136 | 0.094 | 2.89 | 1, 28 | 0.100 |
| Extract | 30 | 1.07 | 0.299 | 0.035 | 0.012 | 0.34 | 1, 28 | 0.565 | |||
| Extract + Honeydew | 30 | 2.10 | 0.497 | 0.331 | 0.485 | 26.40 | 1, 28 | <0.001 * | |||
| 9 | 10/3 to 10/11 | Late-1-to-Late-2 | Control | 30 | 2.30 | 0.484 | −0.087 | 0.017 | 0.49 | 1, 28 | 0.493 |
| Extract | 30 | 0.90 | 0.188 | −0.104 | 0.184 | 6.31 | 1, 28 | 0.018 * | |||
| Extract + Honeydew | 30 | 2.20 | 0.598 | 0.385 | 0.322 | 13.31 | 1, 28 | 0.001 * | |||
| 10 | 10/11 to 10/17 | Late-2 | Control | 30 | 0.90 | 0.218 | −0.025 | 0.010 | 0.30 | 1, 28 | 0.591 |
| Extract | 30 | 1.80 | 0.390 | 0.174 | 0.087 | 2.67 | 1, 28 | 0.114 | |||
| Extract + Honeydew | 30 | 1.70 | 0.500 | 0.349 | 0.365 | 16.09 | 1, 28 | <0.001 * | |||
| 11 | 10/17 to 10/24 | Late-2-to-Late-3 | Control | 30 | 0.57 | 0.196 | 0.001 | 0.002 | 0.06 | 1, 28 | 0.806 |
| Extract | 30 | 0.73 | 0.283 | 0.015 | 0.007 | 0.206 | 1, 28 | 0.654 | |||
| Extract + Honeydew | 30 | 0.50 | 0.133 | 0.017 | 0.038 | 1.11 | 1, 28 | 0.301 | |||
| 12 | 10/24 to 10/31 | Late-3 | Control | 30 | 1.90 | 0.700 | 0.265 | 0.050 | 1.48 | 1, 28 | 0.233 |
| Extract | 30 | 1.20 | 0.240 | −0.104 | 0.194 | 6.77 | 1, 28 | 0.015 * | |||
| Extract + Honeydew | 30 | 0.83 | 0.235 | 0.017 | 0.005 | 0.13 | 1, 28 | 0.719 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cooperband, M.F.; Murman, K.M. Spotted Lanternflies Respond to Natural Pheromone Lures for Mate-Finding and Oviposition. Insects 2024, 15, 447. https://doi.org/10.3390/insects15060447
Cooperband MF, Murman KM. Spotted Lanternflies Respond to Natural Pheromone Lures for Mate-Finding and Oviposition. Insects. 2024; 15(6):447. https://doi.org/10.3390/insects15060447
Chicago/Turabian StyleCooperband, Miriam F., and Kelly M. Murman. 2024. "Spotted Lanternflies Respond to Natural Pheromone Lures for Mate-Finding and Oviposition" Insects 15, no. 6: 447. https://doi.org/10.3390/insects15060447
APA StyleCooperband, M. F., & Murman, K. M. (2024). Spotted Lanternflies Respond to Natural Pheromone Lures for Mate-Finding and Oviposition. Insects, 15(6), 447. https://doi.org/10.3390/insects15060447






